Abstract
Objective
To investigate the poorly understood relationship between the process of urbanization and noncommunicable diseases (NCDs) through the application of a quantitative measure of urbanicity.
Methods
We constructed a measure of the urban environment for seven areas using a seven-item scale based on data from the Census of India 2001 to develop an “urbanicity” scale. The scale was used in conjunction with data collected from 3705 participants in the World Health Organization’s 2003 STEPwise risk factor surveillance survey in Tamil Nadu, India, to analyse the relationship between the urban environment and major NCD risk factors. Linear and logistic regression models were constructed examining the relationship between urbanicity and chronic disease risk.
Findings
Among men, urbanicity was positively associated with smoking (odds ratio, OR: 3.54; 95% confidence interval, CI: 2.4–5.1), body mass index (OR: 7.32; 95% CI: 4.0–13.6), blood pressure (OR: 1.92; 95% CI: 1.4–2.7) and low physical activity (OR: 3.26; 95% CI: 2.5–4.3). Among women, urbanicity was positively associated with low physical activity (OR: 4.13; 95% CI: 3.0–5.7) and high body mass index (OR: 6.48; 95% CI: 4.6–9.2). In both sexes urbanicity was positively associated with the mean number of servings of fruit and vegetables consumed per day (P < 0.05).
Conclusion
Urbanicity is associated with the prevalence of several NCD risk factors in Tamil Nadu, India.
ملخص
الغرض
تقصّي العلاقة التي تفتقر إلى الفهم الواضح بين عملية التحضر والأمراض غير السارية من خلال تطبيق قياس كمي للتحضر.
الطريقة
أعد الباحثون قياساً للبيئة الحضرية لسبع مناطق مستخدمين مقياساً يضم سبعة بنود تستند إلى بيانات الإحصاء الهندي لعام 2001 لإعداد مقياس "التحضر". وقد استُخدِم هذا المقياس مقترناً مع البيانات التي جمعت من 3705 من المشاركين في مسح ترصد عوامل الاختطار التدرجي لمنظمة الصحة العالمية لعام 2003 في تاميل-نادو في الهند، لتحليل العلاقة بين البيئة الحضرية وعوامل الاختطار الكبرى للأمراض غير السارية. وأُعِدَت نماذج خطية ونماذج تحوف لوجستية لفحص العلاقة بين التحضر ومخاطر الأمراض المزمنة.
الموجودات
ارتبط التحضر، بين الرجال، ارتباطاً إيجابياً بالتدخين (نسبة الأرجحية OR: 3.54؛ فاصلة الثقة 95%: 2.4 – 5.1)، وبمنسب كتلة الجسم (نسبة الأرجحية: 7.32؛ فاصلة الثقة 95%: 4.0 - 13.6)، وبضغط الدم (نسبة الأرجحية: 1.92؛ فاصلة الثقة 95%:1.4 – 2.7)، وبالكسل البدني (نسبة الأرجحة: 3.26؛ فاصلة الثقة 95%: 2.5 – 4.3). وارتبط التحضر، بين النساء، ارتباطاً إيجابياً بالكسل البدني (نسبة الأرجحة:4.13 ؛ فاصلة الثقة 95%: 5.7 – 3.0) وبمنسب كتلة الجسم (نسبة الأرجحية 6.48؛ فاصلة الثقة 95%: 4.6 – 9.2). وارتبط التحضر بين كلا الجنسين ارتباطاً إيجابياً بمتوسط عدد حصص الفرد من الاستهلاك اليومي للفاكهة والخضراوات (قيمة الاحتمال P أقل من 0.05).
الاستنتاج
يرتبط التحضر بانتشار العديد من عوامل اختطار الأمراض غير السارية في تاميل-نادو بالهند.
Résumé
Objectif
Étudier la relation encore mal comprise entre le processus d'urbanisation et les maladies non transmissibles (MNT) à travers l'application d'une mesure quantitative de l'urbanisation.
Méthodes
Nous avons construit une mesure de l'environnement urbain pour sept zones, en utilisant une échelle à sept degrés fondée sur les données du recensement indien de 2001, afin d'obtenir une échelle de l'urbanisation. Cette échelle a été employée en association avec les données recueillies auprès de 3705 participants à une enquête de surveillance des facteurs de risque STEPwise, réalisée en 2003 par l'Organisation mondiale de la Santé à Tamil Nadu (Inde) pour analyser la relation entre environnement urbain et facteurs de risque majeurs de MNT. Nous avons élaboré des modèles de régression linéaire et logistique permettant d'étudier la relation entre urbanisation et risque de maladie chronique.
Résultats
Chez les hommes, il existait une association positive entre urbanisation d'une part et tabagisme (Odds ratio, OR : 3,54 ; intervalle de confiance à 95 % : IC : 2,4-5,1), indice de masse corporelle (OR : 7,32 ; IC à 95 % : 4,0-13,6) et pression artérielle (OR : 1,92 ; IC à 95 % : 1,4-2,7), d'autre part, ainsi qu'avec la faiblesse de l'activité physique (OR : 3,26 ; IC à 95 % : 2,5-4,3). Chez les femmes, l'urbanisation était corrélée avec une faible activité physique(OR : 4,13 ; IC à 95 % : 3,0-5,7) et avec un indice de masse corporelle élevé (OR : 6,48 ; IC à 95 % : 4,6-9,2). Chez les deux sexes, l'urbanisation présentait une association positive avec le nombre moyen de portions de fruits ou de légumes consommées par jour (p < 0,05).
