Abstract
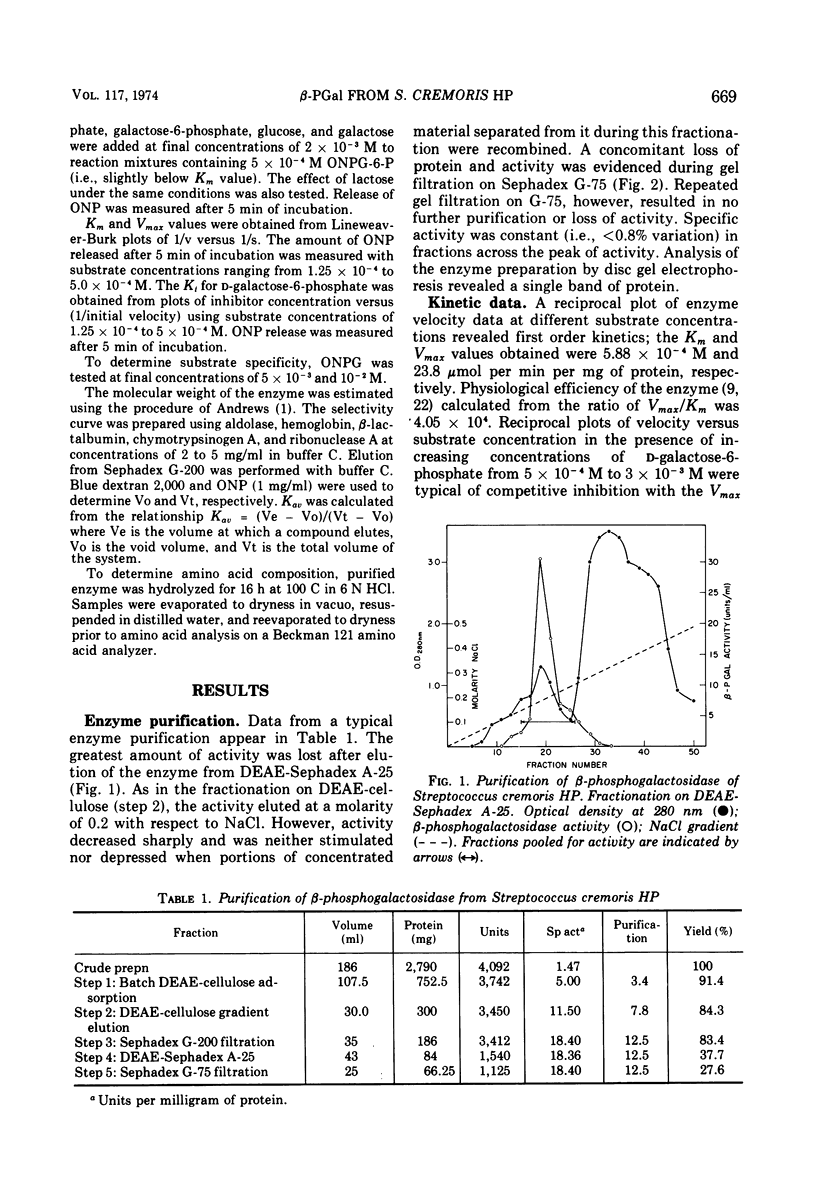
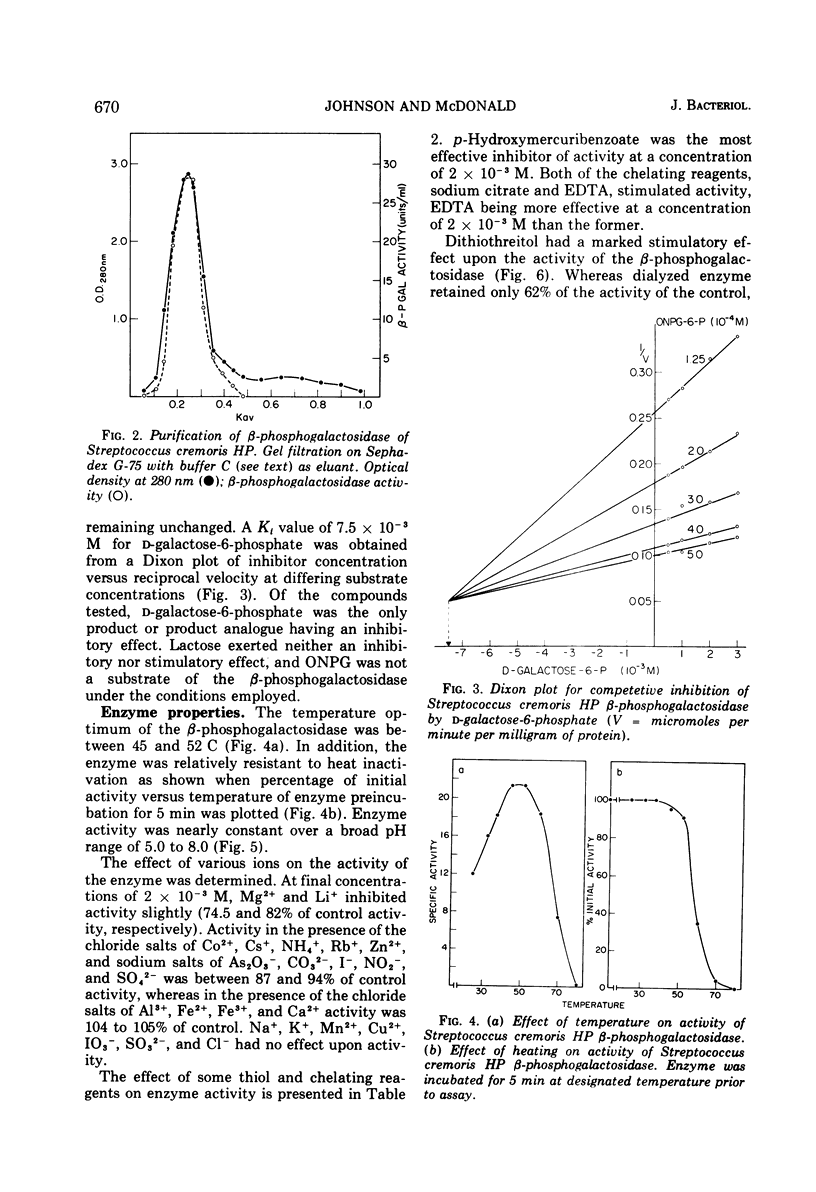
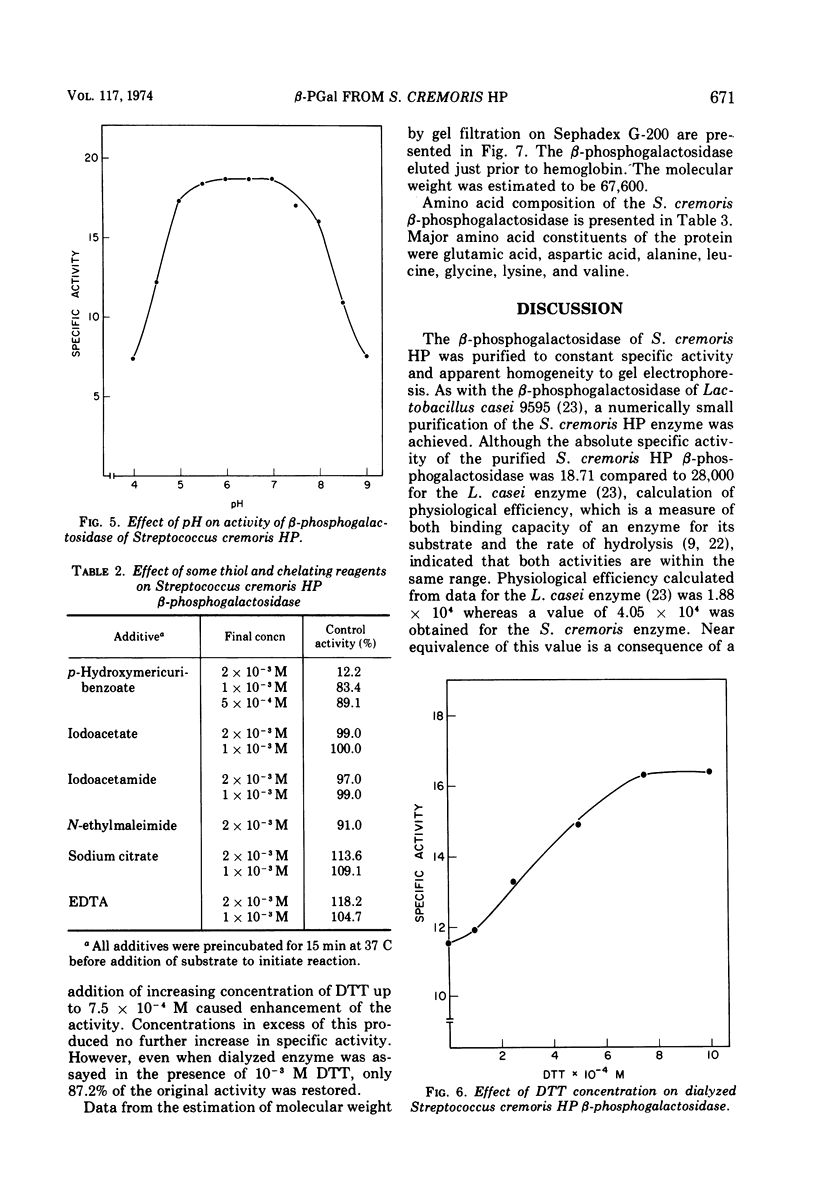
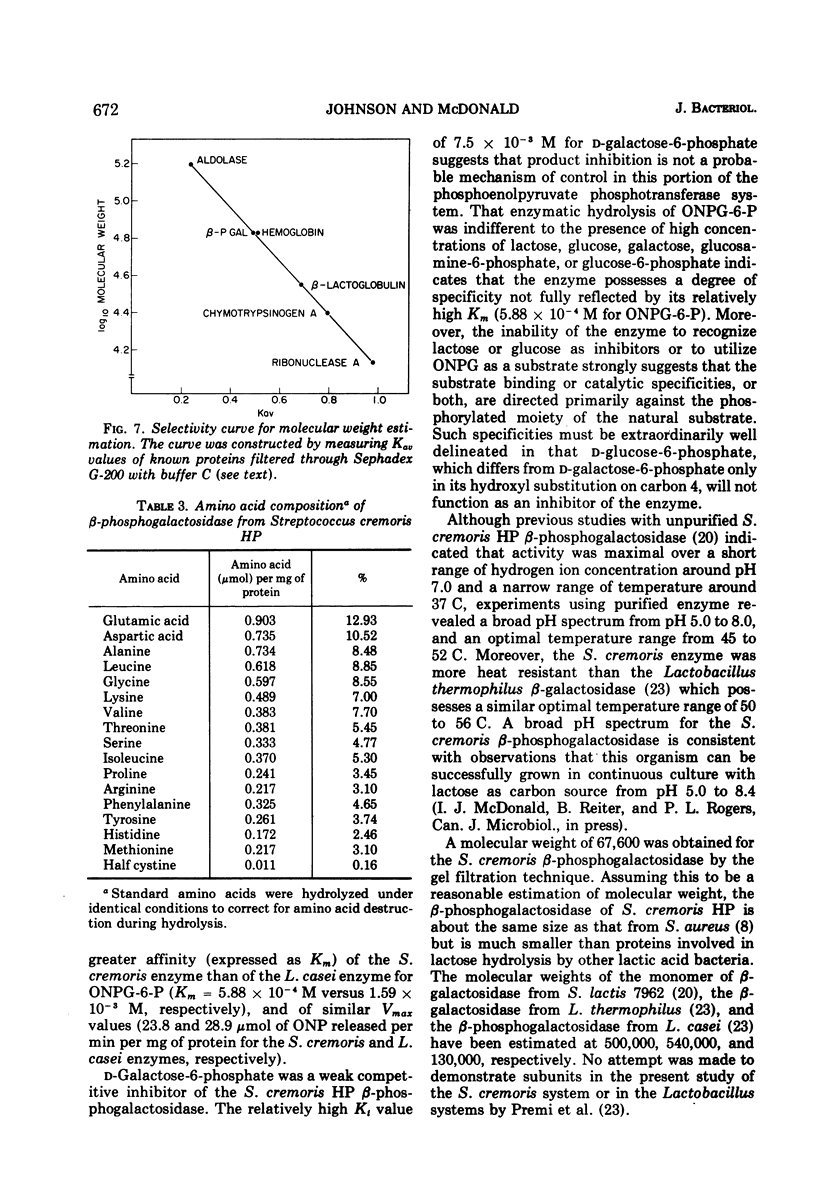
β-d-phosphogalactoside galactohydrolase (β-PGal) was isolated and purified from cell-free extracts of Streptococcus cremoris HP to apparent homogeneity to gel electrophoresis. Using the chromogenic o-nitrophenol-β-d-galactopyranoside-6-phosphate as substrate, the purified enzyme exhibited a specific activity of 18.71 U/mg of protein and Km and Vmax values of 5.88 × 10−4 M and 23.8 μmol of o-nitrophenol liberated per min per mg of protein, respectively. d-Galactose-6-phosphate was a weak competitive inhibitor of β-PGal. Activity was relatively heat resistant and was maximal from pH 5.0 to 8.0 and over a temperature range of 45 to 52 C. Dithiothreitol, ethylenediaminetetraacetic acid, and citrate stimulated β-PGal activity, whereas Mg2+, Li1+, and p-hydroxymercuribenzoate were inhibitory. Molecular weight of the enzyme was estimated at 6.76 × 104. Amino acid composition was similar to other β-phosphogalactosidases previously investigated, with the exception that the S. cremoris enzyme contains a small amount of half cystine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D0galactose metabolism in Staphylococcus aureus: pathway of D-galactose 6-phosphate degradation. Biochem Biophys Res Commun. 