Abstract
Objective
Because studies suggest that ultraviolet radiation (UVR) modulates myositis phenotype and Mi-2 autoantigen expression, we conducted a retrospective investigation to determine if UVR may influence the relative prevalence of dermatomyositis and anti-Mi-2 autoantibodies in the United States.
Methods
We assessed the relationship between surface UVR intensity in the state of residence at the time of onset with the relative prevalence of dermatomyositis and myositis autoantibodies in 380 myositis patients from referral centers in the U.S. Myositis autoantibodies were detected by validated immunoprecipitation assays. Surface UVR intensity was estimated from UV index data collected by the U.S. National Weather Service.
Results
UVR intensity was associated with the relative proportion of patients with dermatomyositis (odds ratio [OR] 2.3, 95% confidence interval [CI] 0.9–5.8) and with the proportion of patients expressing anti-Mi-2 autoantibodies (OR 6.0, CI 1.1–34.1). Modeling of these data showed that these associations were confined to women (OR 3.8, CI 1.3–11.0 and OR 17.3, CI 1.8–162.4, respectively) and suggests that gender influences UVR effects on autoimmune disorders. Significant associations were not seen in men, nor were UVR levels related to the presence of anti-synthetase or anti-signal recognition particle autoantibodies.
Conclusion
This first study of the distribution of myositis phenotypes and UVR exposure in the United States showed that UVR may modulate the clinical and immunologic expression of autoimmune disease in women. Further investigation of the mechanisms by which these effects are produced may give insights into pathogenesis and suggest therapeutic or preventative strategies.
Current evidence suggests that autoimmune diseases result from environmental exposures in genetically susceptible individuals and ultraviolet radiation (UVR) is an environmental exposure of increasing interest (1). Beyond UVR’s recognized association with skin cancer are other immunomodulatory effects possibly related to the development of immune-mediated disorders (2). UVR can promote surface expression of adhesion molecules, alter cytokine expression and decrease resistance to infections. UVR also upregulates the expression, alters the cellular location and induces immune responses to autoantigens. UVR exposure is important to calcium metabolism through effects on metabolically active vitamin D. Moreover, UVR is associated with increases in the clinical expression of conditions characterized by photosensitive rashes, such as lupus and dermatomyositis, in which patients have a lower UV minimal erythemal dose threshold compared to normal controls (3). Furthermore, studies suggest that UVR regulates levels of the dermatomyositis-specific Mi-2 autoantigen via protein translational effects (4).
The idiopathic inflammatory myopathies (IIM) are acquired systemic autoimmune conditions that share chronic muscle weakness due to chronic muscle inflammation. The two major clinical groups, dermatomyositis (DM) and polymyositis (PM), are distinguished by the presence of photosensitive pathognomonic rashes in DM (5). These two forms of IIM share some genetic risk factors but differ in others and appear to have distinct pathogeneses (5). Categorizing myositis patients by the presence or absence of myositis-specific autoantibodies (MSA) results in more homogenous groups in terms of epidemiology, clinical presentations, genetics and prognoses (5). MSA include autoantibodies directed against aminoacyl-tRNA synthetases (anti-synthetases), the signal recognition particle (anti-SRP), and autoantibodies that react with a 240 kD protein called chromodomain helicase DNA binding protein 4 (anti-Mi-2).
To understand possible genetic and environmental risk and protective factors for myositis within the U.S., we studied myositis referral populations to determine the relative prevalence of the clinical and immunologic phenotypes. Because specific photosensitive rashes uniquely characterize DM, and given recent associations of UVR with DM and anti-Mi-2 autoantibodies (6), we focused on assessing if such phenotypic differences may be related to UVR exposure. This first U.S. analysis of myositis phenotypes in different regions has revealed geographic variations in the clinical and immunologic expression of disease in women that are strongly predicted by ambient UVR intensity.
PATIENTS AND METHODS
Clinical and serologic evaluations
A cross-sectional retrospective study of the state of residence at time of onset of myositis was conducted in PM and DM patients from referral centers. Clinical data and sera samples were collected with ethics committee approval and informed consent. DM was distinguished from PM by Gottron’s papules, Gottron’s sign or heliotrope rashes. Consecutive patients who met criteria for definite or probable PM or DM (5), who had data available regarding their state of residence at myositis onset, and for whom myositis autoantibodies had been determined by the Oklahoma Medical Research Foundation, Oklahoma City, OK, were identified from referral centers. The participating centers were: the NIH Clinical Center, Bethesda, MD (N= 218); University of Pittsburgh, Pittsburgh, PA (N=74); the University of Texas, Houston, TX (N=35); and the University of Oklahoma, Oklahoma City, OK (N=8). Another group of myositis patients (n= 45), recruited from other centers in the US (7), were also included in our study. Of the total 380 IIM patients studied (73% of whom were female), 67% were European American, 28% were African American, 2% were Hispanic American, 2% were Asian American and 1% was other ethnic groups. Due to the small numbers in certain ethnic groups, and based upon skin gradients and sensitivity to UVR (8), for our analyses we grouped European Americans, Hispanic Americans and Asian Americans together as non-African Americans.
