Abstract
Background
Although 25% to 44% of patients with heart failure (HF) have diabetes mellitus (DM), the optimal treatment regimen for HF patients with DM is uncertain. We investigated the association between metformin therapy and outcomes in a cohort of advanced, systolic HF patients with DM.
Methods and Results
Patients with DM and advanced, systolic HF (n = 401) were followed at a single university HF center between 1994 and 2008. The cohort was divided into 2 groups based on the presence or absence of metformin therapy. The cohort had a mean age of 56 ± 11 years, left ventricular ejection fraction (LVEF) of 24 ± 7%, with 42% being New York Heart Association (NYHA) III and 45% NYHA IV. Twenty-five percent (n = 99) were treated with metformin therapy. The groups treated and not treated with metformin were similar in terms of age, sex, baseline LVEF, medical history, and baseline glycosylated hemoglobin. Metformin-treated patients had a higher body mass index, lower creatinine, and were less often on insulin. One-year survival in metformin-treated and non-metformin-treated patients was 91% and 76%, respectively (RR = 0.37, CI 0.18–0.76, P = .007). After multivariate adjustment for demographics, cardiac function, renal function, and HF medications, metformin therapy was associated with a non-significant trend for improved survival.
Conclusion
In patients with DM and advanced, systolic HF who are closely monitored, metformin therapy appears to be safe. Prospective studies are needed to determine whether metformin can improve HF outcome.
Keywords: Cardiomyopathy, biguanides, heart transplant, mortality
Diabetes (DM) is prevalent in patients with heart failure (HF), with approximately 25% of chronic HF patients overall,1 and 44% of patients hospitalized having DM.2 Furthermore, several studies have shown that DM and hyperglycemia are associated with new-onset HF,3–5 with a 10% to 15% increased risk of developing HF per every unit increase in glycosylated hemoglobin (HgbA1c, %).5–7 In fact, DM and hyperglycemia are strongly implicated as a cause for progression from asymptomatic left ventricular dysfunction to symptomatic HF, increased hospitalizations for HF, and an overall increased mortality risk in patients with chronic HF.1
Although it is well described that DM is a strong risk factor for increased morbidity and mortality in patients with HF, the optimal medical regimen for glucose control in diabetics with HF is uncertain.8 Many of the medications commonly used to lower serum glucose have theoretic or described adverse effects in HF. For example, insulin and sulfonylureas may cause increased sympathetic nervous system activity and endothelial dysfunction, and thus the potential for an increased mortality risk in patients with HF.8,9 Thiazolidinediones may worsen HF symptoms, especially edema.9,10 Metformin historically had a “black box” warning against use in patients with DM and HF because of the theoretic risk of lactic acidosis. Although this warning on metformin has been recently lifted by the Food and Drug Administration, many physicians are unaware of this change in labeling.11
Recent observational studies of broad-based HF cohorts suggest that metformin has been used in diabetics with HF and may be associated with improved outcomes in these patients. 12,13 However, these findings may not apply to patients with more advanced HF and thus the safety and outcomes of metformin use in diabetics with advanced, systolic HF is still largely unknown. Our study aimed to investigate the association between metformin treatment and outcomes in patients with both DM and advanced, systolic HF, focusing on cardiac function, heart transplant, and mortality.
Methods
Patient Population
The study population initially consisted of 770 patients with a prior diagnosis of type II DM and advanced, systolic HF, referred to the Ahmanson-UCLA Cardiomyopathy center for HF management or transplant evaluation between 1994 and 2008. The start date of 1994 was chosen because this is when metformin use was approved by the Food and Drug Administration in the United States.14 Patients with a left ventricular ejection fraction (LVEF) >40% (n = 43), patients with DM treated by diet alone (n = 95), and those patients without detailed information on DM medications or follow-up (n = 231) were excluded from the analysis. The final cohort consisted of 401 patients with type II DM and advanced, systolic HF. In comparison to the 401 patients included in the study, the 369 patients excluded from the study were similar in terms of New York Heart Association (NYHA) class and etiologies of HF and had a slightly higher baseline LVEF (26 ± 12% vs. 24 ± 7%). All patients were followed in a comprehensive HF management program, as previously described.15 The study was approved by the UCLA Medical Institutional Review Board.
