Abstract
Generally, cancers may undergo the developmental stages of benign proliferation, precancer and invasive cancer. Identification of biomarkers that are expressed throughout the developmental stages will facilitate detection, prevention and therapy of cancer. Piwil2, a member of AGO/PIWI family of proteins, has been suggested to be associated with tumor development. Here we reported that piwil2 can be detected by immunohistochemistry (IHC) in various stages of human cervical squamous cell carcinomas and adenocarcinomas. Interestingly, piwil2 was also detected in some metaplastic epithelial cells as well as histologically “normal” appearing tissues adjacent to malignant lesions. While all the premalignant and malignant lesions expressed varying levels of piwil2, p16INK4a (p16), a surrogate indicator of high-risk human papillomavirus (HR-HPV) infection, was detected in only 84.62% of the specimens. In Papanicolaou (Pap) test, piwil2 was also detected in atypical glandular cells (AGC), low-grade (LSIL) and high-grade squamous intraepithelial lesions (HSIL), whereas p16 was not always concomitantly detected in the same specimens. The results suggest that piwil2 might play important roles throughout the process of cervical cancer development and have the potential to be used as a complementary marker for p16INK4a. It is worth further study to improve the sensitivity and specificity of current screening methods for cervical cancers.
Keywords: Piwil2, precancer, tumor development, field cancerization, cervical cancer, p16INK4a
Introduction
Tumorigenesis denotes a process from initiation, progression to establishment of a tumor. Existing histopathological and clinical data have suggested that the formation of a tumor may undergo the stages of benign proliferation or tumor initiation, precancer and cancer, which might be mediated by tumor-initiating stem cells (TISCs), precancerous stem cells (pCSCs) and cancer stem cells (CSCs), respectively [1-6]. Generally, the stage of tumor initiation is considered to be associated with benign proliferative lesions, such as hyperplasia, metaplasia and/or low grade dysplasia; precancer stages is demonstrated as high grade dysplasia and carcinoma in situ (CIS); and malignant stage exhibits uncontrollable and irreversible proliferation, invasiveness and metastasis of tumor cells [2, 7]. Although numerous cancer genes including oncogenes (ONGs), tumor suppressor genes (TSGs) and stability genes (SGs) have been considered as biomarkers for various types of cancer, most of them are not tumor-specific or not expressed throughout the process of tumor development [2, 8-10]. Therefore, identification of a biomarker(s) that is specific for cancer and expressed throughout the process of cancer development is important for us to better understand the mechanisms underlying tumorigenesis.
Recent identification of precancerous stem cells (pCSCs) in our laboratory [1-4] has led us to discovering pCSC-associated biomarkers, such as piwil2 [1-3], which is not expressed in normal adult stem cells or somatic tissue cells but has been detected in various types of human and animal tumor cell lines as well as human breast cancers [3, 11, 12]. Therefore, we hypothesized that piwil2 is potentially a common biomarker for various types of cancers [1, 2].
The PIWIL2 gene (alias mili in mouse or hili in humans) is a member of the P-element-induced wimpy testis/Argonaute (PIWI/AGO) gene subfamily, which is essential for germ cell development [13-17]. PIWI/AGO genes contain Piwi and PAZ domain (PPD), playing important roles for stem cell self-renewal in drosophila [18], gametogenesis [14], small RNA-mediated gene silencing [19, 20] and/or chromatin remodeling [21, 22]. Recently, piwil2 was found to bind a novel class of RNA called piwi-interacting RNA (piRNA) or repeat-associated small interfering RNAs (rasiRNAs), in mammal testis [23-28]. It may silence the selfish genetic elements, such as retrotransposons, in the germline stem cells (GSCs) of testis [24, 28, 29]. Dysregulated piwi protein expression appears to be associated with tumorigenesis [3, 11, 30]. Therefore, piwil2 might play an important role in tumor development [1, 3], including cervical cancers.
To determine whether piwil2 is a common biomarker for caners, it is a premise to determine whether piwil2 is expressed in various stages of tumor development, including the stages of benign proliferation, precancer and invasive cancer. Recently, we have demonstrated that piwil2 is expressed in various stages of breast cancer [12]. To extend the observation to other cancers, we examined piwil2 expression in various stages of cervical cancers, because the developmental stages of human cervical carcinomas are better defined histopathologically than other cancers.
Cervical cancer remains one of most common cancers threatening woman life, although a prophylactic human papillomavirus (HPV) vaccine may reduce its incidence [31, 32]. HPV infection is a major cause for cervical carcinomas, which can induce the expression of p16INK4a (p16), a tumor suppressor protein [33, 34], in the epithelial cells [32, 35, 36]. The p16, a CDKN2A gene product, is a cyclin-dependent kinase (CDK) inhibitor that decelerates the cell cycle by inactivating the CDKs that phosphorylate retinoblastoma (Rb), an oncogenic protein [32, 35, 36]. HPV E7 protein can inactivate the function of Rb through the induction of p16 expression. Although the effect of p16 expression on cervical cancer development is not clear, HPV plus p16 has sufficient sensitivity to screen cervical lesions caused by high risk (HR)-HPV infection, but lacking high specificity, because only a minority of cases of HPV infection can progress to premalignant or malignant lesions in cervical mucosa [37].
In this study, we have investigated whether piwil2 is expressed in various stages of cervical cancers and compared it with p16 expression. The results demonstrated that piwil2 is expressed in various stages of cervical cancers and has the potential to be used as a complementary biomarker for p16 to improve the sensitivity and specificity of current screening methods for cervical cancers.
Materials and methods
Specimens and reagents
This study was approved by the Institutional Review Board (IRB #2007EO686) at the Ohio State University (OSU). Archival formalin-fixed, paraffin-embedded cervical cancer specimens at various developmental stages were obtained from the Tissue Procurement Shared Resource (TPSR), Comprehensive Cancer Center, Ohio State University. 91 specimens were procured from surgical pathology specimens of the patients with various stages and various types of cervical lesions (Table 1). The specimens were fixed in 10% formalin, embedded in paraffin and prepared for tissue microarrays (TMAs). The TMA cores with 2 mm in diameter were built by the Histological Core Facility, Department of Pathology, OSU. Since tumor tissue were heterogenous in lesions and TMA cores were obtained from a small area of specimens, each H & E stained TMA cores were reassessed double-blindly by two pathologists (Table 1). For experiments from Figures 1 to 5, regular tissue sections were used for analysis of piwil2 expression in various types of lesions, including the histologically “normal” appearing tissues within or near carcinomas. In addition, normal cervical uteri biopsy tissues (NCBT: n=8) were used as normal control for piwil2 staining (Table 1 & Figure 6).
