Abstract
Objective
To describe the magnitude and variation of the epilepsy treatment gap worldwide.
Methods
We conducted a systematic review of the peer-reviewed literature published from 1 January 1987 to 1 September 2007 in all languages using PubMed and EMBASE. The purpose was to identify population-based studies of epilepsy prevalence that reported the epilepsy treatment gap, defined as the proportion of people with epilepsy who require but do not receive treatment. Negative binomial regression models were used to assess trends and associations.
Findings
The treatment gap was over 75% in low-income countries and over 50% in most lower middle- and upper middle-income countries, while many high-income countries had gaps of less than 10%. However, treatment gaps varied widely both between and within countries. They were significantly higher in rural areas (rate ratio, RR: 2.01; 95% confidence interval, CI: 1.40–2.89) and countries with lower World Bank income classification (RR: 1.55; 95% CI: 1.32–1.82). There was no significant trend in treatment gap over time (RR: 0.92; 95% CI: 0.79–1.07).
Conclusion
There is dramatic global disparity in the care for epilepsy between high- and low- income countries, and between rural and urban settings. Our understanding of the factors affecting the treatment gap is limited; future investigations should explore other potential explanations of the gap.
ملخص
الغرض: وصف المقدار والتباين العالمي في فجوة معالجة الصرع.
الطريقة: أجرى الباحثون مراجعة منهجية للمنشورات التي خضعت لمراجعة الزملاء ونشرت خلال الفترة من أول كانون الثاني/يناير 1987 حتى أول أيلول/سبتمبر 2007 بجميع اللغات المستخدمة في موقعي النشريات الطبية على الإنترنت PubMed و EMBASE. وكان الهدف وراء ذلك تحديد الدراسات السكانية لانتشار الصرع التي أبلغت عن وجود فجوة في معالجة الصرع، ويمكن تعريف الفجوة على أنها نسبة المصابين بالصرع الذين يحتاجون إلى العلاج ولكن لا يحصلون عليه. استخدمت نماذج توزيع التحوف ثنائي الحد لتقييم الاتجاهات والارتباطات.
الموجودات: بلغت فجوة المعالجة أكثر من 75% في البلدان المنخفضة الدخل، وأكثر من 50% في أغلب البلدان الواقعة في المرتبة السفلى والمرتبة العليا من البلدان المتوسطة الدخل، في حين بلغت أقل من 10% في أغلب البلدان المرتفعة الدخل، إلا أن فجوة المعالجة تباينت على نحو واسع سواء بين البلدان بعضها البعض أو في داخل البلدان نفسها. وكانت الفجوة أعلى بدرجة يعتد بها في المناطق الريفية (نسبة المعدل rate ratio: 2.01؛ وفاصلة الثقة confidence interval 95%: 1.40 - 2.89) وفي البلدان منخفضة الدخل حسب تصنيف البنك الدولي (نسبة المعدل: 1.55؛ وفاصلة الثقة 95%: 1.32 - 1.82). ولم يكن هنا اتجاه يعتد به في الفجوة العلاجية مع مرور الوقت (نسبة المعدل: 0.92؛ فاصلة الثقة 95%: 0.79 - 1.07).
الاستنتاج: هناك تباين ملحوظ في رعاية الصرع بين البلدان المرتفعة الدخل والبلدان المنخفضة الدخل، وبين المناطق الريفية والمناطق الحضرية. ومازال الإلمام بالعوامل التي تؤثر في الفجوة العلاجية محدوداً؛ وينبغي أن تستكشف الاستقصاءات مستقبلاً سائر التفسيرات المحتملة لهذه الفجوة.
Résumé
Objectif
Décrire l'ampleur et les variations de l'insuffisance du traitement de l'épilepsie dans le monde.
Méthodes
A l'aide de PubMed et d'EMBASE, nous avons réalisé une revue systématique de la littérature examinée par des pairs et publiée entre le 1er janvier 1987 et le 1er septembre 2007 dans toutes les langues. L'objectif était d'identifier des études en population de la prévalence de l'épilepsie indiquant l'insuffisance du traitement de cette maladie, définie comme la proportion des personnes épileptiques ayant besoin d'être traitées, mais ne recevant pas de traitement. Des modèles par régression binomiale négative ont été utilisés pour évaluer les tendances et les associations.
Résultats
L'insuffisance du traitement de l'épilepsie dépassait 75 % dans les pays à faible revenu et 50 % dans la plupart des pays à revenu moyen inférieur et moyen supérieur, alors que dans de nombreux pays à revenu élevé, cette insuffisance était inférieure à 10 %. Néanmoins, l'insuffisance du traitement variait fortement d'un pays à l'autre et au sein d'un même pays. Elle était significativement plus importante dans les zones rurales (risque relatif, RR : 2,01 ; intervalle de confiance à 95 %, IC : 1,40-2,89) et dans les pays appartenant à la classe de revenu inférieure de la Banque mondiale (RR : 1,55 ; IC à 95 % : 1,32-1,82). On n'a relevé aucune tendance significative de l'insuffisance du traitement de l'épilepsie au cours du temps (RR : 0,92 ; IC à 95 % : 0,79-1,07).
Conclusion
Il existe à travers le monde des disparités considérables dans les soins dispensés aux épileptiques, et notamment entre les pays à revenu faible et élevé et entre les environnements ruraux et urbains. Notre compréhension des facteurs influant sur l'insuffisance du traitement est limitée : dans le cadre d'investigations futures, il conviendrait d'étudier d'autres explications possibles de cette insuffisance.
Resumen
Objetivo
Describir la magnitud y las diferencias de la brecha de tratamiento de la epilepsia a nivel mundial.
Métodos
A través de PubMed y EMBASE, se hizo una revisión sistemática de los artículos revisados por homólogos publicados entre el 1 de enero de 1987 y el 1 de septiembre de 2007. La finalidad era encontrar estudios poblacionales sobre la prevalencia de la epilepsia que informaran acerca de la brecha de tratamiento de esa enfermedad, definida como la proporción de personas afectadas que necesitan pero no reciben tratamiento. Las tendencias y relaciones se evaluaron mediante modelos de regresión binomial negativa.
Resultados
La brecha terapéutica era superior al 75% en los países de ingresos bajos, y superior al 50% en la mayoría de los países de ingresos medios bajos y medios altos, mientras que muchos países de ingresos altos presentaban brechas inferiores al 10%. Sin embargo, la magnitud de la brecha terapéutica difería ampliamente tanto entre los países como en cada país. Era significativamente mayor en las zonas rurales (razón de tasas, RT: 2,01, intervalo de confianza del 95%: 1,40–2,89) y en los países incluidos en la categoría de ingresos bajos del Banco Mundial (RT: 1,55, IC95%: 1,32–1,82). No se observó ninguna tendencia significativa de la brecha a lo largo del tiempo (RT: 0,92, IC95%: 0,79–1,07).
Conclusión
En lo referente al tratamiento de la epilepsia, existe una enorme disparidad mundial entre los países de altos y de bajos ingresos, y entre las zonas rurales y las urbanas. Nuestros conocimientos sobre los factores que determinan esa brecha terapéutica son limitados, y en las investigaciones futuras se deberían estudiar otras posibles explicaciones de la misma.
