Abstract
Regulatory T cells (Tregs) play a vital role in autoimmune disorders. Among several markers, forkhead box p3 (Foxp3) is the most specific with regard to Treg activity. Therefore, understanding mechanisms that regulate Foxp3 expression is a critical step for unraveling the complicacy of autoimmune pathophysiology. The present study was undertaken to investigate the crosstalk between NO and Tregs. Interestingly, after myelin basic protein (MBP) priming, the expression of Foxp3 decreased in MBP-primed T cells. However, blocking NO either by inhibiting inducible NO synthase with l-N6-(1-iminoethyl)-lysine hydrochloride or through scavenging with PTIO or by pharmacological drugs, such as pravastatin, sodium benzoate, or gemfibrozil, restored the expression of Foxp3 in MBP-primed T cells. However, this restoration of Foxp3 by pharmacological drugs was reversed by S-nitrosoglutathione, an NO donor. Similarly, NO also decreased the populations of Tregs characterized by CD4+CD25+ and CD25+FoxP3+ phenotypes. We have further confirmed this inverse relationship between NO and Foxp3 by analyzing the mRNA expression of Foxp3 and characterizing CD25+FoxP3+ or CD4+Foxp3+ phenotypes from inducible NO synthase knockout mice. Moreover, this inverse relation between NO and Foxp3 also was observed during priming with myelin oligodendrocyte glycoprotein, another target neuroantigen in multiple sclerosis, as well as collagen, a target autoantigen in rheumatoid arthritis. Finally, we demonstrate that NO inhibited the expression of Foxp3 in MBP-primed T cells via soluble guanylyl cyclase-mediated production of cGMP. Taken together, our data imply a novel role of NO in suppressing Foxp3+ Tregs via the soluble guanylyl cyclase pathway.
Maintenance of tolerance to self Ags is a challenge for our immune system. During development in the thymus, many clones of autoreactive T cells are deleted by programmed cell death (1). However, a few of them escape thymic deletion and therefore survive and pose potential threats during autoimmune attack. Fortunately, the immune system has evolved a regulatory mechanism that restricts the autoreactive T cells in an unresponsive state (1). The key mediators of this regulation are specialized subsets of T cells, known as regulatory T cells (Tregs), that can recognize self-reactive T cells and suppress them by a complex mechanism that is not yet fully understood. There are several kinds of Tregs, for example, naturally occurring, inducible, or IL-10–producing Tregs, and several controversies lie in choosing proper parameters that specifically characterize a particular kind of Treg, but irrespective of these discrepancies, recent advancements in research have established a transcription factor forkhead box p3 (Foxp3) as the most specific marker of Tregs (1-3). Foxp3+ CD4+CD25+ T cells are considered as the most common phenotype of Tregs. Under normal physiological conditions, Tregs are sufficient to suppress self-reactive T cells. However, during autoimmune pathogenesis, the immune system is dysregulated, resulting in a substantial decrease in the activity and number of Tregs, leading to huge proliferation of self-reactive T cells and subsequent autoimmune attack.
Foxp3 is an X chromosome-encoded transcription factor expressed exclusively in Tregs and critically important for the development and function of these cells. The Foxp3 gene was identified originally from mice with scurfy syndrome in which it was found to be mutated (4, 5). Defects in the Foxp3 gene are also responsible for X-linked autoimmunity and allergic dysregulation syndrome, a fatal human disorder that develops in childhood (6). Structurally, Foxp3 belongs to the forkhead family of transcription factors containing a forkhead DNA-binding domain. Foxp3 is considered as a master regulatory molecule in Tregs that acts as a transcriptional repressor as well as an activator (7). IL-2 is one of the targets of Foxp3 that is negatively regulated, whereas surface molecules, such as CD25, glucocorticoid-induced TNF receptor, and CTLA-4, are likely to be positively regulated by Foxp3 (8).
The importance of Tregs in multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE), the animal model of MS, is becoming increasingly significant. MS is also associated with numerical and functional deficiency of Tregs in MS (9, 10). It has been shown that Tregs play a critical role in protection and recovery from EAE. Although the exact mechanism of protection by Tregs is not clearly understood, it is suspected that Tregs exert protection by increasing the Th2 phenotype and decreasing the homing of autoreactive T cells (11). Depletion of CD4+CD25+ cells inhibits natural recovery from EAE, whereas transfer of these cells to recipient mice reduces disease severity (12). These observations imply that regulation of Tregs might play a decisive role in susceptibility to EAE. Recent studies suggest that the expression of Foxp3 and the number of peripheral CD4+CD25+ Foxp3+ T cells are reduced significantly in relapsing-remitting MS patients compared with those in control subjects (13). However, molecular mechanisms by which Tregs are downregulated in MS patients are not well understood. NO, a potent proinflammatory molecule, is strongly implicated in the pathophysiology of autoimmune diseases, such as EAE and MS. Because MS and EAE are autoimmune diseases associated with immune dysregulation and Tregs are critical for immune regulation and maintenance of immune homeostasis, we wondered whether NO plays a role in the regulation of Tregs.
We herein report the first evidence that NO plays a pivotal role in the regulation of Foxp3 in myelin basic protein (MBP)-primed T cells. Although decreasing the level of NO by either inhibitors of inducible NO synthase (iNOS) or scavengers of NO stimulates the expression of Foxp3, increasing the level of NO by an NO donor decreases the expression of Foxp3. Similarly, pharmacological drugs, such as statins, gemfibrozil, or sodium benzoate (NaB), with reported protective roles in EAE (14, 15) can effectively restore Foxp3 in MBP-primed T cells via inhibiting synthesis of NO. In addition to MBP-primed T cells, T cell priming with myelin oligodendrocyte glycoprotein (MOG), another target neuroantigen in MS, as well as collagen, a target autoantigen in rheumatoid arthritis (RA), also reduces the expression of Foxp3 via NO. Furthermore, we demonstrate that NO employs the soluble guanylyl cyclase (sGC) cGMP pathway to inhibit the expression of Foxp3.
Materials and Methods
Reagents
FBS and RPMI 1640 medium were from Invitrogen (Carlsbad, CA). l-N6-(1-iminoethyl)-lysine hydrochloride (l-NIL), carboxy-PTIO, S-nitrosoglutathione (GSNO), NS-2028 (an inhibitor of guanylate cyclase), 8-BrcGMP (a cell-permeable cGMP analogue), and MY-5445 (an inhibitor of cGMP phosphodiesterase) were obtained from BIOMOL (Plymouth Meeting, PA). Gemfibrozil and NaB were purchased from Sigma-Aldrich (St. Louis, MO). Rat anti-Foxp3 was purchased from eBioscience (San Diego, CA). Rabbit anti-iNOS was purchased from Calbiochem (San Diego, CA). FITC-conjugated rat anti-mouse IL-2Rα (CD25) mAb was purchased from Chemicon International (Temecula, CA). PE-labeled rat anti-CD4mAb was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Isolation of MBP-primed T cells
Specific pathogen-free female SJL/J mice (4–6-wk-old) were purchased from Harlan Sprague-Dawley (Indianapolis, IN). B6.129 iNOS−/− mice and their littermate controls were purchased from The Jackson Laboratory (Bar Harbor, ME). MBP-primed T cells were isolated and purified as described earlier (16, 17). Briefly, mice were immunized s.c. with 400 μg bovine MBP and 60 μg Mycobacterium tuberculosis (H37RA; Difco Laboratories, Detroit, MI) in IFA (Calbiochem). Spleens were collected from these mice, and single-cell suspensions were prepared in RPMI 1640 medium containing 10% FBS, 2 mM l-glutamine, 50 μM 2-ME, 100 U/ml penicillin, and 100 μg/ml streptomycin. Splenocytes cultured at a concentration of 0.5–1.0 × 106 cells per ml in 12-well plates were incubated with 50 μg/ml MBP with or without different treatments for 48 or 96 h. The nonadherent splenic T cells were collected and used for RNA isolation and FACS analysis.
Isolation of MOG35–55-primed T cells
B6.129 iNOS−/− mice and their littermate controls were purchased from The Jackson Laboratory. Briefly, micewere immunized s.c. with 100 μg MOG35–55 (Sigma-Aldrich) and 200 μg M. tuberculosis (H37RA; Difco Laboratoies) in IFA (Calbiochem). After 10 d of immunization, spleens were collected from these mice, and single-cell suspensions were prepared in RPMI 1640 medium containing 10% FBS, 2 mM l-glutamine, 50 μM 2-ME, 100 U/ml penicillin, and 100 μg/ml streptomycin. Splenocytes cultured at a concentration of 0.5–1.0 × 106 cells per ml in 12-well plates were incubated with 20 μg/ml MOG35–55 for 48 or 96 h. The nonadherent splenic T cells were collected and used for RNA isolation and FACS analysis.