Table 1.
Pathological Diagnosis and Comparison of Expressions between Piwil2 and p16
| Pathological diagnosis | Piwil2+ | Piwil2 | p16+ | p16 | ||
|---|---|---|---|---|---|---|
| Case No. | (n) | (%) | (n) | (%) | P value* | |
| NCBT (Regular section) | 8 | 2 | 25 | 0 | 0 | ND# |
| HP | 2 | 2 | 100 | 1 | 50 | ND |
| CIN-1 | 2 | 2 | 100 | 8 | 100 | NA$ |
| CIN-2 | 8 | 8 | 100 | 10 | 100 | NA |
| CIN-3 | 16 | 16 | 100 | 16 | 100 | NA |
| Inv.Sqcc | 36 | 36 | 100 | 30 | 83.33 | 0.011 |
| AIS | 5 | 5 | 100 | 5 | 100 | NA |
| Adc | 11 | 11 | 100 | 9 | 81.82 | 0.17 |
| Inv. Adc | 4 | 4 | 100 | 3 | 75 | 0.39 |
| Keratinized sqcc | 1 | 1 | 100 | 0 | 0 | ND |
| Clear cell poor | 1 | 1 | 100 | 0 | 0 | ND |
| Small cells | 3 | 3 | 100 | 3 | 100 | NA |
| Ser adc | 2 | 2 | 100 | 2 | 100 | NA |
| Total TMA cores | 91 | 91 | 100 | 87 | 0.87 | 0.0001 |
Pearson Chi-Square test;
ND, not determinable;
NA, not applicable;
NCBT: normal cervical uteri biopsy tissue; HP: cervical hyperplasia; CIN-1, 2 or 3: cervical intraepithelial neoplasia-1, 2 or 3; Inv. Sqcc: invasive squamous cell carcinoma; AIS: adenocarcinoma in situ; Adc: adenocarcinoma; Inv. Adc: invasive adenocarcinoma; Keratinized sqcc: keratinized squamous cell carcinoma; Clear cell poor: poorly differentiated clear cell carcinoma; Small cell: small cell carcinoma; Ser adc: serous adenocarcinoma.
Figure 1.
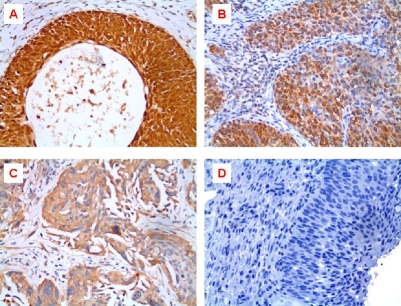
Representative micrographs of IHC staining intensity of piwil2 in cervical cancers. A, Strong intensity: +++; B, Moderate intensity: ++; C, Weak intensity: +; D, Negative control: Rabbit IgG was used as primary antibody instead of polyclonal rabbit anti-piwil2.
Figure 5.
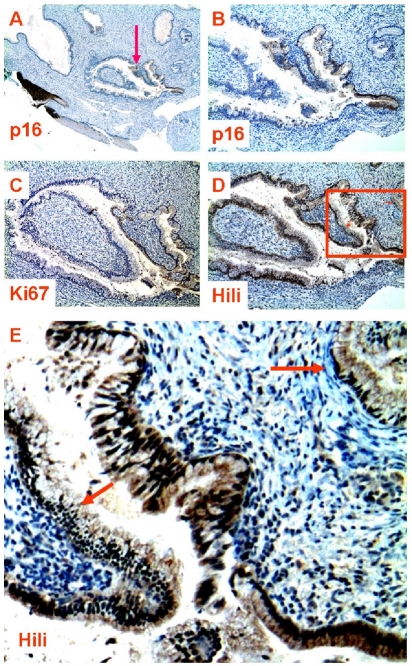
Comparison of the expression between piwil2, p16 and Ki67 in the same cervical neoplasia. The consecutive sections from a patient with CIN-3 were staining with antibody to p16 (A & C), piwil2 (B), or Ki67 (D). A, shown is the micrograph with low magnification showing p16 staining in whole area of CIN-3 (x10). The boxed area was projected as shown in B, C & D (x 100), which were stained by anti-piwil2, p16 and Ki67, respectively. The arrows in the B indicate that the normal appearing glands near CIN-3 were stained by anti-piwil2, but not by anti-p16 (C) and Ki67 (D).
Figure 6.
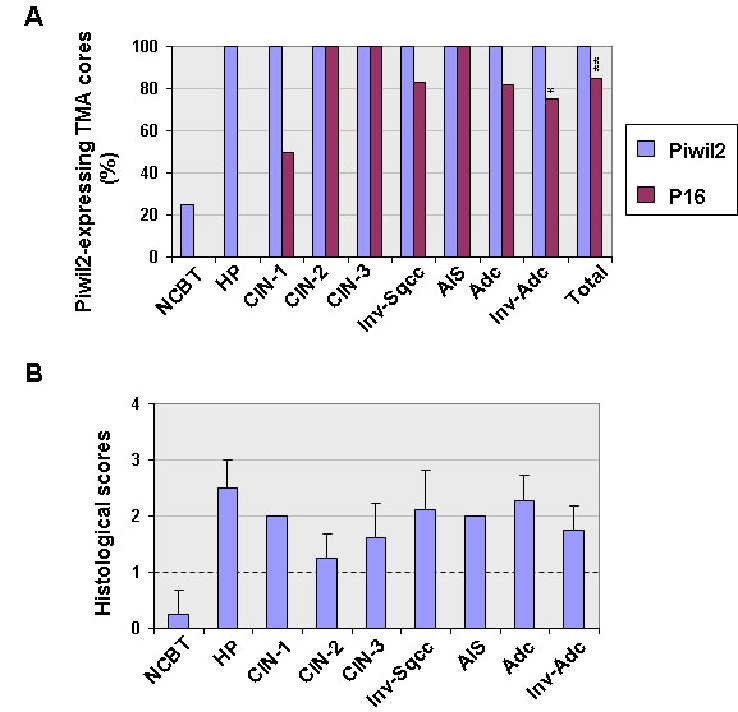
Comparison between piwil2 and p16 expressed in cervical neoplasias. Ninety-one tissue microarray (TMA) cores were prepared from 91 cervical cancer specimens and were examined for piwil2 and p16 expression by IHC. Normal cervical uteri biopsy tissues (NCBT): n=8; Hyperplasia (HP): n=2; cervical intraepithelial neoplasm-1 (CIN-1): n=2; CIN-2: n=8; CIN-3: n=16; invasive squamous cell carcinoma (Inv-sqcc), n=36; adenocarcinoma in situ (AIS), n=5; adenocarcinoma (Adc), n=11; invasive adenocarcinoma (Inv-adc): n=4; Total: n=91. Polyclonal rabbit antibody to piwil2 was used for IHC staining. A, Shown is the prevalence of piwil2 and p16 in various stages and types of cervical neoplasia. Overall, piwil2 but not p16 was expressed in all cervical lesions, especially in the Inv-sqcc. **, p = 0.0001 (Pearson Chi-Square test), and *, p = 0.01(Pearson Chi-Square test) when compared between piwil2 and p16. B, The levels of piwil2 expressed in various developing stages of cervical neoplasia. Shown are the mean score ± SD. In addition, one case of keratinized squamous cell carcinoma and a poorly differentiated clear cell carcinoma expressed piwil2, but not p16. Three cases of small cell carcinoma and two serous adenocarcinomas expressed both piwil2 and p16 (Table 1).
