Abstract
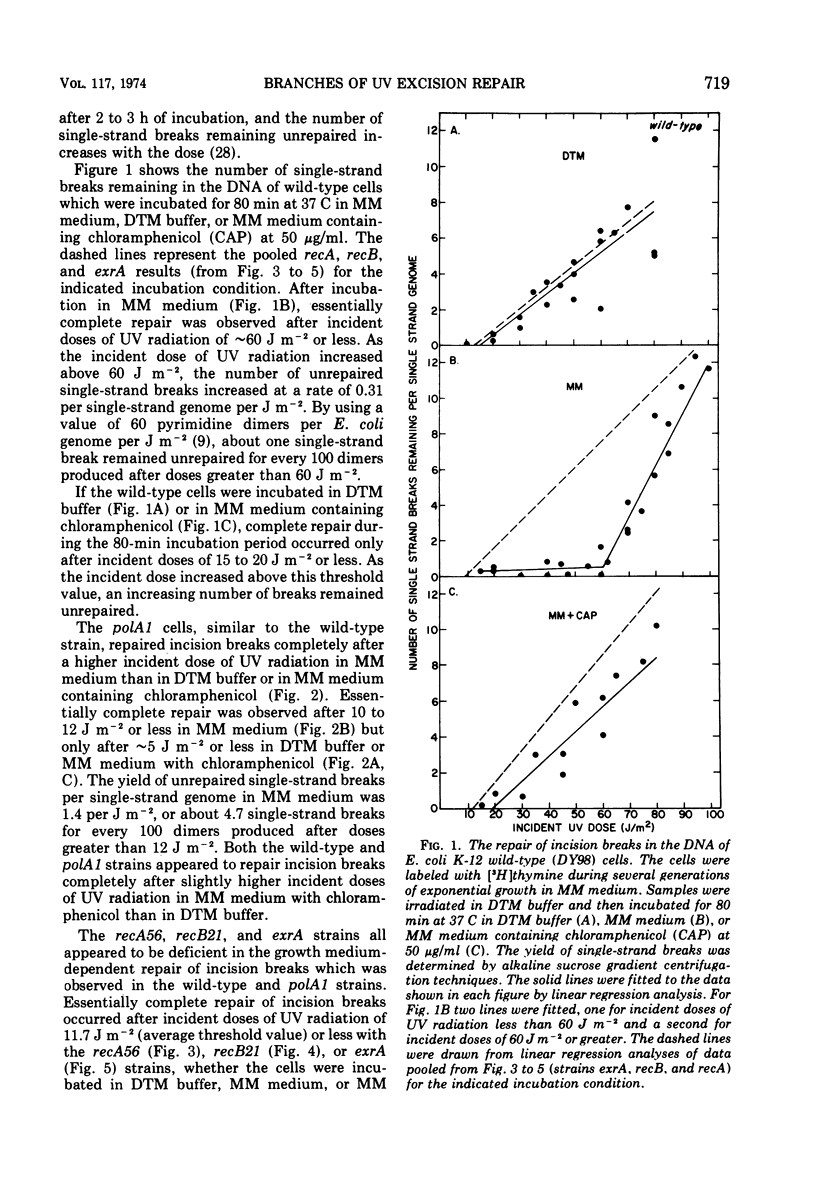
The extent of repair of single-strand breaks (incision breaks) induced in the deoxyribonucleic acid (DNA) of Escherichia coli K-12 cells by the uvr gene-dependent excision repair process after ultraviolet (UV) radiation was determined in the wild-type, polA1, recA56, recB21, and exrA strains. The wild-type strain repaired all incision breaks after incident doses of UV radiation (254 nm) of ∼60 J m−2 or less when incubated in growth medium, or ∼15 J m−2 or less when incubated in buffer. The polA1 strain repaired the incision breaks completely after incident doses of ∼12 J m−2 or less when incubated in growth medium, or after ∼4 J m−2 when incubated in buffer. The recA13, recB21, and exrA strains showed essentially complete repair after incident doses of 10 to 15 J m−2 whether the cells were incubated in buffer or growth medium. These results suggest that the uvr gene-dependent excision repair process may be divided into two branches, one which is dependent on the presence of growth medium and also the rec+exr+ genotype, and a second which can occur in buffer (growth medium-independent) and is largely dependent on DNA polymerase I. The presence of chloramphenicol in the growth medium resulted in an inhibition of the growth medium-dependent repair occurring in wild-type and polA1 cells and had little or no effect on the extent of repair observed in recA56, recB21, or exrA cells. The similarities between the growth medium-dependent and -independent branches of excision repair and two known processes for the repair of X-ray-induced single-strand breaks are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle J. M., Paterson M. C., Setlow R. B. Excision-repair properties of an Escherichia coli mutant deficient in DNA polymerase. Nature. 1970 May 23;226(5247):708–710. doi: 10.1038/226708a0. [DOI] [PubMed] [Google Scholar]
- Campbell J. L., Soll L., Richardson C. C. Isolation and partial characterization of a mutant of Escherichia coli deficient in DNA polymerase II. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2090–2094. doi: 10.1073/pnas.69.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P. K., Hanawalt P. C. Heterogeneity of patch size in repair replicated DNA in Escherichia coli. J Mol Biol. 1972 Jun 14;67(1):1–10. doi: 10.1016/0022-2836(72)90381-6. [DOI] [PubMed] [Google Scholar]
- Cooper P. K., Hanawalt P. C. Role of DNA polymerase I and the rec system in excision-repair in Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1156–1160. doi: 10.1073/pnas.69.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. K., Smith K. C. Dark recovery processes in Escherichia coli irradiated with ultraviolet light. I. Effect of rec mutations on liquid holding recovery. J Bacteriol. 1968 Aug;96(2):365–373. doi: 10.1128/jb.96.2.365-373.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. K., Smith K. C. Requirement for protein synthesis in rec-dependent repair of deoxyribonucleic acid in Escherichia coli after ultraviolet or X irradiation. J Bacteriol. 1972 Aug;111(2):575–585. doi: 10.1128/jb.111.2.575-585.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- KAPLAN H. S., SMITH K. C., TOMLIN P. A. Effect of halogenated pyrimidines on radiosensitivity of E. coli. Radiat Res. 1962 Jan;16:98–113. [PubMed] [Google Scholar]
- Kanner L., Hanawalt P. Repair deficiency in a bacterial mutant defective in DNA polymerase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):149–155. doi: 10.1016/0006-291x(70)90770-9. [DOI] [PubMed] [Google Scholar]
- Kapp D. S., Smith K. C. Repair of radiation-induced damage in Escherichia coli. II. Effect of rec and uvr mutations on radiosensitivity, and repair of x-ray-induced single-strand breaks in deoxyribonucleic acid. J Bacteriol. 1970 Jul;103(1):49–54. doi: 10.1128/jb.103.1.49-54.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T. Excision repair characteristics of recB - res - and uvrC - strains of Escherichia coli. J Bacteriol. 1972 Dec;112(3):1237–1246. doi: 10.1128/jb.112.3.1237-1246.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W., Hanawalt P., Shizuya H. Role of DNA polymerase II in repair replication in Escherichia coli. Nat New Biol. 1973 Aug 22;244(138):242–243. doi: 10.1038/newbio244242a0. [DOI] [PubMed] [Google Scholar]