Isolation of collagen-primed T cells
B6.129 iNOS−/− mice and their littermate controls were immunized intradermally at the base of their tail with 100 μg chicken collagen type II (Sigma-Aldrich) emulsified in CFA containing 200 μg M. tuberculosis (H37RA; Difco Laboratories). The mice received the same dose of injection as the booster injection on day 21. Eight days after booster injection, spleens were collected from these mice, and single-cell suspensions were prepared in RPMI 1640 medium containing 10% FBS, 2 mM l-glutamine, 50 μM 2-ME, 100 U/ml penicillin, and 100 μg/ml streptomycin. Splenocytes cultured at a concentration of 0.5–1.0 × 106 cells per ml in 12-well plates were incubated with 50 μg/ml chicken collagen type II for 48 or 96 h. The nonadherent splenic T cells were collected and used for RNA isolation and FACS analysis.
Treatment with l-NIL and pravastatin
Groups of mice that were immunized with MBP were treated with either l-NIL (5 mg/kg body weight) via i.p. injection or pravastatin (1 mg/kg body weight) via gavage daily for 10 d postimmunization. Control immunized mice received only saline. After 10 d, mice were perfused as described later for immunohistochemical studies.
Assay for NO synthesis
Synthesis of NO was determined by assay of culture supernatants for nitrite, a stable reaction product of NO with molecular oxygen. Briefly, supernatants were centrifuged to remove cells, and 400 μl each supernatant was allowed to react with 200 μl Griess reagent (18) and incubated at room temperature for 15 min. The OD of the assay samples was measured spectrophotometrically at 570 nm. Fresh culture media served as the blank. Nitrite concentrations were calculated from a standard curve derived from the reaction of NaNO2 in the assay.
Semiquantitative RT-PCR analysis
Total RNA was isolated from splenic T cells by using an RNeasy Mini Kit (Qiagen, Valencia, CA) following the manufacturer’s protocol. To remove any contaminating genomic DNA, total RNA was digested with DNase. Semiquantitative RT-PCR was carried out as described earlier (14, 19) using a RT-PCR kit from BD Clontech (Palo Alto, CA). Briefly, 1 μg total RNA was reverse-transcribed using oligo(dT)12–18 as a primer and Moloney murine leukemia virus reverse transcriptase (BD Clontech) in a 20 μl reaction mixture. The resulting cDNA was appropriately diluted, and diluted cDNA was amplified using Titanium Taq DNA polymerase and the following primers: Foxp3, sense, 5′-CAG CTG CCT ACA GTG CCC CTAG-3′, antisense, 5′-CAT TTG CCA GCA GTG GGT AG-3′; CD25, sense, 5′-AGC CAA GTA GGG TGT CTC TCA ACC-3′, antisense, 5′-GCC CAG GATACACAG TGA AGA ACG-3′; CD4, sense, 5′-CCA ACA AGA GCT CAA GGA GAC CAC-3′, antisense, 5′-CGTACC CTC TTT CCTAGC AAA GGA-3′; iNOS, sense, 5′-CCC TTC CGA AGT TTC TGG CAG CAGC-3′, antisense, 5′-GGC TGT CAG AGC CTC GTG GCT TTGG-3′; IFN-γ, sense, 5′-GCTGTTACTGCCACGGCACA-3′, antisense, 5′-GGACCACTCGGATGAGCTCA-3′; GAPDH, sense, 5′-GGT GAA GGT CGG TGT GAA CG-3′, antisense, 5′-TTG GCT CCA CCC TTC AAG TG-3′. Amplified products were electrophoresed on 1.8% agarose gels and visualized by ethidium bromide staining.
Real-time PCR analysis
Real-time PCR analysis was performed using the ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA) as described earlier (15, 20). All of the primers and FAM-labeled probes for mouse genes and GAPDH were obtained from Applied Biosystems. The mRNA expressions of respective genes were normalized to the level of GAPDH mRNA. Data were processed by the ABI Sequence Detection System 1.6 software and analyzed by ANOVA.
Flow cytometry
Surface coexpression of CD4 and CD25 on splenic T cells was checked by two-color flow cytometry, as described previously (16, 21, 22). Approximately 5 × 105 cells suspended in RPMI 1640 medium and FBS were incubated in the dark with appropriately diluted FITC-labeled Abs to CD25 and PE-labeled Abs to CD4 at 4°C for 1 h. Following incubation, the cell suspension was centrifuged, washed three times, and resuspended in 500 μl RPMI 1640 medium and FBS. The cells then were analyzed through FACS (BD Biosciences, San Jose, CA). A minimum of 10,000 cells were accepted for FACS analysis. Cells were gated based on morphological characteristics. Apoptotic and necrotic cells were not accepted for FACS analysis.
Intracellular staining of Foxp3 along with surface staining for CD25 on splenic T cells was performed according to the manufacturer’s protocol. Approximately 1 × 106 cells suspended in flow staining buffer were incubated at 4°C with appropriately diluted FITC-labeled Ab to CD25 for 30 min, washed, and resuspended in fixation and permeabilization solution. Following incubation in dark for 30 min, cells were washed, blocked with test Fc block (anti-mouse CD16/32) in permeabilization buffer, and subsequently incubated with appropriately diluted PE-labeled Abs to Foxp3 at 4°C in the dark. After incubation, the cell suspension was centrifuged, washed three times, and resuspended in an appropriate volume of flow staining buffer. The cells then were analyzed as mentioned above.
Immunofluorescence analysis
Immunofluorescence analysis was performed as described earlier (23, 24). Briefly, 4–6-wk-old MBP-immunized or naive mice were perfused intracardially with PBS (pH 7.4) and then with 4% (w/v) paraformaldehyde solution in PBS. Dissected spleens were postfixed in 4% formaldehyde and PBS for 2-5 d and cryoprotected in 20% sucrose and PBS overnight at 4°C. Splenic tissues were then embedded in OCT (TissueTek, Elkhart, IN) at −50°C, processed for conventional cryosectioning to obtain frozen longitudinal sections (8 μm), and stored at −80°C. Frozen sections were then allowed to cool to at room temperature for 1.5–2 h, washed six times each for 5 min in 1× PBS, blocked in 2% BSA in 1× PBS with 0.5% Triton at room temperature, and incubated with goat anti-CD3 (1:50) (BioSource International, Camarillo, CA) or rabbit anti-iNOS (1:500) and rat anti-Foxp3 (1:200) or rabbit anti-iNOS (1:500) and rat anti-CD11b (1:300) (Chemicon) for overnight at room temperature for dual immunohistochemistry. Sections were then washed six times in 1× PBS and further incubated with Cy2 (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1.5 h at room temperature followed by overnight drying. Next, the sections were rinsed in distilled water, dehydrated successively in ethanol and xylene, and mounted and observed under a LSM 510 confocal microscope (Zeiss, Oberkochen, Germany) using a 63× objective.
Results
Negative regulation of Foxp3 in MBP-primed T cells by NO
Because we have noticed earlier that Tregs are decreased significantly in MBP-primed T cells, we wanted to investigate whether NO is involved in the downregulation of Treg molecules (14). Splenocytes were isolated from MBP-immunized mice, restimulated with MBP (50 μg/ml) with or without different doses of either l-NIL (an inhibitor of iNOS) or PTIO (the scavenger of NO), and subsequently examined for mRNA expression of Foxp3, CD25, and CD4 by semiquantitative RT-PCR and real-time PCR analysis. Interestingly, both l-NIL and PTIO dose-dependently restored the expression of Foxp3 as well as CD25 in MBP-primed T cells without affecting CD4 expression, thus clearly suggesting an essential role of NO in the downregulation of Treg molecules (Fig. 1A, 1B, left and middle panels). Our observation was corroborated further when GSNO, an exogenous source of NO, completely abrogated l-NIL-mediated restoration of Foxp3 and CD25 mRNAs in MBP-primed T cells (Fig. 1A, 1B, right panels). Corresponding estimation of nitrites shows a reciprocal relationship between the production of nitrite and the expression of Treg markers (Foxp3 and CD25) (Fig. 1C). These results suggest that NO plays a pivotal role in the downregulation of Treg markers in MBP-primed splenocytes. Because Tregs are suppressive, a reduction in the number of Tregs may result in enhanced Th1 activity. Therefore, to examine the functional significance of downregulation of Foxp3, we analyzed the mRNA level of IFN-γ, the prototype Th1 cytokine (Fig. 1A). A reciprocal expression level of IFN-γ with respect to Foxp3 strongly suggests that impairment of Treg function due to a decreased level of Foxp3 may drive the Th1 response.
