Abstract
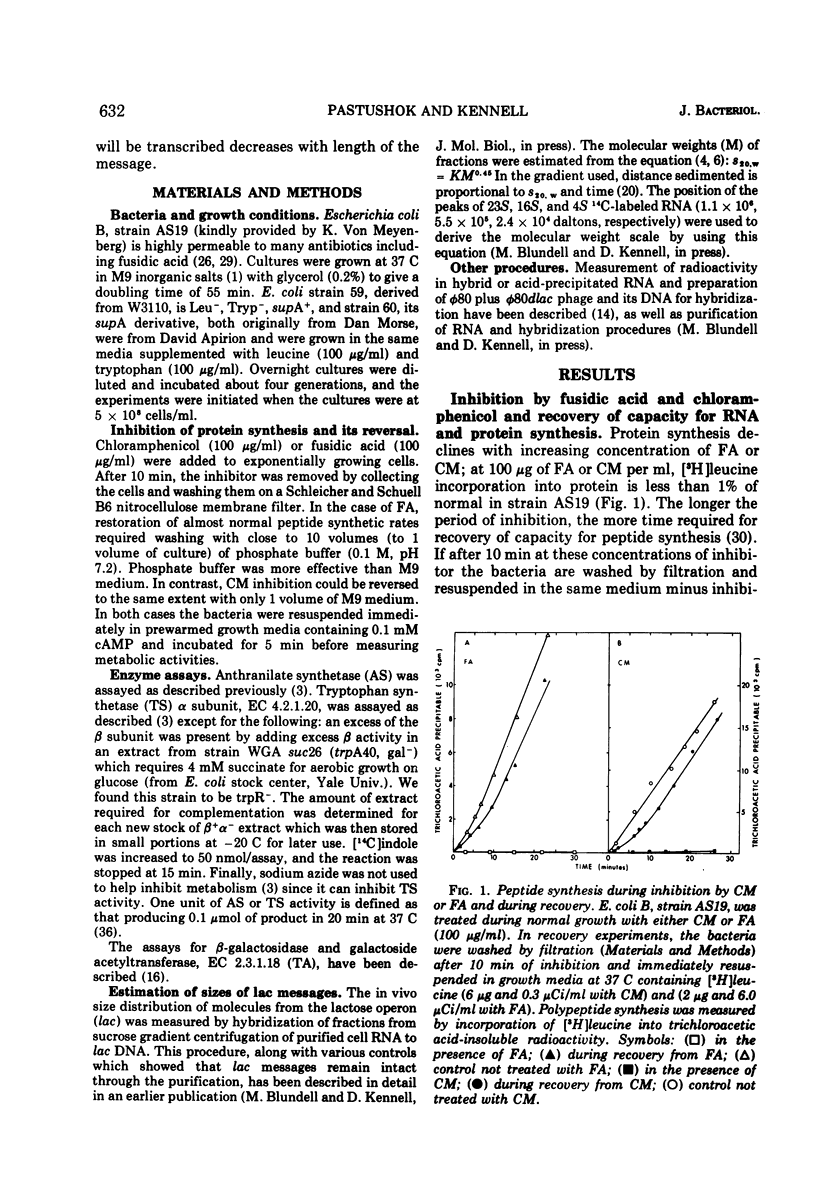
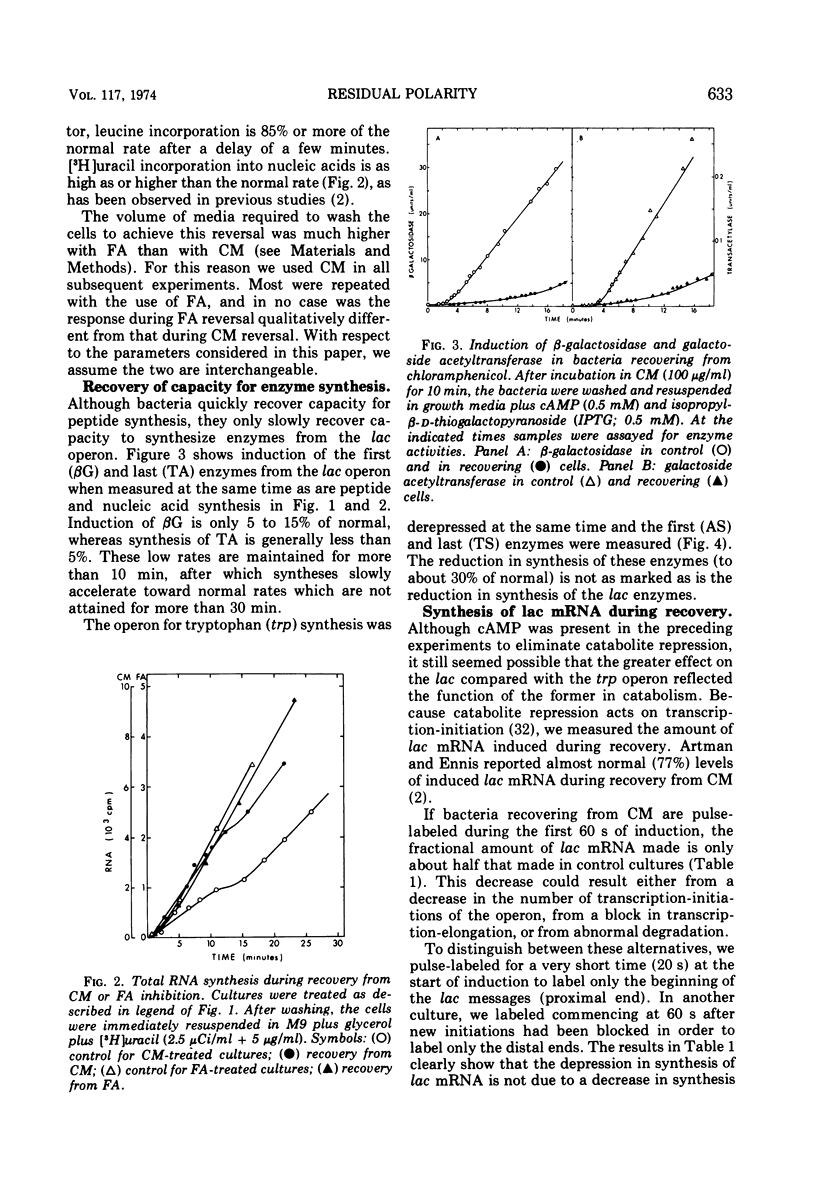
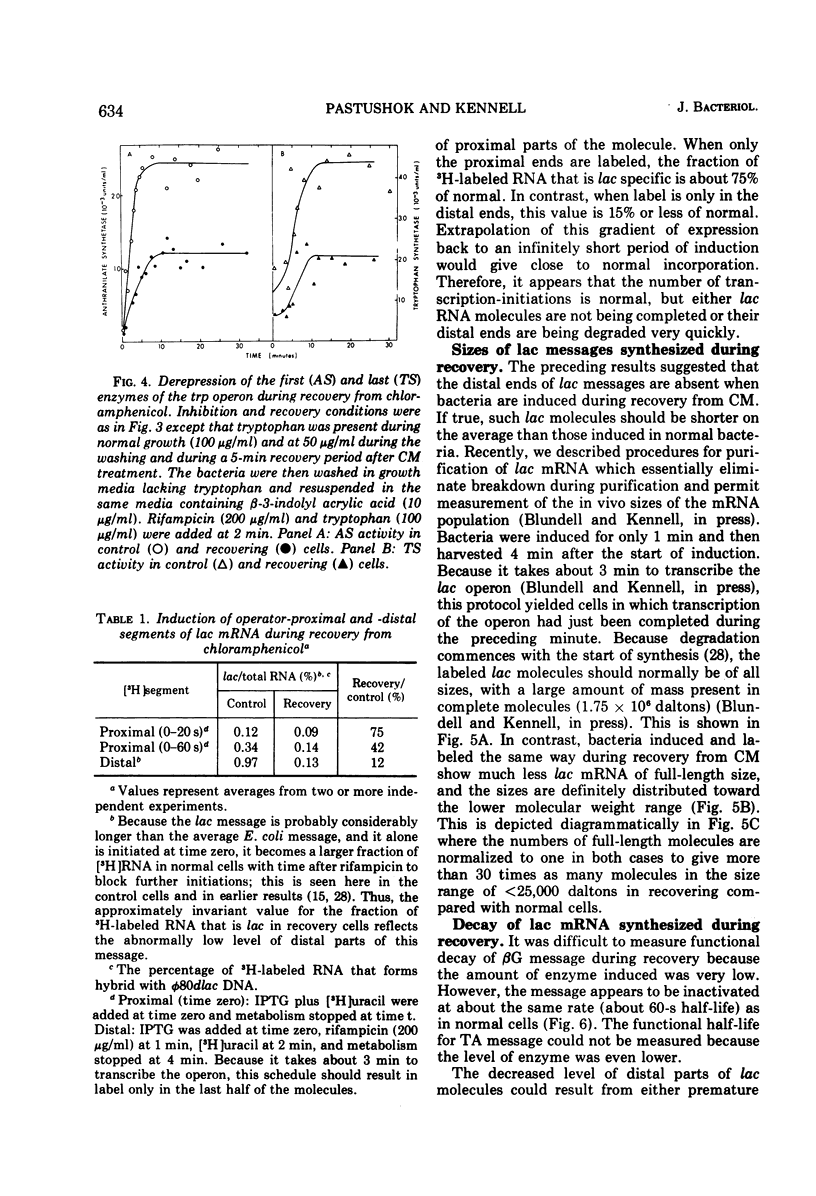
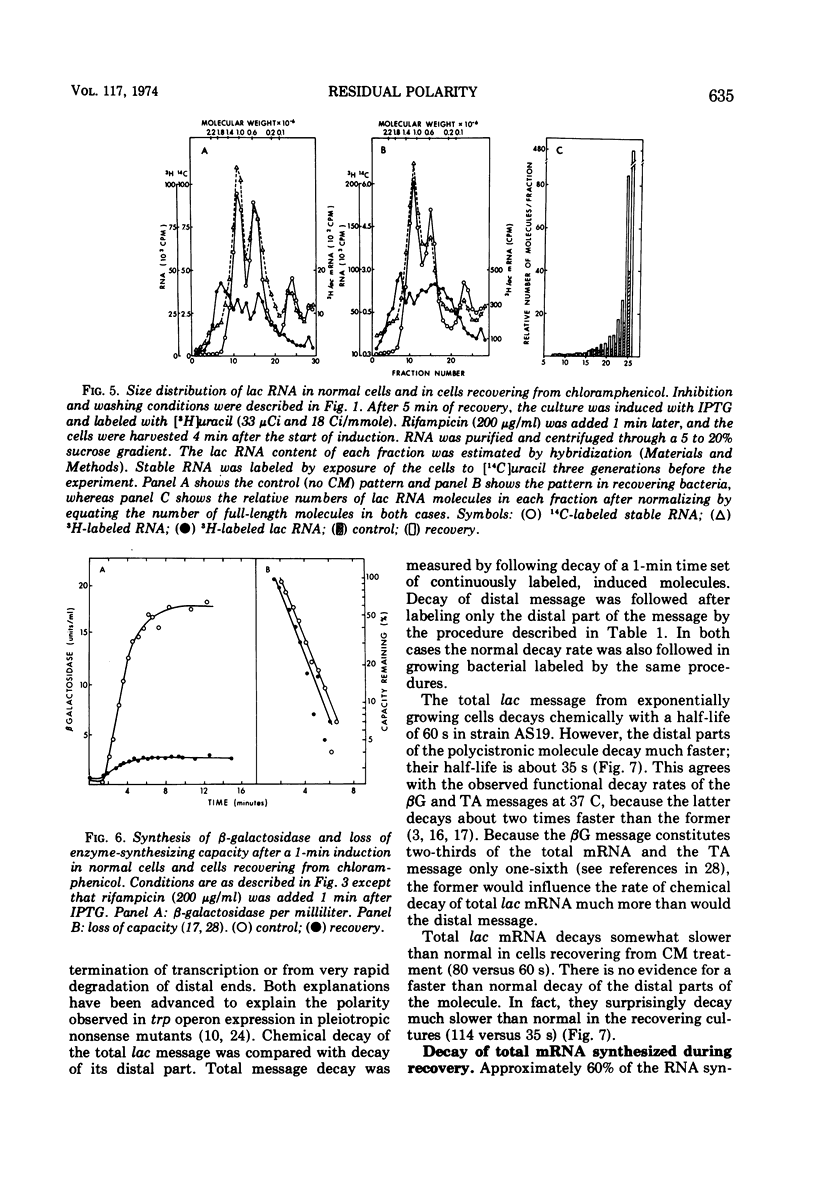
Fusidic acid or chloramphenicol was used to inhibit peptide synthesis to 1% of normal in Escherichia coli B, strain AS19. After 10 min of inhibition, peptide synthesis could be quickly restored to 80% of the normal rate after washing the bacteria on a filter. However, even in the presence of adenosine 3′-5′-cyclic-monophosphoric acid to block catabolite repression, β-galactosidase, the first enzyme of the lactose operon (lac), could only be induced to 10% of normal, and the last enzyme of the operon, galactoside acetyltransferase, even less. The first and last enzymes of the operon for tryptophan synthesis could be derepressed to about 30% of normal. The lac ribonucleic acid (RNA) induced during recovery showed a smaller than normal size distribution on sucrose gradients. The operator-proximal or -distal parts of this RNA were specifically labeled. Hybridization to φ80dlac deoxyribonucleic acid (DNA) suggested that although the distal parts of the lac RNA were barely detectable, initiation was occurring at normal rates in recovery. Either normal levels of distal messenger RNA (mRNA) are made but then rapidly degraded or the mRNA is not completed. The small amount that is made decayed abnormally slowly, probably as a result of slower transcription. Total mRNA decay was multiphasic with all components decaying slower than normal. We propose that there is a residual level of inhibition of peptide synthesis during