Abstract
Alzheimer's disease (AD) is a progressive neurological disorder that causes dementia and poses a major public health crisis as the population ages. Aberrant processing of the amyloid precursor protein (APP) is strongly implicated as a proximal event in AD pathophysiology, but the neurochemical signals that regulate APP processing in the brain are not completely understood. Activation of muscarinic acetylcholine receptors (mAChRs) has been shown to affect APP processing and AD pathology, but less is known about the roles of specific mAChR subtypes. In this study, we used M1 mAChR knock-out mice (M1KO) to isolate the effects of the M1 mAChR on APP processing in primary neurons and on the development of amyloid pathology in a transgenic mouse model of AD. We demonstrate that the loss of M1 mAChRs increases amyloidogenic APP processing in neurons, as evidenced by decreased agonist-regulated shedding of the neuroprotective APP ectodomain APPsα and increased production of toxic Aβ peptides. Expression of M1 mAChRs on the M1KO background rescued this phenotype, indicating that M1 mAChRs are sufficient to modulate nonamyloidogenic APP processing. In APPSwe/Ind transgenic mice, the loss of M1 mAChRs resulted in increased levels of brain Aβ and greater accumulation of amyloid plaque pathology. Analysis of APP metabolites in APPSwe/Ind brain tissue indicates that the loss of M1 mAChRs increases amyloidogenic APP processing. These results indicate that the M1 mAChR is an important regulator of amyloidogenesis in the brain and provide strong support for targeting the M1 mAChR as a therapeutic candidate in AD.
Introduction
Alzheimer's disease (AD) is the most prevalent type of dementia, affecting nearly half of individuals over the age of 85 and causing tremendous emotional distress and economic loss (Evans et al., 1989; Hebert et al., 2003; Alzheimer's Association, 2009). Multiple lines of evidence implicate the amyloid precursor protein (APP) and particularly its aberrant proteolytic processing in the pathophysiology of AD, but less is understood about the exact mechanisms that control APP processing and the production of its neurotoxic β-amyloid (Aβ) derivative in vivo (Selkoe et al., 1996; Thinakaran and Koo, 2008).
Because the accumulation of pathogenic Aβ peptides is implicated as a proximal event in AD, it is important to understand the regulatory mechanisms governing their production. Activation of muscarinic acetylcholine receptors (mAChRs) has been shown to stimulate nonamyloidogenic APP processing in cultured cells and brain slices (Nitsch et al., 1992; Farber et al., 1995), and treatment with cholinergic drugs has shown promise in a range of model systems, including trials in human patients (Farber et al., 1995; Beach et al., 2001b; Hock et al., 2003; Caccamo et al., 2006).
The vast majority of previous studies have relied on agonists and antagonists that are not selective for the five known mAChR subtypes (M1–M5). Multiple “M1-preferring” agonists have shown encouraging results, but they activate other mAChR subtypes in addition to the M1 mAChR (Haring et al., 1994; DeLapp et al., 1998; Nitsch et al., 2000; Hock et al., 2003). Given the diversity in expression patterns of mAChR subtypes in various cell types throughout the brain, cholinergic regulation of APP processing has the potential to be highly mAChR subtype specific (Buckley et al., 1988; Levey et al., 1991, 1995). Thus, determining the mAChR subtypes responsible for regulating APP processing in the brain is critical for optimizing outcomes and limiting off-target effects.
The lack of subtype selective drugs has also hampered progress in the small number of studies performed in vivo. For example, Caccamo et al. (2006) demonstrated that AF267B, claimed to be selective for the M1 mAChR subtype, reduces amyloid pathology in an AD mouse model. However, Jones et al. (2008) recently showed that this compound activates M3 mAChRs as or more potently than M1 mAChRs. Since these two receptors similarly activate signaling pathways and α-secretase processing of APP, the selective role of M1 receptor-specific regulation of amyloidogenesis in vivo remains unknown.
In the present study, we designed experiments to examine the regulation of APP processing by the M1 mAChR subtype. We demonstrate that the genetic deletion of M1 receptors results in a loss of cholinergic regulation of APP processing in primary neurons. By crossing APP-transgenic mice with M1 knock-out mice, we show that M1 receptor deletion exacerbates amyloid pathology in vivo. These results establish the M1 mAChR as a critical regulator of amyloidogenesis in vivo and provide a logical foundation for the development of a new generation of M1-selective drugs for the treatment of AD.
Materials and Methods
Primary neuron culture.
Primary cortical neuron cultures were prepared from wild-type mice and M1 knock-out mice at embryonic day E18. The generation and characterization of these mice has been described previously (Miyakawa et al., 2001). Time-pregnant dams were anesthetized with isoflurane and decapitated. Embryos were dissected and cortical hemispheres were isolated in dissection buffer (HBSS, 10 mm HEPES, 1% penicillin/streptomycin). Tissue was digested with 0.25% trypsin (Invitrogen) and 0.01% DNase in dissection buffer for 15 min at 37°C and rinsed twice with dissection buffer and twice with plating medium [buffered MEM (Invitrogen), 0.6% glucose (Invitrogen), 2 mm l-glutamine (Cellgro), 10% heat-inactivated horse serum (Invitrogen), 1% penicillin/streptomycin]. Tissue was mechanically dissociated by trituration through a fire-polished Pasteur pipette, and viable cells were determined by Trypan blue exclusion. Neurons were plated at a density of 80,000 cells/cm2 on poly-l-lysine-coated 60 mm culture dishes. Cultures were maintained in Neurobasal medium (Invitrogen) containing B-27 supplement (Invitrogen), 2 mm l-glutamine, and 1% penicillin/streptomycin at 37°C, 5% CO2. Lentivirus vectors encoding human APP695swe and human M1 mAChR were added at the time of plating at a multiplicity of infection (MOI) ∼1 and allowed to incubate for 72 h before removal. Cytosine arabinoside was added at a final concentration of 5 μm on day 3 in vitro to control proliferation of non-neuronal cells.
Neuron viability assay.