1973 May 15;52(2):641–647. doi: 10.1016/0006-291x(73)90761-4. [DOI] [PubMed] [Google Scholar]
- CITTI J. E., SANDINE W. E., ELLIKER P. R. BETA-GALACTOSIDASE OF STREPTOCOCCUS LACTIS. J Bacteriol. 1965 Apr;89:937–942. doi: 10.1128/jb.89.4.937-942.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg W., Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. V. The accumulation of phosphorylated carbohydrate derivatives, and evidence for a new enzyme-splitting lactose phosphate. Proc Natl Acad Sci U S A. 1967 Jul;58(1):274–279. doi: 10.1073/pnas.58.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg W., Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. VI. The nature of the derivatives accumulated. J Biol Chem. 1968 Apr 25;243(8):1881–1885. [PubMed] [Google Scholar]
- Hengstenberg W., Penberthy W. K., Hill K. L., Morse M. L. Metabolism of lactose by Staphylococcus aureus. J Bacteriol. 1968 Dec;96(6):2187–2188. doi: 10.1128/jb.96.6.2187-2188.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg W., Penberthy W. K., Morse M. L. Purification of the staphylococcal 6-phospho-beta-D-- galactosidase. Eur J Biochem. 1970 May 1;14(1):27–32. doi: 10.1111/j.1432-1033.1970.tb00256.x. [DOI] [PubMed] [Google Scholar]