Conclusion
L'urbanisation est associée à la prévalence de plusieurs facteurs de risque de MNT à Tamil Nadu en Inde.
Resumen
Objetivo
Investigar la mal entendida relación entre el proceso de urbanización y las enfermedades no transmisibles (ENT) aplicando una medida cuantitativa del grado de urbanización.
Métodos
Hemos desarrollado un indicador del medio ambiente urbano en siete zonas usando una escala de siete puntos a partir de datos del Censo de la India de 2001 para establecer una escala de "urbanicidad". La escala fue utilizada junto con datos referentes a 3705 personas que participaron en la encuesta STEPwise de vigilancia de factores de riesgo llevada a cabo en 2003 por la Organización Mundial de la Salud en Tamil Nadu, India, a fin de analizar la relación entre el entorno urbano y los principales factores de riesgo de ENT. Se construyeron modelos lineales y de regresión logística para estudiar la relación entre urbanicidad y riesgo de enfermedades crónicas.
Resultados
Entre los hombres, la urbanicidad se asoció positivamente al tabaquismo (razón de posibilidades, OR: 3,54, intervalo de confianza del 95%: 2,4-5,1), el índice de masa corporal (OR: 7,32, IC95%: 4,0-13,6), la tensión arterial (OR: 1,92, IC95%: 1,4-2,7) y una baja actividad física (OR: 3,26, IC95%: 2,5-4,3). Entre las mujeres, la urbanicidad se asoció positivamente a una baja actividad física (OR: 4,13, IC95%: 3,0-5,7) y un índice de masa corporal elevado (OR: 6,48, IC95%: 4,6-9,2). En ambos sexos, la urbanicidad se asoció positivamente al número medio de porciones de fruta y verduras consumidas al día (P < 0,05).
Conclusión
La urbanicidad está asociada a la prevalencia de varios factores de riesgo de ENT en Tamil Nadu, India.
Introduction
Over the last few decades, traditional societies in many developing countries have experienced rapid and unplanned urbanization, which has led to lifestyles characterized by unhealthy nutrition, reduced physical activity and tobacco consumption.1 These unhealthy lifestyles are associated with common modifiable risk factors for chronic diseases such as hypertension, diabetes mellitus, dyslipidaemia and obesity.2
It is expected that by 2020 in developing countries, noncommunicable diseases (NCDs) will account for 69% of all deaths, with cardiovascular diseases in the lead.3 The prevalence of diabetes mellitus will almost double in the next 25 years and at least 75% of those affected will be in developing countries. The burden of disease will be worse in these countries, as the majority of sufferers are expected to be relatively young, of lower socioeconomic status and to suffer from severe disease of premature onset.4
Using the dichotomous United Nations definition of urbanization (based on country specific definitions using one or more of population density, population size or administrative division) for more than 100 countries, Ezatti et al.5 found that both body mass index (BMI) and blood cholesterol levels rose rapidly in tandem with increases in national income and level of urbanization. Work undertaken in Sri Lanka shows a greater increase in BMI and other risk factors for cardiovascular disease among urban dwellers than among their rural counterparts.6
Timely interventions in those stages of development in which environmental conditions shift and common modifiable risk factors emerge may help prevent and control chronic disease. It is important to identify these crucial stages and to determine what elements of urbanization are linked to the emergence of risk factors. A greater understanding of these relationships may help us identify interventions that are most likely to be effective in preventing NCDs in countries undergoing rapid urbanization and improve our capacity to stem the rapid increase in NCDs. The objectives of this study were to: establish the feasibility of collecting a multi-component scale of urbanicity in Tamil Nadu, India; and, examine the relationships between urbanicity and chronic disease risk in Tamil Nadu, India.
Methods
The study was based on the working hypothesis that urbanicity, defined as the level of urbanization in a given locality,7 is associated with risk factors for chronic disease. It was conducted in three steps: (i) constructing a measure of urbanicity using a validated scale8 based on data from the Census of India 2001; (ii) calculating the prevalence of NCD risk factors in seven study areas in the state of Tamil Nadu, India, by using data from an NCD risk factor surveillance survey conducted locally in 2003–2004 as part of a larger study9; and (iii) testing for an association between urbanicity and the prevalence of NCD risk factors in the study areas.
Setting
The urban arm of this study was set in Chennai (formerly Madras) and the rural arm was set in six settlements (Agaram, Chunampet, Illeedu, Pudupattu, Puthiram Kottai and Vanniyanallur) in the Kancheepuram district, around 120 km south of Chennai.
Measuring urbanicity
An existing composite continuous measure of urbanicity previously used and validated for the Philippines by Dahly and Adair8,10 was identified through an earlier systematic review.11 It comprised seven elements: population size, population density, access to markets, communications, transport, education and health services. We replicated it in its entirety for three items – population size, population density and education – and modified the remaining four elements to better suit the Indian context. We assigned a maximum of 10 points to each item of the adapted scale, with a resulting range from 0 (no urbanicity) to 70 (high urbanicity) points. The scale is shown in Appendix A (available at: http://www.co-ops.net.au/File.axd?id=1a5a5f74-6be2-4dc4-9f2d-81319c0b8538).