Data Collection
Detailed information on patients’ baseline characteristics, including presence and duration of DM, were collected on initial assessment. DM was defined by patient self-report and medical record documentation of DM. All medications and doses were carefully recorded at initial visit, including detailed documentation of DM medications and doses. Echocardiography, right heart catheterization, and laboratory testing, including HgbA1c levels, occurred within 6 weeks of initial referral date. Echocardiography was also performed at approximately 6 months after initial referral date. Body mass index was calculated (in kg/m2) using the patients’ “dry” weight after empirical or pulmonary artery catheter-guided HF therapy. Prior left heart catheterization reports and angiographic films were reviewed, or if not done previously, left heart catheterization was performed. Significant coronary artery disease was defined as a single stenosis > 70% of the cross-section luminal diameter of the involved artery on angiography.
End Points
There were 2 primary end points considered in this study: all-cause mortality (with all transplants coded as nonfatal events) and all-cause mortality or need for urgent heart transplant (Status IA). For the all-cause mortality endpoint, heart transplantation (Status IA, IB, and II) was coded as a nonfatal end of follow-up at time of transplant, as previously described.16 The composite end point of all-cause mortality/urgent heart transplant (Status IA) included urgent transplant as a fatal event. Change in cardiac function was considered as a secondary end point. Change in cardiac function was defined by the difference in the LVEF at 6-month follow-up as compared with the LVEF on initial visit.
Statistical Analysis
This was an observational, nonrandomized study design in which the total cohort of patients (n = 401) was divided into 2 groups based on metformin use: patients who were taking metformin (n = 99) either as monotherapy (n = 8) or in combination with other DM medications (n = 91), and patients who were not on metformin and using alternative oral hypoglycemic medications and/or insulin (n = 302). Results are presented as mean ± SD for normally distributed continuous variables, median (interquartile range) for non-normally distributed variables, and percentage of total for categorical variables. Independent sample t-test, analysis of variance, and chi-square test were used for comparison of variables, as appropriate. Survival curves were calculated by the Kaplan-Meier method, and differences between the curves were evaluated with the log-rank statistic. Cox proportional hazards modeling was performed to analyze the association between metformin use, in addition to a variety of other prospectively recorded parameters, and survival or urgent transplant-free survival. Variables included in multivariable analyses were age, sex, left ventricular ejection fraction, renal function, and body mass index, hemoglobin, DM duration, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use and β-blocker use. Statistical Package for Social Sciences for Windows, Version 17.0 (Chicago, IL) was used for all analyses.
Results
Baseline Characteristics of the Cohort
The total cohort was 75% men, and ages ranged from 20 to 84 years (mean age 56 ± 11 years). NYHA III and IV HF comprised 42% and 45% of the population, respectively. Mean LVEF was 24 ± 7%. Etiologies of HF were ischemic (60%), idiopathic (20%), and valvular (3%); the remaining etiologies included alcohol-induced, myocarditis, and hypertrophic. Mean duration of DM at the time of referral was 9.9 ± 9.0 years.
There were 99 patients (25%) treated with metformin therapy, and 302 patients (75%) who were treated with alternative oral hypoglycemic agents or insulin that did not take metformin. Differences in patient characteristics among those on or not on metformin treatment were analyzed (Table 1). The 2 groups were similar with regard to age, sex, baseline LVEF, medical history, and baseline HgbA1c. Patients treated with metformin versus those not treated with metformin were more often NYHA III and had lower serum creatinine levels (Table 1).
Table 1.