Polyclonal rabbit anti-mili antibody was generated and purified as previously described [12, 14]. The mAb to Ki67 was purchased from Dako (Carpinteria, California, USA), and mAb to p16 (clone E6H4) was purchased from the CIN-tec (mtm Laboratories, Heidelberg, Germany).
Histological and immunohistochemical (IHC) analysis
IHC analysis was performed as previously described [3, 4, 38]. TMAs were examined for piwil2 and p16 by immunohistochemistry (IHC). Sections (4 ∼ 5 μm) were stained by H & E for pathological analysis, or immunostained with a primary antibody to mili (piwil2) or p16 followed by a horseradish peroxidase (HRP)-conjugated secondary antibody. The immunostained sections were counterstained with hematoxylin and graded for histopathological scores as the follow: cell positive score + staining intensity score/2. The cell positive score was graded: < 5% as 0 (-); 5% - < 25% as 1 (+); 25% - 50% as 2 (++) and > 50% as 3 (+++), and staining intensity score was graded as 0, negative (-); 0.5 -1, faint (+); 1.5 - 2, moderate (++); and 2.5 - 3, strong staining (+++) (Figure 1). The histological score ≥ 1 was designated as positive.
Immunocytochemical ICC analysis of exfoliated cervical cells
Eighteen cervical Pap (SurePath-BD Diagnostics - TriPath, Burlington, NC 27215) slides from women of 16 to 39 years old were selected for the samples diagnosed as low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL) and atypical glandular cells, not otherwise specified (AGC-NOS). Duplicated cervical smear slides were made using the SurePath Prep according to standard procedures. The slides were stained with the Papanicolaou stain and polyclonal rabbit anti-piwil2 antibody or murine mAb to p16. Endometrial cells, piwil2- or p16-expressing cells were identified and photographed.
For antigen retrieval, the slides were first processed with 0.01 mol/L of Citric Buffer (Target Retrieval Solution from Dako, Carpinteria, CA) for 25 minutes in steamer, then incubated sequentially with anti-piwil2 or p16, followed by horseradish peroxidase-conjugated anti-rabbit (LSAB+ from Dako) for piwil2 and anti-mouse (EnVision + from Dako) for p16, following manufacturer's instruction.
Statistical analysis
Pearson Chi-Square test was used to compare the expression in percentage between piwil2 and p16 in each stage of cervical lesion. A value of p ≤ 0.05 was considered significant. Data are represented as mean ± SD or percentage.
Results
Piwil2 is expressed in cervical precancers and cervical carcinomas
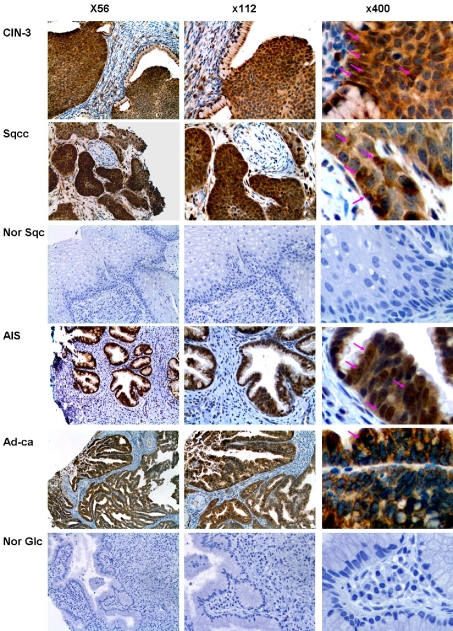
Because piwil2 was detected in pCSCs and various types of tumor cell lines [3, 11], we hypothesized that piwil2 might also be expressed in cervical carcinomas. We examined piwil2 expression in cervical precancers, including cervical squamous cell carcinoma in situ (CIS) or high-grade cervical intraepithelial neoplasia (CIN -3) and adenocarcinoma in situ (AIS), as well as invasive cervical carcinomas such as squamous cell carcinoma (Sqcc) and adenocarcinoma (Adc). As shown in Figure 2, piwil2 was not only detected in all the lesions of CIN-3 and AIS, but also in the Sqcc and Adc. In CIN-3 and invasive Sqcc, piwil2 expression was expressed differentially (Figure 2). While high grade dysplastic cells in CIN-3 expressed piwil2 mainly in cytoplasm, some of them co-expressed piwil2 in the nucleus; invasive Sqcc had fewer piwil2-expressing cells, especially those with nuclear pattern, than CIN-3 (Figure 2, arrows). A similar expression pattern was also observed in AIS and Adc (Figure 2). As controls, normal squamous and glandular epithelial cells did not express piwil2 (Figure 2). The results suggest that piwil2 is expressed in cervical precancers and invasive carcinomas without restriction of histologic types, and its level might be altered with the progression of tumors.
Figure 2.