recovery. The probability that a ribosome is blocked at any codon can be estimated from the data. The longer the message, the less likely its complete translation. We propose that the RNA polymerase can transcribe translatable mRNA for only a finite distance beyond the lead ribosome. Because ribosomes can load at the start of each message in a polycistronic mRNA, the probability that a distal message will be synthesized and translated is a function of the number of more proximal messages and the distances between their ribosome-loading sites.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artman M., Ennis H. L. Dissociation of Lac messenger ribonucleic acid transcription from translation during recovery from inhibition of protein synthesis. J Bacteriol. 1972 May;110(2):652–660. doi: 10.1128/jb.110.2.652-660.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgo C., Apirion D., Schlessinger D. Effects of chloramphenicol and fusidic acid on polyribosome metabolism in escherichia coli. FEBS Lett. 1969 Apr;3(1):34–36. doi: 10.1016/0014-5793(69)80089-x. [DOI] [PubMed] [Google Scholar]
- Hansen M. T., Bennett P. M., von Meyenburg K. Intracistronic polarity during dissociation of translation from transcription in Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):589–604. doi: 10.1016/0022-2836(73)90225-8. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Yanofsky C. Hyper-labile messenger RNA in polar mutants of the tryptophan operon of Escherichia coli. J Mol Biol. 1972 Dec 14;72(1):103–110. doi: 10.1016/0022-2836(72)90072-1. [DOI] [PubMed] [Google Scholar]