FIGURE 1.
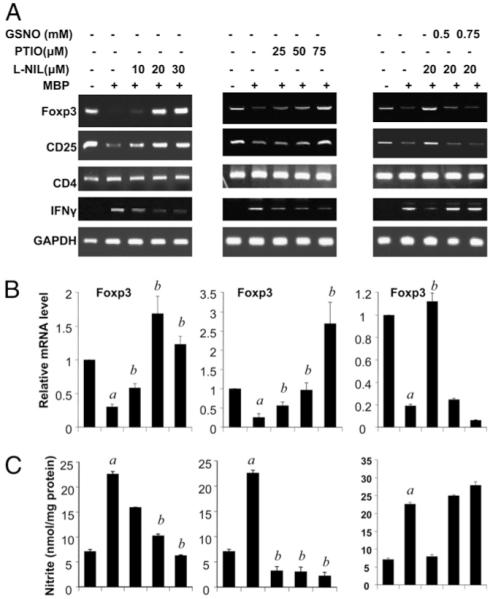
NO negatively regulates the expression of Foxp3 and CD25 in MBP-primed T cells. Splenocytes isolated from MBP-immunized female SJL/J mice were stimulated with MBP in the presence or absence of different doses of l-NIL, PTIO, or GSNO. After 48 h of stimulation, mRNA expression levels of Foxp3, CD25, CD4, and IFN-γ were monitored by semiquantitative RT-PCR (A), and the mRNA expression level of Foxp3 was further confirmed by quantitative real-time PCR (B). After 48 h, supernatants were used for the nitrite assay (C) as described in Materials and Methods. Data are mean ± SD of three different experiments. ap < 0.001 versus control; bp < 0.001 versus MBP only.
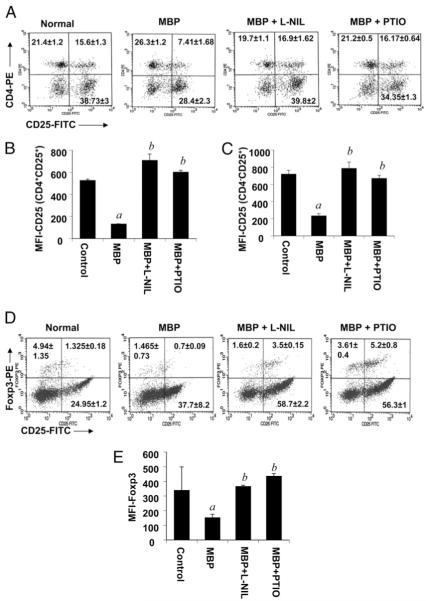
NO negatively regulates CD4+CD25+ as well as CD25+Foxp3+ T cell populations in MBP-primed T cells
Because Tregs are generally CD4+CD25+ T cells, we were further interested to determine whether NO is involved in the regulation of CD4+CD25+ T cells. Because CD4 and CD25 are surface molecules, surface expression of CD4 and CD25 was analyzed by FACS analysis. The result of the FACS analysis demonstrates that MBP priming led to a significant decrease in the population of CD4+CD25+ T cells; however, treatment of splenocytes with either l-NIL (20 μM) or PTIO (50 μM) effectively restored the population of CD4+CD25+Tcells (Fig. 2A). These observations clearly suggest that NO reduces the population of CD4+CD25+ T cells. Although not desirable, the decrease in FL1 intensity in CD25− fractions in MBP-primed T cells (Fig. 2A) is probably the consequence of a loss of CD25 in CD25+ fractions that resulted in a decrease in FL1 intensity. To confirm that the decrease in the proportion of CD4+CD25+ cells is not due to any reduction in FITC intensity, labeling controls with CD3-FITC were analyzed in the presence or absence of l-NIL and PTIO following MBP priming, and the results showed no changes in CD3-FITC intensity (data not shown). However, CD25 also is expressed by many activated T cells that may not express Foxp3, and those Foxp3− CD25+ T cells may lack regulatory properties. Therefore, to confirm the role of NO in the regulation of Treg markers, we analyzed the expression of Foxp3 by intracellular FACS along with surface expression of CD25. We found that both l-NIL and PTIO dramatically increased the population of CD25+Foxp3+ cells in MBP-primed T cells (Fig. 2D). These results strongly suggest that the decrease in Foxp3+CD25+ T cell population in MBP-primed T cells is due to NO.
FIGURE 2.
NO negatively regulates CD4+CD25+ as well as CD25+Foxp3+ T cell populations in MBP-primed T cells. Splenocytes isolated from MBP-immunized female SJL/J mice were stimulated with MBP in the presence or absence of l-NIL or PTIO. After 96 h of stimulation, T cells were incubated with appropriately diluted PE-conjugated anti-CD4 and FITC-conjugated anti-CD25 Abs (A) or PE-conjugated anti-Foxp3 and FITC-conjugated anti-CD25 Abs (D) as described in Materials and Methods followed by FACS analysis. Results represent three independent experiments. MFIs were calculated based on gated populations of CD4+CD25+ (B), CD4−CD25+ (C), and total Foxp3+ (E) cells by using CellQuest software (BD Biosciences). Data are mean ± SD of three different experiments. ap < 0.001 versus control; bp < 0.001 versus MBP only.
Surprisingly, NO also was found to negatively regulate the population of CD25+CD4− cells, as evident from the lower right quadrants of Fig. 2A, although the effect was less pronounced compared with CD4+CD25+ cells. Although most of the Foxp3+ cells are CD4+, there is also another population of Foxp3+ cells that is CD4− but CD8+. Our unpublished observation also shows the presence of CD4−Foxp3+ splenic T cells in normal mice. Therefore, a decrease in the number of CD4−CD25+ cells may be primarily due to the downregulation of Foxp3 by NO, which contributes to a decrease in CD25 in Foxp3+ cells. However, Foxp3−CD25+ T cells are activated T cells, which are not regulated by NO, as evident from the lower right quadrants of Fig. 2D. The mean fluorescence intensities (MFIs) of gated populations of cells further confirm our FACS results (Fig. 2B, 2C, 2E).
Pharmacological inhibitors of NO prevent the attenuation of Treg markers in MBP-primed T cells
To further substantiate our findings and to establish clinical significance as well, we investigated whether pharmacological compounds capable of inhibiting the induction of iNOS and suppressing the disease process of EAE can prevent the decrease in Foxp3 in MBP-primed T cells. Gemfibrozil, NaB, and pravastatin are U.S. Food and Drug Administration-approved drugs for hyperlipidemia, urea cycle disorders, and hypercholesterolemia, respectively. Interestingly, our RT-PCR data show that the treatment of splenocytes with gemfibrozil (200 μM), pravastatin (10 μM), or NaB (1.0 mM) during MBP priming prevented reduction of the mRNA levels of Foxp3 and CD25 but not CD4 (Fig. 3A). The corresponding estimation of nitrite again reflected a reciprocal relationship between NO and Treg markers Foxp3 and CD25 (Fig. 3B). As expected, the prevention of Foxp3 and CD25 expression by pharmacological compounds was dose-dependently abrogated by GSNO, an NO donor (Fig. 3A). This reversal further substantiates the role of NO in the downregulation of Foxp3 and CD25 in MBP-primed T cells.
FIGURE 3.