Viability in lentivirus-transduced neurons was assessed using the CellTiter96 Cell Proliferation (MTS) Assay (Promega). E18 cortical neurons were plated onto poly-l-lysine-coated 96-well culture plates at a density of 50,000 cells/cm2 and either infected with hAPP lentivirus (MOI = 1) or mock-infected, and allowed to incubate for 72 h. The MTS assay was performed according to the manufacturer's instructions, and plates were read on a SpectraMax Plus plate reader (Molecular Devices).
APPSwedish/Indiana × M1KO mice.
Line J20 transgenic mice expressing human amyloid precursor protein incorporating the Swedish and Indiana mutations (APPSwe/Ind) were generously provided by Dr. Lennart Mucke (The Gladstone Institute of Neurological Disease, San Francisco, CA) and have been previously described (Mucke et al., 2000). APPSwe/Ind heterozygous mice were bred to M1−/− mice, and the resulting offspring were then crossed to M1+/− mice to generate M1+/+ and M1−/−, APPSwe/Ind littermates for analysis.
Primary antibodies.
Antibodies used in this study included: 6E10 (APP Aβ domain, Signet), C8 (APP C terminus, gift from Dr. Dennis Selkoe, Harvard Medical School, Boston, MA), Aβ42 [rabbit polyclonal, BioSource (Invitrogen)], β-actin (goat polyclonal, Santa Cruz Biotechnology), and EF1α (Millipore).
Tissue collection.
Animals were killed by sodium pentobarbital overdose and perfused with normal saline. Brains were rapidly removed and sectioned along the sagittal plane. One hemibrain was immersion fixed in 4% buffered paraformaldehyde, and cerebral cortex and hippocampus were isolated from the other hemibrain, snap frozen in liquid nitrogen, and stored at −80°C until analysis. Individual tissue fractions were not subjected to more than one freeze/thaw cycle.
Sequential amyloid extraction.
Cortical hemispheres were homogenized using a Dounce tissue grinder (Kontes) in PBS with protease inhibitor cocktail (Roche) and sonicated (∼30 s at level 7 using a Branson Sonifier 250, Krackeler Scientific) in the presence of 2% SDS, then pelleted by centrifugation for 1 h at 100,000 × g at 8°C in an Optima TLX Ultracentrifuge (Beckman-Coulter). The supernatant was collected and the pellet resuspended in an equal volume of 70% formic acid (FA) and resonicated. FA-soluble fractions were neutralized using 1.0 m Tris, pH 11. SDS-soluble and neutralized FA-soluble fractions were diluted in ELISA sample diluent (50 mm Tris base, 150 mm NaCl, 0.5% Nonidet P-40, 0.5% deoxycholate, 0.1 mg/ml phenylmethylsulfonyl fluoride, protease inhibitor cocktail, pH 7.4).
Tissue fractionation.
Cortical hemispheres were homogenized in PBS with protease inhibitor cocktail using a Dounce tissue grinder at a concentration of 100 mg/ml. Soluble proteins were isolated from membrane fractions by differential centrifugation. Crude homogenates were centrifuged at 1000 × g to remove nuclei and debris (P1). The supernatant (S1) was centrifuged at 10,000 × g for 20 min to isolate larger organelles and membrane proteins (P2), and the resulting supernatant (S2) was subjected to centrifugation at 100,000 × g to enrich for soluble proteins (S3). The P2 fraction was rinsed with 500 mm NaCl to remove membrane associated proteins and further centrifuged at 10,000 × g for 20 min to pellet membrane proteins (P2′). The washed membrane proteins were then incubated with detergent buffer (50 mm Tris, 150 mm NaCl, 0.5% Nonidet P-40, 0.5% deoxycholate) and solubilized proteins recovered by centrifugation at 15,000 × g for 5 min. All steps were performed at 4°C.
Western blotting.
Cell lysates, conditioned media, and fractionated proteins from cortical homogenates were prepared in Laemmli sample buffer (Laemmli, 1970), separated by SDS-PAGE, and transferred to PVDF Immobilon-P membranes (Millipore). Membranes were blocked at room temperature and incubated with primary antibodies overnight at 4°C. Blots were rinsed and incubated with fluorophore-conjugated secondary antibodies (Invitrogen and Rockland) for 1 h at room temperature. Blots were imaged and band intensities were quantified using an Odyssey Image Station (LI-COR).
ELISA measurement of Aβ peptides.
Aβ1-40 and Aβ1-42 levels in conditioned media and tissue homogenates were measured using hAmyloid ELISA (HS) kits (The Genetics Company) according to the manufacturer's instructions. Plates were read at 450 nm on a Spectra Max Plus plate reader (Molecular Devices).
Histochemical amyloid plaque analysis.
Sagittal hemibrains were immersion fixed with 4% paraformaldehyde for 2 h at 4°C, cryoprotected in 30% sucrose, and sectioned at 50 μm on a freezing-sliding microtome. For thioflavin S plaque staining, sections were mounted on glass slides, treated with 1% thioflavin S solution for 10 min, and rinsed in 80% ethanol and water. For Aβ42 immunohistochemistry, free floating sections were fixed with 2% glutaraldehyde, treated with sodium borohydride to quench unreacted glutaraldehyde, and incubated with 70% formic acid to retrieve antigens. Following treatment with hydrogen peroxide, sections were blocked with normal serum and incubated with an anti-Aβ42 antibody overnight at 4°C. Sections were then incubated with a biotinylated secondary antibody and signal was visualized using the avidin-biotin-peroxidase complex method (Vector Laboratories) with diaminobenzidine. Mounted sections were dehydrated with sequential ethanol and Histoclear and images were captured using an Olympus BX51 microscope and Olympus software. Quantitation of extent of amyloid pathology was performed as previously described (Dodson et al., 2008). Briefly, thioflavin-stained plaques were manually counted in a blinded manner using MetaMorph image analysis software (Molecular Devices). Total amyloid burden was quantified by measuring Aβ42-immunopositive surface area in a blinded manner using MetaMorph image analysis software. Plaque quantitation is shown as the mean plaque number or surface area per section as determined from 4 sagittal sections distributed evenly across ∼1 mm of tissue.