Introduction
Epilepsy affects 50 million people worldwide, and 80% of them live in the developing world.1 An individual with epilepsy suffers recurrent seizures unprovoked by acute brain insults or metabolic derangements. Seizures are characterized by a brief period of uncontrolled involuntary shaking. They may be partial, involving only one part of the body, or generalized, involving the entire body, and they may be accompanied by loss of consciousness and of control of bowel or bladder function. Some individuals continue to have frequent seizures despite optimal treatment with anti-epileptic drugs. However, more than 70% of patients who are treated achieve long-term remission or freedom from seizures, usually within 5 years of diagnosis.2
Cost-effective epilepsy treatments are available and an accurate diagnosis can be made without technological equipment. Nonetheless, a vast majority of individuals with epilepsy in many resource-poor regions do not receive treatment.3–5 Untreated epilepsy is a critical public health issue, as people with untreated epilepsy face potentially devastating social consequences and poor health outcomes. Due to stigma, many persons with epilepsy have lower employment and education levels and lower socioeconomic status. For example, children with epilepsy who have a seizure at school may be dismissed, while adults may be barred from marriage or employment.2,6 In addition, persons with epilepsy have poor health outcomes, including greater psychological distress, more physical injuries such as fractures and burns, and increased mortality.7–12
The epilepsy treatment gap, defined as the proportion of people with epilepsy who require treatment but do not receive it, has been proposed as a useful parameter to compare access to and quality of care for epilepsy patients across populations.13,14 Prior anecdotal and descriptive estimates suggest a treatment gap of more than 80% in many low-income countries,13,15 yet one recent systematic review and meta-analysis suggests that the treatment gap in developing countries is as low as 56%.16This intriguing discrepancy may be due to the methodological limitations of the prior systematic review, which had an excessively narrow search strategy, included only English-language articles, and used meta-analytic techniques to generate a population estimate of the treatment gap. First, the search strategy focused on “treatment gap” and “treatment status”. Many epilepsy prevalence studies report treatment data, but as the term “treatment gap” only recently came into usage in the research literature,13 many studies with treatment gap data may have been missed using this search strategy. Second, many studies, particularly from low-income countries, are published in local rather than international journals. By not including languages other than English, many studies with treatment gap data may have been missed. Finally, the use of meta-analytic techniques to generate a unitary estimate of the treatment gap may have biased the estimates for two reasons: first, there was considerable unexplained heterogeneity among treatment gap estimates, and second, included studies were conducted in populations that were not representative of developing countries as a whole.
In this systematic review and analysis of the variation in the epilepsy treatment gap, we have greatly expanded the scope of the systematic review by searching for population-based epilepsy prevalence studies in all languages. We have also described the magnitude of the treatment gap worldwide and conducted some preliminary assessments of its variation.
Methods
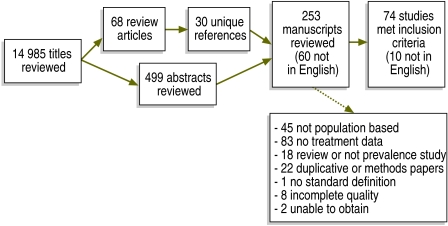
We conducted a systematic review of the peer-reviewed literature published in all languages from 1 January 1987 to 1 September 2007 using PubMed and EMBASE. Search terms included PubMed MeSH terms and keywords “epilepsy” AND “morbidity,” OR “epilepsy” AND “delivery of health care,” OR “treatment gap” AND “epilepsy”. This generated 14 985 titles. Hand searching of 68 reviews of epilepsy prevalence generated an additional 30 unique titles. All titles were reviewed to identify potential epilepsy prevalence studies, then 499 abstracts and 253 full manuscripts were reviewed to identify population-based epilepsy prevalence studies (Fig. 1). Data were extracted and reviewed independently by two authors.
Fig. 1.
Flowchart of study selection for systematic review of population-based studies of epilepsy prevalence and treatment gap
To be included in the analysis, epilepsy prevalence studies had to be based on a population-based sample and apply a standard definition of epilepsy. A population-based sample was defined as a door-to-door or other probability sample of a regional or national population. Studies in which the sample was drawn from a medical care setting were excluded to avoid underestimating the treatment gap. School-based populations in countries where school attendance was low were also excluded. Finally, studies based on methods shown to produce unreliable community-based samples in epilepsy prevalence studies, such as the key informant method, were excluded as well.17,18
The standard definition of epilepsy had to be internally consistent and to differentiate epilepsy from provoked seizures, febrile seizures and isolated seizures. For lifetime epilepsy, acceptable definitions included a history of more than one unprovoked seizure. For active epilepsy, acceptable definitions included a history of more than one unprovoked seizure and either recent seizures (within the previous 5 years) or current use of anti-epilepsy medication. If the treatment gap or other information was missing from the manuscript, we tried to contact the authors to obtain the information before excluding the study.
Further analysis of the variation in epilepsy treatment gap estimates was limited to studies of active epilepsy, as studies using lifetime epilepsy could overestimate the treatment gap. For example, some individuals captured when considering the lifetime prevalence of epilepsy may be in terminal remission and off treatment.19 Including them in the estimates would overestimate the treatment gap because by not being on anti-epileptic drugs, these individuals are receiving the recommended standard of care.
We analysed the variation in the epilepsy treatment gap by study area (urban versus rural), country income category and year. We used negative binomial regression models to examine associations and trends and used separate models to examine the association between treatment gap and study area, country income category and year. Treatment gaps were expressed as the number of untreated persons with active epilepsy, with the number of persons having active epilepsy used as the exposure variable. Studies were classified as rural or not rural based on the site description in the methods section of the manuscripts. Countries were classified as low, lower middle, upper middle or high-income economies using World Bank criteria.20 Prevalence year was extracted from the manuscripts; if no prevalence year was provided, the year of publication was used instead. World Bank income category and prevalence year, arranged in 5-year intervals, were treated as ordered categorical variables. Stata 10 (StataCorp LP, College Station, TX, United States of America) was used for the analysis. Significance level was set at P ≤ 0.05.
Results
Our search yielded 157 epilepsy prevalence studies that met our stated inclusion criteria, but 83 (nearly 53%) of them did not collect treatment gap data. Therefore, our final sample consisted of 74 studies representing 38 countries (Table 1 and Table 2, available at: http://www.who.int/bulletin/volumes/88/4/09-064147/en/index.html). Of note, we reviewed 60 articles in languages other than English (Chinese, English, French, German, Italian, Japanese, Portuguese, Russian, Spanish and Turkish) and 10 of them were included in the study. Manuscripts included in our final sample were published in English, French, Spanish and Turkish.
Table 1. Studies used for estimating epilepsy treatment gap based on the prevalence of active epilepsy, by country.