Baseline Characteristics of the Total Cohort
| Characteristic | Total Cohort (n = 401) |
Metformin Treatment (n = 99) |
No Metformin Treatment (n = 302) |
P Value |
|---|---|---|---|---|
| Age, y | 56 ± 11 | 56 ± 11 | 56 ± 11 | .960 |
| Male sex, % | 75 | 79 | 73 | .266 |
| Left ventricular ejection fraction, % | 24 ±7 | 24 ± 7 | 24 ± 7 | .860 |
| New York Heart Association III/IV, % | 42/45 | 56/29 | 37/49 | .016 |
| Body mass index, kg/m2 | 28.1 ± 5.4 | 29.2 ± 6.1 | 27.7 ± 5.2 | .027 |
| Peak VO2, mL ·kg · min | 12.3 ± 3.9 | 13.2 ± 3.5 | 11.9 ± 4.0 | .030 |
| Coronary artery disease, % | 60 | 59 | 60 | .813 |
| Hypertension, % | 60 | 59 | 60 | .767 |
| Smoking history, % | 57 | 52 | 59 | .192 |
| Diabetes history, y (n = 223) | 9.9 ± 9.0 | 6.1 ± 6.0 | 11.3 ± 9.5 | .0001 |
| Hemoglobin A1c, % | 8.2 ± 2.0 | 8.2 ± 1.8 | 8.1 ± 2.1 | .799 |
| Total cholesterol, mg/dL | 164 ± 61 | 169 ± 54 | 162 ± 63 | .388 |
| Creatinine, mg/dL | 1.5 ± 1.0 | 1.2 ± 0.4 | 1.6 ± 1.1 | .0001 |
| Stage of chronic kidney disease*, % | ||||
| 0–1 | 7.4 | 12.5 | 5.7 | .0001 |
| 2 | 35.4 | 59.4 | 27.3 | |
| 3 | 44.2 | 20.8 | 52.1 | |
| 4 | 11.1 | 7.3 | 12.4 | |
| 5 | 1.9 | 0 | 2.5 | |
| Blood urea nitrogen, mg/dL | 34 ± 22 | 27 ± 19 | 36 ± 22 | .0001 |
| Hemoglobin, g/dL | 12.9 ± 1.9 | 13.6 ± 1.7 | 12.6 ± 2.0 | .0001 |
| Hemoglobin <11 g/dL, % | 17.2 | 5.8 | 20.9 | .001 |
| Albumin, g/dL | 3.7 ± 0.6 | 3.9 ± 0.5 | 3.6 ± 0.6 | .001 |
| Sodium, mmol/L | 136 ± 5 | 137 ± 4 | 136 ± 5 | .029 |
| B-type natriuretic peptide, pg/mL (n = 191) | 534 (199–1200) | 489 (115–925) | 693 (257–1300) | .037 |
| QRS duration, ms | 130 ± 39 | 123 ± 33 | 132 ± 40 | .093 |
| Implantable cardioverter-defibrillator placed, % | 44 | 54 | 41 | .017 |
| Biventricular pacemaker placed, % | 17 | 21 | 16 | .141 |
Stage of chronic kidney disease is based on estimate of glomerular filtration rate (mL/min) from the Modification of Diet in Renal Disease (MDRD) equation: Stage 0–1, >90 mL/min; Stage 2, 60–89 mL/min; Stage 3, 30–59 mL/min; Stage 4, 15–29 mL/min; Stage 5, <15 mL/min.
The frequency of cardiovascular and other DM medication use is listed in Table 2. In the total cohort of patients with DM and HF, 43% were treated with insulin (with or without oral hypoglycemic medications). Patients treated with metformin were more likely to be treated with thiazolidinediones and β-blockers and less likely to be treated with insulin.
Table 2.
Medication Use in the Cohort
| Total Cohort (n = 401) |
Metformin Treatment (n = 99) |
No Metformin Treatment (n = 302) |
P Value | |
|---|---|---|---|---|
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, % |
87 | 93 | 85 | .052 |
| β-blocker, % | 66 | 86 | 59 | .0001 |
| Aldosterone antagonist, % | 44 | 49 | 42 | .245 |
| Statin, % | 65 | 74 | 62 | .036 |
| Antiarrhythmic, % | 30 | 20 | 34 | .007 |
| Insulin, % | 43 | 11 | 53 | .0001 |
| Sulfonylurea, % | 49 | 56 | 47 | .151 |
| Thiazolidinedione, % | 14 | 24 | 11 | .002 |
Relationship Between Metformin Use and Change in Cardiac Function
Baseline LVEF in both the metformin and non-metformin groups was 24 ± 7%. At 6-month follow-up, echocardiography was repeated on 173 patients in this study. The mean 6-month follow-up LVEF of 30 ± 10 % in the metformin therapy group was significantly improved compared with follow-up LVEF in the non-metformin group (27 ± 9%, P = .02 between groups at follow-up). More patients in the metformin group (64%) had improvement in LVEF compared with those not treated with metformin therapy (48%, P = .04). After adjustment for angiotensin-converting enzyme inhibitor/angiotensin receptor blocker therapy and β-blocker therapy, metformin use was associated with a nonsignificant trend toward improvement in LVEF at ± months (relative risk 1.8, 95% confidence interval 0.9–3.6, P = .08).
