Abstract
Healthy older adults were repeatedly exposed to continuous, variable amplitude oscillations of the support surface to determine 1) whether age affects the capacity for postural motor learning under continuous perturbation conditions with limited predictability and 2) whether practice leads to modifications in the control strategy used to maintain balance in older adults. During training, a translating platform underwent 45-second trials of constant frequency (0.5 Hz) and seemingly random amplitude oscillations (range ± 2 to 15 cm). The middle 15 seconds of each trial contained the same sequence of oscillation amplitudes. This repeated middle segment was used for analyses because young adults in Van Ooteghem et al (2008) experienced the same segment, allowing group comparisons to be made in the present study. To examine learning, participants performed a retention test following a 24-hour delay. Kinematic data were used to derive spatial and temporal measures of whole body centre of mass (COM), trunk, thigh, and shank segment orientation, and ankle and knee angle from performance during the repeated middle segment. Results showed that with training, older adults maintained the capacity to learn adaptive postural responses in the form of improved temporal control of the COM and minimization of trunk instability at a rate comparable to young adults. With practice however, older adults maintained a more rigid, ‘platform-fixed’ control strategy which differed from young adults who shifted toward ‘gravity-fixed’ control that minimized their COM motion. This study provides important insight into the ability of older adults to demonstrate longer-term improvements in postural regulation.
Keywords: aging, balance control, platform translation, learning, continuous perturbation, postural coordination
Introduction
It is well documented that the incidence of postural instability increases with advancing age (Horak et al. 1989; Tang and Woollacott 2004) but there is less consensus regarding age-related deficits in motor learning (Seidler 2006). Despite age-related impairment in controllability, balance loss could be reduced if training induced positive changes in the central nervous system’s (CNS) ability to adapt to environmental disturbances. To date, little empirical research has examined the permanency of training-related changes in balance control in older adults, particularly under conditions that lack predictability. The goal of the current study was to determine whether older adults maintain the capacity to learn a novel balance task requiring continuous postural regulation.
Postural instability can result from both self-initiated and externally-imposed perturbations but the greater risk of balance loss exists when perturbations to stability are external and unpredictable (Horak et al. 1997). Young adults exposed to discrete postural disturbances with limited predictability such as a push or slip, generate motor responses that tend toward a default value corresponding to a medium-sized perturbation (Horak et al. 1989) or to a size appropriate to withstand the largest perturbation (Beckley et al. 1991). Responses depend on the degree of unpredictability and the risk associated with an inappropriate response (Pavol et al. 2002; Bhatt and Pai 2005). Studies exploring the effects of age on short-term adaptability of compensatory postural responses to discrete perturbations and to continuous, predictable perturbations (i.e constant amplitude and frequency) suggest that with age, the CNS maintains some ability to modify balance behaviour based on prior experience (Hocherman et al. 1988; Woollacott and Manchester 1993; Horak and Kuo 2000; Bhatt et al. 2006; Fujiwara et al. 2007). These adaptations have been attributed to temporary changes in sensory and motor processes (Bhatt et al. 2006).
Only recently, have studies examined age and adaptability in the context of longer-term changes in balance behaviour (Pavol et al. 2002; Pavol et al. 2004; Pai and Bhatt 2007). In these studies, older participants repeatedly exposed to discrete slip perturbations show decreases in fall occurrence at similar rates as young adults but they also remain more likely than young adults to fall during re-exposure. Such studies of longer-term retention are fundamental to our understanding of the extent to which older adults can reduce their likelihood of falling by learning to recover from a postural perturbation. To date, no study has examined learning capacity in older adults for continuous balance tasks with limited predictability despite the possibility that the responses required for stability under discrete versus continuous perturbation conditions require different adaptive capabilities (Grabiner et al. 2008) or rely on different control systems (Maki and Ostrovski 1993).
Pavol and Pai (2002) proposed that the long-term goal of the central nervous system in unpredictable circumstances is to acquire an optimal movement strategy that decreases the likelihood of losing balance and reduces dependence on reactive responses. Either by choice or by necessity (e.g. age-related functional decline, perception of stability limits), it is possible that older adults will optimize their control using a different movement strategy than young adults or that they will demonstrate a different degree of adaptation. The motor learning literature shows equal rates of performance improvements for young and older adults on some motor tasks but not others (Seidler 2006), so we were uncertain whether rates of improvement would be comparable across groups during the acquisition phase on day one but we hypothesized that default posture control strategies would differ between groups.
In a recent paper (Van Ooteghem et al. 2008), we described the behaviour changes of young participants who maintained balance in response to continuous, variable amplitude motion of a translating platform. With practice, participants improved their balance control by shifting from an ankle strategy toward a multi-segmental control strategy that allowed them to stabilize their centre of mass (COM) in space. Performance improvements were maintained after a 24-hour delay period providing evidence for learning. The purpose of the current study was to explore differences in behaviour between young and older adults on the variable amplitude platform task in an effort to characterize the adaptive capacity of older adults under these conditions.
Methods
Participants
Ten healthy, older adults (7 males, 3 females) ranging in age from 54–80 (mean 66 ± 7.8 years) and height from 157.5 to 183 cm (mean 171 ± 9.2 cm), volunteered to participate. Prior to inclusion in the study, a telephone questionnaire was administered to ensure that participants were free of severe deficits or disorders that could affect postural control. Upon clinical examination, six participants were unable to stand on foam with eyes closed for 30 seconds. One of these participants also exhibited somatosensory loss as determined by reduced Semme-Weinstein monofilament threshold detection on the plantar surface of the foot and by an inability to detect 128 Hz vibration on the great toe. The methods used in the study were approved by the Oregon Health and Science University Institutional Review Board and by the Office of Research Ethics at the University of Waterloo. All participants provided informed consent prior to data collection. For comparison, data from 12 young, healthy adults reported previously in Van Ooteghem et. al., (2008) was used. Young adults ranged in age from 19–29 (mean 24.3 ± 2.8 years) and in height from 160 to183 cm (mean 171 ± 7.4 cm).