Statistical analysis.
All statistical comparisons were performed using Prism 4.0 software (GraphPad). Primary neuron culture APPs, CTF, and Aβ levels were analyzed using paired t tests and repeated-measures ANOVA followed by Dunnett's multiple-comparison test. Two-way ANOVA was performed to detect M1 genotype effect on Aβ accumulation in APPSwe/Ind mice across age groups, followed by Mann–Whitney nonparametric t tests to compare Aβ levels within each age group and histochemical measures of amyloid plaque pathology. Levels of APP metabolites in cortical homogenates were compared using unpaired t tests.
Results
APP is a large type I transmembrane protein that can be proteolytically cleaved by two competing enzymatic processes: a nonamyloidogenic pathway initiated by α-secretase cleavage that results in the shedding of a soluble ectodomain termed “APPsα” and precludes the generation of Aβ, and an amyloidogenic pathway initiated by β-secretase cleavage that gives rise to toxic Aβ peptides, which aggregate and ultimately deposit as amyloid plaques. To test whether the M1 mAChR subtype is necessary for cholinergic regulation of nonamyloidogenic APP processing in cells relevant to AD, we performed a series of experiments in primary neurons. Because AD is an intrinsically human disease, we chose to study the processing of human sequence APP, which differs from the rodent sequence by three amino acid substitutions within the Aβ domain and likely accounts for the development of AD pathology in humans and other species that share this sequence. Cortical neurons from E18 embryonic wild-type and M1KO mice were cultured in vitro and transduced with a lentivirus vector to achieve expression of human sequence APP (hAPP). Because of the high efficiency of retroviral gene delivery, a low copy number of transgene per target cell is sufficient to achieve a high percentage of transduced cells. As shown in Figure 1A, lentiviral transduction of mouse cortical neuron cultures resulted in efficient and consistent hAPP expression at levels approximately twofold to threefold over basal. To assess potential toxicity induced by lentivirus transduction of primary neurons, we performed an MTS [3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay on neurons infected with hAPP lentivirus. Levels of formazan reaction product were not different between treatment groups (mock infection = 100% ± 13.8, hAPP = 104% ± 13.7, p > 0.05), indicating that lentivirus infection does not cause measurable toxicity in our system.
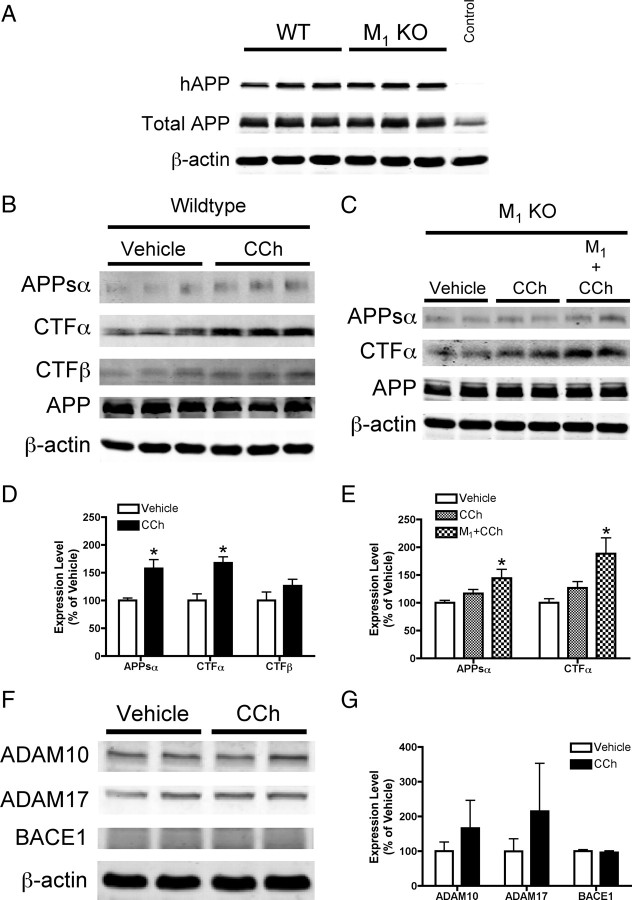
Figure 1.
M1 mAChR regulation of APP processing in primary neurons. A, Western blot analysis of cell lysates from primary cortical neuron cultures derived from wild-type (WT) and M1 mAChR knock-out (M1KO) mice and transduced with a lentiviral vector encoding human sequence amyloid precursor protein (hAPP). A virus encoding green fluorescent protein was used as a control. The human-specific APP antibody 6E10 was used to detect expression of hAPP, and total APP (human plus endogenous murine) was detected using C8 (a C-terminal APP antibody). β-Actin is shown as a loading control. B, D, Representative Western blots and densitometric analysis of conditioned media and cell lysates from WT neuron cultures treated with the mAChR agonist CCh (100 μm). Quantitation of band intensity shows a 66% increase in APPsα shedding into conditioned media of WT neuron cultures treated with CCh (p < 0.05 vs vehicle), and a 68% increase in the level of CTFα (p < 0.01). Data are shown as mean ± SEM from three to five independent experiments. C, E, Western blots and densitometry from conditioned media and cell lysates from M1KO neuron cultures. APPsα and CTFα levels were unchanged in cultures treated with CCh. In M1KO neuron cultures transduced with an M1 mAChR lentivirus, CCh treatment resulted in a 44% increase in APPsα shedding into conditioned media (p < 0.05), and an 88% increase in the levels of CTFα (p < 0.05). Data are shown as mean ± SEM of three independent experiments. F, G, Western blot and densitometric analysis demonstrates no change in levels of ADAM10 (p = 0.16), ADAM17 (p = 0.16), or BACE1 (p = 0.34) with CCh treatment in WT neuron cultures. Data are shown as the mean ± SD.