Piwil2 is expressed in various types of cervical precancers and cancers. The specimens of cervical precancers (CIN-3 and AIS), invasive cancers (Sqcc and Ad-ca), and normal cervical tissues (Nor Sqc and Nor Glc) were stained with rabbit anti piwil2. Shown are representative figures of cervical precancers and invasive cancers stained by anti-piwil2. The arrows in the figures indicate the representative cells that are strongly expressed with piwil2 in the cytoplasm and nucleus (C-N). CIN-3: high-grade cervical intraepithelial neoplasia or squamous cell carcinoma in situ; AIS: Adenocarcinoma in situ; Sqcc: invasive squamous cell carcinoma; Adc: invasive adenocarcinoma; Nor Sqc: normal squamous epithelial cells; Nor Glc: normal glandular epithelial cells.
Piwil2 is expressed in proliferating metaplastic epithelial cells of cervix uteri
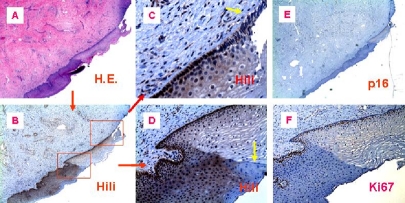
It has been proposed that piwil2 might serve as a barrier gene to tumorigenesis [1, 2]. If this is true, piwil2 should be expressed in very early stages of cancer development. In fact, piwil2 was also detected in some metaplastic epithelial cells adjacent to cervical precancerous or cancerous lesions. Metaplasia is defined as the transformation of one type of mature differentiated cell type into another mature differentiated cell type or as an adaptive response to some insults or injuries, such as carcinogens. During cervical tumorigenesis, cervical metaplasia could be a reversible developmental stage earlier than CIN-1. As shown in Figure 3, in a cervical specimen from a patient with CIN-3, metaplastic squamous epithelial cells close to the neoplastic lesions were observed (Figure 3A). Piwi12 was moderately expressed in the metaplastic squamous epithelia (Figure 3B & D), even in the early premature proliferative parabasal squamous cells (Figure 3B & C). However, not all the cells in these areas expressed piwil2 (Figure 3C & D; yellow arrows). As a control, p16 was not detected in the same sample at all (Figure 3E), implicating that the piwil2-expressing metaplastic cells were not necessarily associated with HR-HPV infection or PIWIL2 gene was activated earlier than CDKN2A in the HR-HPV-infected cells. However, Ki67 (also known as MKI67), a cellular marker for proliferation [39], was concomitantly detected with piwil2 in the area, indicating that these piwil2-expressing cells were proliferating metaplastic cells (Figure 3F). Therefore, the metaplastic cells that expressed piwil2 might be associated with tumor initiation or benign proliferation [5, 40].
Figure 3.
Piwil2 is expressed in the metaplastic proliferating squamous epithelial cells. Shown are the representative figures of cervical metaplastic squamous epithelial cells stained by anti-piwil2, Ki67 or p16 antibody in the morphologically normal appearing area of the consecutive tissue section from a patient with CIN-3 lesion. Piwi12 was moderately expressed in metaplastic squamous epithelial cells, and even in the early parabasal proliferating squamous cells, the piwi12 was faintly or weakly expressed. A, H & E staining; B, Piwil2 was expressed in metaplastic squamous epithelial cells and “normal” cells surrounding the lesion; C, The basal and parabasal cells in the histologically “normal” area projected from expressed piwil2 moderately or weakly. Yellow arrows in C & D indicates the piwil2-negative metaplastic cells. D, Shown is the area projected from B, in which piwil2 was expressed moderately in the epithelial layers of cervical mucosa with newly metaplastic squamous epithelium; E, The section was stained with anti -p16; and F, the section was stained by anti-Ki67. The micrographs were originally taken at x40 (A, B & E) and x100 (F). Figure C & D were projected from B (x400).
Piwil2 is expressed in the histologically “normal” appearing tissues adjacent to cancers
Recently it has been demonstrated that in the colorectal, gastric, and breast cancers, aberrant DNA methylation was detected in histologically “normal” appearing tissues adjacent to cancerous lesions [6, 41-44], suggesting that the occult precancerous alterations at molecular level exist in the histologically “normal” tissues surrounding the malignant lesions. To determine whether piwil2 is expressed in the histologically “normal” tissue adjacent to cervical lesions, we investigated piwil2 expression in the “normal” appearing tissues within or adjacent to premalignant or malignant areas of cervix uteri. As shown in Figure 4 & 5, “normal” appearing tissues adjacent to CIN-3 lesions (Figure 4A & 5A) were strongly stained by anti-piwil2 (Figure 4D and 5B). The glandular epithelial cells with strong piwil2 staining appeared to be hyperplastic (Figure 4E & 5B). This staining is piwil2-specific, because within the same area, some glandular epithelial cells that appeared to be completely normal (without hyperplasia) were not stained by anti-piwil2 at all (Figure 4E: arrows). The size of piwil2-expressing cells was obviously larger than that of piwil2-negative cells (Figure 4E). Thus, the piwil2-expressing cells were sporadically but selectively distributed among normal epithelial cells and might be distinct in origin from those not expressed with piwil2, despite the fact that they were exposed to the same environmental cue.
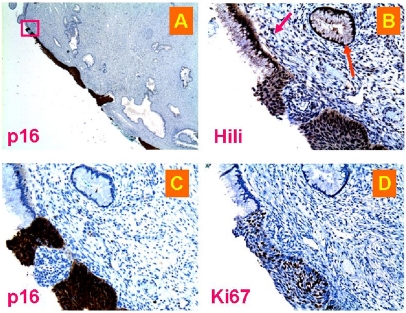
Figure 4.
Piwil2, p16 and Ki67 expression in “normal” appearing glands near neoplasia. The consecutive sections from a patient with cervical neoplasia were staining with antibody to piwil2 (D & E), p16 (A & B), or Ki67 (C). Piwi12 shows the intensity from entirely negative (E, arrows) to weak-moderate-strong positive staining (D & E) in the morphologically normal appearing glands, in which p16 and Ki67 expressions were relatively limited (B & C). A, shown is a CIN-3 lesion stained by anti-p16 and a gland near the lesion (arrow) (x20). B, shown is the p16-stained gland projected from the A (x50). C, shown is Ki67 staining in the same gland (x40). D, the same gland was widely stained by anti-piwil2, demonstrating variable staining intensity as shown in E projected from D (x 200), in which hyperplastic cells were strongly stained by anti-piwil2 and normal cells were negatively stained as indicated by arrows.