Determination of UVR levels
UVR levels were estimated by the UV index, which was obtained from the National Weather Service UV Index Cities Forecast Archive (ftp://ftp.cpc.ncep.noaa.gov/long/uv/cities/). The UV index, a linear variable, is an integration of the UV action spectrum-weighted UVA and UVB irradiances over the 290 to 400 nm range. Because only state locations at time of onset were available for the subjects, an overall average state UV index was calculated from the average annual UV index for 2001 assigned to the state (states with multiple recording sites received the average of the sites’ values).
Statistical Methods
Because accrual of all myositis patients in each area was not possible, geographic variation in the incidence of myositis could not be validly explored. We instead modeled the relative odds for DM using logistic regression (9), modeling the logarithm of the odds for DM versus PM as dependent on possible predictors, which included gender and race. Because of prior evidence that UVR levels were related to the proportion of DM at referral centers (6), we ascertained UV index levels by state and modeled the influence of UVR on the myositis phenotype. Due to the absence or relatively small numbers of subjects in some of the states, we evaluated similar associations using aggregated state data in the seven geoclimatic regions of the US. We also modeled the logarithm of the odds for autoantibody-positivity as a function of the same predictors, using logistic regression. All UVR associations are with the logit of the proportion of DM or the proportion of autoantibodies. All logistic analyses were done using the Generalized Linear Interactive Modeling system package (release 3.77, The Numerical Algorithms Group, Inc., Lisle, IL) and all p values cited are two-sided.
RESULTS
The phenotypes and demographic features of the 380 IIM patients in this study were similar to those reported in other investigations (10). PM accounted for 53% (n=202) of the patients. MSAs were seen in 45% (n=172): of these anti-synthetase autoantibodies were seen in 34% of patients (36% in PM and 31% in DM). Anti-SRP autoantibodies (n=21) were found exclusively in PM patients and anti-Mi-2 autoantibodies (n=23) were found only in DM patients. Significant differences were noted in the ethnic distributions in the clinical and serologic groups. African Americans (n=105) were found to have relatively more PM (66%) than DM compared to non-African Americans, of which only 48% had PM (p=0.002). Ethnic differences were also noted in those with anti-SRP autoantibodies (86% of whom were African American, p< 0.0000005). There were no significant gender differences, however, in the clinical or autoantibody groups.
At the time of myositis onset patients resided in 37 states, which were categorized into seven regions based on shared geoclimatic factors (Table 1). The possible effects of UVR on clinical group and myositis-specific immune responses in these populations were assessed in the total IIM population and in gender and ethnic groups (Table 2). The UV index was positively associated with the proportion of DM patients in the total IIM population, but this did not reach significance at the 0.05 level (OR 2.3, CI 0.9–5.8, p=0.07). The proportion of IIM patients with anti-Mi-2 autoantibodies was significantly associated with the UV index (OR 6.0, CI 1.1–34.1, p=0.05). There were no significant associations between UVR intensity and the proportion of IIM patients with anti-synthetase or anti-SRP autoantibodies, or those without any MSAs (data not shown).
Table 1.