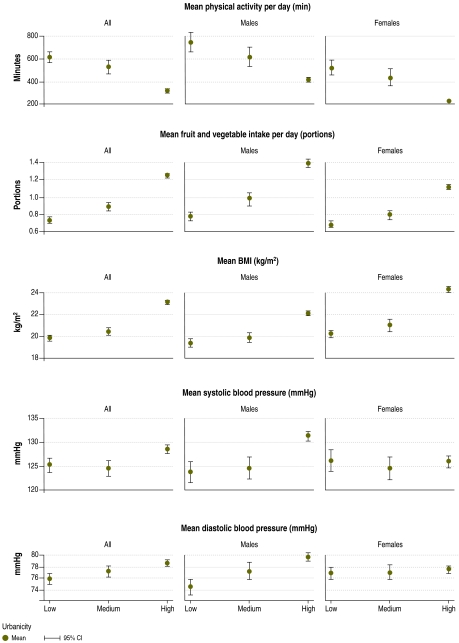
Fig. 1.
Mean of noncommunicable disease risk factor variables in low, middle and high urbanicity groups in seven locationsa in Tamil Nadu, India, 2008
BMI, body mass index; CI, confidence interval.
a Agaram, Chennai, Chunampet, Illeedu, Pudupattu, Puthiram Kottai and Vanniyanallur.
We conducted validity testing on the scale and obtained a Cronbach’s α reliability coefficient of 0.72. Urbanicity as measured by this scale appeared to be consistent with a pre-existing governmental definition of “urban” and “rural”. Results from a full validity study are in preparation.
We took scale data from the 2001 Census of India, which had collected data on amenities in villages and towns throughout the country, including Tamil Nadu.12 It provided data for each administrative area and considered Chennai to be a single administrative area. Data from the survey provided us with information on population size and density, education, health services and access to markets at each study location. As relevant data were collected by the amenities survey for rural, but not urban areas, we took communications and transport data from the relevant municipal authority.
Face validity for the scale was assessed using a photograph taken in the main street of each study location (Appendix B, available at: http://www.co-ops.net.au/File.axd?id=22bb4a17-ca19-4cc5-87a0-e50ccee39d7b). After we quantified urbanicity, each location was classified into three urbanicity groups depending on its score: low (0 to < 24), medium (24 to < 46) and high (46–70). In this way, urbanicity was treated as an ordered categorical variable.
Prevalence of NCD risk factors
We took chronic disease risk factor data from the Indian NCD risk factor surveillance study carried out by the World Health Organization (WHO) and the Indian Council of Medical Research.9 The survey used the validated WHO STEPwise approach to Surveillance questionnaire13 and included information on tobacco use, diet (fruit and vegetable consumption), physical activity, weight and blood pressure. The questionnaire was pretested and modified to fit Indian conditions after pilot testing on a subpopulation in each centre. Data were also collected on the age, sex, level of education and occupation of each participant. The survey also collected address and postcode information from participants and this information was used to link area-level data from the urbanicity scale to individual risk factor data.
Biological and anthropological risk factors were assessed by measuring the height, weight and blood pressure of each participant. The BMI (kg/m2) was calculated from the height and weight of each participant. Blood pressure was recorded in the right arm, in a sitting position, to the nearest 1 mmHg, using an electronic Omron blood pressure monitor. Two readings were taken 5 minutes apart and the mean of the two was taken as the blood pressure.
Study sample groups
For the WHO surveillance project, two samples were required: a rural sample of 2500 individuals and an urban sample of the same size. A 10% non-response rate was permitted.
For the urban component, two of the 155 wards in Chennai were randomly chosen using computer-generated numbers. Next, streets within the ward were randomly allocated using a computer programme and a house-to-house survey was conducted in each selected street until the urban sample of 2500 participants was achieved.
For the rural component, a purposive sample of 33 villages in Kancheepuram district was undertaken to recruit the 2500 rural participants. Of the 33 villages in the original WHO study, six villages and one small rural town (Chunampet) were selected for our study, representing, for us, 1321 possible rural participants. These villages were selected to provide heterogeneity in, and data for, the urbanicity scale.
Participants were eligible if they were aged 15–64 years and had resided in the household for at least 6 months at the time of survey. When more than one individual in the household fulfilled the criteria, lots were drawn to select one participant per home. If the selected household did not have an eligible individual, the next household was contacted. Data on 3705 participants (97%) from the sample population (3821) were obtained, comprising a response rate of 86% within the rural sample and 100% within the urban sample.
Participants were stratified by sex and 10-year age group from both urban and rural areas, with 250 participants in each stratum. Data were collected regardless of what day of the week it was. The study was undertaken based on the methods adapted from the WHO global STEPwise approach for NCD risk factor surveillance.13
Risk factor definition
Tobacco use was defined as reported current daily smoking of tobacco; low fruit and vegetable consumption as < 5 servings of fruit and vegetables per day; low physical activity as < 150 minutes of moderate physical activity per week, high BMI as a BMI ≥ 25 kg/m2; and high blood pressure as a systolic blood pressure ≥ 140 mmHg and/or a diastolic blood pressure ≥ 90 mmHg.
Potential confounders and effect modifiers
Preliminary analysis identified clear differences in risk factor prevalence between men and women, so subsequent analysis was stratified by gender. Age is a well-established potential confounder in chronic disease studies, and crude gender-specific models were subsequently adjusted for age.