| Country | Location | Year | No. of cases | % treatment gap | Setting/population | Study |
|---|---|---|---|---|---|---|
| Argentina | Junin | 1991 | 64 | 22 | Town | Kochen S, Melcon MO. Prognosis of epilepsy in a community-based study: 8 years of follow-up in an Argentine community. Acta Neurol Scand 2005;112:370-4. doi:10.1111/j.1600-0404.2005.00519.x PMID:16281918 |
| Argentina | Buenos Aires | 1991 | 84 | 7 | Urban, primary school | Somoza MJ, Forlenza RH, Brussino M, Licciardi L. Epidemiological survey of epilepsy in the primary school population in Buenos Aires. Neuroepidemiology 2005;25:62-8. doi:10.1159/000086285 PMID:15947492 |
| Brazil | Mato Grosso state | 2000 | 9 | 67 | Rural, Bakairi indians | Borges MA, Barros EP, Zanetta DM, Borges AP. Prevalence of epilepsy in Bakairi indians from Mato Grosso State, Brazil. Arq Neuropsiquiatr 2002;60:80-5 [Portuguese.]. PMID:11965413 |
| Brazil | Rio de Janeiro | 2000 | 5 | 0 | Urban, low-income | Gomes M, Zeitoune R, Kropf L, Van Beeck E. A house-to-house survey of epileptic seizures in an urban community of Rio de Janeiro, Brazil. Arq Neuropsiquiatr 2002;60:708-11. PMID:12364934 |
| Brazil | Barao Geraldo, Campinas; Jaguare and Santo Antonio, Sao Jose do Rio Preto, south-eastern Brazil | 2002 | 290 | 38 | Mixed | Noronha AL, Borges M, Marques L, Zanetta D, Fernandes P, De Boer H, et al. Prevalence and pattern of epilepsy treatment in different socioeconomic classes in Brazil. Epilepsia 2007;48:880-885. doi:10.1111/j.1528-1167.2006.00974.x PMID:17326788 |
| China | 5 provinces: Heilongjiang, Ningxia, Henan, Shanxi, Jiangsu | 2003a | 257 | 63 | Mixed | Wang WZ, Wu JZ, Wang DS, Dai XY, Yang B, Wang TP, et al. The prevalence and treatment gap in epilepsy in China: an ILAE/IBE/WHO study. Neurology 2003;60:1544-5. PMID:12743252 |
| China, Province of Taiwan | 20 districts and townships in Ilan County, NE Taiwan | 1993–95 | 25 | 4 | Mixed, adults > 40 yrs old | Su CL, Chang SF, Chen ZY, Lee CS, Chen RC. Neuroepidemiological survey in Ilan, Taiwan (NESIT): Prevalence of epilepsy. Acta Neurol Taiwan 1998;7:75-84. |
| China | Tibet Autonomous Region | 2006 | 35 | 97 | Rural | Zhao Y, Zhang Q, Tsering T, Sangwan, Hu X, Liu L, et al. Prevalence of convulsive epilepsy and health-related quality of life of the population with convulsive epilepsy in rural areas of Tibet Autonomous Region in China: An initial survey during a verbal episodic memory task. Epilepsy Behav 2008;12:373-81. doi:10.1016/j.yebeh.2007.10.012 PMID:18180204 |
| Ecuador | Atahualpa | 2003 | 18 | 28 | Rural | Del Brutto OH, Santibanez R, Idrovo L, Rodriguez S, Diaz-Calderon E, Navas C, et al. Epilepsy and neurocysticercosis in Atahualpa: a door-to-door survey in rural coastal Ecuador. Epilepsia 2005;46:583-7. doi:10.1111/j.0013-9580.2005.36504.x PMID:15816956 |
| Ecuador | El Carchi and Imbabura regions, northern Ecuador | 1992a | 575 | 79 | Rural | Placencia M, Shorvon SD, Paredes V, Bimos C, Sander JW, Suarez J, et al. Epileptic seizures in an Andean region of Ecuador. Incidence and prevalence and regional variation. Brain 1992;115:771-82. doi:10.1093/brain/115.3.771 PMID:1628201 Placencia M, Sander J, Roman M, Madera A, Crespo F, Cascante S, et al. The characteristics of epilepsy in a largely untreated population in rural Ecuador. J Neurol Neurosurg Psychiatry 1994;57:320-5. doi:10.1136/jnnp.57.3.320 PMID:8158180 |
| Ethiopia | Zay society | 2006a | 34 | 53 | Rural | Almu S, Tadesse Z, Cooper P, Hackett R. The prevalence of epilepsy in the Zay Society, Ethiopia – an area of high prevalence. Seizure 2006;15:211-3. doi:10.1016/j.seizure.2006.01.004 PMID:16488161 |
| Ethiopia | Meskan and Mareko sub-district, Haykoch and Butajira district | 1986–1988 | 306 | 98 | Rural | Tekle-Haimanot R, Forsgren L, Abebe M, Gebre-Mariam A, Heijbel J, Holmgren G, et al. Clinical and electroencephalographic characteristics of epilepsy in rural Ethiopia: a community based study. Epilepsy Res 1990;7:230-9. doi:10.1016/0920-1211(90)90020-V PMID:2289482 |
| Ethiopia | Meskan and Mareko sub-district, Haykoch and Butajira district | 1990 | 139 | 87 | Rural | Tekle-Haimanot R, Forsgren L, Ekstedt J. Incidence of epilepsy in rural central Ethiopia. Epilepsia 1997;38:541-6. doi:10.1111/j.1528-1157.1997.tb01138.x PMID:9184599 |
| France | Paris, Seine et Marne, Seine Saint-Denis, Val de Marne | 1987–1988 | 149 | 7 | Urban | Jallon P. Evaluation of epilepsy prevalence rate in young recruits in a military selection centre. Rev Neurol 1991;147:319-22. PMID:2063084 |
| Gambia | Farafenni | 1997, 1999 | 69 | 100 | Rural | Coleman R, Loppy L, Walraven G. The treatment gap and primary health care for people with epilepsy in rural Gambia. Bull World Health Organ 2002;80:378-83. PMID:12077613 |
| Honduras | Salama County | 1997 | 100 | 58 | Rural | Medina MT, Duron RM, Martinez L, Osorio JR, Estrada AL, Zuniga C, et al. Prevalence, incidence, and etiology of epilepsies in rural Honduras: the Salama Study. Epilepsia 2005;46:124-31. doi:10.1111/j.0013-9580.2005.11704.x PMID:15660778 |
| India | Parsi community of Bombay | 1985 | 50 | 22 | Urban | Bharucha NE, Bharucha E, Bharucha A, Bhise A, Schoenberg B. Prevalence of epilepsy in the Parsi community of Bombay. Epilepsia 1988;29:111-5. doi:10.1111/j.1528-1157.1988.tb04405.x PMID:3258234 |
| India | Calicut district, Kerala | 1997a | 26 | 50 | Mixed | Hackett RJ, Hackett L, Bhakta P. The prevalence and associated factors of epilepsy in children in Calicut District, Kerala, India. Acta Paediatr 1997;86:1257-60. doi:10.1111/j.1651-2227.1997.tb14857.x PMID:9401524 |
| India | Kuthar Valley, Anantnag district, South Kashmir | 1986 | 157 | 75 | Rural | Koul R, Razdan S, Motta A. Prevalence and pattern of epilepsy (Lath/Mirgi/Laran) in Rural Kashmir, India. Epilepsia 1988;29:116-22. doi:10.1111/j.1528-1157.1988.tb04406.x PMID:3258235 Razdan S, Kaul R, Motta A, Kaul S, Bhatt R. Prevalence and pattern of major neurological disorders in rural Kashmir (India) in 1986. Neuroepidemiology 1994;13:113-9. doi:10.1159/000110368 PMID:8015664 |
| India | Yelandur, Karnataka, south India | 1990–1991 | 457 | 78 | Rural | Mani KS, Rangan G, Srinivas HV, Kalyanasundaram S, Narendran S, Reddy AK. The Yelandur study: a community-based approach to epilepsy in rural South India – epidemiological aspects. Seizure 1998;7:281-8. doi:10.1016/S1059-1311(98)80019-8 PMID:9733402 |
| India | West Bengal | 1995–1996 | 90 | 90 | Rural | Pal DK, Das T, Sengupta S. Comparison of key informant and survey methods for ascertainment of childhood epilepsy in West Bengal, India. Int J Epidemiol 1998;27:672-6. doi:10.1093/ije/27.4.672 PMID:9758124 |
| India | Thrissur, Palakkad, Malappuram districts, Kerala, south India; High literacy and health awareness | 1996 | 1175 | 38 | Semi-urban | Radhakrishnan K, Pandian JD, Santhoshkumar T, Thomas SV, Deetha TD, Sarma PS, et al. Prevalence, knowledge, attitude, and practice of epilepsy in Kerala, South India. Epilepsia 2000;41:1027-35. doi:10.1111/j.1528-1157.2000.tb00289.x PMID:10961631 |
| India | Baruipur block, west Bengal, east India | 1992–1993 | 75 | 65 | Rural | Saha SP, Bhattacharya S, Das SK, Maity B, Roy T, Raut DK. Epidemiological study of neurological disorders in a rural population of Eastern India. J Indian Med Assoc 2003;101:299-300. PMID:14575218 |
| India | Churu Tehsil, Rajasthan | 2005 | 517 | 40 | Rural | Sureka RK, Sureka R. Prevalence of epilepsy in rural Rajasthan – a door-to-door survey. J Assoc Physicians India 2007;55:741-2. PMID:18173034 |
| Kenya | Kilifi district | 2003 | 408 | 85 | Rural | Edwards T, Scott AG, Munyoki G, Odera VM, Chengo E, Bauni E, et al. Active convulsive epilepsy in a rural district of Kenya: a study of prevalence and possible risk factors. Lancet Neurol 2008;7:50-6. doi:10.1016/S1474-4422(07)70292-2 PMID:18068520 |
| Lao People's Democratic Republic | District Hinheub | 2003–2004 | 33 | 97 | Rural | Tran DS, Odermatt P, Le TO, Huc P, Druet-Cabanac M, Barennes H, et al. Prevalence of epilepsy in a rural district of central Lao People's Democratic Republic PDR. Neuroepidemiology 2006;26:199-206. doi:10.1159/000092407 PMID:16569936 |