State and regional locations of the myositis subjects in the study
| Region | State | UV Index | DM | PM | Anti-Mi-2 | Total cases |
|---|---|---|---|---|---|---|
| California | CA | 5.32 | 4 | 5 | 2 | 9 |
| Industrial Midwest | IL | 3.61 | 12 | 8 | 2 | 20 |
| IN | 3.86 | 0 | 3 | 0 | 3 | |
| KY | 4.28 | 1 | 0 | 0 | 1 | |
| MI | 3.42 | 0 | 4 | 0 | 4 | |
| OH | 3.68 | 5 | 9 | 1 | 14 | |
| WI | 3.44 | 1 | 1 | 0 | 2 | |
| WV | 4.12 | 2 | 4 | 0 | 6 | |
| Northeast | CT | 3.51 | 1 | 1 | 0 | 2 |
| DE | 4.03 | 2 | 1 | 0 | 3 | |
| MA | 3.46 | 2 | 5 | 1 | 7 | |
| MD | 4.05 | 26 | 21 | 4 | 47 | |
| NH | 3.31 | 2 | 3 | 0 | 5 | |
| NJ | 3.97 | 6 | 5 | 1 | 11 | |
| NY | 3.50 | 9 | 14 | 0 | 23 | |
| PA | 3.75 | 33 | 50 | 2 | 83 | |
| RI | 3.56 | 1 | 0 | 0 | 1 | |
| VT | 3.04 | 0 | 2 | 0 | 2 | |
| Northwest | OR | 2.97 | 1 | 2 | 0 | 3 |
| WA | 2.69 | 0 | 1 | 0 | 1 | |
| Southeast | AL | 5.68 | 2 | 3 | 1 | 5 |
| GA | 5.19 | 3 | 3 | 0 | 6 | |
| LA | 5.79 | 0 | 3 | 0 | 3 | |
| MS | 5.49 | 1 | 1 | 0 | 2 | |
| NC | 4.73 | 6 | 6 | 0 | 12 | |
| SC | 5.39 | 5 | 3 | 0 | 8 | |
| TN | 4.83 | 1 | 2 | 0 | 3 | |
| VA | 4.44 | 21 | 16 | 2 | 37 | |
| Southwest | AZ | 6.10 | 2 | 0 | 1 | 2 |
| NM | 6.12 | 1 | 1 | 0 | 2 | |
| NV | 5.58 | 0 | 1 | 0 | 1 | |
| OK | 5.22 | 2 | 5 | 2 | 7 | |
| TX | 5.61 | 22 | 15 | 3 | 37 | |
| Upper Midwest | MN | 3.21 | 0 | 1 | 0 | 1 |
| MO | 4.19 | 1 | 3 | 1 | 4 | |
| ND | 3.88 | 1 | 0 | 0 | 1 | |
| SD | 3.56 | 2 | 0 | 0 | 2 | |
Table 2.
Gender and racial associations of the UV Index by state with the proportion of subjects with dermatomyositis and with anti-Mi-2 autoantibodies*
| Myositis groups | Odds ratio (95% confidence interval) for development of: | |
|---|---|---|
| Dermatomyositis (n=178) | Anti-Mi-2 autoantibody (n=23) | |
| All subjects | 2.3 (0.9, 5.8) | 6.0 (1.1, 34.1) |
| Non-African Americans | 3.2 (1.0, 9.8) | 6.4 (0.8, 49.9) |
| African Americans | 2.4 (0.4, 13.1) | 6.0 (0.2, 175.8) |
| Females | 3.8 (1.3, 11.0) | 17.3 (1.8, 162.4) |
| Non-African Americans | 6.9 (1.7, 28.1) | 37.1 (1.9, 713.1) |
| African Americans | 2.4 (0.4, 15.0) | 4.4 (0.1, 129.0) |
| Males | 0.6 (0.1, 3.6) | 1.1 (0.0, 27.3) |
| Non-African Americans | 0.6 (0.1, 4.4) | 1.1 (0.0, 27.0) |
| African Americans | 0.3 (0.0, 117.4) | Not calculable due to small numbers |
Significant associations are bolded; the odds ratio is for the UV Index range across the U.S. states in the study
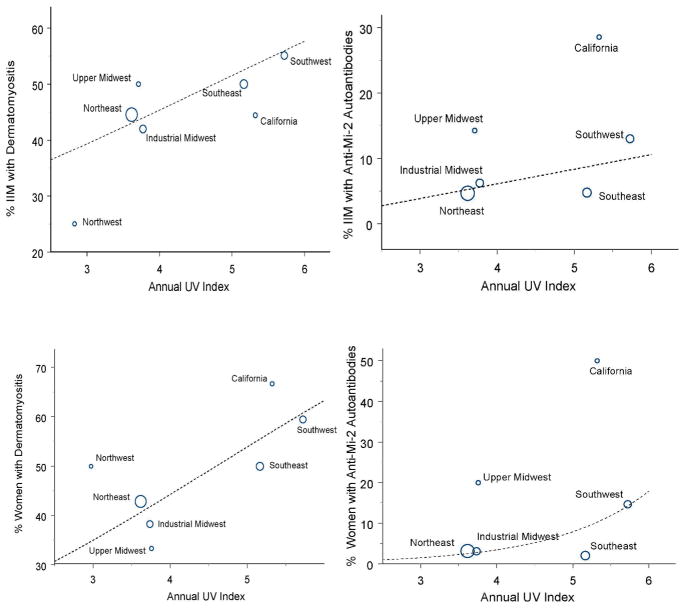
Surprisingly, the UVR effects above appeared to be the result of associations in women (OR 3.8, CI 1.3–11.0, p=0.014 for the association of the UV index with the proportion of DM, and OR 17.3, CI 1.8–162.4, p=0.012 for the association of the UV index with the proportion of anti-Mi-2 autoantibodies). These associations in women were stronger in non-African Americans than African Americans, but the fitted linear relationships were similar and the smaller sample sizes in African Americans limited the power to detect possible associations (Table 2). Nonetheless, there was no evidence for such relationships with the UV index in men, whether African American or not. Since some states had no or relatively few patients, these associations were displayed graphically after aggregating state data into seven regions of the U.S. and analyses of these data gave similar results (Figure 1).