Task and Procedures
Participants stood on a hydraulically driven, servo-controlled platform that could be translated horizontally forward and backward. To prevent falls without restricting motion, subjects wore an industrial safety harness tethered to a sliding hook on an overhead rail. They were instructed to maintain balance while standing with eyes focused on a poster approximately 2m straight ahead and arms crossed at the chest; aiming to avoid stepping if possible. The platform oscillated at a fixed frequency of 0.5 Hz and variable amplitude ranging from ± 2 cm to the largest amplitude that participants could withstand without taking a step (maximum ±15 cm). The maximum amplitude ranged from 80–100% of the 15 cm maximum delivered to young adults in a previous study (Van Ooteghem et al. 2008). Only two participants were unable to maintain balance with their feet in place at this magnitude. For these two participants, platform oscillations were scaled to their maximum (12 and 13 cm). To decrease the likelihood of a step or fall, the platform was offset forward by 6 cm at the start of each trial and the first movement of the platform was in the backward direction.
Trials were composed of three, 15-second segments containing seemingly random oscillations; however, the middle segment included a sequence of platform movements that occurred in every trial. Participants were not informed of this repetition. The repeated sequence of platform oscillations was embedded in the middle of each trial to conceal the repetition and improve the likelihood that participants would deem the perturbation environment unpredictable. The middle segment contained the same sequence of oscillations as the middle segment in Van Ooteghem et al. (2008) and was therefore used for analyses. The first and third segments in the present study were matched for average velocity of translation by deriving the sequences from the pool of amplitudes that defined the middle segment. This method decreased the possibility that the segments would present different degrees of challenge to participants or that the repeated sequence of oscillations would be detected. Combined, the three segments produced a 45-second trial.
Data collection began with a 20-second trial of constant amplitude translation (8 cm), which served to familiarize participants with continuous platform motion. Testing consisted of six blocks of seven trials with a 2-minute rest period between blocks. To separate temporary performance effects from more permanent changes in behaviour that would reflect learning, participants returned for a seven-trial retention test approximately 24 hours following practice.
Data Recording
A Motion Analysis System (Santa Rosa, CA) with six cameras captured three-dimensional spatial coordinate information about body segment displacements and the movement of the platform. Reflective markers were placed bilaterally on the following anatomical landmarks: fifth metatarsophalangeal, lateral malleolus, lateral femoral condyle, greater trochanter, anterior superior iliac spine, iliac crest, styloid process, olecranon, acromion process, lateral mandibular joint and on the xyphoid process. A marker was also placed on the back of the platform. Data were sampled at 60 Hz and low pass filtered using a 2nd order, dual pass Butterworth filter with a cut-off frequency of 5 Hz. The position of the centre of mass (COM) of each body segment in the antero-posterior (AP) direction was calculated using the kinematic data and anthropometric data provided by Winter (1990). Whole body COM position (in space) in the AP direction was derived from the weighted sum of the individual segment COM locations using a custom-designed MATLAB program (Mathworks, Natick, MA). Right side marker data were also used to determine trunk, thigh, and shank segment orientation in the sagittal plane. The trunk segment was defined from the acromium process to the greater trochanter, the thigh segment from the lateral femoral condyle to the greater trochanter, and the shank segment from the lateral malleolus to the lateral femoral condyle. Ankle and knee angles were calculated from foot, thigh and shank segments.
Outcome Measures
Mean gain of the COM (COM peak displacement/platform peak displacement) and mean relative phase of the COM (COM time peak/platform time peak) were derived using the methods described in Van Ooteghem et al. (2008). Together, gain and phase were quantified to examine spatial and temporal control of the COM. Theoretically, a COM gain value of 1.0 would occur if participants adopted a “platform-fixed” control strategy that allowed their COM to travel as far as the platform. Alternatively, a small COM gain would be achieved if participants stabilized their trunk in space (termed “gravity-fixed”) and allowed their lower limbs to travel with the platform. Temporally, positive relative phase values would occur under conditions of COM phase lead relative to platform motion and would indicate predictive control of COM motion. In addition to COM measures, alignment of the trunk relative to gravitational vertical was calculated. The decision to use this measure was driven by the trunk’s significant contribution to the COM and is supported by evidence that the ability to limit undesirable motion of the HAT segment (head, arms, and trunk) is the key factor distinguishing older adults who fall from those who don’t (Grabiner et al. 2008). Tilt (in space) was determined for each time point and these values were averaged for each segment within a trial to determine mean tilt and mean tilt variability. Positive values indicated forward trunk tilt. We considered low variability to reflect more consistent, stable posture of the trunk segment. COM gain, COM phase, and trunk tilt variability were defined as primary outcome measures for balance control. To further describe the COM control strategy, secondary analyses of lower limb postures were conducted by examining time series for thigh and shank segments and by calculating mean ankle and knee joint angle position and variability. Negative thigh segment orientation, positive shank segment orientation, and smaller knee joint angle indicated a flexed-knee control strategy while lower ankle and knee joint variability reflected more rigid postures of the lower limbs.