Furthermore, we observed that piwil2-expressing cells in the histologically “normal” tissues did not always concomitantly express p16 and Ki67 (Figure 4B & C and Figure 5C & D). While piwil2 was widely expressed in most glandular epithelial cells of a gland (Figure 4D), p16 was only detected in limited area of the same gland (Figure 4B). The p16 expression is implicated that the involved cells were infected by HPV [35, 36] and had already developed into dysplastic cells undergoing proliferation. This appeared to be true, because the Ki67, a molecule that can be easily detected in proliferating cells within a tumor, was also detected in the same area where p16 was detected with the cell numbers being comparable (Figure 4C). Similar phenomenon was also observed in the cervical squamous epithelium (Figure 5). The piwi12 was detected in the CIN-3 (Figure 5B) with a level being comparable to p16 (Figure 5C) and Ki67 (Figure 5D). However, piwi12 proteins but not Ki67 and p16 were also detected in the adjacent columnar and metaplastic squamous epithelia and in a “normal” appearing gland nearby (Figure 5B: arrows). These results suggest that piwil2 expression may but not necessarily correlated with p16 and Ki67 expression in metaplastic epithelia, which might convey a distinct biological signal from p16 and Ki67. Concomitant expression of piwil2 with Ki67 might represent aggravated cell transformation (Figure 3 & [3, 12]). Therefore, PIWIL2 gene activation might be an event earlier than p16 and Ki67 activation at the initial stage of cervical lesions.
Piwil2 is expressed in p16-negative cervical carcinomas
To further verify that piwil2 expression is associated cell transformation but not necessarily correlated with HPV infection, we extended the observations above to up to another 91 cases of cervical lesions at various developmental stages (Figure 6 & Table 1). TMA cores were prepared and stained by anti-piwil2 or anti-p16. In addition, 8 cases of normal cervical biopsy tissues (NCBT) with colposcopy without expressing p16 were used as normal tissue controls (Figure 6). As a result, piwil2 was detected in all types and all stages of cervical lesions (Figure 6). While all benign proliferation (HP, CIN-1 and GA), precancers (CIN-2, CIN-3, and AIS), and invasive cancers (Inv-Sqcc and Inv-Adc) were expressed with piwil2 (100%), p16 was detected in only about 84.62% of the TMA cores (p= 0.0001), especially, the percentage of piwil2 expression in cervical squamous carcinoma was significantly higher than p16 expression (p=0.01) (Figure 6A). However, the histological score of piwil2 was individually different but had no significant difference between the lesions in average (Figure 6B). In contrast, no significant levels of piwil2 were detected in NCBT in average (score: < 0.5; Figure 6B), although about 25% (2/8) of NCBT were detected with low levels of piwil2 (Figure 6A). While not all TMA cores were expressed with p16 in the lesions of CIN-1, AIS, invasive Sqcc, invasive Adc, keratinized squamous cell carcinoma and poorly differentiated clear cell carcinoma (Figure 6A, and Table 1), p16 was detected in all lesions of CIN-2/3, CIS and glandular atypia (GA) (Figure 6A). These results suggest that piwil2 is also expressed in non-HPV-infected cervical neoplasia or p16-negative cervical neoplasia.
Piwil2-expessing cells were detected in exfoliated abnormal cervical epithelial cells
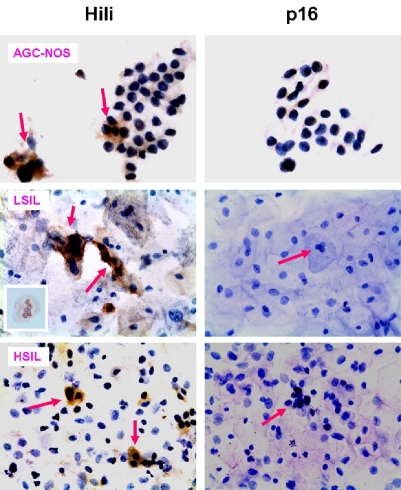
Papanicolaou (Pap smear) is used to detect abnormal cells in the cervix uteri. Since piwil2 can be detected in the cervical tissues with various types of lesion, we hypothesized that piwil2 can also be detected in exfoliated abnormal epithelial cells. As shown in Figure 7, piwil2 was detected by immunocytochemistry (ICC) in exfoliated abnormal epithelial cells from all abnormal lesions of the 18 patients examined, including AGC-NOS, LSIL and HSIL, but not in normal cells (Figure 7). In contrast, p16 was detected in only about 50% of the samples (9/18) of Pap smear samples with abnormal cervical cells. Atypical glandular cells (AGC) on cervical smears are often associated with clinically significant uterine lesions [45]. The results suggest that piwil2 has the potential to be used as a complementary biomarker for p16 to promote the sensitivity and specificity of Pap smear screening.
Figure 7.
Piwil2 is expressed in abnormal cervical epithelial cells. Unstained SurePath cervical smear slides from all abnormal lesions of the 18 patients were prepared and stained with rabbit anti-piwil2 or mAb to p16 followed by HRP-conjugated secondary antibody. The data show that piwil2 (hili) was specifically expressed in the various types of atypical cells and precancerous lesions, such as AGC-NOS (atypical glandular cells, not otherwise specified), LSIL (low-grade squamous intraepithelial lesion) and HSIL (high-grade squamous intraepithelial lesion). In contrast, p16 was not detected in the matched specimens. The result suggests that piwil2 is more sensitive than p16 in Sure-Path prepared cervical smear slides as a biomarker for cervical lesion. An inset in the figure of LSIL (left panel) shows Koilocytes expressing piwil2. Original magnification of the micrographs: x600.
Discussion
In this study, we have demonstrated that piwil2, like in breast cancers [12], is expressed in various stages of human cervical squamous cell carcinomas and adenocarcinomas. Interestingly, while varying levels of piwil2 were detected by IHC in all the premalignant and malignant lesions, p16 was detected in only 84.62% of the specimens. In Papanicolaou (Pap) test, piwil2 was also consistently detected in all the atypical glandular cells (AGC), low-grade (LSIL) and high-grade squamous intraepithelial lesions (HSIL), whereas p16 was not always concomitantly detected in the same specimens. The results suggest that piwil2 is more widely expressed in various stages of cervical lesions than p16. To our surprising, the frequency of p16-positive lesions in the selected Pap test is obviously lower than we expected. This may be related to quality control of ICC in different laboratory. Whether p16 expression in cervical cancer screening is traditionally overestimated needs further investigation.