Outcome variables
Following WHO guidelines, we calculated risk factor prevalence for each outcome within each study area. In addition, we calculated the mean number of servings of fruit and vegetables per day (based on the WHO STEPwise approach), mean time (minutes) spent in physical activity per week, mean BMI, mean systolic blood pressure and mean diastolic blood pressure.13
Statistical methods
Initial analysis produced descriptive statistics for each group. For continuous exposure data of normal distribution, a one way analysis of variance (ANOVA) test was used to analyse the relationship between outcome variables from each of the urbanicity groups. A Kruskal-Wallis one-way ANOVA test was used for continuous exposure data not normally distributed. Normality was assessed by reviewing histograms and normal probability–probability plots of the data. A Mantel-Haenszel χ2 test and regression analyses were used to estimate the effect of urbanicity controlled for potential confounders and to assess for effect modification. Linear (in the case of continuous outcome variables) and logistic (in the case of binary outcome variables) regression methods were used to explore differences between the groups, with adjustment for confounding variables. Data assumptions related to linear regression methods were checked. All statistical analyses were performed using STATA, version 10.0 (StataCorp LP, College Station, TX, USA).
Results
Urbanicity scores
Urbanicity scores ranged from 14 to 68. Table 1 shows the grouping of the seven study sites into three levels of urbanicity. Only Chennai had a high population density. Most areas had access to various modes of communication and transport. Education was evenly distributed and most localities had poor access to health services.
Table 1. Urbanicity scores for seven locations in Tamil Nadu, India, 2001.
| Locality | Size | Density | Access to markets | Communication | Transport | Education | Health | Total points | Urbanicity |
|---|---|---|---|---|---|---|---|---|---|
| llleedu | 3 | 1 | 1 | 5 | 4 | 0 | 0 | 14 | Low |
| Agaram | 2 | 1 | 1 | 5 | 6 | 2 | 0 | 17 | Low |
| Pudupattu | 2 | 1 | 0 | 5 | 8 | 2 | 0 | 18 | Low |
| Vanniyanallur | 2 | 1 | 0 | 8 | 6 | 2 | 0 | 19 | Low |
| Puthiram Kottai | 3 | 1 | 2 | 8 | 6 | 4 | 1 | 25 | Medium |
| Chunampet | 6 | 1 | 3 | 9 | 6 | 6 | 6 | 37 | Medium |
| Chennai | 10 | 8 | 10 | 10 | 10 | 10 | 10 | 68 | High |
Demographic characteristics
For age, each group had a similar mean (38.5–39.4 years), standard deviation (14.2–14.9 years) and range (15–64 years) (Table 2). The educational status of the groups increased with increasing urbanicity: 47.6% of those in the low urbanicity group had some schooling compared with 62.0% in the medium urbanicity group and 82.0% in the high urbanicity group. There were also differences in the work status of the groups. There were a higher proportion of homemakers (those whose daily work typically involves unpaid duties within the home) and people in professional or skilled working categories in the most urban areas when compared with the least urban areas. The proportion of unskilled and landless labourers was lower in the most urban areas when compared to the least urban areas.
Table 2. Demographic characteristics of participants in study of noncommunicable disease risk factors, by urbanicity group, in seven locationsa in Tamil Nadu, India, 2003.
| Characteristic | Urbanicity group | |||
|---|---|---|---|---|
| Low | Medium | High | Total | |
| Participants, no. (%) | 639 (17.2) | 500 (13.5) | 2566 (69.3) | 3705 |
| Age in years | ||||
| Mean | 38.9 | 38.5 | 39.4 | 39.2 |
| Standard deviation | 14.6 | 14.9 | 14.2 | 14.4 |
| Range | 15–64 | 15–64 | 15–64 | 15–64 |
| Sex, no. (%) | ||||
| Male | 269 (42.1) | 257 (51.4) | 1282 (50.0) | 1808 (48.8) |
| Female | 370 (57.9) | 243 (48.6) | 1284 (50.0) | 1897 (51.2) |
| Educational status, no. (%) | ||||
| No formal schooling | 325 (50.9) | 169 (33.8) | 327 (12.7) | 821 (22.2) |
| Some schooling | 304 (47.6) | 310 (62.0) | 2104 (82.0) | 2718 (73.4) |
| Graduate and above b | 10 (1.6) | 21 (4.2) | 135 (5.3) | 166 (4.5) |
| Work status, no. (%) | ||||
| Professional/clerical/business person | 8 (1.3) | 23 (4.6) | 178 (6.9) | 209 (5.6) |
| Self-employed/skilled | 20 (3.1) | 42 (8.4) | 389 (15.2) | 451 (12.2) |
| Unskilled/landless labourer | 394 (61.7) | 204 (40.8) | 537 (20.9) | 1135 (30.6) |
| Homemaker | 106 (16.6) | 121 (24.2) | 1040 (40.5) | 1267 (34.2) |
| Other (retired/student/unemployed) | 111 (17.4) | 110 (22.0) | 422 (16.5) | 643 (17.4) |
a Agaram, Chennai, Chunampet, Illeedu, Pudupattu, Puthiram Kottai and Vanniyanallur.
b Completed college/university or had a postgraduate degree
Urbanicity and risk factors
Smoking
There was an association between smoking prevalence and urbanicity for men but not for women (Table 3). Men in the high urbanicity group were three and a half times more likely to smoke daily than those in the low urbanicity group (Table 4). This trend remained significant after adjustment for age (P < 0.05). Only two women in the survey smoked daily and there was no association between female smoking prevalence and urbanicity (Table 4).