Relationship Between Metformin Use and Mortality
There were 71 deaths during the first year of follow-up and 106 total deaths by 2 years. Of the 71 deaths by 1 year, progressive HF deaths accounted for 22 (31%), whereas 15 (21%) deaths were sudden, 3 (4%) occurred secondary to myocardial infarction, and 31 (44%) occurred from unknown or other causes. At 2 years, 82 subjects received heart transplants: 41 urgent and 41 elective status.
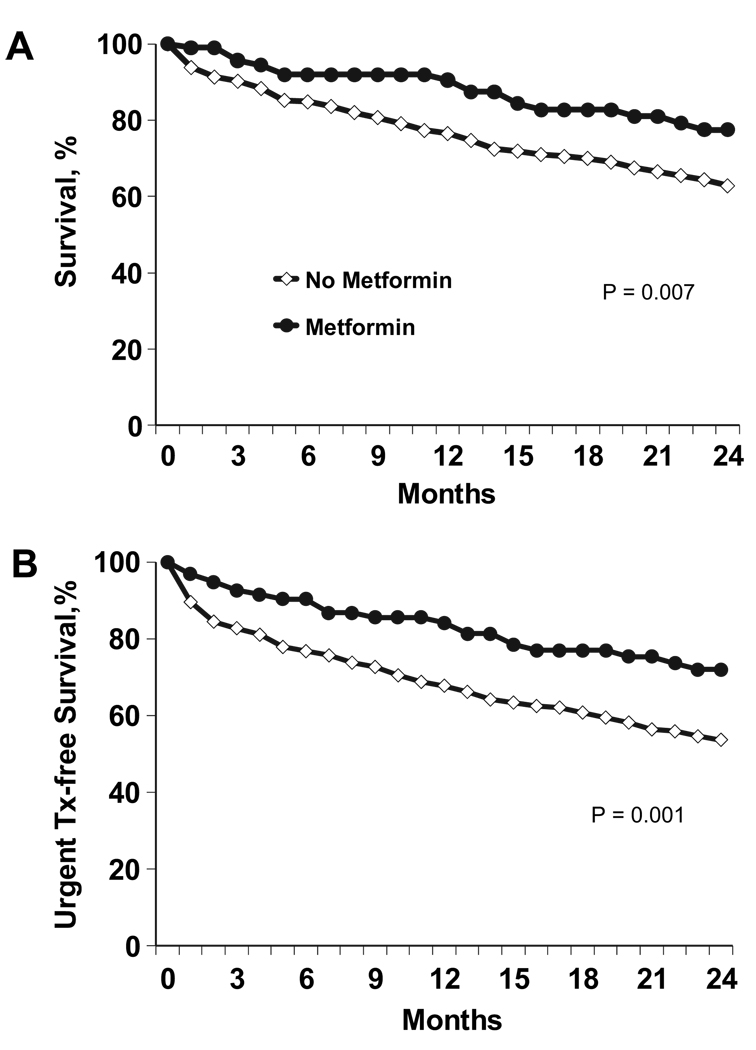
Patients treated with metformin were found to have a significantly longer survival compared with patients not treated with metformin. Survival rates at 1- and 2-year follow-up for patients treated with metformin versus those not treated with metformin were 91% and 76% (P = .007) and 78% and 63% (P = .007), respectively (Fig. 1A). Patients not receiving metformin treatment were also at significantly increased risk for the combined end point of death or urgent transplantation. Survival free from death or urgent heart transplant was 84% versus 67% at 1 year (P = .004) and 72% versus 54% at 2 years (P = .001) for patients treated with metformin and for patients not treated with metformin, respectively (Fig. 1B).
Fig. 1.