- Johnson K., Dusart J., Campbell J. N., Ghuysen J. M. Exocellular beta-lactamases of Streptomyces albus G and strains R39 and K11. Antimicrob Agents Chemother. 1973 Feb;3(2):289–298. doi: 10.1128/aac.3.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue P., MacDonald R. E. Identification of thiomethyl-beta-D-galactoside 6-phosphate accumulated by Staphylococcus aureus. J Biol Chem. 1968 Feb 10;243(3):680–682. [PubMed] [Google Scholar]
- Laue P., MacDonald R. E. Studies on the relation of thiomethyl-beta-D-galactoside accumulation to thiomethyl-beta-D-galactoside phosphorylation in Staphylococcus aureus HS1159. Biochim Biophys Acta. 1968 Oct 15;165(3):410–418. doi: 10.1016/0304-4165(68)90220-1. [DOI] [PubMed] [Google Scholar]
- McDonald I. J. Filamentous forms of Streptococcus cremoris and Streptococcus lactis. Observations on structure and susceptibility to lysis. Can J Microbiol. 1971 Jul;17(7):897–902. doi: 10.1139/m71-143. [DOI] [PubMed] [Google Scholar]
- McFeters G. A., Sandine W. E., Becker R. R., Elliker P. R. Some factors affecting association-dissociation of beta-galactosidase from Streptococcus lactis 7962. Can J Microbiol. 1969 Jan;15(1):105–110. doi: 10.1139/m69-016. [DOI] [PubMed] [Google Scholar]