| Madagascar | Grand Antananarivo | 2001 | 25 | 32 | Urban | Andriantseheno L, Ralaizandriny D. Prevalence communautaire de l'epilepsie chez les Malgaches. Epilepsies 2004;16:83-6. |
| Mali | 18 villages in Tyenfala, Baguineda | 2000 | 70 | 65 | Rural | Farnarier G, Diop S, Coulibaly B, Arborio S, Dabo A, Diakite M, et al. [Onchocerciasis and epilepsy. Epidemiological survey in Mali]. Med Trop (Mars) 2000;60:151-5. PMID:11100441 |
| Mali | Comune IV and Comune VI, Bamako district | 1998 | 46 | 76 | Urban | Traore M, Tahny R, Sacko M. Prevalence de l'epilepsie chez les enfants de 3 a 15 ans dans 2 communes du district de Bamako. Rev Neurol (Paris) 2000;156: Suppl 1:S18. |
| Nigeria | Igbo-Ora | 1982 | 101 | 96 | Town | Osuntokun BO, Adeuja A, Nottidge V, Bademosi O, Olumide A, Ige O, et al. Prevalence of the epilepsies in Nigerian Africans: a community based study. Epilepsia 1987;28:272-9. doi:10.1111/j.1528-1157.1987.tb04218.x PMID:3582291 |
| Norway | Vaga community, southern Norway | 1995–1997 | 12 | 0 | Rural | Brodtkorb E, Sjaastad O. Epilepsy prevalence by individual interview in a Norwegian community. Seizure 2008;17:646-50. doi:10.1016/j.seizure.2008.03.005 PMID:18434213 |
| Norway | Oppland county | 2001 | 35 | 3 | Mixed | Svendsen T, Lossius M, Nakken KO. Age-specific prevalence of epilepsy in Oppland County, Norway. Acta Neurol Scand 2007;116:307-11. doi:10.1111/j.1600-0404.2007.00909.x PMID:17922724 |
| Pakistan | Sind Province, Mirpur Sakro | 1987 | 126 | 98 | Rural | Aziz H, Ali S, Frances P, Khan M, Hasan K. Epilepsy in Pakistan: a population-based epidemiologic study. Epilepsia 1994;35:950-8. doi:10.1111/j.1528-1157.1994.tb02539.x PMID:7925166 |
| Pakistan | Karachi | 1987 | 115 | 73 | Urban | Aziz H, Ali S, Frances P, Khan M, Hasan K. Epilepsy in Pakistan: a population-based epidemiologic study. Epilepsia 1994;35:950-8. doi:10.1111/j.1528-1157.1994.tb02539.x PMID:7925166 |
| Panama | Changuinola, Bocas del Toro province | 1988 | 19 | 100 | Small town, Guaymi Indians | Gracia F, deLao S, Castillo L, Larreategui M, Archbold C, Brenes M, et al. Epidemiology of epilepsy in Guaymi Indians from Bocas del Toro Province, Republic of Panama. Epilepsia 1990;31:718-23. doi:10.1111/j.1528-1157.1990.tb05512.x PMID:2245802 |
| Senegal | Pikine Health District, suburb of Dakar | 2005 | 64 | 23 | Suburban | Ndoye NF, Sow AD, Diop AG, Sessouma B, Sene-Diouf F, Boissy L, et al. Prevalence of epilepsy its treatment gap and knowledge, attitude and practice of its population in sub-urban Senegal an ILAE/IBE/WHO study. Seizure 2005;14:106-11. doi:10.1016/j.seizure.2004.11.003 PMID:15694563 |
| South Africa | Bushbuckridge, Northern Province | 2000 | 45 | 51 | Rural, children | Christianson AL, Zwane ME, Manga P, Rosen E, Venter A, Kromberg JG. Epilepsy in rural South African children – prevalence, associated disability and management. S Afr Med J 2000;90:262-6. PMID:10853404 |
| South Africa | Nkalukeni village | 2003 | 38 | 88 | Rural | Del Rio-Romero A, Foyaca-Sibat H, Ibanez-Valdes L, Vega-Novoa E. Prevalence of Epilepsy and General Knowledge about Neurocysticercosis at Nkalukeni Village, South Africa. Internet Journal of Neurology 2005;3:1-12. |
| Spain | Madrid | 1984 | 9 | 56 | Urban | Cruz Gutierrez-del-Olmo M, Schoenberg BS, Portera-Sanchez A. Prevalence of neurological diseases in Madrid, Spain. Neuroepidemiology 1989;8:43-7. doi:10.1159/000110164 PMID:2643061 |
| Togo | 10 of 13 villages in Nadoba, Batamariba district; Batamariba or Tamberma tribe | 2002 | 92 | 100 | Rural | Balogou AA, Grunitzky K, Belo M, Sankaredja M, Djagba D, Tatagan-Agbi K, et al. Managment of Epilepsy Patients in Batamariba District, Togo. Acta Neurol Scand 2007;116:211-6. doi:10.1111/j.1600-0404.2007.00871.x PMID:17824896 |
| Togo | Tone | 1995 | 170 | 78 | Mixed | Balogou AA, Doh A, Grunitzky K. Affections neurologiques et endemie goitreuse: analyse comparative de deux provinces du Togo. Bull Soc Pathol Exot 2001;94:406-10. PMID:11889943 |
| Turkey | Central Anatolia (Elmadag township and Kutludugun village) and Demirlirbahce district of Anakara city | 1987 | 59 | 70 | Mixed | Aziz H, Guvener A, Akhtar SW, Hasan KZ. Comparative epidemiology of epilepsy in Pakistan and Turkey: population-based studies using identical protocols. Epilepsia 1997;38:716-22. doi:10.1111/j.1528-1157.1997.tb01242.x PMID:9186255 |
| Turkey | Sivas | 1997 | 33 | 76 | Urban | Topalkara K, Akyuz A, Sumer H, Bekar D, Topaktas S, Dener S, et al. Siva il Merkezinde Tabakali Ornekleme Yontemi ile Gerceklestirilen Epilepsi Prevalans Calismasi. Epilepsia 1999;5:24-9. |
| Turkey | Bursa city center | 2004–2005 | 18 | 50 | Urban | Calişir N, Bora I, Irgil E, Boz M. Prevalence of Epilepsy in Bursa City Center, an Urban Area of Turkey. Epilepsia 2006;47:1691-9. doi:10.1111/j.1528-1167.2006.00635.x PMID:17054692 |
| Turkey | Silivri | 1994 | 49 | 45 | Mixed | Karaagaç N, Yeni SN, Senocak M, Bozluolcay M, Savrun FK, Ozdemir H, et al. Prevalence of epilepsy in Silivri, a rural area of Turkey. Epilepsia 1999;40:637-42. doi:10.1111/j.1528-1157.1999.tb05567.x PMID:10386534 |
| Turkey | 47 villages in Ulas town, Sivas city, middle Anatolia region of Turkey | 125 | 78 | Mixed | Sahin A, Bolayir E, Sumer H, Tas A, Mollaoglu M, Dener S. Epidemiologic evaluation of epileptic and nonepileptic seizures in Sivas region of Middle Anatolia. Neurol Psychiatry Brain Res 2004;11:97-102. | |
| Uganda | Kabende parish, Kabarole district | 1994 | 61 | 100 | Rural | Kaiser C, Kipp W, Asaba G, Mugisa C, Kabagambe G, Rating D, et al. The prevalence of epilepsy follows the distribution of onchocerciasis in a west Ugandan focus. Bull World Health Organ 1996;74:361-7. PMID:8823957 |
| United Kingdom of Great Britain and Northern Ireland | National child survey | 1988 | 124 | 2 | National, children only | Kurtz Z, Tookey P, Ross E. Epilepsy in young people: 23 year follow up of the British national child development study. BMJ 1998;316:339-42. PMID:9487166 |
| United Republic of Tanzania | Nachingwea district | 1999 | 42 | 95 | Rural | Dent W, Helbok R, Matuja WB, Scheunemann S, Schmutzhard E. Prevalence of active epilepsy in a rural area in South United Republic of Tanzania: a door-to-door survey. Epilepsia 2005;46:1963-9. doi:10.1111/j.1528-1167.2005.00338.x PMID:16393163 |
| United Republic of Tanzania | Ulanga district | 1989–1990 | 185 | 100 | Rural | Rwiza H, Kilonzo G, Haule J, Matuja W, Mteza I, Mbena P, et al. Prevalence and incidence of epilepsy in Ulanga, a rural Tanzanian district: a community-based study. Epilepsia 1992;33:1051-6. doi:10.1111/j.1528-1157.1992.tb01758.x PMID:1464263 Rwiza H. The Muhimbili epilepsy project, a three pronged approach. Trop Geogr Med 1994;46 Suppl;22-4. |
| United States of America | Washington Heights, Inwood, New York City | 2004–2005 | 42 | 7 | Urban | Kelvin EA, Hesdorffer DC, Bagiella E, Andrews H, Pedley TA, Shih TT, et al. Prevalence of self-reported epilepsy in a multiracial and multiethnic community in New York City. Epilepsy Res 2007;77:141-50. doi:10.1016/j.eplepsyres.2007.09.012 PMID:18023147 |
| United States of America | California | 2003 | 322 | 10 | Mixed | Kobau R, Zahran H, Grant D, Thurman DJ, Price PH, Zack MM. Prevalence of active epilepsy and health-related quality of life among adults with self-reported epilepsy in California: California Health Interview Survey, 2003. Epilepsia 2007;48:1904-13. doi:10.1111/j.1528-1167.2007.01161.x PMID:17565591 |
| United States of America | 19 states | 2005 | 919 | 7 | Mixed | Kobau R, Zahran H, Thurman DJ, Zack MM, Henry TR, Schachter SC, et al. Epilepsy surveillance among adults — 19 States, Behavioural Risk Factor Surveillance System, 2005. MMWR Surveill Summ 2008;57:1-20. PMID:18685554 |
| Zambia | Chikankata catchment area | 2000–2001 | 799 | 97 | Rural | Birbeck GL, Kalichi EM. Epilepsy prevalence in rural Zambia: a door-to-door survey. Trop Med Int Health 2004;9:92-5. doi:10.1046/j.1365-3156.2003.01149.x PMID:14728612 |
a Prevalence year not reported in manuscript, therefore publication year substituted.