Figure 1.
Associations between the annual UV Index in seven U.S. regions and the proportion of patients with dermatomyositis (DM) and anti-Mi-2 autoantibodies in each region. Modeling of these data for all myositis patients based on state of residence showed a non-significant trend for association between the UV index and the logit of the proportion of DM patients (p=0.07, upper left panel), but a significant association with the logit of the proportion of those with anti-Mi-2 autoantibodies (p=0.05, upper right panel). The data suggest that these associations are driven by women (for DM, p=0.014, lower left panel; for anti-Mi-2 autoantibodies, p=0.012, lower right panel). Because no patients with anti-Mi-2 autoantibodies were in the Northwest region, this region is not represented in the right two panels. The size of the circle representing each region is proportional to the number of patients residing in that region at the time of myositis onset.
DISCUSSION
Variations in IIM clinical features and serologic expression were noted across U.S. geographic regions and were found to correlate with the intensity of UVR. These findings suggest that gradients of DM and anti-Mi-2 autoantibodies exist in the U.S., as they do elsewhere, and are related to UVR intensity (6). This is the first investigation to show evidence of a gender influence on UVR associations in autoimmune disorders.
There were certain limitations in the approaches we used that could have influenced our results. Because data to assess the population-based incidence or prevalence of DM and PM at each location are not available, we used information from referral centers that evaluate myositis patients in the region as a practical approach. It is likely that myositis patients seen at referral centers are not representative of the larger myositis population. While we are not aware of any systematic referral biases based on autoantibodies or clinical phenotype to these centers that could account for these findings, referral bias remains a possibility. Another limitation is that we had only state of residence at the time of myositis onset available to us for this study, and UVR intensities can vary considerably from one part of a state to another. Environmental exposures or factors other than UVR may also account for these findings. Moreover, we have no way to take individual-level exposure, or differences in UVR exposure at different locations over time, into account. Finally, the use of personal photoprotective measures, as well as occupations and avocations, can greatly influence an individual’s cumulative UVR exposure. Future investigations should address these issues and possible alternative explanations.
The significant associations of ethnic groups with the clinical and autoantibody phenotypes in IIM are also potentially revealing in terms of pathogenesis. While known risk factors for these phenotypes include selected polymorphisms in immune response genes, they do not fully account for these differences. Of course, the increased skin pigmentation in African Americans could alter their circulating levels of vitamin D and also reduce the damaging effects of UVR, thus limiting UVR-induced alterations that may lead to DM and anti-Mi-2 autoantibodies (11). It is not known why African Americans are more likely to produce anti-SRP autoantibodies, but since these do not seem to be influenced by UVR, mechanisms other than skin pigmentation are likely responsible.
The reasons for the female gender effects seen in this investigation remain unclear, although some studies have suggested differential impacts of UVR in males and females. It is known that 17β-estradiol prevents UVB-induced suppression of the contact hypersensitivity response caused by immunosuppressive cytokines produced by keratinocytes in mice (12). Also, men were immunosuppressed by solar-simulated UVR doses three times lower than those required to immunosuppress women (13). Since these studies imply a greater impact of UVR on immune effects in men than women, other mechanisms would need to be operative to potentially explain how UVR preferentially results in the development of DM and anti-Mi-2 autoantibodies in women.
How UVR might result in DM or anti-Mi-2 autoantibodies is also unknown, however, many potential mechanisms are suggested from the published UVR effects on immunosuppression and promotion of autoimmunity (14). UVR induces a cascade of events involving type I interferon signatures, which have been associated with DM. Additionally, there is a wide range of effects of UVR involving many other immune networks involved in the pathogenesis of myositis, including alterations in stress proteins, IL-1, IL-6, TNFα, nuclear factor (NF)-kappaB and adhesion molecule pathways (15). Another recent finding is the rapid upregulation of Mi-2 antigen following UVR, which is mediated via more efficiently translated messages and increased stability of the protein (4). Given these data, it is tempting to speculate that the development of DM and DM-specific anti-Mi-2 autoantibodies, which are associated with certain major histocompatibility loci, are related to UV-induced increased expression of target autoantigens, combined with altered immune responses, in genetically susceptible individuals. Alternatively, our data are consistent with the possibility that UVR exposure, or a correlate of UVR exposure, is protective for the development of PM. Understanding these mechanisms more fully, as well as the gender and ethnic influences on them, should allow for insights into the pathogenesis of myositis and could have implications for deciphering the causes of other autoimmune diseases.