To measure mAChR-regulated APP processing, wild-type neurons were allowed to condition medium for 8 h in the presence or absence of the nonselective mAChR agonist carbachol (CCh, 100 μm). Western blot analysis of conditioned media samples and cell lysates showed a significant increase in levels of APPsα (66%) and the APP C-terminal fragment resulting from α-secretase cleavage (CTFα) (68%) following carbachol stimulation (Fig. 1B,D). Pretreatment with the muscarinic receptor antagonist atropine (1 μm) blocked the CCh-induced increase in APPsα (vehicle = 100 ± 13%, atropine plus CCh = 102 ± 29%). Levels of CTFβ were unchanged following CCh treatment (p = 0.22). Expression of full-length APP was not altered between treatment groups (vehicle = 100% ± 20.5, CCh = 110% ± 9.59; p = 0.28). This result is consistent with other reports from cultured cells and brain slices (Nitsch et al., 1992; Farber et al., 1995), and indicates that the cellular machinery required for mAChR-mediated signaling and APP processing is intact and functional in the primary neuron culture system.
Having established regulated APP cleavage in wild-type neurons, we performed a similar experiment using neurons from M1KO mice. As shown in Figure 1, C and E, deletion of the M1 receptor results in a significant reduction in the amount of CCh-stimulated APP processing, with no statistically significant difference in APPsα or CTFα between CCh- and vehicle-treated neurons. To test whether M1 receptor activation is sufficient to regulate nonamyloidogenic APP processing, we exogenously expressed the M1 mAChR subtype on the M1 mAChR knock-out background. Total mAChR levels in M1 mAChR KO cultures measured by [3H]-NMS binding were reduced by 69% compared to WT levels, consistent with previous studies quantifying the relative abundance of mAChR subtypes in rodent brain (Levey et al., 1991). Total mAChR levels in M1KO neurons transduced with M1 lentivirus were increased by 156% compared to WT. In M1KO neurons transduced with both human APP and M1 mAChR viruses, carbachol stimulation resulted in significant increases in levels of APPsα (increased by 44%, p < 0.05) and CTFα (increased by 88%, p < 0.05) compared to vehicle-treated neurons (Fig. 1C,E). Expression of full-length APP was not altered across treatment groups (vehicle = 100% ± 15.8, CCh = 106% ± 7.74, M1 + CCh = 112% ± 6.45; one-way ANOVA p = 0.33). We also measured levels of the putative α-secretase enzymes ADAM10 and ADAM17, as well as BACE1, the enzyme responsible for β-secretase cleavage of APP, in WT neuron cultures treated with CCh. Levels of all three enzymes were statistically similar in CCh-treated versus vehicle-treated neurons, although there was a greater degree of variability in ADAM levels (Fig. 1F,G).
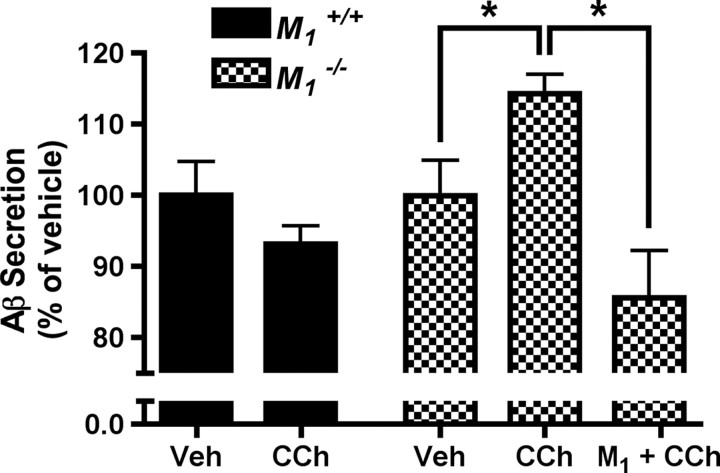
We next examined the effects of manipulating M1 mAChR signaling on the production of Aβ. In wild-type neuron cultures, CCh treatment resulted in a trend toward reduction of Aβ secretion, but this effect did not reach statistical significance (Fig. 2). In M1KO neurons, CCh treatment resulted in a small but significant increase in secretion of total Aβ (increased by 14%, p < 0.05 vs vehicle). The fact that CCh stimulation caused a decrease in Aβ secretion in wild-type neurons but an increase in secretion in M1KO neurons indicates that not only is M1 mAChR activation necessary for regulated nonamyloidogenic APP processing, but that there are also other mAChR subtypes capable of promoting amyloidogenic APP processing. This result is consistent with a previous report using an M2/M4-preferring antagonist to modulate release of APPs in brain slices (Farber et al., 1995). In M1KO neuron cultures rescued with M1 lentivirus and stimulated with CCh, Aβ levels were reduced to 85% of baseline levels and were significantly lower than Aβ levels in CCh-treated M1KO cultures (p < 0.01). Together, these data indicate that the M1 mAChR is essential for carbachol-mediated nonamyloidogenic APP processing in cortical neurons.
Figure 2.
Aβ peptide levels in conditioned media from WT and M1KO primary neuron cultures. In WT neurons, CCh treatment (100 μm) resulted in a trend toward decreased Aβ production, although this difference did not reach statistical significance. In M1KO neurons, CCh treatment caused an increase in Aβ production (p < 0.05 vs vehicle). Following rescue of M1KO neuron cultures by transduction with an M1 lentivirus, CCh treatment resulted in a significant reduction in Aβ levels compared to CCh-treated M1KO neurons (p < 0.01). Data are shown as mean ± SEM from three independent experiments.