Table 2. Studies used for estimating epilepsy treatment gap based on the lifetime prevalence of epilepsy, by country.
| Country | Location | Year | No. of cases | % treatment gap | Setting/population | Author |
|---|---|---|---|---|---|---|
| Bangladesh | Attempts to be representative of country; 3 rural, 2 urban areas | 1992a | 67 | 86 | Mixed, children | Durkin MS, Davidson L, Hasan K, Hasan Z, Hauser W, Khan N, et al. Estimates of the prevalence of childhood seizure disorders in communities where professional resources are scarce: results from Bangladesh, Jamaica and Pakistan. Paediatr Perinat Epidemiol 1992;6:166-80. doi:10.1111/j.1365-3016.1992.tb00758.x PMID:1584719 |
| Bolivia | Cordillera Province, Santa Cruz Department | 1994 | 124 | 100 | Rural | Nicoletti A, Reggio A, Bartoloni A, Failla G, Sofia V, Bartalesi F, et al. Prevalence of epilepsy in rural Bolivia: a door-to-door survey. Neurology 1999;53:2064-9. PMID:10599782 |
| Cameroon | Bilomo, west bank of Mbam river in Centre Province of Cameroon | 1998 | 93 | 32 | Rural | Njamnshi AK, Sini V, Djientcheu VDP, Ongolo-Zogo P, Mapoure Y, Yepnjio FN, et al. Risk factors associated with epilepsy in a rural area in Cameroon: A preliminary study. African J Neurol Sci 2007;26:18-26. |
| China | 4 towns, 6 villages in Dongning County, Mundanjiang City, Heilongjiang Province | 2000 | 81 | 36 | Rural | Ma GY, Li ZQ, Lu S, Wang LH, Wen SR, Li GZ, et al. Survey of etiological factors of epilepsy in Mudanjian rural population by randomly cluster sampling. Chin J Clin Rehabil 2004;8:3178-9. Tang Y, Li GZ, Ma GY, Wang DS. Epidemiological survey of epilepsy in Dongning county, a rural area in Heilongjiang Province of China. Chin J Clin Rehabil 2004;8:770-1. |
| Colombia | Medellin | 1983 | 77 | 73 | Urban | Zuloaga L, Soto C, Jaramillo D, Mora O, Betancur C, Londono R. [Prevalence of epilepsy in Medellin, Colombia, 1983]. Bol Oficina Sanit Panam 1988;104:331-44. PMID:2971372 |
| France | Haute-Vienne, Limousin region | 1986–87 | 20 | 55 | Mixed | Munoz M, Boutros-Toni F, Preux PM, Chartier JP, Ndzanga E, Boa F, et al. Prevalence of neurological disorders in Haute-Vienne department (Limousin region-France). Neuroepidemiology 1995;14:193-8. doi:10.1159/000109796 PMID:7643954 |
| Guatemala | Small rural village | 1996a | 16 | 31 | Rural | Mendizabal JE, Salguero LF. Prevalence of epilepsy in a rural community of Guatemala. Epilepsia 1996;37:373-6. doi:10.1111/j.1528-1157.1996.tb00574.x PMID:8603643 |
| India | PHC cachement area, Haryana, North India | 1992–4 | 126 | 53 | Rural | Singh A, Kaur A. Epilepsy in rural Haryana – prevalence and treatment seeking behaviour. J Indian Med Assoc 1997;95:37-9, 47. PMID:9357239 |
| Italy | Riposto (Catania Province); Santa Teresa di Riva (Messina Province); Terrasini (Palermo Province) Sicily | 1987 | 111 | 39 | Semi-urban | Rocca WA, Savettieri G, Anderson DW, Meneghini F, Grigoletto F, Morgante L, et al. Door-to-door prevalence survey of epilepsy in three Sicilian municipalities. Neuroepidemiology 2001;20:237-41. doi:10.1159/000054796 PMID:11684899 |
| Jamaica | May Pen and Lionel Town, Clarendon parish | 1992a | 32 | 62 | Rural, children | Durkin MS, Davidson L, Hasan K, Hasan Z, Hauser W, Khan N, et al. Estimates of the prevalence of childhood seizure disorders in communities where professional resources are scarce: results from Bangladesh, Jamaica and Pakistan. Paediatr Perinat Epidemiol 1992;6:166-80. doi:10.1111/j.1365-3016.1992.tb00758.x PMID:1584719 |
| Netherlands | Elderly, Rotterdam Study | 1991–1993 | 85 | 52 | Suburb | de la Court A, Breteler MM, Meinardi H, Hauser WA, Hofman A. Prevalence of epilepsy in the elderly: the Rotterdam Study. Epilepsia 1996;37:141-7. doi:10.1111/j.1528-1157.1996.tb00005.x PMID:8635424 |
| Pakistan | Greater Karachi; 43 urban and 16 rural | 1992a | 99 | 62 | Mixed, children | Durkin MS, Davidson L, Hasan K, Hasan Z, Hauser W, Khan N, et al. Estimates of the prevalence of childhood seizure disorders in communities where professional resources are scarce: results from Bangladesh, Jamaica and Pakistan. Paediatr Perinat Epidemiol 1992;6:166-80. doi:10.1111/j.1365-3016.1992.tb00758.x PMID:1584719 |
| Singapore | National | 1995 | 89 | 6 | National; 18 year old men | Kun LN, Ling LW, Wah YW, Lian TT. Epidemiologic study of epilepsy in young Singaporean men. Epilepsia 1999;40:1384-7. doi:10.1111/j.1528-1157.1999.tb02009.x PMID:10528933 |
| Spain | Guillena municipality | 1981 | 40 | 18 | Mixed, children | Nieto Barrera M. Neuroepidemiology of epilepsy. An Esp Pediatr 1988;29:59-63. PMID:3250297 |
| Sri Lanka | 218 villages belonging to 12 Gramodaya Centres | 1983 | 690 | 49 | Mixed | Senanayake N. Epilepsy control in a developing country-the challenge of tomorrow. Ceylon Med J 1987;32:181-99. PMID:3506450 |
| United States of America | Copiah County | 1978 | 246 | 40 | Rural | Haerer AF, Anderson D, Schoenberg B. Prevalence and clinical features of epilepsy in a biracial United States population. Epilepsia 1986;27:66-75. doi:10.1111/j.1528-1157.1986.tb03503.x PMID:3948820 |
| United States of America | US population | 2004 | 123 | 50 | Mixed | Kobau R, Gilliam F, Thurman DJ. Prevalence of self-reported epilepsy or seizure disorder and its associations with self-reported depression and anxiety: results from the 2004. Health Styles Survey Epilepsia 2006;47:1915-21. doi:10.1111/j.1528-1167.2006.00612.x |
| United States of America | South Carolina | 2003–5 | 379 | 50 | Mixed | Prevalence of epilepsy and health-related quality of life and disability among adults with epilepsy – South Carolina, 2003 and 2004. MMWR Morb Mortal Wkly Rep 2005;54:1080-2. PMID:16251865 Ferguson PL, Chiprich J, Smith G, Dong B, Wannamaker BB, Kobau R, et al. Prevalence of self-reported epilepsy, health care access, and health behaviours among adults in South Carolina. Epilepsy Behav 2008;13:529-34. doi:10.1016/j.yebeh.2008.05.005 PMID:18585962 |
a Prevalence year not reported in manuscript, therefore publication year substituted.