- Monk M., Peacey M., Gross J. D. Repair of damage induced by ultraviolet light in DNA polymerase-defective Escherichia coli cells. J Mol Biol. 1971 Jun 14;58(2):623–630. doi: 10.1016/0022-2836(71)90376-7. [DOI] [PubMed] [Google Scholar]
- Nishioka H., Doudney C. O. Different modes of loss of photoreversibility of ultraviolet light-induced true and suppressor mutations to tryptophan independence in an auxotrophic strain of Escherichia coli. Mutat Res. 1970 Apr;9(4):349–358. doi: 10.1016/0027-5107(70)90017-5. [DOI] [PubMed] [Google Scholar]
- Paterson M. C., Boyle J. M., Setlow R. B. Ultraviolet- and X-ray-induced responses of a deoxyribonucleic acid polymerase-deficient mutant of Escherichia coli. J Bacteriol. 1971 Jul;107(1):61–67. doi: 10.1128/jb.107.1.61-67.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G., Bridges B. A. Survival, mutation and capacity to repair single-strand DNA breaks after gamma irradiation in different Exr - strains of Escherichia coli. Mol Gen Genet. 1972;119(2):93–102. doi: 10.1007/BF00269129. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Johansen I. Incisions in ultraviolet irradiated circular bacteriophage lambda DNA molecules in excision proficient and deficient lysogens of E. coli. Mol Gen Genet. 1973;123(2):173–184. doi: 10.1007/BF00267333. [DOI] [PubMed] [Google Scholar]
- Shimada K., Ogawa H., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. II. Breakage and repair of ultraviolet irradiated intracellular DNA of phage lambda. Mol Gen Genet. 1968 May 3;101(3):245–256. doi: 10.1007/BF00271626. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Meun D. H. Repair of radiation-induced damage in Escherichia coli. I. Effect of rec mutations on post-replication repair of damage due to ultraviolet radiation. J Mol Biol. 1970 Aug;51(3):459–472. doi: 10.1016/0022-2836(70)90001-x. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa H. Structural genes of ATP-dependent deoxyribonuclease of Escherichia coli. Nat New Biol. 1972 Sep 6;239(88):14–16. doi: 10.1038/newbio239014a0. [DOI] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. DNA polymerase required for rapid repair of x-ray--induced DNA strand breaks in vivo. Science. 1971 May 21;172(3985):851–854. doi: 10.1126/science.172.3985.851. [DOI] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. Influence of ultrafast repair processes (independent of DNA polymerase I) on the yield of DNA single-strand breaks in Escherichia coli K-12 x-irradiated in the presence of or absence of oxygen. Radiat Res. 1972 Oct;52(1):99–114. [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. Production and repair of radiochemical damage in Escherichia coli deoxyribonucleic acid; its modification by culture conditions and relation to survival. J Bacteriol. 1971 Jan;105(1):127–135. doi: 10.1128/jb.105.1.127-135.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. The rapair of DNA single-strand breaks in E. coli K-12 x-irradiated in the presence or absence of oxygen; the influence of repair on cell survival. Radiat Res. 1973 Aug;55(2):334–345. [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Evidence for the control by exrA and polA genes of two branches of the uvr gene-dependent excision repair pathway in Escherichia coli K-12. J Bacteriol. 1973 Oct;116(1):175–182. doi: 10.1128/jb.116.1.175-182.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Involvement of DNA polymerase 3 in excision repair after ultraviolet irradiation. Nat New Biol. 1973 Aug 22;244(138):240–241. doi: 10.1038/newbio244240a0. [DOI] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Sensitivity to x-radiation of strains of Escherichia coli K-12 which lack DNA polymerase II. Mol Gen Genet. 1973 May 9;122(3):287–290. doi: 10.1007/BF00278604. [DOI] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. X-ray sensitivity and repair capacity of a polA1 exrA strain of Escherichia coli K-12. J Bacteriol. 1973 Apr;114(1):121–127. doi: 10.1128/jb.114.1.121-127.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