Data Analyses
To evaluate the effects of age on skill acquisition, outcome measures for the middle (repeated) segment of each trial were compared to previously collected data for young adults (Van Ooteghem et al. 2008). Mixed model ANOVAs with 2 (group) × 6 (training block) were used to analyze performance improvements on day one. Levene’s test for homogeneity of variance was conducted prior to the analysis of each variable. Linear regression was used to determine the slope of mean COM gain and phase (log transform) for individual participants during the six blocks of training on day one. This data was analyzed using one-sample t-tests (p=0.01). To examine retention in older adults, the block of retention trials completed on day two was compared to early (block one) and late (block six) training on day one using one-way repeated measures ANOVAs. Retention comparisons were restricted to primary outcome measures that showed substantial changes in older adults during training on day one (COM phase, trunk tilt and trunk tilt variability). Post hoc analyses were conducted using one-way repeated measures ANOVAs for significant interactions between group and training block, or Tukey’s studentized range (Honestly Significant Difference (HSD)) tests.
An acceptable significance level was 0.05 unless otherwise noted and only those trials in which participants did not take a step were included. In total, 33/504 trials were omitted from training data in young adults and 31/490 trials were omitted from training and retention data for older adults due to stepping.
Results
Acquisition Performance
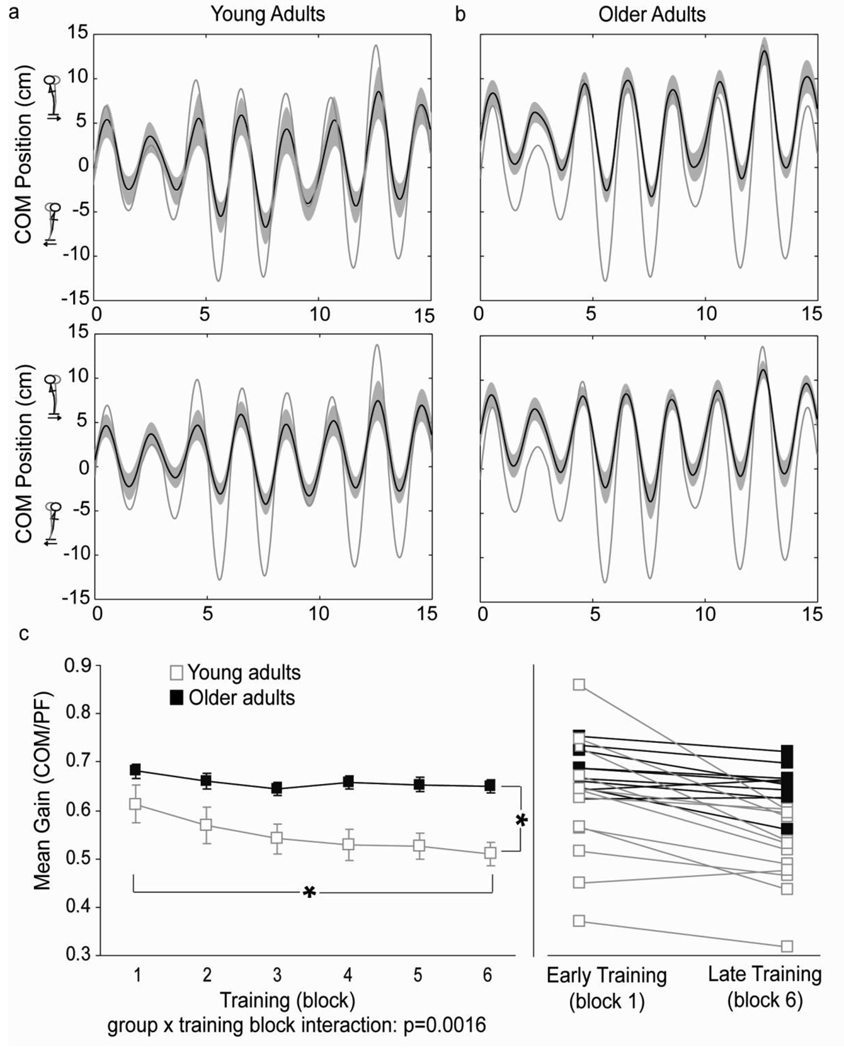
Participants in the current study showed differences in both spatial and temporal control of their COM relative to young adults. Larger COM-to-surface displacement ratios (COM gains) in older adults indicated that they had poorer postural stability in space because they allowed their body COM to be displaced farther with surface displacements, particularly during forward translations (Fig. 1a). Statistically, COM gain differences were revealed by an interaction between group and training block (F(2,39)=4.59; p=0.016 (Greenhouse-Geisser); Fig. 1b) accompanied by a main effect of group (F(1,20)=9.239; p=0.006). Post hoc analysis indicated that young adults had significantly lower gains than older adults as early as block one (p<0.0001). Main effects analysis of training block for older adults revealed that they did decrease their gain significantly (F(5,45)=6.23; p=0.0002) with practice however, these reductions were modest relative to young adults (average 4.6 ± 4.7% versus 15.6 ± 10.4%). Examination of individual participants showed a significant change in gain for 7/12 young adults but only 1/10 older adults as measured by a slope that was significantly different from zero (p<0.01). It should be noted, that three of the young participants who did not show significant gain reductions with training were those who had the smallest gain in early training (range: 0.3645 to 0.5105) indicating a possible floor effect for these participants. Reanalyzing the COM gain data without these three participants further strengthened the interaction between group and training block (F(2,32)=8.37; p=0.001; Greenhouse Geisser). Further, post hoc tests showed that changes in gain for older adults occurred during early exposures to the task as evidenced by significant differences between block one and the remaining training blocks which did not differ from one another.