Although large numbers of genes that contribute to cancer development, including ONG, TSG and SG, have been identified and extensively investigated [8-10], the mechanisms underlying tumor development remain elusive. This is due to the current failure to identify a causal gene that is ubiquitously responsible throughout the whole process of cancer development [1, 2, 8]. Recently, we have reported that piwil2 is expressed in pCSCs as well as in various stages of breast cancers without the restriction of their classification [3, 12]. Consistently in this study, we have also demonstrated that piwil2 is expressed in various stages and various types of cervical neoplasia. These results strongly suggest that piwil2 might play important roles throughout the process of tumor development without restriction of tissue origin. Because piwil2 is a carrier of tens of thousands of piRNAs and play crucial roles in germline development, especially for the maintenance of GSCs [46], its abnormal or ectopic expression might disturb the many molecular pathways of a normal cell, leading to cell transformation or carcinogenesis [1, 2, 46]. In supporting of the hypothesis, we have observed that overexpression of piwil2 in adult stem cells led to transformation-associated cell death (TACD) [3]. Moreover, the fact that expression of piwil2 in various stages of both human breast and cervical cancers provides us an important avenue to explore the mechanism underlying piwil2 regulating tumor development.
Field cancerization is an important phenomenon associated with cancer recurrence [47]. Like in the breast cancers [12], piwil2 was also detected in the histologically “normal” appearing tissue adjacent to cancerous lesions of cervix uteri. This may be related to the “field effect” of cancerization [48]. The epigenetic and/or genetic alterations in the “normal” appearing tissues are often the risk factors of cancerization [41, 42], and thus this change in the normal tissues distant from cancerous lesions could be a cause of tumor recurrence [49]. In this study, we have found that piwil2-expressing epithelial cells within the “normal” appearing tissues usually exhibited hyperplastic and/or metaplastic morphology, suggesting a different origin of these cells from real normal cells or varying fates of the epithelial cells exposing to the same environment. Such fates are certainly supposed to be determined by cell-intrinsic factors. Moreover, we have also observed that not all hyperplastic and metaplastic epithelial cells expressed piwil2 in the same area, suggesting that piwil2-expressing hyperplastic and metaplastic cells are qualitatively distinct from their piwil2-negative counterparts. Taken together, these findings implicate that piwil2 expression is likely associated with tumor initiation. A large cohort of experiment is warranted to delineate the significance of piwil2-expressing hyperplastic and metaplastic cells in tumor initiation.
Since piwil2 is expressed in various stages of cervical neoplasia, it seems feasible that piwil2 be used as a biomarker for early detection and intervention of cervical cancers. There is no doubt that piwil2 has the potential to be a target of cancer intervention. However, the fact that piwil2 appears in all grades of precancer (LSIL and HSIL), makes it less likely that it alone could be used as a biomarker for diagnosis of cervical cancer. A major challenge for CIN diagnosis is that CIN -2 and its mimics are difficult to be diagnosed based on morphology alone with poor inter-pathologist agreement [50, 51]. It might be possible to distinguish CIN-2 from its mimics with the help of piwil2 and or piwil2 in combination with other biomarkers such as p16 and HR-HPV.
HR-HPV testing is very sensitive, and could be excellent for screening, but it is not specific enough. P16 is one marker being explored to improve specificity. In addition, CIN is also underestimated in women positive for HR-HPV with negative biopsy, and addition of p16 may offset the underestimation [52]. While piwil2 was detected in all tissue specimens and Pap smears with cervical lesions, p16 was only detected in up to 50% - 80% of the specimens [53], suggesting that piwil2 is more widely expressed than p16 in cervical lesions. Thus, HR-HPV + p16 might still underestimate CIN in women positive for HR-HPV with negative biopsy, and addition of piwil2 might overcome the deficiency.
Piwil2 expression in various stages of cervical lesions is not only confirmed in histopathology as discussed above but also in cytology. In Pap smears, piwil2 but not p16 was detected in all cervical epithelial lesions, such as AGC-NOS, LSIL and HSIL. Atypical glandular cells (AGC) in cervical smears are often associated with clinically significant uterine lesions, as revealed by a study that showed that ∼ 56% of the AGC-diagnosis are associated with significant cancerous or precancerous lessons [45]. However, subjective variations between cytopathologists and the lack of p16 expression might lead to underestimation of AGCs. Addition of piwil2 as a complementary marker might overcome the difficulty and promote sensitivity and specificity of Pap test [11, 54].
In conclusion, piwil2 is expressed in various stages of cervical cancer including benign proliferative lesions, precancerous lesions and malignant lesions regardless of lesion classification. Since it is more widely expressed in cervical lesions than p16; it has the potential to be used as a complementary biomarker with HR-HVP and p16 to promote their sensitivity and specificity for detection of cervical neoplasias. A further large study is warranted to consolidate the findings.
Acknowledgments
The authors are grateful to the excellent comments on the manuscript from Dr. Elizabeth R. Unger at the Centers for Disease Control and Prevention, Atlanta, Georgia, USA. This work is supported from Department of Pathology, OSU, Strategy Initiative (SIG), 2006/2007, Immunology Program Award 2008 (OSUCCC), and American Cancer society IRG-112367 (JXG).