Given the decreased APPsα shedding and increased Aβ production in primary neurons from M1KO mice, we designed an in vivo experiment to determine the impact of loss of M1 signaling on the development of amyloid pathology in the brain. Cohorts of M1+/+ and M1−/− littermates carrying the APPSwe/Ind transgene were generated and aged to 3, 6, 12, and 16 months. Cerebral cortex homogenates were subjected to sequential extraction with SDS and formic acid (FA) to recover Aβ peptides, and levels of Aβ1-40 and Aβ1-42 were determined using sandwich ELISA. Aβ1-40 and Aβ1-42 levels in M1+/+ and M1−/− mice are shown in Table 1. We observed an age-dependent increase in both Aβ1-40 and Aβ1-42 in both M1+/+ and M1−/− mice beginning between 6 and 12 months of age, consistent with previous reports of amyloid plaque accumulation in this line of transgenic mice (Mucke et al., 2000). The loss of M1 mAChRs had a significant effect on the accumulation of Aβ (two-way ANOVA genotype effect p < 0.0001). By 16 months of age, Aβ1-40 and Aβ1-42 levels in M1−/− mice were increased by 488 and 293%, respectively, as compared to M1+/+ littermates (p < 0.05 for both effects), indicating that the loss of M1 receptors exacerbates the accumulation of Aβ in the brain. This effect was consistent for both SDS- and FA-soluble fractions of Aβ.
Table 1.
ELISA measurements of Aβ1-40 and Aβ1-42 peptide levels in M1+/+ and M1−/− APPSwe/Ind cortex at 3, 6, 12, and 16 months of age
| Age |
||||
|---|---|---|---|---|
| 3 months(mean ± SD) | 6 months(mean ± SD) | 12 months(mean ± SD) | 16 months(mean ± SD) | |
| Aβ1-40 | ||||
| M1+/+ | 318 ± 90 | 301 ± 49 | 4221 ± 2369 | 7050 ± 9668 |
| M1−/− | 287 ± 63 | 309 ± 161 | 4002 ± 1726 | 41,435 ± 19806 |
| p value | 0.7396 | 0.2284 | 1.0000 | 0.0190 |
| Aβ1–42 | ||||
| M1+/+ | 72 ± 10 | 55 ± 20 | 97,038 ± 77,335 | 148,472 ± 175,542 |
| M1−/− | 69 ± 17 | 92 ± 108 | 100,188 ± 46,472 | 583,435 ± 321,325 |
| p value | 0.5362 | 0.8518 | 0.9048 | 0.0381 |
Values include both SDS- and formic acid-soluble Aβ fractions and are shown as the mean ± SD of the absolute concentrations (pg/ml). At 16 months, levels of both Aβ1-40 and Aβ1-42 are increased in M1−/− mice compared to M1+/+ littermates (Aβ1-40 increased by 488%, p < 0.05; Aβ1-42 increased by 293%, p < 0.05).
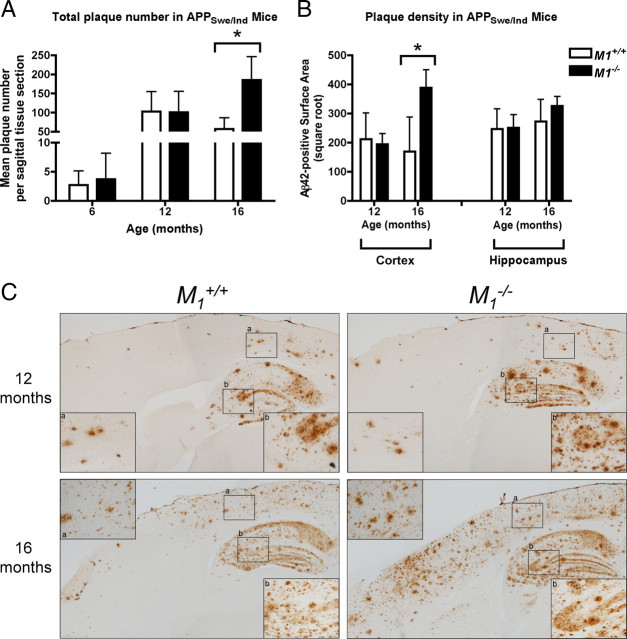
To determine whether the loss of M1 mAChRs affects the development of amyloid plaque pathology, we performed histochemical evaluation of plaque burden on brain sections from APPSwe/Ind/M1+/+ and APPSwe/Ind/M1−/− mice. Plaque counts of thioflavin S-positive amyloid deposits (Fig. 3A) and surface area measurements of Aβ42-immunopositive brain sections (Fig. 3B,C) were used to quantify amyloid pathology. Consistent with our results from biochemical measurement of Aβ, deletion of M1 had a significant effect on amyloid plaque pathology at 16 months of age. Plaque counts demonstrated a 227% increase in amyloid plaque number in M1−/− mice compared to M1+/+ littermates (p = 0.0159) at this age (Fig. 3A). This effect was consistent for both cortex and hippocampus. Quantitation of amyloid plaque burden by measuring the surface area of Aβ42-immunopositive tissue revealed a 129% increase in cortical plaque load in M1−/− mice compared to M1+/+ littermates (Fig. 3B) (p = 0.0381). In cortex, the increased accumulation of cortical plaque pathology in M1−/− mice was most striking in anterior regions (Fig. 3C). In the hippocampus, there was no statistically significant difference in total Aβ42-immunopositive surface area between M1+/+ and M1−/− mice.
Figure 3.
Amyloid pathology in M1+/+ and M1−/− APPSwe/Ind mice. A, Total number of thioflavin S-positive plaques is significantly increased at 16 months of age in M1−/− mice (mean ± SD, p < 0.01). B, Amyloid plaque density (mean surface area of Aβ42-positive immunoreactivity (pixels) per tissue section) is significantly increased in the cortex of 16-month-old M1−/− mice (mean ± SD, p < 0.05). C, Light micrographs of Aβ42-immunopositive plaques (brown deposits) in M1+/+ and M1−/− APPSwe/Ind mice at 12 and 16 months of age. High-power magnification corresponding to boxed regions of cortex (a) and hippocampus (b) are shown as insets.