Fig. 1.
Group average tracings of a) young adult COM motion in early (top) and late (bottom) training and b) older adult COM motion in early (top) and late (bottom) training. Black trace denotes COM motion. Shaded bands represent standard deviation of the mean. Grey trace denotes platform motion. c) Group (left) and individual (right) changes in COM gain with training. Error bars represent standard error of the mean. Young adult data taken from Van Ooteghem et al. (2008)
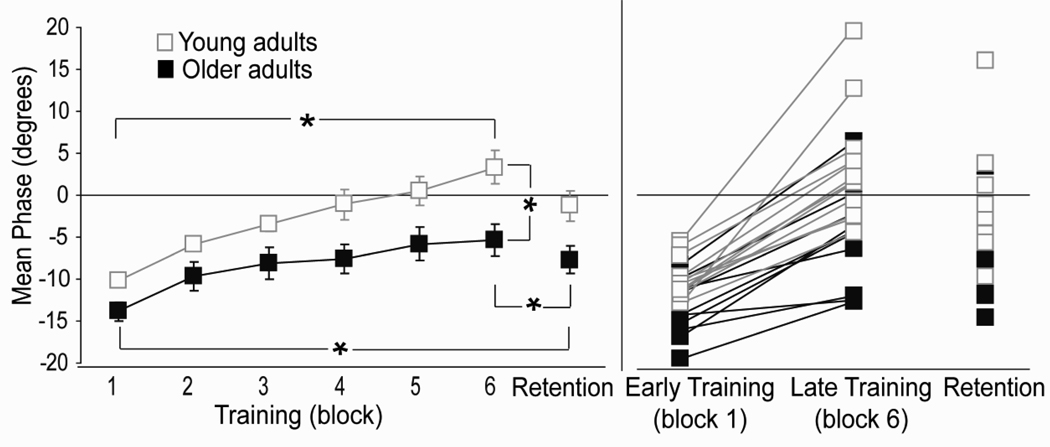
The temporal control of the COM also differed between young and older adults. Analysis of mean relative phase between COM and platform displacements revealed a main effect of block (F(2, 36)=42.990; p<0.001(Greenhouse-Geisser) and group (F(1,20)=8.433; p=0.009; Fig. 2). Examination of individual participants showed a significant change in phase for all young adults and 8/10 older adults (p<0.01). Unlike young adults however, most older adults (7/10) did not achieve temporal phase lock (defined as less than two degrees phase lag) between COM and platform displacements.
Fig. 2.
Group (left) and individual (right) changes in COM phase during training and retention testing. Positive values represent COM phase lead relative to platform motion. Error bars represent standard error of the mean. Asterisks indicate main effects significant at p<0.05. Young adult data taken from Van Ooteghem et al. (2008)
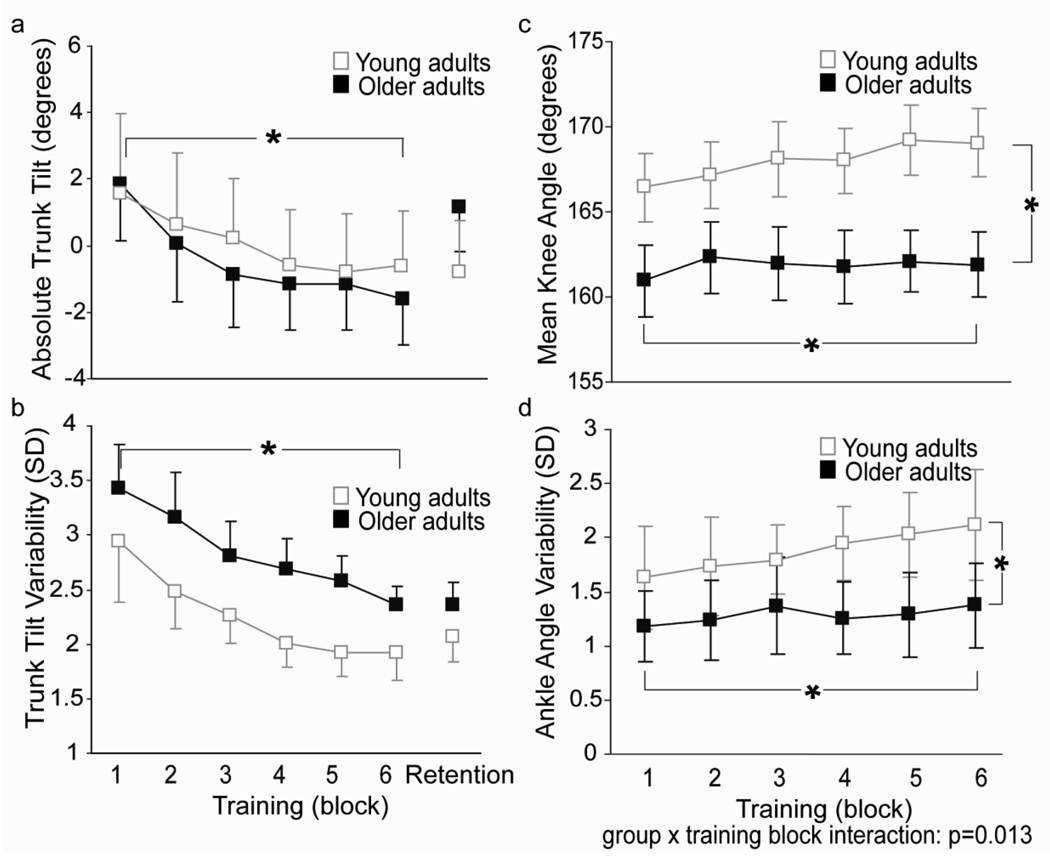
Changes in mean trunk tilt and trunk tilt variability with training were similar for young and older adults (Fig. 3) despite group differences in COM gain and phase. For both groups, mean trunk position shifted from a slightly flexed to upright posture (from 1.58° ± 6.73° to −0.58° ± 4.82° for young adults and 1.86° ± 5.57° to −1.60° ± 4.24° for older adults) as evidenced by a main effect of block (F(1,29)=9.73; p=0.002 (Greenhouse-Geisser); Fig. 3a). Young and older adults also showed comparable decreases in amount of trunk motion with training as indicated by a main effect of block for trunk tilt variability (F(1, 27)=11.13; p=0.001 (Greenhouse-Geisser); Fig.3b). These results suggest that the ability to improve trunk control was preserved with age regardless of the strategy used to maintain balance on the platform.