References
- 1.Gao JX. Cancer stem cells: the lessons from precancerous stem cells. J Cell Mol Med. 2008;12:67–96. doi: 10.1111/j.1582-4934.2007.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao JX, Zhou Q. Epigenetic progenitors in tumor initiation and development. Drug Discovery Today: Disease Models. 2009;6:5–12. [Google Scholar]
- 3.Chen L, Shen R, Ye Y, Pu XA, Liu X, Duan W, Wen J, Zimmerer J, Wang Y, Liu Y, Lasky LC, Heerema NA, Perrotti D, Ozato K, Kuramochi-Miyagawa S, Nakano T, Yates AJ, Carson Iii WE, Lin H, Barsky SH, Gao JX. Precancerous Stem Cells Have the Potential for both Benign and Malignant Differentiation. PLoS ONE. 2007;2:e293. doi: 10.1371/journal.pone.0000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen R, Ye Y, Chen L, Yan Q, Barsky SH, Gao JX. Precancerous stem cells can serve as tumor vasculogenic progenitors. PLoS ONE. 2008;3:e1652. doi: 10.1371/journal.pone.0001652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werbowetski-Ogilvie TE, Bhatia M. Pluripotent human stem cell lines: what we can learn about cancer initiation. Trends in Molecular Medicine. 2008;14:323–332. doi: 10.1016/j.molmed.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Shen R, Tao L, Xu Y, Chang S, Brocklyn JV, Gao JX. Reversibility of aberrant global DNA and estrogen receptor-a gene methylation distinguishes the colorectal precancer from cancer. Int J Clin Exp Pathol. 2009;2:21–33. [PMC free article] [PubMed] [Google Scholar]
- 7.Berman JJ, Albores-Saavedra J, Bostwick D, Delellis R, Eble J, Hamilton SR, Hruban RH, Mutter GL, Page D, Rohan T, Travis W, Henson DE. Precancer: a conceptual working definition - results of a Consensus Conference. Cancer Detect Prev. 2006;30:387–394. doi: 10.1016/j.cdp.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 9.Solé X, Bonifaci Nr, LÃ3pez-Bigas Nr, Berenguer A, HernÃjndez P, Reina O, Maxwell CA, Aguilar H, Urruticoechea A, de Sanjosé S, Comellas F, CapellAi G, Moreno Vc, Pujana MA. Biological Convergence of Cancer Signatures. PLoS ONE. 2009;4:e4544. doi: 10.1371/journal.pone.0004544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–724. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JH, Schutte D, Wulf G, Fuzesi L, Radzun HJ, Schweyer S, Engel W, Nayernia K. Stem-cell protein Piwil2 is widely expressed in tumors and inhibits apoptosis through activation of Stat3/Bcl-XL pathway. Hum Mol Genet. 2006;15:201–211. doi: 10.1093/hmg/ddi430. [DOI] [PubMed] [Google Scholar]
- 12.Liu JS, R, Gang H, Chen L, Yin Y, Hua K, Jarjoura D, Nakano T, Ramesh GK, Shapiro CL, Barsky SH, Gao JX. Piwil2 is expressed in various stages of breast cancers and has the potential to be used as a novel biomarker. Int J Clin Exp Pathol. 2010;3:328–337. [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki T, Shiohama A, Minoshima S, Shimizu N. Identification of eight members of the Argo-naute family in the human genome small star, filled. Genomics. 2003;82:323–330. doi: 10.1016/s0888-7543(03)00129-0. [DOI] [PubMed] [Google Scholar]
- 14.Kuramochi-Miyagawa S, Kimura T, Ijiri TW, Isobe T, Asada N, Fujita Y, Ikawa M, Iwai N, Okabe M, Deng W, Lin H, Matsuda Y, Nakano T. Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development. 2004;131:839–849. doi: 10.1242/dev.00973. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Engel W, Nayernia K. Stem cell protein Piwil2 modulates expression of murine spermatogonial stem cell expressed genes. Mol Reprod Dev. 2006;73:173–179. doi: 10.1002/mrd.20391. [DOI] [PubMed] [Google Scholar]
- 16.Unhavaithaya Y, Hao Y, Beyret E, Yin H, Kuramochi-Miyagawa S, Nakano T, Lin H. MILI, a PIWI-interacting RNA-binding Protein, Is Required for Germ Line Stem Cell Self-renewal and Appears to Positively Regulate Translation. Journal of Biological Chemistry. 2009;284:6507–6519. doi: 10.1074/jbc.M809104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Saxe JP, Tanaka T, Chuma S, Lin H. Mili Interacts with Tudor Domain-Containing Protein 1 in Regulating Spermatogenesis. Current Biology. 2009;19:640–644. doi: 10.1016/j.cub.2009.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox DN, Chao A, Baker J, Chang L, Qiao D, Lin H. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998;12:3715–3727. doi: 10.1101/gad.12.23.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmell MA, Xuan Z, Zhang MQ, Hannon GJ. The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev. 2002;16:2733–2742. doi: 10.1101/gad.1026102. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 21.Buhler M, Verdel A, Moazed D. Tethering RITS to a nascent transcript initiates RNAi- and heterochromatin-dependent gene silencing. Cell. 2006;125:873–886. doi: 10.1016/j.cell.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 22.Yin H, Lin H. An epigenetic activation role of Piwi and a Piwi-associated piRNA in Drosophila melanogaster. Nature. 2007;450:304–308. doi: 10.1038/nature06263. [DOI] [PubMed] [Google Scholar]
- 23.Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ, Kuramochi-Miyagawa S, Nakano T, Chien M, Russo JJ, Ju J, Sheridan R, Sander C, Zavolan M, Tuschl T. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–207. doi: 10.1038/nature04916. [DOI] [PubMed] [Google Scholar]
- 24.Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science. 2007;316:744–747. doi: 10.1126/science.1142612. [DOI] [PubMed] [Google Scholar]
- 25.Grivna ST, Beyret E, Wang Z, Lin H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006;20:1709–1714. doi: 10.1101/gad.1434406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grivna ST, Pyhtila B, Lin H. MIWI associates with translational machinery and PIWI-interacting RNAs (piRNAs) in regulating spermatogenesis. Proc Natl Acad Sci U S A. 2006;103:13415–13420. doi: 10.1073/pnas.0605506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, Bartel DP, Kingston RE. Characterization of the piRNA complex from rat testes. Science. 2006;313:363–367. doi: 10.1126/science.1130164. [DOI] [PubMed] [Google Scholar]