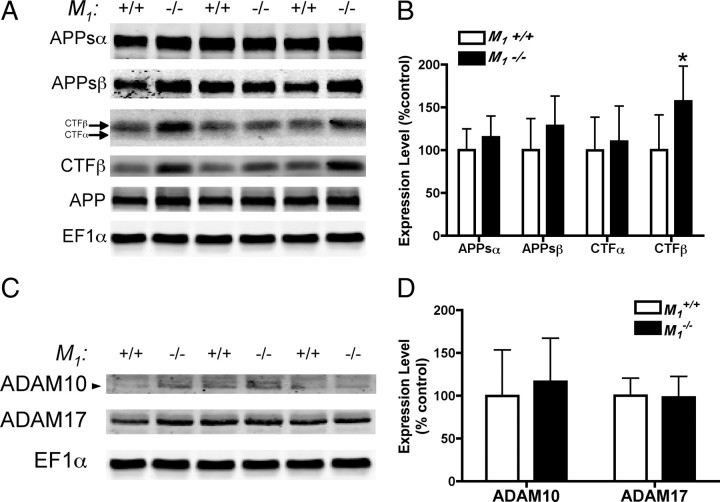
To investigate the mechanism underlying the observed increased in amyloid pathology in M1−/− mice, we performed Western blot analysis of APP fragments in cortical tissue homogenates from 16-month-old APPSwe/Ind mice. Full-length APP levels were unchanged (M1+/+ = 100.0 ± 13.7% vs M1−/− = 99.7 ± 12.5%), as were levels of CTFα (p = 0.65). Levels of CTFβ were significantly increased in M1−/− mice compared to M1+/+ mice (Fig. 4A) (increased to 157% of wild-type; p < 0.05). In contrast to our results from primary neuron experiments, we did not observe significant differences in levels of APPsα (p = 0.2954) or APPsβ (p = 0.1830). One likely explanation for this discrepancy is that APPs derivatives secreted into brain tissue are presumably cleared into CSF and thus are not retained in the tissue homogenate used for APP metabolite analysis in this study. It is also possible that there is a difference in steady-state brain levels of APP metabolites in aged animals as compared to dynamic changes induced by CCh-treatment in regulated APP processing experiments in primary neurons. Nevertheless, our results from both in vitro and in vivo experiments strongly indicate that the loss of M1 mAChRs results in increased accumulation of amyloidogenic APP derivatives, leading to increased Aβ production and amyloid pathology.
Figure 4.
A, B, Immunoblot analysis of APP metabolites in M1+/+ and M1−/− APPSwe/Ind cortex. Membrane and soluble proteins from cortical homogenates of M1+/+ (n = 7) and M1−/− (n = 6) mice were fractionated by differential centrifugation and subjected to SDS-PAGE and Western blotting with antibodies to multiple APP metabolites. Representative immunoblots are shown probed with 6E10 to detect full-length APP, APPsα, and CTFβ, 192swe to detect the Swedish mutation form of APPsβ, and C8 to detect both CTFα and CTFβ. EF1α is shown as a loading control. For quantitation, APPs levels were normalized to EF1α, and CTF levels were normalized to full-length APP. Densitometric quantitation revealed a 57% increase in CTFβ in M1−/− mice (mean ± SD, p < 0.05). C, D, Immunoblot analysis of ADAM10 and ADAM17 in M1+/+ and M1−/− APPSwe/Ind cortex. Levels of both proteins were unchanged in M1+/+ compared to M1−/− (data shown as mean ± SD).
As chronic mAChR stimulation has been reported to promote α-secretase cleavage of APP by increasing levels of the α-secretase candidate ADAM17 (Caccamo et al., 2006), we examined the effect of M1 deletion on steady-state levels of both ADAM10 and ADAM17 in cortex from 16 month-old M1+/+ and M1−/− mice. As shown in Figure 4, C and D, we found no difference in expression levels of ADAM10 (p = 0.56) and ADAM17 (p = 0.89) between M1+/+ and M1−/− mice.
Discussion
In the present study, we investigated the role of the M1 mAChR in regulating amyloidogenesis in primary neurons and in the development of amyloid pathology in a transgenic mouse model of AD. While previous studies have established that M1 mAChR overexpression and semiselective agonists with preferential activation of M1 and other mAChR subtypes enhance α-secretase processing of APP, our study is the first to use genetic models to definitively isolate the M1 mAChR subtype, thus avoiding the ambiguity associated with nonselective agonists. Here we show that the M1 mAChR is necessary and sufficient to regulate nonamyloidogenic APP processing in primary cortical neurons. Furthermore, we demonstrate that APPSwe/Ind transgenic mice lacking M1 mAChRs develop increased amyloid pathology as measured by increased brain Aβ levels and amyloid plaque burden. APP metabolite analysis in brain tissue from aged APPSwe/Ind mice suggests that this exacerbation of pathology is due to increased amyloidogenic processing of APP. Together, these data validate the M1 mAChR as a critical regulator of amyloidogenesis in vivo.
Our analysis of APP metabolites in CCh-stimulated primary neuron cultures has important implications regarding the mechanism by which M1 mAChR signaling may influence neuronal physiology. We observed the largest changes in levels of APPsα and CTFα, the products of α-secretase cleavage. The APPsα ectodomain has been shown to be neuroprotective in some systems, and may play a role in memory enhancement, possibly by facilitating synapse formation (Mattson et al., 1993; Meziane et al., 1998; Bell et al., 2008). A recent study has also proposed a role for APPsα in the disruption of APP dimers on the cell surface, which the authors argue is important for regulating cell survival (Gralle et al., 2009). Regardless of the combination of mechanisms by which APPsα exerts a beneficial effect in the CNS, it is logical to conclude that signaling pathways that promote its secretion may be important for normal physiological brain function.
One potential explanation for our observed effects of M1 activation on APP processing is through modulation of one or more of the secretase enzymes. As M1 activation had the largest effect on α-secretase-mediated processing events, it is likely that the mechanism involves α-secretase, either through direct activation or by regulating traffic of ADAM enzymes and/or APP substrate. The cell biology of APP trafficking has been the focus of much study (for review, see (Thinakaran and Koo, 2008)), but further work will be required to appreciate exactly how M1 activation may participate in this process. We did not observe an acute effect of M1 activation on regulation of ADAM expression levels, nor did we find that deletion of M1 altered ADAM levels in vivo, but it is possible that chronic mAChR stimulation may induce ADAM upregulation, as suggested by Caccamo et al. (2006).