Table 3. Noncommunicable disease risk factor prevalence (%), by urbanicity group, in seven locationsa in Tamil Nadu, India, 2003.
| Risk factor | Urbanicity group | All |
Male |
Female |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | 95% CI | No. | % | 95% CI | No. | % | 95% CI | ||||
| Daily smoking | Low | 639 | 5.6 | 4.0–7.7 | 269 | 13.4 | 9.6–18.0 | 370 | 0.0 | – | ||
| Medium | 500 | 12.8 | 10.0–16.0 | 257 | 24.5 | 19.4–30.2 | 243 | 0.4 | 0–2.3 | |||
| High | 2566 | 17.7 | 16.2–19.2 | 1282 | 35.3 | 32.7–38.0 | 1284 | 0.1 | 0–0.4 | |||
| Low physical activityb | Low | 639 | 65.0 | 61.1–68.6 | 269 | 50.6 | 44.4–56.7 | 370 | 75.4 | 70.7–79.7 | ||
| Medium | 500 | 72.0 | 67.8–75.9 | 257 | 64.2 | 58.0–70.1 | 243 | 80.3 | 74.7–85.1 | |||
| High | 2566 | 84.8 | 83.4–86.2 | 1282 | 76.9 | 74.5–79.2 | 1284 | 92.7 | 91.1–94.0 | |||
| Low fruit and vegetable intakec | Low | 639 | 100.0 | – | 269 | 100.0 | – | 370 | 100.0 | – | ||
| Medium | 500 | 100.0 | – | 257 | 100.0 | – | 243 | 100.0 | – | |||
| High | 2566 | 99.9 | 99.7–100.0 | 1282 | 99.8 | 99.4–100.0 | 1284 | 100.0 | – | |||
| High BMId | Low | 639 | 7.7 | 5.7–10.0 | 269 | 4.1 | 2.1–7.2 | 370 | 10.3 | 7.4–13.8 | ||
| Medium | 500 | 13.8 | 10.9–17.1 | 257 | 10.1 | 6.7–14.5 | 243 | 17.7 | 13.1–23.1 | |||
| High | 2566 | 33.2 | 31.4–35.1 | 1282 | 23.8 | 21.5–26.2 | 1284 | 42.6 | 39.9–45.4 | |||
| High blood pressuree | Low | 639 | 19.1 | 16.1–22.4 | 269 | 17.8 | 13.5–23.0 | 370 | 20.0 | 16.0–24.4 | ||
| Medium | 500 | 20.2 | 16.8–24.0 | 257 | 21.0 | 16.2–26.5 | 243 | 19.3 | 14.6–24.9 | |||
| High | 2566 | 26.6 | 24.9–28.3 | 1282 | 29.4 | 26.9–32.0 | 1284 | 23.8 | 21.4–26.2 | |||
BMI, body mass index; CI, confidence interval.
a Agaram, Chennai, Chunampet, Illeedu, Pudupattu, Puthiram Kottai and Vanniyanallur.
b Defined as < 150 minutes of moderate physical activity per week.
c Defined as < 5 servings of fruit and vegetables per day (blank here since there was zero reporting for this category in this case).
d Defined as a BMI ≥ 25 kg/m2.
e Defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg.
Table 4. Crude odds ratios and age-adjusted odds ratios for noncommunicable disease risk factors, by urbanicity group, in seven locationsa in Tamil Nadu, India, 2003.
| Risk factor | Urbanicity group | Crude OR |
Age-adjusted OR |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | P for trend | OR | 95% CI | P for model | P for trend | |||
| Men | ||||||||||
| Daily smoking | Low | 1.0 | – | – | ** | 1.0 | – | – | ** | |
| Medium | 2.10 | 1.3–3.3 | * | – | 1.96 | 1.2–3.1 | * | – | ||
| High | 3.54 | 2.4–5.1 | ** | – | 3.43 | 2.4–5.0 | ** | – | ||
| Low physical activityb | Low | 1.0 | – | – | ** | 1.0 | – | – | ** | |
| Medium | 1.75 | 1.2–2.5 | * | – | 1.74 | 1.2–2.48 | * | – | ||
| High | 3.26 | 2.5–4.3 | ** | - | 3.22 | 2.5–4.2 | ** | – | ||
| Low fruit and vegetable intakec | Low | – | – | – | NS | – | – | – | ||
| Medium | – | – | – | – | – | – | ||||
| High | – | – | – | – | – | – | ||||
| High BMId | Low | 1.0 | – | – | ** | 1.0 | – | – | ** | |
| Medium | 2.64 | 1.3–5.5 | * | – | 2.38 | 1.1–5.0 | * | – | ||
| High | 7.32 | 4.0–13.6 | ** | – | 6.95 | 3.7–12.9 | ** | – | ||
| High blood pressuree | Low | 1.0 | – | – | ** | 1.0 | – | – | ** | |
| Medium | 1.22 | 0.8–1.9 | NS | – | 1.20 | 0.8–1.9 | NS | – | ||
| High | 1.92 | 1.4–2.7 | ** | – | 1.90 | 1.3–2.7 | ** | – | ||
| Women | ||||||||||
| Daily smoking | Low | – | – | – | – | – | – | – | – | |
| Medium | – | – | – | – | – | – | – | |||
| High | – | – | – | – | – | – | – | |||
| Low physical activityb | Low | 1.0 | – | – | ** | 1.0 | – | – | ** | |
| Medium | 1.33 | 0.9–2.0 | NS | – | 1.27 | 0.9–1.9 | ** | – | ||
| High | 4.13 | 3.0–5.7 | ** | – | 4.16 | 3.0–5.7 | * | – | ||
| Low fruit and vegetable intakec | Low | – | – | – | – | – | – | – | ||
| Medium | – | – | – | – | – | – | – | |||
| High | – | – | – | – | – | – | – | |||
| High BMId | Low | 1.0 | – | – | ** | 1.0 | – | – | ** | |
| Medium | 1.88 | 1.2–3.0 | * | – | 2.16 | 1.3–3.5 | * | – | ||
| High | 6.48 | 4.6–9.2 | ** | – | 7.34 | 5.1–10.5 | ** | – | ||
| High blood pressuree | Low | 1.0 | – | – | NS | 1.0 | – | – | * | |
| Medium | 0.96 | 0.6–1.4 | NS | – | 0.98 | 0.6–1.6 | NS | – | ||
| High | 1.25 | 0.9–1.7 | NS | – | 1.38 | 1.0–1.9 | NS | – | ||
*P < 0.05; **P < 0.001.