Fig. 3.
Group changes in a) trunk tilt with respect to gravity during training and retention b) trunk tilt variability during training and retention, c) knee angle during training, and d) ankle angle variability during training. Error bars represent standard error of the mean. Asterisks indicate main effects significant at p<0.05. Young adult data taken from Van Ooteghem et al. (2008)
Ankle and knee joint angle analyses indicated that young and older adults approached the task by adopting different behaviours in their lower limbs. A group effect for mean knee joint position revealed that older adults showed significantly greater knee flexion throughout the task (F(1,20)=5.11; p=0.035; Fig. 3c). A main effect of block however, indicated that both groups decreased knee flexion with training (F(2,45)=4.68; p=0.012 (Greenhouse-Geisser)). Both groups also showed comparable decreases in knee joint variability with training (F(3,53)=5.23; p=0.004; not shown). Although there was no main effect of group or training block for mean ankle joint position, an interaction between training group and block existed for ankle joint variability (F(2,47)=4.42; p = 0.013 (Greenhouse-Geisser); Fig. 3d). Post hoc analyses revealed that ankle angle variability was significantly less for older adults in both early (p<0.0001) and late training (p<0.0001) and that despite group differences, older adults did show modest increases in ankle angle variability (F(5,45)=5.25; p=0.001).
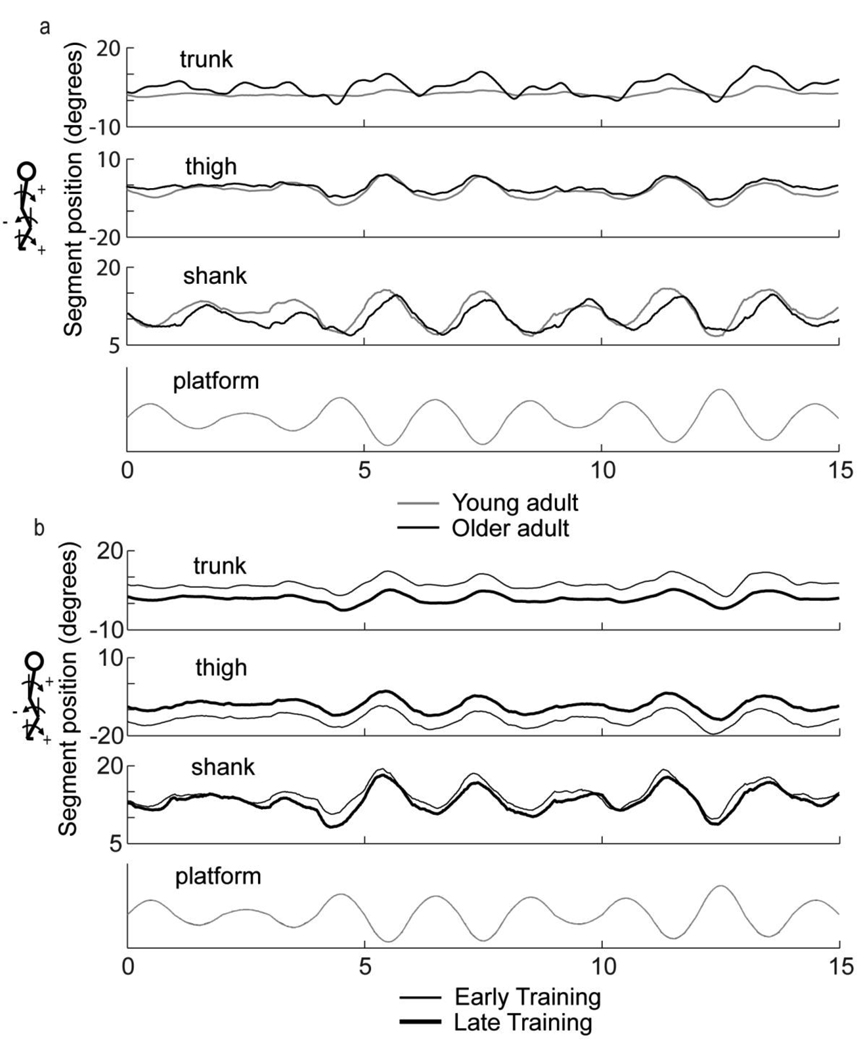
Despite significant group effects for knee joint position, examination of time series for trunk, thigh, and shank segment motion in individual participants revealed that after training, three older adults adopted lower limb motions comparable to young adults (Fig. 4a) while five others were characterized by adjustments in segment alignment with persistent negative tilt of the thigh segment (Fig. 4b). Another two participants showed negligible change in limb motion with training. The participant shown in Fig. 4b was also characterized by the smallest change in COM gain with training.