Active epilepsy was used to estimate the treatment gap in 54 populations from 28 countries (Table 1) and lifetime epilepsy was used to estimate the treatment gap in 18 populations from 16 countries (10 of which were not among the countries for which the active epilepsy gap was estimated). (Table 2). Studies spanned nearly 30 years, from 1978 to 2006, and originated across the globe, including Africa, Asia, Europe and North and South America. Study populations differed markedly in terms of type of study area (urban versus rural), sample size and degree to which they represented the entire country. Nearly 47% (34/72) of the included studies were drawn from rural populations. Treatment gaps were calculated from samples ranging from 5 to 1175 epilepsy cases. Samples were drawn from many different populations; some were nationally representative, while others represented small ethnic groups, indigenous groups, schoolchildren or military recruits.
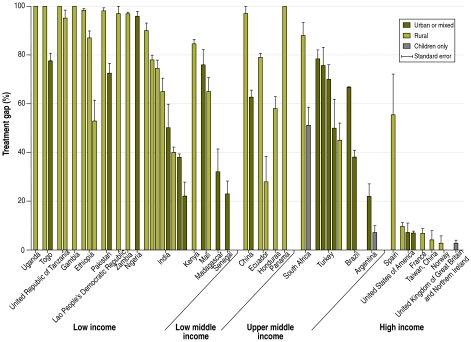
Treatment gaps estimated from active epilepsy prevalence ranged widely between countries. Gaps were 10% or less in China (Province of Taiwan), Norway, Singapore, the United Kingdom of Great Britain and Northern Ireland, the United States of America, and select populations in Argentina, Brazil and France. In sharp contrast, treatment gaps were greater than 95% in China, Ethiopia, the Gambia, the Lao People’s Democratic Republic, Nigeria, Pakistan, Panama, Togo, Uganda, the United Republic of Tanzania and Zambia (Fig. 2). A wide range of treatment gaps was observed within countries as well. For example, treatment gaps in India ranged from 22% in an urban middle- income population to 90% in a sample of rural villages.18,21
Fig. 2.
Epilepsy treatment gap (%) and standard errors, by country and World Bank income category
Like treatment gaps estimated from active epilepsy prevalence, the gaps estimated from lifetime prevalence also ranged widely, from 6% in Singapore to 100% in Bolivia (Fig. 3).22,23 In most cases, gaps estimated from lifetime prevalence were larger than those estimated from active epilepsy prevalence. However, paradoxically, in a few low-income countries such as Pakistan and India, the treatment gap estimated from lifetime prevalence was smaller than some or all of the gap estimates based on active epilepsy prevalence.
Fig. 3.
Epilepsy treatment gap (%) and standard errors calculated from lifetime prevalence estimates
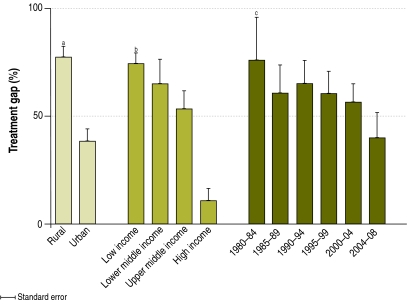
For the analysis of the variation in the treatment gap, only studies estimating the gap from individuals with active epilepsy were used. In these studies, rural populations had treatment gaps nearly twice as high as populations from towns or from suburban, semi-urban or urban locations (rate ratio, RR: 2.01; 95% confidence interval, CI: 1.40–2.89; Z: 3.77; P < 0.001) (Fig. 4). For example, in India the treatment gap ranged from 40 to 90% in rural areas and from 22 to 50% in mixed, suburban and urban populations.18,21,24,25 Similar trends were observed in Brazil, China, Pakistan and Togo. However, there were a few exceptions: in a rural population from Rajasthan, India, the treatment gap was 40% (the third lowest in India),25 while in a rural population of Mali it was 65% (versus 76% in an urban population).26,27
Fig. 4.
Mean epilepsy treatment gap (%) and standard errors by rural/urban status, World Bank income category and year data collected
a RR = 2.01; 95% CI: 1.40–2.89, P < 0.001
b RR = 1.55; 95% CI: 1.32–1.82, P < 0.001
c RR = 0.92; 95% CI: 0.79–1.07, P = 0.28
There was a significant trend towards larger epilepsy treatment gaps in countries with lower incomes; for every one-level decrease in World Bank income category, the treatment gap increased by a factor of 1.55 (95% CI: 1.32–1.82; Z: 5.34; P < 0.001) (Fig. 4). However, within high-income countries, larger gaps were found in select populations. In a small sample from Spain, the treatment gap was greater than 50%, while among the Guaymi Indians in Panama the gap was 100%.28,29 Similarly, select populations in low-income countries had surprisingly small gaps; suburban and urban populations in India, Madagascar and Senegal had treatment gaps of less than 30%.21,30–32
Direct comparisons of treatment gaps over time were difficult to carry out because of differences in study methods and populations. In Ethiopia, two studies in the same population in which the same methods were used showed a gap of 98% in 1986–1988 and a gap of 87% among new cases identified during a repeat survey in 1990.33,34 Overall, treatment gaps decreased from 1980 to the present, but no significant trend over time was detected (RR: 0.92; 95% CI: 0.79–1.07; Z: −1.08; P = 0.28).
Discussion
The results of this systematic review of the literature suggest that there are dramatic global disparities in the care and treatment of epilepsy patients. Treatment gaps for active epilepsy exceeded 75% in most low-income countries and 50% in most lower middle- and upper middle-income countries. In stark contrast, many high-income countries had gaps of less than 10%. However, treatment gaps varied widely, both between and within countries.
Our search methods resulted in more comprehensive estimates of the epilepsy treatment gap than those employed in previous studies. First, our systematic and thorough search strategy and rigorous inclusion criteria ensured the quality of included studies. Second, our wider search strategy, which focused on epilepsy prevalence rather than on the treatment gap, captured 26 more studies than did a recent systematic review,16 even when we applied the same inclusion criteria. Third, our search of the non-English-language literature led to an additional 10 studies.
The subsequent analysis of the variation in the treatment gap showed significantly higher gaps in rural areas and lower-income countries. These findings are consistent with those for other health indicators, such as the rates of vaccination coverage and of maternal, infant and under-five mortality, which suggest wide disparities in care between rural and urban areas and between high- and low-income countries.35–40 On the other hand, epilepsy treatment gaps have decreased from 1980 to the present, though the trend is not statistically significant.
While intriguing, these preliminary analyses do not fully explain the variation in the treatment gap, which may additionally reflect local or regional differences in access to and quality of epilepsy care or in the availability of individual or regional economic resources.13,16 In addition, cultural differences in the stigma associated with epilepsy may determine whether an individual seeks care for epilepsy or not.2,6
In our analysis, we found that the treatment gap varied widely both within and between countries and that it was significantly associated with country income classification and a population’s status as urban or rural. Similarly, prior studies of the gap demonstrated significant heterogeneity in treatment gap estimates.16 The wide variation among estimates as well as the systematic variation as a function of selected covariates suggests that meta-analytic techniques may not be appropriate for obtaining overall population estimates of the epilepsy treatment gap. Further study into the influence of macroeconomic and microeconomic factors and of resources for the care of people with epilepsy and other neurologic disorders will be critical to understanding the reasons for this heterogeneity. Accounting for the systematic variation in the gap is essential to creating summary estimates of the gap. Combining demographic approaches with multiple imputation techniques could generate more representative gap estimates.