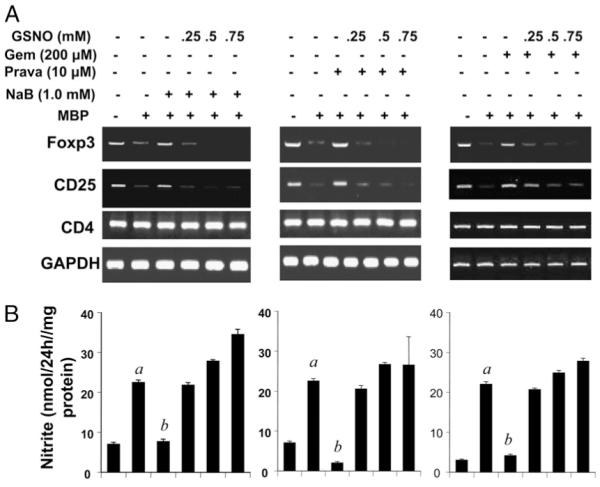
Pharmacological inhibitors of iNOS upregulate the expression of Foxp3 and CD25 in MBP-primed T cells. Splenocytes isolated from MBP-immunized female SJL/J mice were stimulated with MBP in the presence or absence of NaB (1.0 mM), pravastatin (10 μM), or gemfibrozil (200 μM) only or along with different doses of GSNO. After 48 h of stimulation, mRNA expression levels of Foxp3, CD25, and CD4 were monitored by semiquantitative RT-PCR (A), and supernatants were used for the nitrite assay (B). Data are mean ± SD of three different experiments. ap < 0.001 versus control; bp < 0.001 versus MBP only.
Pharmacological inhibitors of NO enrich CD4+CD25+ populations of cells in MBP-primed T cells
We next wanted to see whether these pharmacological inhibitors also prevent the reduction of the number of CD4+CD25+ cells in MBP-primed T cells. Consistent with RT-PCR results, our FACS data reveal that pravastatin and NaB effectively prevented the reduction in the number of CD4+CD25+ T cells during MBP priming and that GSNO completely abrogated this prevention (Fig. 4A). These data further corroborate the role of NO in the attenuation of Tregs. The results were confirmed by the MFI of the gated population of cells (Fig. 4B, 4C). As mentioned earlier, the decrease in FL1 intensity in CD25− fractions in MBP-primed T cells (Fig. 4A) is probably the consequence of a loss of CD25 in CD25+ fractions that resulted in a decrease in FL1 intensity.
FIGURE 4.
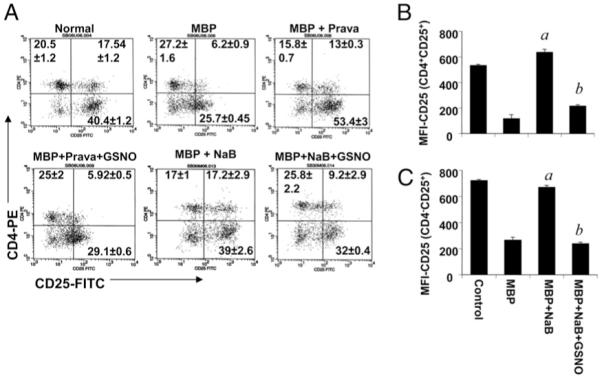
Pharmacological inhibitors of iNOS enrich CD4+CD25+ T cell populations in MBP-primed T cells. Splenocytes isolated from MBP-immunized female SJL/J mice were stimulated with MBP in the presence or absence of NaB (1.0 μM) or pravastatin (10 μM) only or along with GSNO (500 μM) or DETA-NONOate (50 μM). After 96 h of stimulation, T cells were incubated with appropriately diluted PE-conjugated anti-CD4 and FITC-conjugated anti-CD25 Abs (A) as described in Materials and Methods followed by FACS analysis. Results represent three independent experiments. MFIs were calculated based on gated populations of CD4+CD25+ (B) and CD4−CD25+ (C) cells by using CellQuest software (BD Biosciences). Data are mean ± SD of three different experiments. ap < 0.001 versus MBP; bp < 0.001 versus MBP and NaB.
Knockdown of iNOS prevents the suppression of Foxp3 expression in MBP-primed T cells
To confirm an essential role of NO in the attenuation of Foxp3, we further analyzed the mRNA level of Foxp3 in splenic T cells from wildtype and iNOS−/− mice with or without MBP immunization and priming. As expected, both semiquantitative RT-PCR (Fig. 5A) and real-time PCR (Fig. 5B) analyses demonstrate that MBP priming reduced the expression of Foxp3 in T cells of wild-type B6.129 mice but not that of iNOS−/− mice. The role of NO was further substantiated by FACS analysis, which demonstrated that the NO donor DETA-NONOate significantly reduced the population of CD25+Foxp3+ cells in MBP-primed T cells isolated from iNOS−/−B6.129 mice (Fig. 5C). These results further support our finding that NO is involved in the suppression of Foxp3 in T cells during MBP priming. Consistent with our above observations (Fig. 2D), here we found that NO had no effect on the CD25+Foxp3− population (Fig. 5C, lower right quadrants). These data further confirm that the downregulatory effect of NO on CD25 is limited to Foxp3+CD25+ cells and that Foxp3−CD25+ cells are not downmodulated by NO.
FIGURE 5.
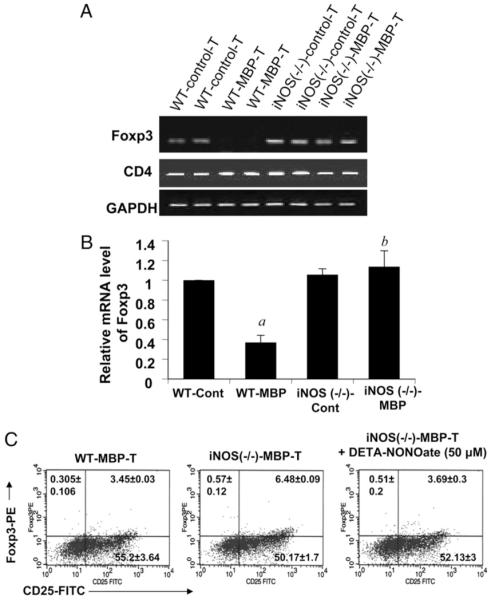
Knockdown of iNOS prevents the suppression of Foxp3 expression in T cells during MBP priming. Splenocytes isolated from MBP-immunized B6.129 wild-type and iNOS−/− mice were stimulated with MBP. After 48 h, mRNA expression levels of Foxp3 and CD4 were monitored by semiquantitative RT-PCR (A), and mRNA expression level of Foxp3 was monitored by quantitative real-time PCR (B). Data are mean ± SD of three different experiments. ap < 0.001 versus wild-type control; bp < 0.001 versus wild-type MBP only. C, Splenocytes isolated from MBP-immunized B6.129 wild-type, stimulated with MBP only, and iNOS−/− mice were stimulated with MBP in the presence or the absence of DETA-NONOate (50 μM). After 96 h of stimulation, T cells were incubated with appropriately diluted PE-conjugated anti-Foxp3 and FITC-conjugated anti-CD25 Abs followed by FACS analysis. Results represent three independent experiments.
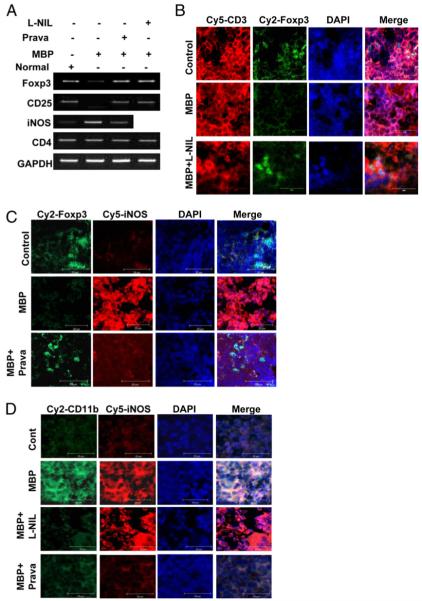
l-NIL and pravastatin restore the expression of Treg markers in spleens of MBP-immunized mice
We further investigated whether NO also is involved in the downregulation of Foxp3 in vivo. Groups of mice were immunized with MBP with or without treatment with l-NIL (5 mg/kg body weight) or pravastatin (1 mg/kg body weight), and spleens were analyzed for mRNA expression levels of Foxp3, CD25, CD4, and iNOS as well as the protein levels of Foxp3 and iNOS by immunohistochemical analysis. Our semiquantitative RT-PCR data suggest that both l-NIL and pravastatin completely abrogated the attenuation of Foxp3 and CD25 in MBP-immunized spleens (Fig. 6A). However, we did not observe any change in the expression of CD4 under any of these treatment conditions (Fig. 6A). Analysis of iNOS mRNA reveals that MBP immunization induced the expression of iNOS in vivo in the spleen and that pravastatin treatment markedly inhibited splenic expression of iNOS in MBP-immunized mice (Fig. 6A). Consistently, our dual-immunohistochemical studies with CD3 and Foxp3 further substantiate the role of NO in the expression of Foxp3 in vivo at the protein level (Fig. 6B). Similarly, a dual-immunohistochemical analysis with Foxp3 and iNOS also reveals that the expression of Foxp3 inversely correlated with the expression of iNOS in the spleens of MBP-immunized mice (Fig. 6C). These results indicate that NO derived from iNOS suppresses Tregs in vivo in the spleens of MBP-immunized mice.