Metformin and outcomes over 2-year follow-up. (A) 2-year survival, (B) 2-year survival free from death or urgent heart transplant
We performed multivariate analyses to adjust for several potential confounders of the relationship between metformin and outcomes in this cohort of diabetic HF patients. After adjustment for age, sex, LVEF, renal function, body mass index, DM duration, hemoglobin, use of angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers, and use of β-blockers, metformin treatment was not significantly associated with improved survival outcomes (Table 3). When the only medication entered into the multivariate analysis was β-blocker use, the results were not significantly changed.
Table 3.
Outcomes in Metformin-treated vs. Non-metformin-treated HF patients with DM
| Metformin Treatment (n = 99) |
No Metformin Treatment (n = 302) |
P Value | |
|---|---|---|---|
| All-cause mortality | |||
| 1 y | |||
| Mortality (%) | 9 | 24 | .007 |
| Unadjusted HR (95% CI) | 0.37 (0.18–0.76) | 1.00 | .007 |
| Multivariate HR (95% CI) | 0.63 (0.21–1.89) | 1.0 | .40 |
| 2 y | |||
| Mortality (%) | 22 | 37 | .007 |
| Unadjusted HR (95% CI) | 0.51 (0.30–0.87) | 1.00 | .01 |
| Multivariate HR (95% CI) | 0.79 (0.36–1.71) | 1.00 | .54 |
| All-cause mortality/urgent heart transplant | |||
| 1 y | |||
| Mortality/urgent heart transplant (%) | 16 | 33 | .004 |
| Unadjusted HR (95% CI) | 0.43 (0.25–0.76) | 1.00 | .004 |
| Multivariate HR (95% CI) | 0.85 (0.38–1.92) | 1.00 | .70 |
| 2 y | |||
| Mortality/urgent heart transplant (%) | 28 | 46 | .002 |
| Unadjusted HR (95% CI) | 0.51 (0.32–0.79) | 1.00 | .001 |
| Multivariate HR (95% CI) | 0.80 (0.42–1.53) | 1.00 | .82 |
HF, heart failure; HR, hazard ratio; CI, confidence interval.
Multivariate model adjusted for age, sex, left ventricular ejection fraction, renal function, body mass index, diabetes duration, hemoglobin, use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and use of β-blockers.
Discussion
This study suggests that metformin is a safe therapy for DM in patients with DM and advanced, systolic HF, followed in a university HF disease management program. We observed that presence of metformin therapy, as compared with absence of metformin therapy, was associated with better survival in this patient population. However, this association was no longer statistically significant after adjustment for multiple covariates. Whether metformin therapy is protective in DM and systolic HF, or whether its use is merely associated with better overall health status or better medical care will require further study. Patients treated with metformin had significantly fewer comorbidities including less renal dysfunction and less anemia, were more likely to be treated with β-blockers, and were less likely to be treated with insulin and antiarrhythmics (Table 1, Table 2); thus, there are many confounding factors, which may explain the relationship observed between metformin therapy and beneficial outcomes.
DM is a well-recognized independent risk factor for the development of HF.3,4 Both HF and DM are believed to share pathophysiologic processes such as neurohormonal activation, endothelial dysfunction, and oxidative stress.17,18 The reported prevalence of DM is 4% to 6% in the general population,19 approximately 25% in patients with chronic HF,1 and may be close to 44% in hospitalized HF patients.2 With an overall HF prevalence of 5.7 million, there are an estimated 1 to 2 million patients with both HF and DM in the United States.19
Metformin, DM, HF, and Mortality
DM has been associated with increased mortality in patients with established systolic HF. Analyses from the Studies of Left Ventricular Dysfunction trial have demonstrated that DM serves as an independent risk factor for progression from asymptomatic left ventricular dysfunction to symptomatic HF as well as a risk factor for all-cause mortality in patients with symptomatic HF (relative risk [RR] 1.4).20 In an analysis of the Danish Investigations of Arrhythmias and Mortality on Dofetalide Study Group study, DM predicted mortality independent of HF etiology (RR 1.5).21 Despite this well-described relationship between mortality, DM, and HF, the optimal medical regimen for serum glucose control in diabetic patients with HF remains uncertain.8