Our data set had several limitations. First, our sample was limited because we excluded epilepsy prevalence studies that did not collect treatment information (nearly half of those identified) or that calculated the gap from a potentially biased sample, such as clinic or hospital patients. Using lifetime prevalence to calculate the gap could have resulted in an overestimate, so we only included data on lifetime prevalence for descriptive purposes.
Furthermore, our ability to generate national treatment gap estimates was limited. Most treatment gap estimates were based on data from selected populations that were not representative of the nation as a whole. A sample not representative of the population was not a criterion for exclusion because it was a limitation of nearly all the studies reviewed. Among the studies we included were several performed in a rural or urban area only,34,41 among the elderly or children exclusively,27,42,43 in areas with a high prevalence of epilepsy,44 in military22,45 or school populations,46 or in regions populated by only one or a few ethnic groups.7,47 Likewise, several included studies had been conducted in ethnic or social groups that differed from the population of the country as a whole. Examples include the Parsi community in India,21 the Bakairi indians from Brazil,44 the Zay society in Ethiopia48 or the Guaymi indians of Panama.28 Therefore, caution should be exercised in extrapolating treatment gap estimates from such select populations to the country as a whole without proper adjustment.
Although we tried to minimize variation by means of our inclusion criteria, study methods – case ascertainment, sampling technique, the definitions of active epilepsy and of adequate treatment, etc. – differed widely among studies. The quality and comparability of treatment gap data could be improved by applying standard definitions for adequate treatment and active epilepsy and by using more nationally representative population-based samples to generate active epilepsy prevalence and estimate the treatment gap. Better insight into the causes of this gap would be obtained if epilepsy prevalence studies routinely collected information on other sociodemographic characteristics, the availability and accessibility of local or regional health services and treatment, and the stigma associated with seeking care.
Conclusion
In summary, our systematic review of the epilepsy treatment gap worldwide shows a dramatic global disparity in the care of epilepsy patients between high- and low-income countries and between rural and urban settings. Epilepsy is a common and potentially serious neurological disorder that can be diagnosed and treated inexpensively. Historically, epilepsy has received little public health attention despite poor health outcomes and potentially devastating social consequences from untreated disease. In recent years, many countries have undertaken initiatives to decrease the epilepsy treatment gap, notably the demonstration projects such as the Global Campaign Against Epilepsy, conducted jointly by the International League against Epilepsy, the International Bureau for Epilepsy and the World Health Organization. Large community based trials in Brazil and China have demonstrated that epilepsy can be treated with inexpensive and effective drugs at the community level by primary health professionals with basic training.5,49 Increased commitment by the global health community is needed to reduce the treatment gap and thereby reduce the potentially devastating social consequences and poor health outcomes resulting from untreated epilepsy.
Acknowledgements
We thank John Boscardin, Associate Professor of Medicine and Biostatistics, for his help with the statistical analysis.
Funding:
Ana-Claire Meyer: Veterans Affairs/Robert Wood Johnson Clinical Scholars Program, American Academy of Neurology Practice Research Training Fellowship. Gretchen Birbeck: The Global Burden of Diseases, Injuries, and Risk Factors Study.
Competing interests:
None declared.
References
- 1.Leonardi M, Ustun T. The global burden of epilepsy. Epilepsia. 2002;43(Suppl 6):21–5. doi: 10.1046/j.1528-1157.43.s.6.11.x. [DOI] [PubMed] [Google Scholar]
- 2.de Boer HM, Mula M, Sander JW. The global burden and stigma of epilepsy. Epilepsy Behav. 2008;12:540–6. doi: 10.1016/j.yebeh.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Kwan P, Brodie M. Phenobarbital for the treatment of epilepsy in the 21st century: a critical review. Epilepsia. 2004;45:1141–9. doi: 10.1111/j.0013-9580.2004.12704.x. [DOI] [PubMed] [Google Scholar]
- 4.Chisholm D, WHO-CHOICE Cost-effectiveness of first-line antiepileptic drug treatments in the developing world: a population-level analysis. Epilepsia. 2005;46:751–9. doi: 10.1111/j.1528-1167.2005.52704.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang WZ, Wu J, Ma G, Dai X, Yang B, Wang T, et al. Efficacy assessment of phenobarbital in epilepsy: a large community-based intervention trial in rural China. Lancet Neurol. 2006;5:46–52. doi: 10.1016/S1474-4422(05)70254-4. [DOI] [PubMed] [Google Scholar]
- 6.Baskind R, Birbeck GL. Epilepsy-associated stigma in sub-Saharan Africa: the social landscape of a disease. Epilepsy Behav. 2005;7:68–73. doi: 10.1016/j.yebeh.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Birbeck G, Chomba E, Atadzhanov M, Mbewe E, Haworth A. The social and economic impact of epilepsy in Zambia: a cross-sectional study. Lancet Neurol. 2007;6:39–44. doi: 10.1016/S1474-4422(06)70629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birbeck GL. Seizures in rural Zambia. Epilepsia. 2000;41:277–81. doi: 10.1111/j.1528-1157.2000.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 9.Jilek-Aall L, Rwiza H. Prognosis of epilepsy in a rural African community: a 30 year follow-up of 164 patients in an outpatient clinic in rural Tanzania. Epilepsia. 1992;33:645–50. doi: 10.1111/j.1528-1157.1992.tb02341.x. [DOI] [PubMed] [Google Scholar]
- 10.Amoroso C, Zwi A, Somerville E, Grove N. Epilepsy and stigma. Lancet. 2006;367:1143–4. doi: 10.1016/S0140-6736(06)68503-6. [DOI] [PubMed] [Google Scholar]
- 11.Jacoby A, Snape D, Baker G. Epilepsy and social identity: the stigma of a chronic neurological disorder. Lancet Neurol. 2005;4:171–8. doi: 10.1016/S1474-4422(05)01014-8. [DOI] [PubMed] [Google Scholar]
- 12.Ding D, Wang W, Wu J, Ma G, Dai X, Yang B, et al. Premature mortality in people with epilepsy in rural China: a prospective study. Lancet Neurol. 2006;5:823–7. doi: 10.1016/S1474-4422(06)70528-2. [DOI] [PubMed] [Google Scholar]
- 13.Kale R. Global Campaign against Epilepsy: the treatment gap. Epilepsia. 2002;43(Suppl 6):31–3. doi: 10.1046/j.1528-1157.43.s.6.13.x. [DOI] [PubMed] [Google Scholar]
- 14.Begley CE, Baker GA, Beghi E, Butler J, Chisholm D, Langfitt J, et al. Cross-country measures for monitoring epilepsy care. Epilepsia. 2007;48:990–1001. doi: 10.1111/j.1528-1167.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- 15.Diop AG, de Boer HM, Mandlhate C, Prilipko L, Meinardi H. The global campaign against epilepsy in Africa. Acta Trop. 2003;87:149–59. doi: 10.1016/S0001-706X(03)00038-X. [DOI] [PubMed] [Google Scholar]