- 28.Saito K, Nishida KM, Mori T, Kawamura Y, Miyoshi K, Nagami T, Siomi H, Siomi MC. Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes Dev. 2006;20:2214–2222. doi: 10.1101/gad.1454806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, Hannon GJ. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–1103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 30.Qiao D, Zeeman AM, Deng W, Looijenga LH, Lin H. Molecular characterization of hiwi, a human member of the piwi gene family whose overexpression is correlated to seminomas. Oncogene. 2002;21:3988–3999. doi: 10.1038/sj.onc.1205505. [DOI] [PubMed] [Google Scholar]
- 31.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 32.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 33.Holladay EB, Logan S, Arnold J, Knesel B, Smith GD. A comparison of the clinical utility of p16(INK4a) immunolocalization with the presence of human papillomavirus by hybrid capture 2 for the detection of cervical dysplasia/neoplasia. Cancer. 2006;108:451–461. doi: 10.1002/cncr.22284. [DOI] [PubMed] [Google Scholar]
- 34.Liman AK, Giampoli EJ, Bonfiglio TA. Should women with atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion, receive reflex human papillomavirus-DNA testing? Cancer. 2005;105:457–460. doi: 10.1002/cncr.21387. [DOI] [PubMed] [Google Scholar]
- 35.Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153:1741–1748. doi: 10.1016/S0002-9440(10)65689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JS, Dong SM, Kim HS, Lee JY, Um SJ, Park IS, Kim SJ, Namkoong SE. Detection of p16 gene alteration in cervical cancer using tissue microdissection and LOH study. Cancer Lett. 1999;136:101–108. doi: 10.1016/s0304-3835(98)00366-8. [DOI] [PubMed] [Google Scholar]
- 37.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 38.Gao JX, Liu X, Wen J, Zhang H, Durbin J, Liu Y, Zheng P. Differentiation of Monocytic Cell Clones into CD8alpha(+) Dendritic Cells (DC) Suggests that Monocytes Can Be Direct Precursors for Both CD8alpha(+) and CD8alpha(-) DC in the Mouse. J Immunol. 2003;170:5927–5935. doi: 10.4049/jimmunol.170.12.5927. [DOI] [PubMed] [Google Scholar]
- 39.Thomas S, Johannes G. The Ki-67 protein: From the known and the unknown. Journal of Cellular Physiology. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Gao J. Cancer stem cells: the lessons from pre-cancerous stem cells. J Cell Mol Med. 2007;11 doi: 10.1111/j.1582-4934.2007.00170.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, Arii K, Kaneda A, Tsuka-moto T, Tatematsu M, Tamura G, Saito D, Sugimura T, Ichinose M, Ushijima T. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989–995. doi: 10.1158/1078-0432.CCR-05-2096. [DOI] [PubMed] [Google Scholar]
- 42.Lewis CM, Cler LR, Bu DW, Zochbauer-Muller S, Milchgrub S, Naftalis EZ, Leitch AM, Minna JD, Euhus DM. Promoter hypermethylation in benign breast epithelium in relation to predicted breast cancer risk. Clin Cancer Res. 2005;11:166–172. [PubMed] [Google Scholar]
- 43.Jass JR. Hyperplastic polyps and colorectal cancer: is there a link? Clin Gastroenterol Hepatol. 2004;2:1–8. doi: 10.1016/s1542-3565(03)00284-2. [DOI] [PubMed] [Google Scholar]
- 44.Shen L, Kondo Y, Rosner GL, Xiao L, Hernandez NS, Vilaythong J, Houlihan PS, Krouse RS, Prasad AR, Einspahr JG, Buckmeier J, Alberts DS, Hamilton SR, Issa JP. MGMT promoter methylation and field defect in sporadic colorectal cancer. J Natl Cancer Inst. 2005;97:1330–1338. doi: 10.1093/jnci/dji275. [DOI] [PubMed] [Google Scholar]
- 45.Scheiden R, Wagener C, Knolle U, Dippel W, Capesius C. Atypical glandular cells in conventional cervical smears: incidence and follow-up. BMC Cancer. 2004;4:37. doi: 10.1186/1471-2407-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomson T, Lin H. The Biogenesis and Function of PIWI Proteins and piRNAs: Progress and Prospect. Annual Review of Cell and Developmental Biology. 2009;25:355–376. doi: 10.1146/annurev.cellbio.24.110707.175327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dakubo GD, Jakupciak JP, Birch-Machin MA, Parr RL. Clinical implications and utility of field cancerization. Cancer Cell Int. 2007;7:2. doi: 10.1186/1475-2867-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giovannucci E, Ogino S. DNA methylation, field effects, and colorectal cancer. J Natl Cancer Inst. 2005;97:1317–1319. doi: 10.1093/jnci/dji305. [DOI] [PubMed] [Google Scholar]
- 49.Yan PS, Venkataramu C, Ibrahim A, Liu JC, Shen RZ, Diaz NM, Centeno B, Weber F, Leu YW, Shapiro CL, Eng C, Yeatman TJ, Huang TH. Mapping geographic zones of cancer risk with epigenetic biomarkers in normal breast tissue. Clin Cancer Res. 2006;12:6626–6636. doi: 10.1158/1078-0432.CCR-06-0467. [DOI] [PubMed] [Google Scholar]
- 50.Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. Jama. 2002;287:2120–2129. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 51.Steinau M, Rajeevan MS, Lee DR, Ruffin MT, Horowitz IR, Flowers LC, Tadros T, Birdsong G, Husain M, Kmak DC, Longton GM, Vernon SD, Unger ER. Evaluation of RNA markers for early detection of cervical neoplasia in exfoliated cervical cells. Cancer Epidemiol Biomarkers Prev. 2007;16:295–301. doi: 10.1158/1055-9965.EPI-06-0540. [DOI] [PubMed] [Google Scholar]
- 52.Ordi J, Garcia Sn, del Pino M, Landolfi S, Alonso I, QuintÃ3 L, Torné A. p16INK4a Immu-nostaining Identifies Occult CIN Lesions in HPV-positive Women. International Journal of Gynecologic Pathology. 2009;28:90–97. doi: 10.1097/PGP.0b013e31817e9ac5. 10.1097/PGP.1090b1013e31817e31819ac31815. [DOI] [PubMed] [Google Scholar]
- 53.Tsoumpou I, Arbyn M, Kyrgiou M, Wentzensen N, Koliopoulos G, Martin-Hirsch P, Malamou-Mitsi V, Paraskevaidis E. p16INK4a immunostaining in cytological and histological specimens from the uterine cervix: A systematic review and metaanalysis. Cancer Treatment Reviews. 2009;35:210–220. doi: 10.1016/j.ctrv.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuramochi-Miyagawa S, Kimura T, Yomogida K, Kuroiwa A, Tadokoro Y, Fujita Y, Sato M, Matsuda Y, Nakano T. Two mouse piwi-related genes: miwi and mili. Mech Dev. 2001;108:121–133. doi: 10.1016/s0925-4773(01)00499-3. [DOI] [PubMed] [Google Scholar]