- Imamoto F. Diversity of regulation of genetic transcription. I. Effect of antibiotics which inhibit the process of translation on RNA metabolism in Escherichia coli. J Mol Biol. 1973 Feb 25;74(2):113–136. doi: 10.1016/0022-2836(73)90102-2. [DOI] [PubMed] [Google Scholar]
- Imamoto F. Evidence for premature termination of transcription of the tryptophan operon in polarity mutants of Escherichia coli. Nature. 1970 Oct 17;228(5268):232–235. doi: 10.1038/228232a0. [DOI] [PubMed] [Google Scholar]
- Imamoto F., Tani S. Diversity of regulation of genetic transcription. Nat New Biol. 1972 Dec 6;240(101):172–175. doi: 10.1038/newbio240172a0. [DOI] [PubMed] [Google Scholar]
- Imamoto F., Yanofsky C. Transcription of the tryptophan operon in polarity mutants of Escherichia coli. I. Characterization of the tryptophan messenger RNA of polar mutants. J Mol Biol. 1967 Aug 28;28(1):1–23. doi: 10.1016/s0022-2836(67)80073-1. [DOI] [PubMed] [Google Scholar]
- Kennell D., Bicknell I. Decay of messenger ribonucleic acid from the lactose operon of Escherichia coli as a function of growth temperature. J Mol Biol. 1973 Feb 15;74(1):21–31. doi: 10.1016/0022-2836(73)90351-3. [DOI] [PubMed] [Google Scholar]
- Kennell D. Inhibition of host protein synthesis during infection of Escherichia coli by bacteriophage T4. II. Induction of host messenger ribonucleic acid and its exclusion from polysomes. J Virol. 1970 Aug;6(2):208–217. doi: 10.1128/jvi.6.2.208-217.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D., Simmons C. Synthesis and decay of messenger ribonucleic acid from the lactose operon of Escherichia coli during amino-acid starvation. J Mol Biol. 1972 Oct 14;70(3):451–464. doi: 10.1016/0022-2836(72)90552-9. [DOI] [PubMed] [Google Scholar]