- 16.Mbuba CK, Ngugi AK, Newton CR, Carter JA. The epilepsy treatment gap in developing countries: a systematic review of the magnitude, causes, and intervention strategies. Epilepsia. 2008 doi: 10.1111/j.1528-1167.2008.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaamugisha J, Feksi AT. Determining the prevalence of epilepsy in the semi-urban population of Nakuru, Kenya, comparing two independent methods not apparently used before in epilepsy studies. Neuroepidemiology. 1988;7:115–21. doi: 10.1159/000110144. [DOI] [PubMed] [Google Scholar]
- 18.Pal DK, Das T, Sengupta S. Comparison of key informant and survey methods for ascertainment of childhood epilepsy in West Bengal, India. Int J Epidemiol. 1998;27:672–6. doi: 10.1093/ije/27.4.672. [DOI] [PubMed] [Google Scholar]
- 19.Haerer AF, Anderson DW, Schoenberg BS. Prevalence and clinical features of epilepsy in a biracial United States population. Epilepsia. 1986;27:66–75. doi: 10.1111/j.1528-1157.1986.tb03503.x. [DOI] [PubMed] [Google Scholar]
- 20.World development indicators Washington, DC: The World Bank; 2006. [Google Scholar]
- 21.Bharucha NE, Bharucha EP, Bharucha AE, Bhise AV, Schoenberg BS. Prevalence of epilepsy in the Parsi community of Bombay. Epilepsia. 1988;29:111–5. doi: 10.1111/j.1528-1157.1988.tb04405.x. [DOI] [PubMed] [Google Scholar]
- 22.Kun LN, Ling LW, Wah YW, Lian TT. Epidemiologic study of epilepsy in young Singaporean men. Epilepsia. 1999;40:1384–7. doi: 10.1111/j.1528-1157.1999.tb02009.x. [DOI] [PubMed] [Google Scholar]
- 23.Nicoletti A, Reggio A, Bartoloni A, Failla G, Sofia V, Bartalesi F, et al. Prevalence of epilepsy in rural Bolivia: a door-to-door survey. Neurology. 1999;53:2064–9. doi: 10.1212/wnl.53.9.2064. [DOI] [PubMed] [Google Scholar]
- 24.Hackett RJ, Hackett L, Bhakta P. The prevalence and associated factors of epilepsy in children in Calicut District, Kerala, India. Acta Paediatr. 1997;86:1257–60. doi: 10.1111/j.1651-2227.1997.tb14857.x. [DOI] [PubMed] [Google Scholar]
- 25.Sureka RK, Sureka R. Prevalence of epilepsy in rural Rajasthan — a door-to-door survey. J Assoc Physicians India. 2007;55:741–2. [PubMed] [Google Scholar]
- 26.Farnarier G, Diop S, Coulibaly B, Arborio S, Dabo A, Diakite M, et al. Onchocerciasis and epilepsy. Epidemiological survey in Mali Med Trop (Mars) 200060151–5.French [PubMed] [Google Scholar]
- 27.Traoré M, Tahny R, Sacko M.Prevalence de l'epilepsie chez les enfants de 3 a 15 ans dans 2 communes du district de Bamako Rev Neurol (Paris) 2000156Suppl 1S18French [Google Scholar]
- 28.Gracia F, de Lao SL, Castillo L, Larreategui M, Archbold C, Brenes MM, et al. Epidemiology of epilepsy in Guaymi Indians from Bocas del Toro Province, Republic of Panama. Epilepsia. 1990;31:718–23. doi: 10.1111/j.1528-1157.1990.tb05512.x. [DOI] [PubMed] [Google Scholar]
- 29.Cruz Gutierrez-del-Olmo M, Schoenberg BS, Portera-Sanchez A. Prevalence of neurological diseases in Madrid, Spain. Neuroepidemiology. 1989;8:43–7. doi: 10.1159/000110164. [DOI] [PubMed] [Google Scholar]
- 30.Ndoye NF, Sow AD, Diop AG, Sessouma B, Sene-Diouf F, Boissy L, et al. Prevalence of epilepsy its treatment gap and knowledge, attitude and practice of its population in sub-urban Senegal an ILAE/IBE/WHO study. Seizure. 2005;14:106–11. doi: 10.1016/j.seizure.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Andriantseheno L, Ralaizandriny D. Prevalence communautaire de l'epilepsie chez les Malgaches. Epilepsies. 2004;16:83–6. [Google Scholar]
- 32.Radhakrishnan K, Pandian JD, Santhoshkumar T, Thomas SV, Deetha TD, Sarma PS, et al. Prevalence, knowledge, attitude, and practice of epilepsy in Kerala, South India. Epilepsia. 2000;41:1027–35. doi: 10.1111/j.1528-1157.2000.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 33.Tekle-Haimanot R, Forsgren L, Abebe M, Gebre-Mariam A, Heijbel J, Holmgren G, et al. Clinical and electroencephalographic characteristics of epilepsy in rural Ethiopia: a community based study. Epilepsy Res. 1990;7:230–9. doi: 10.1016/0920-1211(90)90020-V. [DOI] [PubMed] [Google Scholar]
- 34.Tekle-Haimanot R, Forsgren L, Ekstedt J. Incidence of epilepsy in rural central Ethiopia. Epilepsia. 1997;38:541–6. doi: 10.1111/j.1528-1157.1997.tb01138.x. [DOI] [PubMed] [Google Scholar]
- 35.Bender DE, Rivera T, Madonna D. Rural origin as a risk factor for maternal and child health in periurban Bolivia. Soc Sci Med. 1993;37:1345–9. doi: 10.1016/0277-9536(93)90164-Y. [DOI] [PubMed] [Google Scholar]
- 36.Anand S, Barnighausen T. Human resources and health outcomes: cross-country econometric study. Lancet. 2004;364:1603–9. doi: 10.1016/S0140-6736(04)17313-3. [DOI] [PubMed] [Google Scholar]
- 37.Anand S, Barnighausen T. Health workers and vaccination coverage in developing countries: an econometric analysis. Lancet. 2007;369:1277–85. doi: 10.1016/S0140-6736(07)60599-6. [DOI] [PubMed] [Google Scholar]
- 38.Hobcraft JN, McDonald J, Rutstein S. Socio-economic factors in infant and child mortality: a cross-national comparison. Popul Stud. 1984;38:193–223. doi: 10.2307/2174073. [DOI] [PubMed] [Google Scholar]
- 39.Senior M, Williams H, Higgs G. Urban-rural mortality differentials: controlling for material deprivation. Soc Sci Med. 2000;51:289–305. doi: 10.1016/S0277-9536(99)00454-2. [DOI] [PubMed] [Google Scholar]
- 40.Sastry N. What explains rural-urban differentials in child mortality in Brazil? Soc Sci Med. 1997;44:989–1002. doi: 10.1016/S0277-9536(96)00224-9. [DOI] [PubMed] [Google Scholar]
- 41.Gomes Md Mda M, Zeitoune RG, Kropf LA, Beeck Ed Eda S. A house-to-house survey of epileptic seizures in an urban community of Rio de Janeiro, Brazil. Arq Neuropsiquiatr. 2002;60(3-B):708–11. doi: 10.1590/s0004-282x2002000500004. [DOI] [PubMed] [Google Scholar]
- 42.Kurtz Z, Tookey P, Ross E. Epilepsy in young people: 23 year follow up of the British national child development study. BMJ. 1998;316:339–42. doi: 10.1136/bmj.316.7128.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de la Court A, Breteler MM, Meinardi H, Hauser WA, Hofman A. Prevalence of epilepsy in the elderly: the Rotterdam Study. Epilepsia. 1996;37:141–7. doi: 10.1111/j.1528-1157.1996.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 44.Borges MA, Barros EP, Zanetta DM, Borges AP. Prevalence of epilepsy in Bakairi indians from Mato Grosso State, Brazil. Arq Neuropsiquiatr. 2002;60:80–5. doi: 10.1590/s0004-282x2002000100014. [DOI] [PubMed] [Google Scholar]
- 45.Jallon P. Evaluation du taux de prevalence de l'epilepsie dans un centre de selecion de l'armee. Rev Neurol. 1991;147:319–22. [PubMed] [Google Scholar]
- 46.Somoza MJ, Forlenza RH, Brussino M, Licciardi L. Epidemiological survey of epilepsy in the primary school population in Buenos Aires. Neuroepidemiology. 2005;25:62–8. doi: 10.1159/000086285. [DOI] [PubMed] [Google Scholar]
- 47.Edwards T, Scott AG, Munyoki G, Odera VM, Chengo E, Bauni E, et al. Active convulsive epilepsy in a rural district of Kenya: a study of prevalence and possible risk factors. Lancet Neurol. 2008;7:50–6. doi: 10.1016/S1474-4422(07)70292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almu S, Tadesse Z, Cooper P, Hackett R. The prevalence of epilepsy in the Zay Society, Ethiopia, an area of high prevalence. Seizure. 2006;15:211–3. doi: 10.1016/j.seizure.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Li LM, Fernandes P, Noronha A, Marques L, Borges M, Borges K, et al. Demonstration project on epilepsy in Brazil: outcome assessment. Arq Neuropsiquiatr. 2007;65(Suppl 1):58–62. doi: 10.1590/s0004-282x2007001000010. [DOI] [PubMed] [Google Scholar]