BMI, body mass index; CI, confidence interval; NS, not significant; OR, odds ratio.
a Agaram, Chennai, Chunampet, Illeedu, Pudupattu, Puthiram Kottai and Vanniyanallur.
b Defined as < 150 minutes of moderate physical activity per week.
c Defined as < 5 servings of fruit and vegetables per day (blank here, since there was zero reporting for this category in this case).
d Defined as a BMI ≥ 25 kg/m2.
e Defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg.
Low physical activity
For both men and women, urbanicity was negatively associated with physical activity, although the latter was low even in the least urbanized group. The odds of being physically inactive increased with urbanicity among both men and women, with urban men three times and urban women four times more likely to be inactive than men and women in the low urbanicity group. This relationship remained consistent in both sexes after age adjustment (Table 4).
Low fruit and vegetable consumption
Only two participants in the survey consumed at least five portions of fruit and vegetables daily, on average. We found no significant difference in the prevalence of low fruit and vegetable consumption across urbanicity groups, either for men or for women. However, we did find a positive association (P < 0.05) between urbanicity and the mean number of portions of fruit and vegetables consumed per day by both men and women, before (Table 4) and after age adjustment (Table 5, available at: http://www.who.int/bulletin/volumes/88/4/09-065847/en/index.html).
Table 5. Values for noncommunicable disease risk factors, per urbanicity group, in seven locationsa in Tamil Nadu, India, 2003.
| Outcome | Urbanicity group | No. | Mean | SD | 95% CI | ANOVA or K-W testbP-value |
|---|---|---|---|---|---|---|
| Men | ||||||
| Time spent in physical activity per week (minutes) | Low | 269 | 738.0 | 2.4 | 660.6–823.9 | 0.0001* |
| Medium | 257 | 610.3 | 2.7 | 529.9–702.9 | ||
| High | 1282 | 416.6 | 2.3 | 394.9–439.1 | ||
| No. of servings of fruit and vegetables per day | Low | 269 | 0.78 | 0.4 | 0.7–0.8 | 0.0001* |
| Medium | 257 | 0.98 | 0.6 | 0.9–1.1 | ||
| High | 1282 | 1.39 | 0.8 | 1.4–1.4 | ||
| BMI (kg/m2) | Low | 269 | 19.4 | 3.1 | 189.0–19.7 | 0.0001* |
| Medium | 257 | 19.9 | 3.7 | 19.4–20.3 | ||
| High | 1282 | 22.1 | 4.3 | 21.8–22.3 | ||
| Systolic blood pressure (mmHg) | Low | 269 | 123.8 | 18.0 | 121.6–126.0 | < 0.0001 |
| Medium | 257 | 124.6 | 18.1 | 122.4–126.8 | ||
| High | 1282 | 131.2 | 19.9 | 130.1–132.3 | ||
| Mean diastolic blood pressure (mmHg) | Low | 269 | 74.6 | 10.6 | 73.2–76.0 | < 0.0001 |
| Medium | 257 | 77.3 | 10.4 | 75.9–78.7 | ||
| High | 1282 | 79.7 | 11.6 | 79.0–80.4 | ||
| Women | ||||||
| Time spent in physical activity per week (minutes) | Low | 370 | 514.4 | 2.8 | 456.1–580.0 | 0.0001* |
| Medium | 243 | 430.4 | 2.8 | 365.8–506.2 | ||
| High | 1284 | 234.0 | 2.3 | 221.0–248.3 | ||
| Number of servings of fruit and vegetables per day | Low | 370 | 0.7 | 0.4 | 0.7–0.7 | 0.0001* |
| Medium | 243 | 0.8 | 0.4 | 0.7–0.8 | ||
| High | 1284 | 1.1 | 0.6 | 1.1–1.2 | ||
| BMI (kg/m2) | Low | 370 | 20.2 | 3.6 | 19.9–21.0 | 0.0001* |
| Medium | 243 | 21.0 | 4.4 | 20.5–21.6 | ||
| High | 1284 | 24.3 | 5.0 | 24.0–24.6 | ||
| Systolic blood pressure (mmHg) | Low | 370 | 126.1 | 22.9 | 123.8–128.5 | 0.6226 |
| Medium | 243 | 124.6 | 19.1 | 122.2–127.0 | ||
| High | 1284 | 126.0 | 21.6 | 124.8–127.2 | ||
| Diastolic blood pressure (mmHg) | Low | 370 | 77.0 | 11.6 | 75.9–78.0 | < 0.0001 |
| Medium | 243 | 77.1 | 11.6 | 75.8–78.4 | ||
| High | 1284 | 77.6 | 12.3 | 77.0–78.2 |
* P-value for K-W test.