- Kepes A. Sequential transcription and translation in the lactose operon of Escherichia coli. Biochim Biophys Acta. 1967 Mar 29;138(1):107–123. doi: 10.1016/0005-2787(67)90591-6. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Kawano G., Tanaka N. Association of fusidic acid sensitivity with G factor in a protein-synthesizing system. Biochem Biophys Res Commun. 1968 Dec 9;33(5):769–773. doi: 10.1016/0006-291x(68)90226-x. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Morse D. E., Mosteller R. D., Yanofsky C. Dynamics of synthesis, translation, and degradation of trp operon messenger RNA in E. coli. Cold Spring Harb Symp Quant Biol. 1969;34:725–740. doi: 10.1101/sqb.1969.034.01.082. [DOI] [PubMed] [Google Scholar]
- Morse D. E. Polarity induced by chloramphenicol and relief by suA. J Mol Biol. 1971 Jan 14;55(1):113–118. doi: 10.1016/0022-2836(71)90285-3. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. Polarity and the degradation of mRNA. Nature. 1969 Oct 25;224(5217):329–331. doi: 10.1038/224329a0. [DOI] [PubMed] [Google Scholar]
- NATHANS D., von EHRENSTEIN, MONRO R., LIPMANN F. Protein synthesis from aminoacyl-soluble ribonucleic acid. Fed Proc. 1962 Jan-Feb;21:127–133. [PubMed] [Google Scholar]
- Schwartz T., Craig E., Kennell D. Inactivation and degradation of messenger ribnucleic acid from the lactose operon of Escherichia coli. J Mol Biol. 1970 Dec 14;54(2):299–311. doi: 10.1016/0022-2836(70)90431-6. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M., Iida S. Mutants of Escherichia coli permeable to actinomycin. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2315–2320. doi: 10.1073/pnas.58.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone A. E., Magasanik B., Reznikoff W. S., Miller J. H., Beckwith J. R. Catabolite sensitive site of the lac operon. Nature. 1969 Mar 15;221(5185):1012–1014. doi: 10.1038/2211012b0. [DOI] [PubMed] [Google Scholar]
- Soreq H., Kaplan R. Inducible and constitutive -galactosidase formation in cells recovering from protein synthesis inhibition. J Bacteriol. 1971 Dec;108(3):1147–1153. doi: 10.1128/jb.108.3.1147-1153.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szybalski W. Initiation and patterns of transcription during phage development. Proc Can Cancer Conf. 1969;8:183–215. [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac messenger ribonucleic acid synthesis by cyclic adenosine 3',5'-monophosphate and glucose. J Biol Chem. 1970 May 10;245(9):2259–2267. [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac transcription in antibiotic-treated E. coli. Nat New Biol. 1971 Mar 10;230(10):41–44. doi: 10.1038/newbio230041a0. [DOI] [PubMed] [Google Scholar]
- Vazquez D. Binding of chloramphenicol to ribosomes. The effect of a number of antibiotics. Biochim Biophys Acta. 1966 Feb 21;114(2):277–288. doi: 10.1016/0005-2787(66)90309-1. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Ito J. Nonsense codons and polarity in the tryptophan operon. J Mol Biol. 1966 Nov 14;21(2):313–334. doi: 10.1016/0022-2836(66)90102-1. [DOI] [PubMed] [Google Scholar]