Metformin had previously been contraindicated for use in patients with DM and HF. This contraindication was introduced to the label soon after the release of the drug in the United States, when case reports surfaced of HF patients taking metformin who developed lactic acidosis.22 It was never certain, however, whether the relationship between metformin use and the development of lactic acidosis was causative. By the end of 2006, the Food and Drug Administration eliminated the HF contraindication on product labeling for metformin,11,22,23 although HF is still in the label’s “Warnings” section.22 Subsequent analyses revealed a miniscule risk of lactic acidosis associated with metformin therapy, as compared with that of an earlier biguanide, phenformin.22,24
Recent studies of metformin use in diabetic patients with HF have shown that metformin may be beneficial to these patients. In a retrospective study of Medicare beneficiaries with DM discharged after hospitalization with the principal diagnosis of HF, metformin treatment was associated with a significantly lower risk of death after 1 year (HR 0.87, 95% CI 0.78–0.97) and a lower risk of hospital readmission (HR 0.92, 95% CI 0.92–0.99).12 In another retrospective study of new users of oral antidiabetic agents who also had HF, metformin monotherapy was again associated with a significantly lower risk of death or hospitalization as compared to sulfonylurea monotherapy (HR 0.83, 95% CI 0.70–0.99).13 It has also been observed that metformin therapy in patients with DM without HF may in fact decrease incidence of HF. In a recent study of 20,450 type II DM patients, metformin therapy was associated with a reduced risk of HF (HR 0.76, 95% CI 0.64– 9.91) when compared with sulfonylurea therapy.25 In a retrospective study from the Saskatchewan Heath databases of adults without HF who were being newly treated with oral antidiabetic agents, the incidence of HF was greater in patients using sulfonylurea monotherapy (4.4 cases per 100 patient years) as compared with patients using metformin monotherapy (3.3 cases per 100 patient years).26 In a separate retrospective study of subjects with DM and no prior history of HF from the Kaiser Permanente Northwest DM registry, HF incidence rates were highest in therapeutic managements that included insulin and lowest in regimens that included metformin.27
In the current study, metformin in patients with DM and advanced, systolic HF was not associated with worsened survival or survival free from death or urgent heart transplant throughout the 2 years of follow-up. Thus the current study is consistent with previous findings concerning metformin treatment in DM and HF in other patient populations. The observation that metformin is not dangerous in HF is now extended to patients with advanced, systolic HF.
There are potential mechanisms by which metformin may improve cardiac function and overall survival. There is increasing evidence that activity of adenosine monophosphate (AMP)-activated protein kinase, an enzyme that plays a central role in energy homeostasis in the heart and in many other tissues, is implicated in the pathophysiology of cardiovascular and metabolic diseases. 28 Metformin is thought to have an important mechanistic role in the activity of AMP-activated protein kinase. In a recent study of murine models of HF, metformin significantly improved left ventricular function and survival via activation of AMP-activated protein kinase and its downstream mediators.29 This evidence suggests that metformin may be cardioprotective independent of antihyperglycemic effects,29 perhaps by augmenting left ventricular myocardial efficiency at the molecular level. Metformin may also reduce morbidity and mortality in HF via intensive serum glucose control. In the UK Prospective Diabetes Study of overweight patients with type 2 DM, metformin-treated patients had better blood glucose control as compared to non-emetformin-treated patients (median HgbA1c 7.4% vs. 8.0%), and had a risk reduction of 32% (95% CI 13–47%, P = .002) for any DM-related end point including cardiovascular mortality.30 Whether metformin may improve cardiac function and overall survival because of mechanisms unrelated to hyperglycemia or better glycemic control is unknown and needs to be further investigated.