ANOVA, analysis of variance; BMI, body mass index; CI, confidence interval; K-W, Kruskal-Wallis; SD, standard deviation.
a Agaram, Chennai, Chunampet, Illeedu, Pudupattu, Puthiram Kottai and Vanniyanallur.
b Means were compared using one-way ANOVA or the K-W test when assumptions for ANOVA were not met.
High body mass index
The prevalence of high BMI was greater among both males and females in the high urbanicity group (Table 3).The odds of having a high BMI were seven times higher among men and more than six times higher among women in the most urbanized group compared with the least urbanized. The OR remained significant in the age-adjusted models, although a slight reduction was noted among men and a slight increase among women. Mean BMI was positively associated with urbanicity among both men and women (Table 5). The BMI of men and women living in the most urbanized areas was an average of 2.7 kg/m2 and 4.1 kg/m2 higher, respectively, than that of their counterparts in the low urbanicity group. These findings remained consistent after adjustment for age.
High blood pressure
The prevalence of high blood pressure was associated with urbanicity in men but not in women (Table 3). Men in the most urban group were almost twice as likely to have high blood pressure as those in the low urbanicity group (Table 4). No association was found among women, and the results for both sexes remained consistent after adjustment for age.
Urbanicity was positively associated with diastolic blood pressure in both males and females (Table 5). Among men, urbanicity group was positively associated with blood pressure, even after adjusting for age (Fig. 1).
Discussion
The hypothesis that a relationship exists between urbanicity and NCD risk factors has been supported by the findings of this study. Among men, urbanicity was positively associated with smoking, BMI, blood pressure and low physical activity; among women, urbanicity was associated with low physical activity and BMI.
The 2003 NCD risk factor study was carried out by WHO and the Indian Council for Medical Research using a validated WHO survey instrument. Aspects of the measure relied on self-reported behaviour and were therefore susceptible to social desirability bias as well as other biases. The collection of biometric and anthropometric data provides more objective measures of NCD risk. We observed similar directions in the association between urbanicity and self-reported risk behaviours and urbanicity and anthropometric measures. More detailed risk factor information should be collected in future studies. Dietary information in the WHO STEPwise study was limited to fruit and vegetable consumption, whereas information on fat, oil, sugar and processed food consumption would provide greater information on overall diet.
The external validity of the study may be affected by the small number of wards surveyed in Chennai and the purposive sampling of a small number of villages in Kancheepuram district. It is difficult to say with certainty that these areas are representative of all areas in Tamil Nadu with similar levels of urbanicity. While we established cross-sectional associations with this measure of urbanicity and chronic disease, we cannot provide evidence of a temporal relationship between living in an urban area and acquiring risk factors for NCD. Some items included in the scale demonstrated possible “floor” and “ceiling” effects, with responses grouped at the top or bottom of the scale (notably population density and access to health services). The similarity in values for these items suggests that they provide little discriminatory ability. This observation needs further examination across different contexts to understand whether these findings are specific to this sample of Tamil Nadu, India, or whether they would be observed in studies in other settings.
Data from a range of countries have shown differences in health in urban and rural areas.14–17 Several studies in India have demonstrated a higher prevalence of NCD risk factors in urban than in rural areas.18–22 Epidemiological studies have shown that in rural adults, the prevalence of coronary heart disease is lower (3–5%) than in urban adults (7–10%).23 However, this relationship is not straightforward and there is no universally accepted definition of urban, urbanization or urbanicity and therefore no definitive measure. This study has demonstrated that it is possible to construct and apply a quantitative measure of the urban environment which allows for a more refined examination of the relationship between urbanicity and risk factors for NCD. It identified significant variation in the urbanicity of areas classified as rural by the Census of India. These differences in urbanicity are associated with an increase in prevalence of several common modifiable risk factors for NCDs.
We applied an adaptation of a recently used scale7 that had certain omissions (including access to water, sanitation facilities, electricity, housing construction, income, etc.). Further work is also needed to understand the implications of scaling different elements of the urbanizing environment to develop an exposure measure for NCDs. Ranking according to population size and density has some inherent logic, although this is less true for items reflecting access to transport and communication. Further development is required to understand the relative contribution of each scale element for chronic disease risk, develop weights where appropriate, and include other items that may be relevant, including access to mobile telephones, television and the internet.
Acknowledgements
The authors would like to thank WHO and the Indian Council of Medical Research for supporting the previous study of NCD risk factors and for allowing us to use the data for this analysis. Dr Mohan’s Diabetes Specialties Centre, Chennai, is a WHO Collaborating Centre for Noncommunicable Diseases Prevention and Control.