Acknowledgments
The authors thank Dr. Paul Plotz for clinical assistance and useful discussions, Dr. Kakali Sarkar for help in the preparation of the manuscript and Drs. Kathy Coyle and Mark Gourley for critical comments after reviewing the manuscript. This work was supported in part by the Intramural Research programs of the National Institute of Environmental Health Sciences, NIH.
Reference List
- 1.Gourley M, Miller FW. Mechanisms of disease: Environmental factors in the pathogenesis of rheumatic disease. Nat Clin Pract Rheumatol. 2007;3(3):172–80. doi: 10.1038/ncprheum0435. [DOI] [PubMed] [Google Scholar]
- 2.Gallagher RP, Lee TK. Adverse effects of ultraviolet radiation: a brief review. Prog Biophys Mol Biol. 2006;92(1):119–31. doi: 10.1016/j.pbiomolbio.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Dourmishev L, Meffert H, Piazena H. Dermatomyositis: comparative studies of cutaneous photosensitivity in lupus erythematosus and normal subjects. Photodermatology, Photoimmunology and Photomedicine. 2004;20(5):230–4. doi: 10.1111/j.1600-0781.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- 4.Burd CJ, Kinyamu HK, Miller FW, Archer TK. UV radiation regulates Mi-2 through protein translation and stability. J Biol Chem. 2008;283(50):34976–82. doi: 10.1074/jbc.M805383200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller FW. Inflammatory Myopathies: Polymyositis, dermatomyositis, and related conditions. In: Koopman W, Moreland L, editors. Arthritis and Allied Conditions, A Textbook of Rheumatology. 15. Philadelphia: Lippincott, Williams and Wilkins; 2005. pp. 1593–620. [Google Scholar]
- 6.Okada S, Weatherhead E, Targoff IN, Wesley R, Miller FW. Global surface ultraviolet radiation intensity may modulate the clinical and immunologic expression of autoimmune muscle disease. Arthritis Rheum. 2003;48(8):2285–93. doi: 10.1002/art.11090. [DOI] [PubMed] [Google Scholar]
- 7.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23(5):581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 8.Tadokoro T, Yamaguchi Y, Batzer J, Coelho SG, Zmudzka BZ, Miller SA, et al. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J Invest Dermatol. 2005;124(6):1326–32. doi: 10.1111/j.0022-202X.2005.23760.x. [DOI] [PubMed] [Google Scholar]
- 9.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley and Sons; 1989. [Google Scholar]
- 10.Chinoy H, Fertig N, Oddis CV, Ollier WE, Cooper RG. The diagnostic utility of myositis autoantibody testing for predicting the risk of cancer-associated myositis. Ann Rheum Dis. 2007;66(10):1345–9. doi: 10.1136/ard.2006.068502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi Y, Takahashi K, Zmudzka BZ, Kornhauser A, Miller SA, Tadokoro T, et al. Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006;20(9):1486–8. doi: 10.1096/fj.06-5725fje. [DOI] [PubMed] [Google Scholar]
- 12.Hiramoto K, Tanaka H, Yanagihara N, Sato EF, Inoue M. Effect of 17beta-estradiol on immunosuppression induced by ultraviolet B irradiation. Arch Dermatol Res. 2004;295(8–9):307–11. doi: 10.1007/s00403-003-0437-0. [DOI] [PubMed] [Google Scholar]
- 13.Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol. 2008;128(2):447–54. doi: 10.1038/sj.jid.5701058. [DOI] [PubMed] [Google Scholar]
- 14.Murphy GM. Ultraviolet radiation and its effects on the immune system. Clin Exp Dermatol. 2000;25(2):162–3. doi: 10.1046/j.1365-2230.2000.0604k.x. [DOI] [PubMed] [Google Scholar]
- 15.Grundtman C, Lundberg IE. Pathogenesis of idiopathic inflammatory myopathies. Curr Rheumatol Rep. 2006;8(3):188–95. doi: 10.1007/s11926-996-0024-4. [DOI] [PubMed] [Google Scholar]