FIGURE 6.
l-NIL and pravastatin restore the expression of Treg markers in vivo in the spleens of MBP-immunized mice. Female SJL/J mice immunized with MBP were treated with saline, l-NIL (5 mg/kg body weight), or pravastatin (1 mg/kg body weight) daily for 10 d postimmunization followed by isolation of RNA from spleens and RT-PCR analysis for Foxp3, CD25, iNOS, and CD4 (A). Splenic sections were dual-immunostained for CD3 and Foxp3 (B), iNOS and Foxp3 (C), or iNOS and CD11b (D). DAPI was used to visualize the nucleus. The settings of the microscope remained strictly unaltered during the entire study. Results represent three independent experiments. Original magnification ×40 (B–D).
To investigate whether NO is produced by APCs, dual immunohistochemistry with iNOS and CD11b (a marker of macrophages) was performed. The colocalization of iNOS in CD11b+ cells strongly suggests that NO is produced primarily from macrophages of MBP-treated spleens (Fig. 6D). Both l-NIL and pravastatin significantly inhibited the expression of CD11b; however, iNOS expression was not inhibited in l-NIL-treated sections (Fig. 6D). Being a competitive inhibitor of iNOS, l-NIL inhibited the enzymatic activity of iNOS without altering the expression of iNOS protein and thereby suppressed the production of NO. Because NO is directly involved in the upregulation of CD11b (20), inhibition of NO resulted in the suppression of CD11b (Fig. 6D).
Knockdown of iNOS prevents the suppression of Foxp3 expression in vivo in the spleens of MBP-immunized mice
Next, to confirm the role of NO in the attenuation of Treg molecules invivo, spleens of wild-type and iNOS−/− mice with or without MBP immunization were analyzed for the mRNA level of Foxp3 by semiquantitative RT-PCR and real-time PCR as well as the protein level by immunohistochemical analysis. Consistent with our finding in isolated splenocytes, the semiquantitative RT-PCR and real-time PCR data as well as dual-immunohistochemical analysis with CD3 and Foxp3 demonstrate that MBP immunization markedly inhibited the expression of Foxp3 mRNA (Fig. 7A,7B) and protein (Fig. 7C) in vivo in the spleens of wild-type but not iNOS−/− mice. However, under similar experimental conditions, the expression of CD4 remained unchanged (Fig. 7A). These results further confirm the fact that iNOS-derived NO is critically required for the attenuation of Foxp3 during MBP immunization.
FIGURE 7.
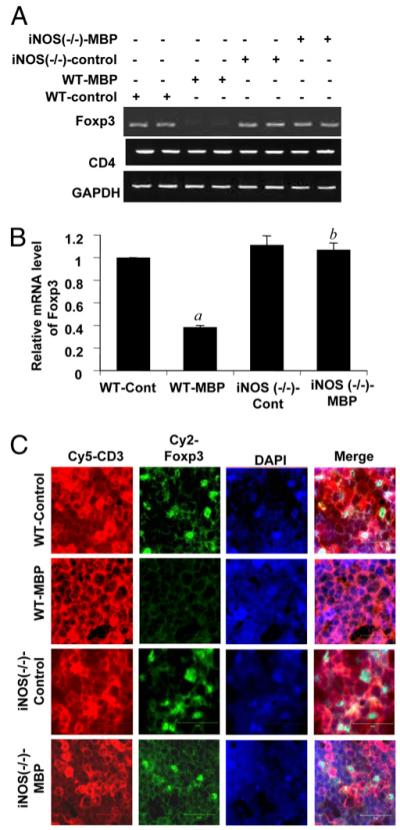
Knockdown of iNOS prevents the suppression of Foxp3 expression invivo in the spleens of MBP-immunized mice: Spleens were isolated from control or MBP-immunized B6.129 wild-type and iNOS knockout mice, and the mRNA expression levels of Foxp3 and CD4 were monitored by semiquantitative RT-PCR (A). The mRNA expression of Foxp3 was estimated by quantitative real-time PCR (B). Data are mean ± SD of three different experiments. ap < 0.001 versus wild-type control; bp < 0.001 versus wild-type MBP only. Splenic sections were dual-immunostained for CD3 and Foxp3. DAPI was used to visualize the nucleus (C) (original magnification ×40). The settings of the microscope remained strictly unaltered during the entire study. Results represent three independent experiments.
Knockdown of iNOS prevents the suppression of Foxp3 expression in T cells during priming with MOG
MOG is another target Ag in MS, and MOG-induced EAE has been a well-known animal model of MS. Here, we investigated the status of Foxp3 level in MOG-primed T cells and whether it also was regulated by NO. Therefore, we analyzed the mRNA level of Foxp3 in splenic T cells from wild-type and iNOS−/− mice with or without MOG immunization and priming. As evident from semiquantitative RT-PCR (Fig. 8A) and real-time PCR (Fig. 8B) analyses, similar to MBP, MOG priming also reduced the expression of Foxp3 in T cells of wild-type but not iNOS−/− mice. The role of NO in the suppression of Foxp3 in MOG-primed T cells was further substantiated by FACS analysis (Fig. 8C). These results suggest that the downregulation of Foxp3 by NO is not specific to MBP priming but rather that it could be a response triggered by the priming of T cells with any MS-specific Ags.
FIGURE 8.
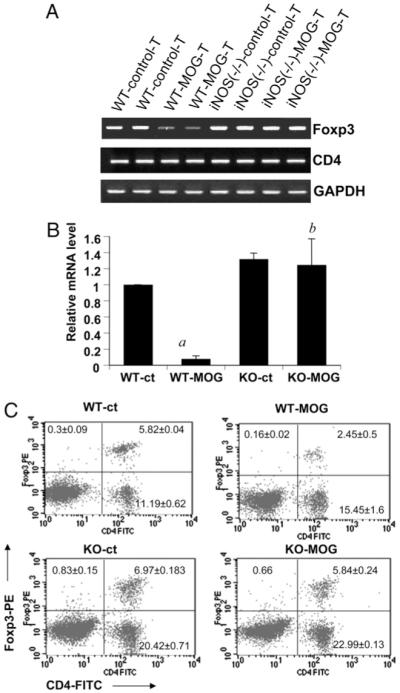
Knockdown of iNOS prevents the suppression of Foxp3 expression in T cells during priming with MOG. Splenocytes isolated from MOG-immunized B6.129 wild-type and iNOS−/− mice were stimulated with MOG. After 48 h, mRNA expression levels of Foxp3 and CD4 were monitored by semiquantitative RT-PCR (A), and mRNA expression level of Foxp3 was monitored by quantitative real-time PCR (B). Data are mean ± SD of three different experiments. ap < 0.001 versus wild-type control; bp < 0.001 versus wild-type MOG only. C, Splenocytes isolated from MOG-immunized B6.129 wild-type and iNOS−/− mice were stimulated with MOG. After 96 h of stimulation, T cells were incubated with appropriately diluted PE-conjugated anti-Foxp3 and FITC-conjugated anti-CD4 Abs followed by FACS analysis. Results represent three independent experiments.
Knockdown of iNOS prevents the suppression of Foxp3 expression in T cells during priming with collagen Ag
To further examine whether priming of only MS-related Ags or Ags of other autoimmune disorders also downregulate Foxp3 via NO, we analyzed the effect of NO on the level of Foxp3 in T cells from a mouse model of RA, an autoimmune inflammatory disorder primarily affecting the joints. Therefore, the mRNA level of Foxp3 was examined in splenic T cells from wild-type and iNOS−/− mice with or without immunization and priming with collagen, an autoantigen for RA. Both semiquantitative RT-PCR (Fig. 9A) and real-time PCR (Fig. 9B) analyses demonstrate that, similar to MBP and MOG, collagen priming also reduced the expression of Foxp3 in T cells of wild-type but not iNOS−/− mice. The role of NO in the reduction of Foxp3 in collagen-primed T cells was further substantiated by FACS analysis (Fig. 9C). These results strongly suggest that the downregulation of Foxp3 by NO is not specifically responsive to any particular autoantigen but rather a more generalized downstream response of autoantigenic stimulation.