Competing interests:
None declared.
References
- 1.Reddy KS. Cardiovascular diseases in the developing countries: dimensions, determinants, dynamics and directions for public health action. Public Health Nutr. 2001;5:231–7. doi: 10.1079/phn2001298. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJL, Lopez AD. The global burden of disease: a comprehensive assessment of mortality and disability from disease, injury, and risk factors in 1990 and projected for 2020 Cambridge: Harvard University Press; 1996 (Global Burden of Disease and Injury Series, vol. 1). [Google Scholar]
- 3.Boutayeb A, Boutayeb S. The burden of non communicable diseases in developing countries. Int J Equity Health. 2005;4:2. doi: 10.1186/1475-9276-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97:596–601. doi: 10.1161/01.cir.97.6.596. [DOI] [PubMed] [Google Scholar]
- 5.Ezzati M, Vander Hoorn S, Lawes CMM, Leach R, James WPT, Lopez AD, et al. Rethinking the “diseases of affluence” paradigm: global patterns of nutritional risks in relation to economic development. PLoS Med. 2005;2:e133. doi: 10.1371/journal.pmed.0020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arambepola C, Allender S, Ekanayake R, Fernando D. The association between urban living and obesity independent of population and lifestyle characteristics in an urban area. Trop Med Int Health. 2008;13:448–57. doi: 10.1111/j.1365-3156.2008.02021.x. [DOI] [PubMed] [Google Scholar]
- 7.Vlahov D, Galea S. Urbanization, urbanicity, and health. J Urban Health. 2002;79:S1–12. doi: 10.1093/jurban/79.suppl_1.S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahly DL, Adair LS. Quantifying the urban environment: a scale measure of urbanicity outperforms the urban-rural dichotomy. Soc Sci Med. 2007;64:1407–19. doi: 10.1016/j.socscimed.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohan V, Mathur P, Deepa R, Deepa M, Shukla DK, Menon GR, et al. Urban rural differences in prevalence of self-reported diabetes in India: the WHO–ICMR Indian NCD risk factor surveillance. Diabetes Res Clin Pract. 2008;80:159–68. doi: 10.1016/j.diabres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Mendez MA, Popkin BM. Globalization, urbanization and nutritional change in the developing world. Electronic Journal of Agricultural and Development Economics. 2004;1:220–41. [Google Scholar]
- 11.Allender S, Foster C, Hutchinson L, Arambepola C. Quantification of urbanization in relation to chronic diseases in developing countries: a systematic review. J Urban Health. 2008;85:938–51. doi: 10.1007/s11524-008-9325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Census of India. Village and town amenities directory. Directory of census operations. New Delhi: Office of the Registrar General, India; 2001.
- 13.STEPwise approach to surveillance(STEPS) [Internet site]. Geneva: World Health Organization. Available at: http://www.who.int/chp/steps/en/ [accessed 15 October 2009].
- 14.Misch KA Ischaemic heart disease in urbanised Papua New Guinea: an autopsy study. Cardiology. 1988;75:71–75. doi: 10.1159/000174351. [DOI] [PubMed] [Google Scholar]
- 15.Vorster HH, Venter CS, Wissing MP, Margetts BM. The nutrition and health transition in the North West Province of South Africa: a review of the THUSA (Transition and Health during Urbanisation of South Africans) study. Public Health Nutr. 2005;8:480–90. doi: 10.1079/PHN2005784. [DOI] [PubMed] [Google Scholar]
- 16.Lin HC, Lin YJ, Liu TC, Chen CS, Chiu WT. Urbanization and Stroke Prevalence in Taiwan: Analysis of a Nationwide Survey. Journal of Urban Health. 2007;84:604–14. doi: 10.1007/s11524-007-9195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray CJL, Lopez AD. Mortality by cause for eight regions of the world: Global burden of disease study. Lancet. 1997;349:1269–76. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 18.Chadha SL, Gopinath N, Shekawat S. Urban–rural differences in the prevalence of coronary heart disease and its risk factors in Delhi. Bull World Health Organ. 1997;75:31–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Shetty PS. Nutrition transition in India. Public Health Nutr. 2002;5(1A):175–82. doi: 10.1079/PHN2001291. [DOI] [PubMed] [Google Scholar]
- 20.Lubree HG, Rege SS, Bhat DS, Raut KN, Panchanadikar AN, Joglekar CV, et al. Body fat and cardiovascular risk factors in Indian men in three geographical locations. Food Nutr Bull. 2002;23(Suppl):146–9. [PubMed] [Google Scholar]
- 21.Ramachandran A. Epidemiology of non insulin dependant diabetes mellitus in India. In: Shetty PS, Gopalan C, eds. Diet, nutrition and chronic disease: an Asian perspective. London: Smith Gordon; 1998. pp. 38-41. [Google Scholar]
- 22.Gopalan C. Diet related non-communicable diseases in South and South East Asia. In: Shetty PS, McPherson K, eds. Diet, nutrition and chronic disease: lessons from contrasting worlds. London: John Wiley & Sons; 1997. pp. 10-23. [Google Scholar]
- 23.Gupta R. Burden of coronary heart disease in India. Indian Heart J. 2005;57:632–8. [PubMed] [Google Scholar]