It is important to note that precise goals for glycemic control in HF have not been established. The conventional wisdom states that better control of DM (ie, lower serum glucose and HgbA1c levels) should improve morbidity and mortality in patients with DM and HF. However, in a recent study of patients with DM and advanced HF, an inverse relationship between HgbA1c levels and mortality was observed, with higher HgbA1c levels correlating with improved survival in this cohort of patients.31 Furthermore, in a large cohort of patients hospitalized with HF, there was no significant association found between admission glucose levels and mortality, suggesting that the relationship between hyperglycemia and adverse outcomes cannot be automatically extended to patients hospitalized with HF.32 Prospective studies of antidiabetic therapy and glucose control are warranted; however, the heterogeneity of current diabetic care in HF may make this type of trial difficult to complete.33
Study Limitations
We acknowledge several potential limitations of our study. The cohort is a select population of patients with advanced systolic HF and DM, referred to a single university center. Glycemic control outcomes and re-hospitalizations were not tracked. Most importantly, this was an observational study and there were significant baseline differences between the 2 patient groups, most notably in creatinine and blood urea nitrogen levels, NYHA class, and duration of DM. This introduces the potential for a number of residual measured and unmeasured confounding factors to influence the analyses. It is possible that metformin was previously prescribed to patients who were perceived as being less likely to experience an adverse reaction or with greater HF clinical stability. In addition, the relationship between metformin use and improved outcomes was no longer significant with the addition of HF medication use to the multivariate analyses, also suggesting that metformin was prescribed to patients whose HF was being better managed. It should also be noted that these patients were closely monitored in a comprehensive HF management program and these findings may not apply to patients who are followed in other settings. We do not have data on all laboratory, echo, and medication data at time of follow-up. We also do not have information on the time course of metformin therapy in all subjects. Nonetheless, this study has identified a potential course of management of DM in patients with advanced HF.
Conclusions
In this cohort of patients with advanced systolic HF and DM, metformin appears to be safe and may be associated with favorable clinical outcomes. HF patients with DM treated with metformin more often had an improvement in LVEF, and had better survival free from death or urgent heart transplantation, as compared to patients not treated with metformin, although the associations did not remain significant after multivariable adjustment. However, this study is merely observational and thus cannot imply a causative relationship. The present findings warrant future well-designed, randomized clinical trials to determine the safety and efficacy of metformin, as well as other insulin sensitizing agents, in patients with DM and advanced, systolic HF.
Acknowledgments
Dr. Horwich was supported by NIH/NHLBI 1K23HL085097. Dr. Fonarow was supported by the Ahmanson Foundation (Los Angeles, CA) and holds the Eliot Corday Chair in Cardiovascular Medicine and Science.
References
- 1.MacDonald MR, Petrie MC, Hawkins NM, Petrie JR, Fisher M, McKelvie R, et al. Diabetes, left ventricular systolic dysfunction, and chronic heart failure. Eur Heart J. 2008;10:1224–1240. doi: 10.1093/eurheartj/ehn156. [DOI] [PubMed] [Google Scholar]
- 2.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 4.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 5.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27:1879–1884. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- 6.Stratton IM, Adler AI, Neil HA, Matthews DR, Cull CA, Wright AD, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iribarren C, Karter AJ, Go AS, Ferrara A, Liu JY, Sidney S, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103:2668–2673. doi: 10.1161/01.cir.103.22.2668. [DOI] [PubMed] [Google Scholar]
- 8.Eurich DT, McAlister FA, Blackburn DF, Majumdar SR, Tsuyuki RT, Varney J, et al. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. BMJ. 2007;335:497–507. doi: 10.1136/bmj.39314.620174.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koro CE, Bowlin SJ, Weiss SR. Antidiabetic therapy and the risk of heart failure in type 2 diabetic patients: an independent effect or confounding by indication. Pharmacoepidemiol Drug Safe. 2005;14:697–703. doi: 10.1002/pds.1069. [DOI] [PubMed] [Google Scholar]