Fig. 4.
Trunk, thigh, and shank segment time series for a) representative young and older adult showing similar lower limb motion during late training and b) representative older adult during early and late training characterized by persistent flexed postural alignment. Grey trace denotes platform motion. Young adult data taken from Van Ooteghem et al. (2008)
Retention Performance
Retention performance was analyzed for the three variables (COM phase, trunk tilt and trunk tilt variability) that showed substantial changes with training on day one. For all measures, older adults demonstrated some maintenance of performance improvements providing evidence for learning. A main effect of test block (early training, late training, retention) revealed that older adults showed some loss of the temporal shifts in COM demonstrated during late training on day one (F(2,18)=36.19; p<0.0001; Fig. 2). Post hoc comparisons however, revealed that phase lag during retention testing remained significantly less than the lag observed in early training. Analysis of trunk tilt and trunk tilt variability also produced a main effects of test block (F(2,18)=6.472; p=0.008 and F(1,10)=18.87; p=001 (Greenhouse-Geisser) respectively) but post hoc comparisons revealed no significant loss in trunk control from late training on day one to retention testing on day two (Fig 3a and 3b).
Discussion
The results presented here demonstrate that older adults possess an ability to learn adaptive postural responses to continuous, variable amplitude postural perturbations. Adaptations included improved temporal control of their COM in response to the constant frequency of platform motion and minimization of trunk instability at a rate comparable to young adults. Longer-term learning was demonstrated by improved retention test performances relative to early practice. The two groups however, differed in their approach to the task. In general, older adults adopted a more rigid, flexed knee posture throughout training. This strategy differed from young adults who gradually shifted toward a straight-legged, multi-segmental control strategy that enabled them to minimize their COM motion (Van Ooteghem et al. 2008). To our knowledge, this is the first study to describe the effects of age on the ability to learn a continuous balance task with perturbations that have limited predictability; in this case, with constant frequency but variable amplitude.
The main finding of the study is that age did not affect the ability of participants to show some improvement in compensatory posture control with practice under conditions requiring continuous postural regulation, or to show longer-term retention of these improvements. The predominant change in older adults occurred in their ability to control trunk motion. With training, both young and older adults aligned their trunk more vertically and reduced overall trunk motion. These changes occurred at similar rates for the two groups however; young adults exhibited an accompanying shift from an ankle strategy to a multi-segment, gravity-fixed control strategy (Van Ooteghem et al. 2008). In contrast, older adults showed a more platform-fixed strategy evidenced by greater COM gain most notably during forward translations (Fig. 1a and 1b), and less ankle angle variability (Fig. 3d). Differences in COM gain behaviour between young and older adults during forward translations suggest that the control strategies adopted by older adults were driven in part, by efforts to avoid backward balance loss.
Our results agree with previous studies showing that the general response of older adults to constant amplitude/frequency perturbations is to adopt a rigid movement strategy (Hocherman et al. 1988; Wu 1998). Of greater interest for describing practice-related changes in older adults, we show that this platform-fixed strategy persists with training. Unlike the results of Hocherman et al. (1988) however, most older adults (7/10) in the current study did not stand on the platform with fully extended knees. We propose that differences in knee position in the current study reflect the need to limit transfer of reactive forces in a variable amplitude perturbation environment, perhaps to compensate for a decreased ability to control trunk movement.
Evidence for similar rigid response strategies in both predictable and non-predictable perturbation conditions could support the theory that postural equilibrium under various conditions can be achieved by a limited repertoire of response strategies (Horak and Nashner 1986). We suggest however, that modest training-related changes in control strategy amongst older adults reflect loss of CNS flexibility with age. As a group, older adults also showed less between-subject variability in COM gain than young adults (Fig 1c). This finding differs from results of clinical balance tests which typically report increases in variability with age (Era et al. 2006). In the current task, age-related limitations in joint and sensory system function might have constrained the number of options available to older adults. Alternatively, young adults could have possessed a larger range of reference experiences to assist in task performance, enabling some participants to anticipate the consequences of their movements (e.g. the young participant who had the lowest COM gain in early training was a surfer).
Larger COM gains in early training for most older adults provided an opportunity for this group to demonstrate greater practice-related change. Only one older adult however, showed a significant reduction in COM gain in response to variable amplitude platform motion. In this study, the frequency of platform motion was constant which may have served as a regulatory feature of the task (Magill 1998). All subjects demonstrated significant improvement in temporal control of the COM with practice at a rate comparable to young adults suggesting that with age, the CNS maintains an ability to tune into temporal regularities in the perturbation environment. It is important to note that given the same amount of training, only three older adults achieved COM phase lock similar to a majority of young adults. An inability to achieve predictive control of COM like young adults could have been caused by age-related functional impairment in response latencies and reflex loop time (Woollacott et al. 1986; Maurer et al. 2006). In early training, older adults also showed a significantly greater phase lag providing support for age-related response limitations.
Possible reasons for strategy differences between groups and limited practice-related changes in strategy amongst older adults
Older adults exposed to the variable amplitude balance task adopted a rigid, flexed knee posture with their trunk fixed to the surface rather than to gravity, even after practice. Age-related changes both in joint mobility (i.e. joint stiffness, decreased range of motion) and sensory system function (i.e. visual, vestibular, or proprioceptive decline) could have forced older adults to persist with a simplified, default control strategy by limiting the CNS’s ability to develop a robust internal representation of postural control. The effect of these changes could have been exacerbated by threat of falling, prompting older adults to self-select a different goal in response to variable amplitude platform motion (Horak and Kuo 2000) or to refrain from exploring alternate control strategies.