We also found that M1 mAChR activation by CCh induced a trend toward decreased Aβ secretion in wild-type neurons, and that CCh actually increased Aβ secretion in M1KO neurons. This finding suggests that other mAChR subtypes may stimulate amyloidogenic APP processing and agrees with a previous report showing that an M2/M4-preferring antagonist can potentiate CCh-stimulated APPs release from brain slices (Farber et al., 1995). These data indicate that mAChR signaling may be important for regulating multiple aspects of APP processing and amyloidogenesis in neurons, and therefore, that a loss of M1 mAChR signaling may have multiple deleterious consequences in the context of AD pathogenesis. Previous work has also demonstrated that nicotinic acetylcholine receptors (nAChRs) can modulate APP processing, and carbachol is capable of activating nAChRs at high concentrations. However, nAChR stimulation has been associated with decreased, not increased, Aβ secretion, indicating that nAChR activation is not responsible for the increase in Aβ secretion we observed in CCh-stimulated M1KO neurons (Kim et al., 1997; Lenzken et al., 2007; Nie et al., 2008).
Our data from APPSwe/Ind mice represent the first assessment of M1 mAChR loss on the development of amyloid pathology in vivo. We demonstrate that M1 mAChR deletion results in increased levels of pathogenic Aβ peptides in brain, as well as increased accumulation of amyloid plaque pathology. These findings are consistent with the important role that the M1 mAChR plays in regulating APP processing as well as reports from several model systems, including human data, demonstrating that manipulation of mAChR signaling can modulate the development of amyloid pathology in vivo (Beach et al., 2000, 2001a,b; Nitsch et al., 2000; Perry et al., 2003; Caccamo et al., 2006). Our results from analysis of APP metabolites in aged APPSwe/Ind mice support the conclusions drawn from cellular models, implicating the M1 mAChR as a pivotal regulator of nonamyloidogenic APP processing in the brain. What is less clear, however, is the mechanism by which loss of M1 signaling results in increased brain Aβ levels and amyloid plaque pathology. Increased steady-state levels of Aβ in the brain could be the result of either increased Aβ production, or decreased Aβ clearance and/or degradation. Clearance of Aβ peptides, and the effects of this process on amyloid pathology, are areas of intense focus in AD research, and it is not known at this point how cholinergic signaling may participate in this process.
Further research, including follow-up studies in APP transgenic mice, will be required to more fully understand the implications of M1 mAChR regulation of amyloidogenesis in the brain. In addition to the observed effects on amyloidogenesis, it will be important to investigate whether loss of M1 mAChRs has an effect on learning and memory impairment in APP-transgenic mice. Accumulation of neurotoxic Aβ species impairs synaptic function (Walsh et al., 2005) and multiple lines of APP-transgenic mice show deficits in learning and memory tasks (Woodruff-Pak, 2008), so it is logical to hypothesize that the increase in amyloid pathology induced by deletion of M1 would exacerbate cognitive deficits. Given the role that the M1 mAChR plays in certain aspects of working memory and memory consolidation (Anagnostaras et al., 2003), the loss of M1 mAChR signaling accompanied by increased accumulation of amyloid pathology may have an additive detrimental effect on cognition.
In conjunction with studies examining the effects of M1 mAChR deletion on amyloid pathology and memory impairment, it will be important to evaluate the potential for M1-selective agonists in reducing amyloid pathology and promoting cognitive processes. All of the mAChR-targeted therapies tried to date have shown only modest efficacy for AD symptoms, but it remains to be seen whether newer generations of M1-selective agonists will be able to offer more meaningful therapeutic benefit. Our data from cultured cells indicates that M1-selective agonists are effective at promoting nonamyloidogenic APP processing and are therefore excellent candidates for therapies aimed at reducing amyloid pathology in vivo (Jones et al., 2008).
The findings of the present study validate the long-standing hypothesis that the M1 mAChR is an important regulator of APP processing in the brain. Our approach using M1KO mice is the first of its kind to genetically isolate the M1 mAChR subtype, circumventing the limitations imposed by the use of semiselective mAChR agonists in previous studies. We observed that M1 mAChR loss decreased the shedding of the neuroprotective APPsα molecule in primary neurons, and increased the production of neurotoxic Aβ in primary neurons and in vivo, ultimately exacerbating amyloid pathology in a transgenic mouse model of AD. These data suggest that the M1 mAChR may regulate multiple aspects of neuronal physiology and AD pathology, emphasizing the importance of drug development to target molecules with disease-modifying potential, including M1. The intimate relationship between the M1 mAChR and higher cognitive functions including working memory and consolidation further underscores the potential benefit of M1-based therapies for AD and other cognitive disorders.
Footnotes
This work was supported by National Institutes of Health Grants NS030454 (A.I.L.) and F30 AG029731 (A.A.D.) and a predoctoral fellowship from the PhRMA Foundation (A.A.D.). We are grateful to Yinghong Cui, Guofu Fang, Xinping Huang, Howard Rees, and Zoe White for expert technical assistance.