- McFeters G. A., Sandine W. E., Elliker P. R. Involvement of sulfhydryl groups in the -galactosidase of Streptococcus lactis. J Bacteriol. 1971 Oct;108(1):599–600. doi: 10.1128/jb.108.1.599-600.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Sandine W. E., Elliker P. R. Purification and properties of Streptococcus lactis beta-galactosidase. J Bacteriol. 1967 Mar;93(3):914–919. doi: 10.1128/jb.93.3.914-919.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Walter L. A., Sandine W. E., Elliker P. R. Involvement of phosphoenolpyruvate in lactose utilization by group N streptococci. J Bacteriol. 1969 Aug;99(2):603–610. doi: 10.1128/jb.99.2.603-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L., Miller A., 3rd, Sandine W. E., Elliker P. R. Mechanisms of lactose utilization by lactic acid streptococci: enzymatic and genetic analyses. J Bacteriol. 1970 Jun;102(3):804–809. doi: 10.1128/jb.102.3.804-809.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molskness T. A., Lee D. R., Sandine W. E., Elliker P. R. -D-phosphogalactoside galactohydrolase of lactic streptococci. Appl Microbiol. 1973 Mar;25(3):373–380. doi: 10.1128/am.25.3.373-380.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M. L., Hill K. L., Egan J. B., Hengstenberg W. Metabolism of lactose by Staphylococcus aureus and its genetic basis. J Bacteriol. 1968 Jun;95(6):2270–2274. doi: 10.1128/jb.95.6.2270-2274.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. PURIFICATION AND PROPERTIES OF PENICILLINASES FROM TWO STRAINS OF BACILLUS LICHENIFORMIS: A CHEMICAL, PHYSICOCHEMICAL AND PHYSIOLOGICAL COMPARISON. Biochem J. 1965 Mar;94:666–675. doi: 10.1042/bj0940666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premi L., Sandine W. E., Elliker P. R. Lactose-hydrolyzing enzymes of Lactobacillus species. Appl Microbiol. 1972 Jul;24(1):51–57. doi: 10.1128/am.24.1.51-57.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Romano A. H., Eberhard S. J., Dingle S. L., McDowell T. D. Distribution of the phosphoenolpyruvate: glucose phosphotransferase system in bacteria. J Bacteriol. 1970 Nov;104(2):808–813. doi: 10.1128/jb.104.2.808-813.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S., Malamy A. Taxonomic investigations on expressed and cryptic phospho-beta-glucosidases in Enterobacteriaceae. J Bacteriol. 1969 Aug;99(2):422–433. doi: 10.1128/jb.99.2.422-433.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