Generally, both groups aimed to decrease trunk motion but as suggested in our previous paper, the multi-segmental control strategy adopted by young adults may reflect efforts to improve efficiency during training (Van Ooteghem et al. 2008). It is possible instead, that the primary goal for older adults was to maintain a safe margin of stability between their COM and base-of-support in an effort to avoid stepping. In both studies, participants were instructed to maintain balance by keeping their feet in place. Previous research shows that older adults prefer to use a stepping strategy, even when the COM is well within the boundaries of the base-of-support (Maki and McIlroy 2005). Adopting a gravity-fixed control strategy similar to young adults would have decreased their margin of stability, particularly at amplitude extremes. Further, separation of the upper and lower body as observed in the gravity-fixed control strategy adopted by young adults in late training requires good joint mobility, particularly at the hips and ankles, appropriate timing of muscle activation to control the trunk, and intact vestibular function to keep the trunk relatively stable with respect to gravity. All of these requirements can become limited with age (Buchanan and Horak 2002; Tang and Woollacott 2004).
Age-related declines in sensory system function could have negatively affected older adults’ ability to gather information about the perturbation characteristics or their body orientation; restricting their ability to evolve their control strategy with practice. If older adults experienced loss in vestibular or proprioceptive sensitivity, they might have shifted sensory system weighting toward vision (Lord and Menz 2000, Speers et al. 2002). The platform-fixed control strategy adopted by older adults produced a stable head position with respect to the trunk and head displacement which might have generated rich, optic flow information. Since the frequency of platform motion was constant, temporal regularity in the approach and retreat of a stable reference point might have provided helpful cues regarding body motion. Studies of the influence of static and dynamic visual cues on posture control have shown that dynamic visual cues contribute to fast stabilization of the whole body (Amblard et. al. 1985).
Learning in a variable amplitude environment
For both COM phase and trunk tilt variability, retention test outcomes were better than pre-practice performance demonstrating older adults’ capacity for postural motor learning in a variable amplitude environment. Some loss in the ability to exploit the temporal regularity in platform motion from late training to retention testing on day two did occur but this was also observed in young adults (Van Ooteghem et al. 2008). The decline in performance could be attributed in part, to a warm up decrement but this possibility needs to be explored further. The ability to control trunk motion however, was maintained across days of testing. Evidence for longer-term retention of these performance improvements in older adults provides important insight into the potential for sustainable changes in continuous postural regulation, despite kinematic strategies that were different from younger adults. More work must be done however, to examine older adults’ persistence with a simplified control strategy that could offer less stability and be more energy demanding.
Acknowledgements
The authors would like to thank Edward King and John Buchanan for technical assistance. Funded by NSERC grant RGPIN2278502, NIH grant AG006457, and ONF grant 2005-PREV-MS-352.
Contributor Information
Karen Van Ooteghem, Department of Kinesiology, University of Waterloo, 200 University Ave. W., Waterloo, Ontario, Canada N2L 3G1.
James S. Frank, Faculty of Graduate Studies and Research, University of Windsor, 401 Sunset Ave., Windsor, Ontario, Canada N9B 3P4, jsfrank@uwindsor.ca, t: (519) 253-3000 ext. 2107, f: (519) 971-3667.
Fay B Horak, Department of Neurology, Oregon Health and Sciences University, 505 NW 185th Ave., Portland, Oregon, USA 97006.
References
- 1.Amblard B, Cremieux J, Marchand AR, Carblanc A. Lateral orientation and stabilization of human stance: static versus dynamic visual cues. Exp Brain Res. 1985;61:21–37. doi: 10.1007/BF00235617. [DOI] [PubMed] [Google Scholar]
- 2.Beckley DJ, Bloem BR, Remler MP, Roos RA, Van Dijk JG. Long latency postural responses are functionally modified by central set. Electroencephalogr Clin Neurophysiol. 1991;81(5):353–358. doi: 10.1016/0168-5597(91)90024-r. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt T, Pai YC. Long-term retention of gait stability improvements. J Neurophysiol. 2005;94(3):1971–1979. doi: 10.1152/jn.00266.2005. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan JJ, Horak FB. Vestibular loss disrupts control of head and trunk on a sinusoidally moving platform. J Vest Res. 2002;11(6):371–389. [PubMed] [Google Scholar]
- 5.Era P, Sainio P, Koskinen S, Haavisto P, Vaara M, Aromaa A. Postural balance in a random sample of 7,979 subjects aged 30 years and over. Gerontology. 2006;52(4):204–213. doi: 10.1159/000093652. [DOI] [PubMed] [Google Scholar]
- 6.Fujiwara K, Kiyota T, Maeda K, Horak FB. Postural control adaptability to floor oscillation in the elderly. J Physiol Anthropol. 2007;26(4):485–493. doi: 10.2114/jpa2.26.485. [DOI] [PubMed] [Google Scholar]