FIGURE 9.
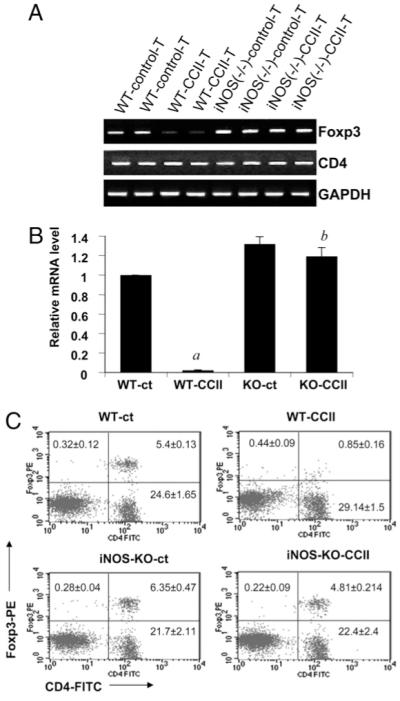
Immunization with collagen suppresses the expression of Foxp3 in T cells from wild-type but not the iNOS knockout mice. Splenocytes isolated from collagen-immunized B6.129 wild-type and iNOS−/− mice were stimulated with collagen. After 48 h, mRNA expression levels of Foxp3 and CD4 were monitored by semiquantitative RT-PCR (A), and mRNA expression level of Foxp3 was monitored by quantitative real-time PCR (B). Data are mean ± SD of three different experiments. ap < 0.001 versus wild-type control; bp < 0.001 versus wild-type collagen only. C, Splenocytes isolated from collagen-immunized B6.129 wild-type and iNOS−/− mice were stimulated with collagen. After 96 h of stimulation, T cells were incubated with appropriately diluted PE-conjugated anti-Foxp3 and FITC-conjugated anti-CD4 Abs followed by FACS analysis. Results represent three independent experiments.
Involvement of sGC in the suppression of Foxp3 in MBP-primed T cells
Next, we investigated mechanisms by which NO may suppress the expression of Foxp3 in Tregs. Because sGC is intimately coupled to NO-induced downstream signaling events (25, 26), we attempted to explore the role of sGC in the expression of Foxp3 in MBP-primed T cells. Splenocytes were incubated with different concentrations of NS-2028, a very specific inhibitor of sGC, during MBP priming followed by analysis of Foxp3 expression in MBP-primed T cells. As evident from semiquantitative RT-PCR analyses (Fig. 10A) and real-time PCR analysis (Fig. 10B), MBP priming suppressed the expression of Foxp3 in T cells; however, NS-2028 dosedependently abrogated the inhibitory effect of MBP priming on the expression of Foxp3. Because NO is produced during MBP priming, these results suggest that NO decreases Foxp3 via sGC.
FIGURE 10.
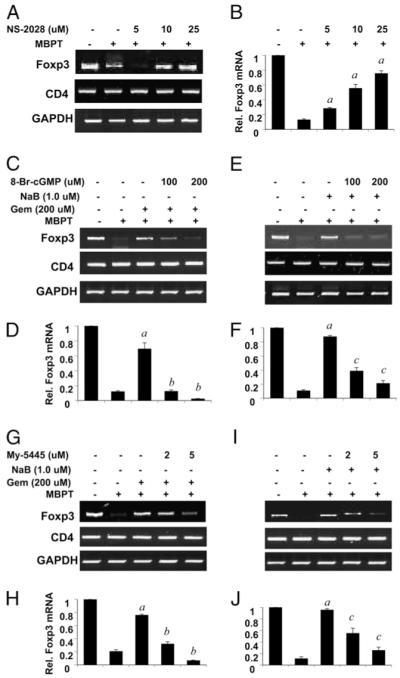
Attenuation of Foxp3 expression in MBP-primed T cells is via the sGC-cGMP pathway. Splenocytes isolated from MBP-immunized female SJL/J mice were stimulated with MBP in the presence or absence of different doses of NS-2028 (A, B), gemfibrozil, or NaB with or without different doses of 8-Br-cGMP (C–F) or MY-5445 (G–J). After 48 h of stimulation, the mRNA expression levels of Foxp3 and CD4 were monitored by semiquantitative RT-PCR (A, C, E, G, I) and quantitative real-time PCR (B, D, F, H, J). Data are mean ± SD of three different experiments. ap < 0.001 versus MBP; bp < 0.001 versus gemfibrozil; cp < 0.001 versus NaB.
Does cGMP mimic the effect of NO in inhibiting the expression of Foxp3 in MBP-primed T cells?
Because sGC catalyzes the formation of cGMP from GTP, we investigated whether cGMP alone is sufficient to inhibit the expression of Foxp3 in MBP-primed T cells. The level of cGMP in a cell could be increased by either a cell-permeable cGMP derivative (8-Br-cGMP) or an inhibitor of cGMP phosphodiesterase (MY5445). As evident from RT-PCR and real-time PCR analyses, treatment of splenocytes with NaB (Fig. 10C, 10D) and gemfibrozil (Fig. 10E, 10F) during MBP priming restored the expression of Foxp3 in T cells. Interestingly, 8-Br-cGMP alone abrogated the restoration of Foxp3 expression by NaB (Fig. 10C, 10D) and gemfibrozil (Fig. 10E, 10F) in MBP-primed T cells. However, the expression of CD4 remained unchanged under similar treatment conditions (Fig. 10C, 10E), suggesting that the inhibitory effect of cGMP on the expression of Foxp3 is specific and that this effect is not due to any cell death of T cells. Next, the effect of MY5445 on the expression of Foxp3 was tested in MBP-primed T cells. Splenocytes were treated with either NaB or gemfibrozil in the presence or absence of MY-5445 during MBP priming. Similar to 8-Br-cGMP, MY-5445 also dose-dependently suppressed the restoration of Foxp3 in NaB- (Fig. 10G, 10H) and gemfibrozil-treated (Fig. 10I, 10J) splenocytes. These results suggest that MBP priming decreases the expression of Foxp3 in T cells via the NO-sGC-cGMP pathway.
Discussion
MS is an autoimmune disease resulting from the activation and proliferation of myelin-reactive T cells, which cross the blood-brain barrier to enter into the CNS where these cells initiate, promote, and aggravate multifaceted inflammatory and degenerative insults, ultimately leading to demyelination and axonal injury. There are many hypotheses, including microbial and viral infection, molecular mimicry, etc., to explain how autoreactive T cells might be activated (27). However, no consensus has been reached due to the lack of convincing evidence. The real breakthrough came after the concept of Tregs became clear. Although a few different types of Tregs with specific surface or secretory molecules have been identified, Foxp3 is generally considered as the signature molecule that is associated with Treg properties (1, 28). The primary role of Tregs in the immune system is to suppress unwanted activation of responder cells, including Th1 and Th2, and maintain immune homeostasis (28-30). Therefore, it is likely that dysfunction of Tregs could be a major cause for the activation of myelin-reactive T cells in MS.
Accordingly, there is a significant decrease in the number of CD4+ Foxp3+ T cells as well as the expression level of Foxp3 in relapsing remitting MS and other lymphoproliferative autoimmune disorders (9, 10, 13). However, the molecular mechanism by which Tregs are suppressed in MS and other autoimmune disorders is poorly understood. Recently, Dominitzki et al. (31) have demonstrated that trans-signaling via soluble IL-6 receptors might play a key role in the regulation of Foxp3 by augmenting the expression of SMAD7, the inhibitor of TGF-β signaling, and hence Foxp3 expression. Although activation of an innate immune response by microbial infection may result in the production of IL-6 by dendritic cells, which in turn may suppress the expression of Foxp3, this mechanism is unable to explain why only myelin-reactive T cells are specifically activated in MS (32). Several lines of evidence presented in this article demonstrate that NO is critically required for the attenuation of Foxp3 in MBP-primed T cells as well as spleens of MBP-immunized donor mice and that this suppression is mediated by the activation of the sGC-cGMP pathway.