References
- Alzheimer's Association. 2009 Alzheimer's disease facts and figures. Alzheimers Dement. 2009;5:234–270. doi: 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51–58. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- Beach TG, Potter PE, Kuo YM, Emmerling MR, Durham RA, Webster SD, Walker DG, Sue LI, Scott S, Layne KJ, Roher AE. Cholinergic deafferentation of the rabbit cortex: a new animal model of Abeta deposition. Neurosci Lett. 2000;283:9–12. doi: 10.1016/s0304-3940(00)00916-2. [DOI] [PubMed] [Google Scholar]
- Beach TG, Kuo YM, Schwab C, Walker DG, Roher AE. Reduction of cortical amyloid beta levels in guinea pig brain after systemic administration of physostigmine. Neurosci Lett. 2001a;310:21–24. doi: 10.1016/s0304-3940(01)02076-6. [DOI] [PubMed] [Google Scholar]
- Beach TG, Walker DG, Potter PE, Sue LI, Fisher A. Reduction of cerebrospinal fluid amyloid beta after systemic administration of M1 muscarinic agonists. Brain Res. 2001b;905:220–223. doi: 10.1016/s0006-8993(01)02484-2. [DOI] [PubMed] [Google Scholar]
- Bell KF, Zheng L, Fahrenholz F, Cuello AC. ADAM-10 over-expression increases cortical synaptogenesis. Neurobiol Aging. 2008;29:554–565. doi: 10.1016/j.neurobiolaging.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buckley NJ, Bonner TI, Brann MR. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci. 1988;8:4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A, LaFerla FM. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- DeLapp N, Wu S, Belagaje R, Johnstone E, Little S, Shannon H, Bymaster F, Calligaro D, Mitch C, Whitesitt C, Ward J, Sheardown M, Fink-Jensen A, Jeppesen L, Thomsen C, Sauerberg P. Effects of the M1 agonist xanomeline on processing of human beta-amyloid precursor protein (FAD, Swedish mutant) transfected into Chinese hamster ovary-m1 cells. Biochem Biophys Res Commun. 1998;244:156–160. doi: 10.1006/bbrc.1998.8235. [DOI] [PubMed] [Google Scholar]
- Dodson SE, Andersen OM, Karmali V, Fritz JJ, Cheng D, Peng J, Levey AI, Willnow TE, Lah JJ. Loss of LR11/SORLA enhances early pathology in a mouse model of amyloidosis: evidence for a proximal role in Alzheimer's disease. J Neurosci. 2008;28:12877–12886. doi: 10.1523/JNEUROSCI.4582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- Farber SA, Nitsch RM, Schulz JG, Wurtman RJ. Regulated secretion of beta-amyloid precursor protein in rat brain. J Neurosci. 1995;15:7442–7451. doi: 10.1523/JNEUROSCI.15-11-07442.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralle M, Botelho MG, Wouters FS. Neuroprotective secreted amyloid precursor protein acts by disrupting amyloid precursor protein dimers. J Biol Chem. 2009;284:15016–15025. doi: 10.1074/jbc.M808755200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring R, Gurwitz D, Barg J, Pinkas-Kramarski R, Heldman E, Pittel Z, Wengier A, Meshulam H, Marciano D, Karton Y, Fisher A. Amyloid precursor protein secretion via muscarinic receptors: reduced desensitization using the M1-selective agonist AF102B. Biochem Biophys Res Commun. 1994;203:652–658. doi: 10.1006/bbrc.1994.2232. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Hock C, Maddalena A, Raschig A, Müller-Spahn F, Eschweiler G, Hager K, Heuser I, Hampel H, Müller-Thomsen T, Oertel W, Wienrich M, Signorell A, Gonzalez-Agosti C, Nitsch RM. Treatment with the selective muscarinic m1 agonist talsaclidine decreases cerebrospinal fluid levels of A beta 42 in patients with Alzheimer's disease. Amyloid. 2003;10:1–6. doi: 10.3109/13506120308995249. [DOI] [PubMed] [Google Scholar]
- Jones CK, Brady AE, Davis AA, Xiang Z, Bubser M, Tantawy MN, Kane AS, Bridges TM, Kennedy JP, Bradley SR, Peterson TE, Ansari MS, Baldwin RM, Kessler RM, Deutch AY, Lah JJ, Levey AI, Lindsley CW, Conn PJ. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci. 2008;28:10422–10433. doi: 10.1523/JNEUROSCI.1850-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim YK, Jeong SJ, Haass C, Kim YH, Suh YH. Enhanced release of secreted form of Alzheimer's amyloid precursor protein from PC12 cells by nicotine. Mol Pharmacol. 1997;52:430–436. doi: 10.1124/mol.52.3.430. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenzken SC, Lanni C, Govoni S, Lucchelli A, Schettini G, Racchi M. Nicotinic component of galantamine in the regulation of amyloid precursor protein processing. Chem Biol Interact. 2007;165:138–145. doi: 10.1016/j.cbi.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ. Expression of m1–m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci. 1995;15:4077–4092. doi: 10.1523/JNEUROSCI.15-05-04077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron. 1993;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- Meziane H, Dodart JC, Mathis C, Little S, Clemens J, Paul SM, Ungerer A. Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proc Natl Acad Sci U S A. 1998;95:12683–12688. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci. 2001;21:5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of Aβ1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Li Z, Lukas RJ, Shen Y, Song L, Wang X, Yin M. Construction of SH-EP1-alpha4beta2-hAPP695 cell line and effects of nicotinic agonists on beta-amyloid in the cells. Cell Mol Neurobiol. 2008;28:103–112. doi: 10.1007/s10571-007-9218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Deng M, Tennis M, Schoenfeld D, Growdon JH. The selective muscarinic M1 agonist AF102B decreases levels of total Abeta in cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 2000;48:913–918. [PubMed] [Google Scholar]
- Perry EK, Kilford L, Lees AJ, Burn DJ, Perry RH. Increased Alzheimer pathology in Parkinson's disease related to antimuscarinic drugs. Ann Neurol. 2003;54:235–238. doi: 10.1002/ana.10639. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Yamazaki T, Citron M, Podlisny MB, Koo EH, Teplow DB, Haass C. The role of APP processing and trafficking pathways in the formation of amyloid beta-protein. Ann N Y Acad Sci. 1996;777:57–64. doi: 10.1111/j.1749-6632.1996.tb34401.x. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Shankar GM, Townsend M, Fadeeva JV, Betts V, Podlisny MB, Cleary JP, Ashe KH, Rowan MJ, Selkoe DJ. The role of cell-derived oligomers of Abeta in Alzheimer's disease and avenues for therapeutic intervention. Biochem Soc Trans. 2005;33:1087–1090. doi: 10.1042/BST20051087. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS. Animal models of Alzheimer's disease: therapeutic implications. J Alzheimers Dis. 2008;15:507–521. doi: 10.3233/jad-2008-15401. [DOI] [PubMed] [Google Scholar]