- 10.Aguilar D, Bozkurt B, Pritchett A, Petersen NJ, Deswal A. The impact of thiazolidinedione use on outcomes in ambulatory patients with diabetes mellitus and heart failure. J Am Coll Cardiol. 2007;50:32. doi: 10.1016/j.jacc.2007.01.096. [DOI] [PubMed] [Google Scholar]
- 11.Inzucchi SE, Masoudi FA, McGuire DK. Metformin therapy in patients with type 2 diabetes complicated by heart failure. Am Heart J. 2007;154:45. doi: 10.1016/j.ahj.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation. 2005;111:583–590. doi: 10.1161/01.CIR.0000154542.13412.B1. [DOI] [PubMed] [Google Scholar]
- 13.Eurich DT, Majumdar SR, McAlister FA, Tsuyuki RT, Johnson JA. Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diabetes Care. 2005;28:2345–2351. doi: 10.2337/diacare.28.10.2345. [DOI] [PubMed] [Google Scholar]
- 14.Melchior WR, Jaber LA. Metformin: an antihyperglycemic agent for treatment of type 2 diabetes. Ann Pharmacother. 1996;30:158–164. doi: 10.1177/106002809603000210. [DOI] [PubMed] [Google Scholar]
- 15.Fonarow GC, Stevenson LW, Walden JA, Livingston NA, Steimle AE, Hamilton MA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol. 1997;30:725–731. doi: 10.1016/s0735-1097(97)00208-8. [DOI] [PubMed] [Google Scholar]
- 16.Aaronson KD, Mancini DM. Mortality remains high for outpatient transplant candidates with prolonged (>6 months) waiting list time. J Am Coll Cardiol. 1999;33:1189–1195. doi: 10.1016/s0735-1097(98)00697-4. [DOI] [PubMed] [Google Scholar]
- 17.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 18.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities- the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistic—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 20.Shindler DM, Kostis JB, Yusuf S, Quinones MA, Pitt B, Stewart D, et al. Diabetes mellitus, a predictor of morbidity and mortality in the studies of left ventricular dysfunction (SOLVD) trials and registry. Am J Cardiol. 1996;77:1017–1020. doi: 10.1016/s0002-9149(97)89163-1. [DOI] [PubMed] [Google Scholar]
- 21.Gustafsson I, Brendorp B, Seibaek M, Burchardt H, Hildebrandt P, Kober L, et al. Influence of diabetes and diabetes-gender interaction on the risk of death in patients hospitalized with congestive heart failure. J Am Coll Cardiol. 2004;43:771–777. doi: 10.1016/j.jacc.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 22.Inzucchi SE, Masoudi FA, McGuire DK. Metformin in heart failure. Diabetes Care. 2007;30:129. doi: 10.2337/dc07-1686. [DOI] [PubMed] [Google Scholar]
- 23.Tahrani AA, Varughese GI, Scarpello JH, Hanna FW. Metformin, heart failure, and lactic acidosis: is metformin absolutely contraindicated? BMJ. 2007;335:508–512. doi: 10.1136/bmj.39255.669444.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misbin RI. The phantom of lactic acidosis due to metformin in patients with diabetes. Diabetes Care. 2004;27:1791–1793. doi: 10.2337/diacare.27.7.1791. [DOI] [PubMed] [Google Scholar]
- 25.Pantalone KM, Kattan MW, Yu C, Wells BJ, Arrigain S, Jain A, et al. The risk of developing coronary artery disease or congestive heart failure, and overall mortality, in type 2 diabetic patients receiving rosiglitazone, pioglitazone, metformin, or sulfonylureas: a retrospective analysis. Acta Diabetol. 2009;46:145–154. doi: 10.1007/s00592-008-0090-3. [DOI] [PubMed] [Google Scholar]
- 26.McAlister FA, Eurich DT, Majumdar SR, Johnson JA. The risk of heart failure in patients with type 2 diabetes treated with oral agent monotherapy. Eur Heart J. 2008;10:703. doi: 10.1016/j.ejheart.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Nichols GA, Koro CE, Gullion CM, Ephross SA, Brown JB. The incidence of congestive heart failure associated with antidiabetic therapies. Diabetes Metab Res Rev. 2005;21:51–57. doi: 10.1002/dmrr.480. [DOI] [PubMed] [Google Scholar]
- 28.Wong AK, Howie J, Petrie JR, Lang CC. AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin Sci (London) 2009;116:607–620. doi: 10.1042/CS20080066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 31.Eshagian S, Horwich TB, Fonarow GC. An unexpected inverse relationship between HbA1c levels and mortality in patients with diabetes and advanced systolic heart failure. Am Heart J. 2006;151:91. doi: 10.1016/j.ahj.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Kosiborod M, Inzucchi SE, Spertus JA, Wang Y, Masoudi FA, Havranek EP, et al. Elevated admission glucose and mortality in elderly patients hospitalized with heart failure. Circulation. 2009;119:1899–1907. doi: 10.1161/CIRCULATIONAHA.108.821843. [DOI] [PubMed] [Google Scholar]
- 33.Eurich D, Tsuyuki R, Majumdar S, et al. Metformin treatment in diabetes and heart failure: when academic equipoise meets clinical reality. Trials. 2009;10:12. doi: 10.1186/1745-6215-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]