NO is an extremely critical molecule implicated in the pathogenesis of MS. There are some studies demonstrating that NO may enhance switching to Th1 differentiation and expansion, but it is not sufficient to explain how Th1 cells escape suppression by Tregs (33). In this article, we provide the first evidence that Foxp3 is negatively regulated by NO. First, we presented the evidence that NO is involved in the attenuation of Foxp3 and CD25 but not CD4 expression in MBP-primed T cells as demonstrated by the abrogation of this attenuation by l-NIL, the competitive inhibitor of iNOS, PTIO, the scavenger of NO, or pharmacological drugs inhibiting iNOS, such as NaB, gemfibrozil, and pravastatin (14, 15). As expected, these pharmacological drugs remained unable to restore the expression of Foxp3 in MBP-primed T cells in the presence of GSNO, thus providing direct evidence of the involvement of NO in the suppression of Foxp3 and CD25. Therefore, it is possible that NO-mediated switching to Th1 could be dependent on NO-mediated suppression of Tregs.
Our results strongly implicate that the suppression of Foxp3 in MBP-primed T cells is due to the direct effect of NO signaling, whereas the suppression of CD25 is a secondary consequence of NO, which was evident from the inability of donor NO to decrease the population of CD25+Foxp3− cells in iNOS−/− T cells (Fig. 5C). According to our unpublished observations, the expression of CD25 was abrogated by antisense knockdown of Foxp3, suggesting that Foxp3 may play an important role in positive regulation of CD25 in Foxp3+ T cells. There are also reports that suggest that Foxp3, once expressed, can reinforce the expression of CD25 (8, 34). Therefore, in our studies, reduction in the mRNA level of CD25 after MBP stimulation (Figs. 1A, 3A) is probably due to the direct effect of downregulation of Foxp3. However, many activated T cells express CD25, but because mRNA analysis was performed only after 48 h of stimulation, expression of CD25 in activated T cells probably was not high enough to compensate for the loss due to the downregulation of Foxp3. In Foxp3− T cells, the expression of CD25 is certainly independent of Foxp3 (35). However, the decrease in populations of CD4+CD25+ and CD4−CD25+ T cells by NO is probably due to the direct effect of NO on Foxp3+ T cells, resulting in the impairment of Foxp3-dependent transcriptional activation of CD25. Interestingly, CD4−CD25+ cells may also express Foxp3. These cells may be CD8+, and CD8+Foxp3+ Tregs also have been reported (36). Because Foxp3 is a more accurate and specific marker of Tregs than CD25 (37), our emphasis was to study the role of NO in the expression of Foxp3, and therefore the significance of our findings should be related to Foxp3+ Tregs that also express CD25.
Our in vivo results further support the role of NO in the attenuation of Foxp3 as demonstrated by the restoration of Foxp3 by l-NIL or pravastatin in the spleens of MBP-immunized mice. Severe upregulation of iNOS and its colocalization with CD11b, the marker of macrophages and monocytes, in the spleens of MBP-immunized mice strongly suggest that it is the iNOS-derived NO, expressed by splenic APCs, which is responsible for the suppression of Foxp3. The unperturbed expression of CD4 probably implies that the suppression of Foxp3 is not due to any reduction of CD4+ cells but rather due to the selective effect of NO on the expression of Foxp3.
The essential role of NO in the attenuation of Foxp3 was confirmed when we demonstrated that knockdown of iNOS completely abrogated the suppression of Foxp3 in MBP-primed T cells as well as spleens of MBP-immunized mice. Although our results clearly signify an essential role of NO in the suppression of Foxp3, complete knockdown of iNOS may not be beneficial, because increases in clinical symptoms and mortality rates in iNOS−/− mice with EAE have been observed compared with those of the wild-type mice, suggesting a protective role of NO in EAE (38). Moreover, Tregs are not the single factor that regulates the disease process of EAE. Therefore, for therapeutic purposes, instead of knocking down iNOS completely, partial inhibition of the expression of iNOS by suitable doses of pharmacological compounds to abrogate the suppression of Foxp3 would likely have a larger beneficial effect.
MBP is not the only autoantigen targeted in MS. Among the other neuroantigens, MOG is an important candidate, and MOG-reactive T cells also play significant roles in the pathogenesis of MS (39, 40). We therefore analyzed the level of Foxp3 in MOG-primed T cells and whether NO had any effect on it. Substantial reduction of Foxp3 in MOG-primed T cells from wild-type but not iNOS−/− mice strongly suggests that attenuation of Tregs by NO is not specific to any particular neuroantigen but rather a possible mode of reduction of Tregs in MS following activation against any of the myelin Ags targeted in the disease.
The above findings instigated us to examine whether NO also could suppress Tregs in other autoimmune diseases. RA is an autoimmune inflammatory disease that mostly affects joints. The role of Tregs in the pathogenesis of RA is currently a highly debated issue. The status of Tregs in the disease is also unclear. Some studies did not find any defect in Tregs from the patients of RA, whereas others reported defective Tregs in RA (41-44). Interestingly, our results clearly show that T cell priming with collagen, an autoantigen targeted in RA, dramatically decreased the expression of Foxp3 in T cells from wild-type but not iNOS−/− mice. Hence, our observation strongly indicates that in addition to MS, in other autoimmune diseases, such as RA, Tregs may be attenuated and that attenuation also is critically governed by NO.
NO most commonly employs sGC as its immediately downstream effector molecule, which in turn catalyzes the formation of cGMP to modulate downstream functions (25, 26). Interestingly, our findings suggest that the attenuation of Foxp3 in MBP-primed T cells involves the activation of the sGC-cGMP pathway. In contrast, an unparallel observation has been reported recently that suggests that NO induces CD4+CD25+ Tregs (45). However, these Tregs are Foxp3− and induced in a cGMP-independent manner. Therefore, it might be possible that NO differentially modulates two different kinds of Tregs. Although NO suppresses CD25+ Foxp3+ Tregs via the activation of sGC-cGMP, it induces CD4+ CD25+Foxp3− Tregs independent of the GC-cGMP pathway. The resultant outcome thus will depend on the relative predominance of any of the pathways.
In summary, our study delineates a novel role of NO in the downregulation of Foxp3. There are significant numbers of APCs (e.g., macrophages and dendritic cells) in spleens that can induce the expression of iNOS upon stimulation (46). Following immunization with myelin Ags, the Ags are presented by APCs via MHC class II to prime T cells against myelin. We propose that during first encounter with myelin Ag both naive Tregs and Th cells are primed against myelin Ag and as a result myelin-reactive memory Tregs and memory Th cells are produced. During subsequent encounter with myelin Ag presented by APCs, activation of costimulatory molecules along with MHC class II-TCR interaction induces upregulation of iNOS in APCs, resulting in huge production of NO, which in turn attenuates the expression of Foxp3 via activation of the sGC-cGMP pathway, leading to a reduction in myelin-reactive Foxp3-expressing T cells. We hypothesize that a reduction in myelin-reactive Foxp3-expressing Tregs may abrogate its suppressive action on myelin-reactive encephalitogenic Th1 cells, thereby facilitating their activation and proliferation and leading to CNS autoimmune disease. Therefore, specific targeting of NO by either pharmacological inhibitors of iNOS (statins, gemfibrozil, and NaB) or NO scavengers may be an important step to restore Foxp3+ Tregs and attenuate autoreactive T cells in MS and other autoimmune disorders.
Acknowledgments
This work was supported by National Institutes of Health Grants NS39940 and NS48923.