- 7.Grabiner MD, Donovan S, Bareither ML, Marone JR, Hamstra-Wright K, Gatts S, Troy KL. Trunk kinematics and fall risk of older adults: translating biomechanical results to the clinic. J Electromyogr Kinesiol. 2008;18:197–204. doi: 10.1016/j.jelekin.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Hocherman S, Dickstein R, Hirschbiene A, Pillar T. Postural responses of normal geriatric and hemiplegic patients to a continuing perturbation. Exp Neurol. 1988;99:388–402. doi: 10.1016/0014-4886(88)90156-2. [DOI] [PubMed] [Google Scholar]
- 9.Horak FB, Diener HC, Nashner LM. Influence of central set on human postural responses. J Neurophysiol. 1989;62(4):841–853. doi: 10.1152/jn.1989.62.4.841. [DOI] [PubMed] [Google Scholar]
- 10.Horak FB, Henry SM, Shumway-Cook A. Postural perturbations: new insights for treatment of balance disorders. Phys Ther. 1997;77(5):517–533. doi: 10.1093/ptj/77.5.517. [DOI] [PubMed] [Google Scholar]
- 11.Horak FB, Kuo A. Postural adaptation for altered environments, tasks, and intentions. In: Winter JM, Crago PE, editors. Biomechanics and Neural Control of Posture and Movement. New York: Springer-Verlag; 2000. pp. 267–281. [Google Scholar]
- 12.Horak FB, Nashner L. Central programming of postural movements: adaptation to altered support surface configurations. J Neurophysiol. 1986;55(6):1369–1381. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- 13.Lord SR, Menz HB. Visual contributions to postural stability in older adults. Gerontol. 2000;46(6):306–310. doi: 10.1159/000022182. [DOI] [PubMed] [Google Scholar]
- 14.Magill RA. 1997 C.H. McCloy research lecture: knowing is more than we can talk about: implicit learning in motor skill acquisition. Res Q Exerc Sport. 1998;69(2):104–110. doi: 10.1080/02701367.1998.10607676. [DOI] [PubMed] [Google Scholar]
- 15.Maki BE, Ostrovski G. Do postural responses to transient and continuous perturbations show similar vision and amplitude dependence? J Biomech. 1993;26(10):1181–1190. doi: 10.1016/0021-9290(93)90066-n. [DOI] [PubMed] [Google Scholar]
- 16.Maki BE, McIlroy WE. Change-in-support balance reactions in older persons: an emerging research area of clinical importance. Neurologic Clinics. 2005;23(3):751–783. doi: 10.1016/j.ncl.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Maurer C, Mergner T, Peterka RJ. Multisensory control of human upright stance. Exp Brain Res. 2006;171(2):231–250. doi: 10.1007/s00221-005-0256-y. [DOI] [PubMed] [Google Scholar]
- 18.Pai YC, Bhatt T. Repeated-slip training: an emerging paradigm for prevention of slip-related falls among older adults. Phys Ther. 2007;87(11):1478–1491. doi: 10.2522/ptj.20060326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavol MJ, Pai YC. Feedforward adaptations are used to compensate for potential loss of balance. Exp Brain Res. 2002;145(4):528–538. doi: 10.1007/s00221-002-1143-4. [DOI] [PubMed] [Google Scholar]
- 20.Pavol MJ, Runtz EF, Edwards BJ, Pai YC. Age influences outcome of a slipping perturbation during initial but not repeated exposures. J Gerontol A Biol Sci Med Sci. 2002;57(8):M496–M503. doi: 10.1093/gerona/57.8.m496. [DOI] [PubMed] [Google Scholar]
- 21.Pavol MJ, Runtz EF, Pai YC. Young and older adults exhibit proactive and reactive adaptations to repeated slip exposure. J Gerontol A Biol Sci Med Sci. 2004;59(5):494–502. doi: 10.1093/gerona/59.5.m494. [DOI] [PubMed] [Google Scholar]
- 22.Seidler RD. Differential effects of age on sequence learning and sensorimotor adaptation. Brain Res Bull. 2006;70:337–346. doi: 10.1016/j.brainresbull.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Speers RA, Kuo AD, Horak FB. Contributions of altered sensation and feedback responses to changes in coordination of postural control due to aging. Gait Posture. 2002;16(1):20–30. doi: 10.1016/s0966-6362(02)00003-6. [DOI] [PubMed] [Google Scholar]
- 24.Tang PF, Woollacott M. Balance control in older adults. In: Bronstein AM, Brandt T, Woollacott MJ, Nutt JG, editors. Clinical Disorders of Balance, Posture, and Gait. 2nd Ed. New York: Oxford University Press; 2004. pp. 385–403. [Google Scholar]
- 25.Van Ooteghem K, Frank JS, Allard F, Buchanan JJ, Oates AR, Horak FB. Compensatory postural adaptations during continuous, variable amplitude perturbations reveal generalized rather than sequence-specific learning. Exp Brain Res. 2008;187(4):603–611. doi: 10.1007/s00221-008-1329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winter DA. Biomechanics and Motor Control of Human Movement. 2nd Ed. New York: John Wiley and Sons, Inc; 1990. pp. 56–57. [Google Scholar]
- 27.Woollacott MH, Manchester DL. Anticipatory postural adjustments in older adults: are changes in response characteristics due to changes in strategy? J. Gerontol. 1993;48:64–70. doi: 10.1093/geronj/48.2.m64. [DOI] [PubMed] [Google Scholar]
- 28.Woollacott MH, Shumway-Cook A, Nashner L. Aging and posture control: changes in sensory organization and muscular coordination. Int J Aging Hum Dev. 1986;23(2):97–114. doi: 10.2190/VXN3-N3RT-54JB-X16X. [DOI] [PubMed] [Google Scholar]
- 29.Wu G. Age-related differences in body segmental movement during perturbed stance in humans. Clin Biomech. 1998;13(4–5):300–307. doi: 10.1016/s0268-0033(98)00068-0. [DOI] [PubMed] [Google Scholar]