Abbreviations used in this paper
- DETA-NONOate
diethylenetriamine NONOate
- EAE
experimental autoimmune encephalomyelitis
- Foxp3
forkhead box p3
- GSNO
S-nitrosoglutathione
- iNOS
inducible NO synthase
- l-NIL
l-N6-(1-iminoethyl)-lysine hydrochloride
- MBP
myelin basic protein
- MFI
mean fluorescence intensity
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- NaB
sodium benzoate
- PTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethyl imidazoline-1-oxy 3-oxide
- RA
rheumatoid arthritis
- sGC
soluble guanylyl cyclase
- Treg
regulatory T cell
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat. Rev. Immunol. 2004;4:889–899. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- 2.Beissert S, Schwarz A, Schwarz T. Regulatory T cells. J. Invest. Dermatol. 2006;126:15–24. doi: 10.1038/sj.jid.5700004. [DOI] [PubMed] [Google Scholar]
- 3.Shevach EM, Stephens GL. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat. Rev. Immunol. 2006;6:613–618. doi: 10.1038/nri1867. [DOI] [PubMed] [Google Scholar]
- 4.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 5.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 6.Powell BR, Buist NR, Stenzel P. An X-linked syndrome of diarrhea, polyendocrinopathy, and fatal infection in infancy. J. Pediatr. 1982;100:731–737. doi: 10.1016/s0022-3476(82)80573-8. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler SF. FOXP3: of mice and men. Annu. Rev. Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 8.Marson A, Kretschmer K, Frampton GM, Jacobsen ES, Polansky JK, MacIsaac KD, Levine SS, Fraenkel E, von Boehmer H, Young RA. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huan J, Culbertson N, Spencer L, Bartholomew R, Burrows GG, Chou YK, Bourdette D, Ziegler SF, Offner H, Vandenbark AA. Decreased FOXP3 levels in multiple sclerosis patients. J. Neurosci. Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 11.Paust S, Cantor H. Regulatory T cells and autoimmune disease. Immunol. Rev. 2005;204:195–207. doi: 10.1111/j.0105-2896.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 12.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J. Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 13.Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, Medaer R, Hupperts R, Stinissen P. Compromised CD4+ CD25high regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahmachari S, Pahan K. Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis. J. Immunol. 2007;179:275–283. doi: 10.4049/jimmunol.179.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy A, Fung YK, Liu X, Pahan K. Up-regulation of microglial CD11b expression by nitric oxide. J. Biol. Chem. 2006;281:14971–14980. doi: 10.1074/jbc.M600236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dasgupta S, Jana M, Liu X, Pahan K. Role of very-late antigen-4 (VLA-4) in myelin basic protein-primed T cell contact-induced expression of proinflammatory cytokines in microglial cells. J. Biol. Chem. 2003;278:22424–22431. doi: 10.1074/jbc.M301789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dasgupta S, Jana M, Liu X, Pahan K. Myelin basic protein-primed T cells of female but not male mice induce nitric-oxide synthase and proinflammatory cytokines in microglia: implications for gender bias in multiple sclerosis. J. Biol. Chem. 2005;280:32609–32617. doi: 10.1074/jbc.M500299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jana M, Liu X, Koka S, Ghosh S, Petro TM, Pahan K. Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. J. Biol. Chem. 2001;276:44527–44533. doi: 10.1074/jbc.M106771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasgupta S, Roy A, Jana M, Hartley DM, Pahan K. Gemfibrozil ameliorates relapsing-remitting experimental autoimmune encephalomyelitis independent of peroxisome proliferator-activated receptor-α. Mol. Pharmacol. 2007;72:934–946. doi: 10.1124/mol.106.033787. [DOI] [PubMed] [Google Scholar]
- 20.Jana M, Jana A, Liu X, Ghosh S, Pahan K. Involvement of phosphatidylinositol 3-kinase-mediated up-regulation of IκBα in antiinflammatory effect of gemfibrozil in microglia. J. Immunol. 2007;179:4142–4152. doi: 10.4049/jimmunol.179.6.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dasgupta S, Jana M, Liu X, Pahan K. Myelin basic protein-primed T cells induce nitric oxide synthase in microglial cells. Implications for multiple sclerosis. J. Biol. Chem. 2002;277:39327–39333. doi: 10.1074/jbc.M111841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy A, Liu X, Pahan K. Myelin basic protein-primed T cells induce neurotrophins in glial cells via αvβ3 integrin. J. Biol. Chem. 2007;282:32222–32232. doi: 10.1074/jbc.M702899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahmachari S, Fung YK, Pahan K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J. Neurosci. 2006;26:4930–4939. doi: 10.1523/JNEUROSCI.5480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-κB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2007;104:18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawkins RD, Son H, Arancio O. Nitric oxide as a retrograde messenger during long-term potentiation in hippocampus. Prog. Brain Res. 1998;118:155–172. doi: 10.1016/s0079-6123(08)63206-9. [DOI] [PubMed] [Google Scholar]
- 26.Bredt DS. Nitric oxide signaling in brain: potentiating the gain with YC-1. Mol. Pharmacol. 2003;63:1206–1208. doi: 10.1124/mol.63.6.1206. [DOI] [PubMed] [Google Scholar]
- 27.McCoy L, Tsunoda I, Fujinami RS. Multiple sclerosis and virus induced immune responses: autoimmunity can be primed by molecular mimicry and augmented by bystander activation. Autoimmunity. 2006;39:9–19. doi: 10.1080/08916930500484799. [DOI] [PubMed] [Google Scholar]
- 28.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 29.Fantini MC, Becker C, Tubbe I, Nikolaev A, Lehr HA, Galle P, Neurath MF. Transforming growth factor β induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut. 2006;55:671–680. doi: 10.1136/gut.2005.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakaguchi S, Powrie F. Emerging challenges in regulatory T cell function and biology. Science. 2007;317:627–629. doi: 10.1126/science.1142331. [DOI] [PubMed] [Google Scholar]
- 31.Dominitzki S, Fantini MC, Neufert C, Nikolaev A, Galle PR, Scheller J, Monteleone G, Rose-John S, Neurath MF, Becker C. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4+CD25− T cells. J. Immunol. 2007;179:2041–2045. doi: 10.4049/jimmunol.179.4.2041. [DOI] [PubMed] [Google Scholar]
- 32.Shu SA, Lian ZX, Chuang YH, Yang GX, Moritoki Y, Comstock SS, Zhong RQ, Ansari AA, Liu YJ, Gershwin ME. The role of CD11c+ hepatic dendritic cells in the induction of innate immune responses. Clin. Exp. Immunol. 2007;149:335–343. doi: 10.1111/j.1365-2249.2007.03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niedbala W, Wei X, Liew FY. IL-15 induces type 1 and type 2 CD4+ and CD8+ T cells proliferation but is unable to drive cytokine production in the absence of TCR activation or IL-12/IL-4 stimulation in vitro. Eur. J. Immunol. 2002;32:341–347. doi: 10.1002/1521-4141(200202)32:2<341::AID-IMMU341>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 34.Curiel TJ. Regulatory T-cell development: is Foxp3 the decider? Nat. Med. 2007;13:250–253. doi: 10.1038/nm0307-250. [DOI] [PubMed] [Google Scholar]
- 35.Nakano A, Watanabe M, Iida T, Kuroda S, Matsuzuka F, Miyauchi A, Iwatani Y. Apoptosis-induced decrease of intrathyroidal CD4+CD25+ regulatory T cells in autoimmune thyroid diseases. Thyroid. 2007;17:25–31. doi: 10.1089/thy.2006.0231. [DOI] [PubMed] [Google Scholar]
- 36.Sobhani I, Le Gouvello S. Critical role for CD8+FoxP3+ regulatory T cells in colon cancer immune response in humans. Gut. 2009;58:743–744. doi: 10.1136/gut.2008.175521. [DOI] [PubMed] [Google Scholar]
- 37.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 38.Fenyk-Melody JE, Garrison AE, Brunnert SR, Weidner JR, Shen F, Shelton BA, Mudgett JS. Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J. Immunol. 1998;160:2940–2946. [PubMed] [Google Scholar]
- 39.Shao H, Huang Z, Sun SL, Kaplan HJ, Sun D. Myelin/oligodendrocyte glycoprotein-specific T-cells induce severe optic neuritis in the C57BL/6 mouse. Invest. Ophthalmol. Vis. Sci. 2004;45:4060–4065. doi: 10.1167/iovs.04-0554. [DOI] [PubMed] [Google Scholar]
- 40.Bullard DC, Hu X, Adams JE, Schoeb TR, Barnum SR. p150/95 (CD11c/CD18) expression is required for the development of experimental autoimmune encephalomyelitis. Am. J. Pathol. 2007;170:2001–2008. doi: 10.2353/ajpath.2007.061016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esensten JH, Wofsy D, Bluestone JA. Regulatory T cells as therapeutic targets in rheumatoid arthritis. Nat. Rev. Rheumatol. 2009;5:560–565. doi: 10.1038/nrrheum.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4+CD25+ regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–2785. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 43.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J. Exp. Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niedbala W, Cai B, Liu H, Pitman N, Chang L, Liew FY. Nitric oxide induces CD4+CD25+ Foxp3− regulatory T cells from CD4+CD25− T cells via p53, IL-2, and OX40. Proc. Natl. Acad. Sci. USA. 2007;104:15478–15483. doi: 10.1073/pnas.0703725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gal A, Tamir S, Tannenbaum SR, Wogan GN. Nitric oxide production in SJL mice bearing the RcsX lymphoma: a model for in vivo toxicological evaluation of NO. Proc. Natl. Acad. Sci. USA. 1996;93:11499–11503. doi: 10.1073/pnas.93.21.11499. [DOI] [PMC free article] [PubMed] [Google Scholar]