Abstract
Background
Participants who are instructed to use reappraisal to downregulate negative emotion show decreased amygdala responses and increased prefrontal responses. However, it is not known whether individual differences in the tendency to use reappraisal manifests in similar neural responses when individuals are spontaneously confronted with negative situations. Such spontaneous emotion regulation might play an important role in normal and pathological responses to the emotional challenges of everyday life.
Methods
Fifty-six healthy women completed a blood oxygenation-level dependent functional magnetic resonance imaging challenge paradigm involving the perceptual processing of emotionally negative facial expressions. Participants also completed measures of typical emotion regulation use, trait anxiety, and neuroticism.
Results
Greater use of reappraisal in everyday life was related to decreased amygdala activity and increased prefrontal and parietal activity during the processing of negative emotional facial expressions. These associations were not attributable to variation in trait anxiety, neuroticism, or the use of another common form of emotion regulation, namely suppression.
Conclusions
These findings suggest that, like instructed reappraisal, individual differences in reappraisal use are associated with decreased activation in ventral emotion generative regions and increased activation in prefrontal control regions in response to negative stimuli. Such individual differences in emotion regulation might predict successful coping with emotional challenges as well as the onset of affective disorders.
Keywords: Amygdala, cognitive control, emotion, fMRI, regulation, reappraisal
Emotions play a crucial role in adaptation (1), shaping a wide range of cognitive (2) and behavioral (3) responses. However, even as emotions influence how we respond to adaptive challenges, they are themselves subject to regulation. The past decade has seen a dramatic increase in research on emotion regulation (4), and it is now clear that emotion regulation influences emotional experience, peripheral physiology, and neural dynamics (5–7) and impacts a wide range of physical (8) and mental health (9) outcomes. One type of emotion regulation that has been a particular research focus is “reappraisal,” which involves altering the meaning of a potentially emotion-eliciting situation in order to change its emotional impact (6).
Instructed Reappraisal
To examine the effects of reappraisal, one common approach has been to instruct participants to use reappraisal to downregulate responses to emotion-eliciting stimuli such as films (10) or slides (11–15). Behaviorally, instructed reappraisal decreases emotion experience (12,13). Neurally, instructed reappraisal decreases activation in emotion-generative brain regions such as the amygdala (11–15) and increases activation in a distributed network of brain regions associated with verbal processing, attention, and cognitive control such as the anterior cingulate cortex (ACC), ventral lateral prefrontal cortex (vlPFC), dorsal lateral prefrontal cortex (dlPFC), medial prefrontal cortex (mPFC), and orbitofrontal cortex (OFC) (see [7] for review). Functional connectivity analyses suggest that activity in prefrontal regions is inversely related to amygdala activation (10,16).
Spontaneous Reappraisal
Although reappraisal is occasionally triggered by explicit instructions in everyday life (such as when a parent coaches a child about how to think about an upcoming exam), it more commonly arises spontaneously, without explicit instructions from another person. Studies of spontaneous (automatic) emotion regulation have typically adopted an individual-difference strategy (17,18), considering individual differences in the use of regulation strategies such as reappraisal, as indexed by the Emotion Regulation Questionnaire (ERQ) (18). This measure has been widely used across a large number of cultures to examine emotion regulation in everyday life (19). Such studies have found that individuals who use reappraisal more frequently have lower levels of negative affect, greater interpersonal functioning, and greater psychological and physical well-being (18). These studies suggest but do not directly demonstrate that spontaneous use of reappraisal engages the same neural systems revealed by studies of instructed regulation.
Three studies have provided initial evidence that spontaneous emotion regulation engages the same neural systems as instructed regulation. Jackson et al. (20) found that individual differences in left-sided frontal electroencephalogram activation negatively correlated with startle magnitude, suggesting that individuals automatically engaged left-sided PFC regions to decrease emotional reactivity. Haas et al. (21) found that individual differences in agreeableness predicted activation in lateral PFC in response to threatening faces, suggesting that agreeable individuals might be more likely than less agreeable individuals to engage in self-regulation. Abler et al. (22) found that depressed individuals who frequently use reappraisal in everyday life showed decreased amygdala responses during the anticipation of emotion.
These findings are suggestive, but these studies have key limitations. First, these studies did not consider individual differences in emotional reactivity. This is an important limitation, because differences in reactivity (as indexed by neuroticism and trait anxiety) have been shown to predict greater amygdala reactivity (23–26). Second, these studies did not examine both emotion-generative and cognitive-control brain regions. Third, the first two studies did not assess individual differences in the tendency to use different regulation strategies and instead indirectly inferred regulation from other types of individual differences. Only the third study (22) directly assessed individual differences in reappraisal. However, the sample was small (n = 12), and the role of cortical control regions was not reported. Furthermore, the sample contained only depressed individuals, who might not exhibit typical neural correlates of reappraisal (27), and examined anticipation rather than emotion processing per se.
The Present Study
The goal of the present functional magnetic resonance imaging (fMRI) study was to examine the relationship between individual differences in self-reported use of reappraisal and the spontaneous response of the amygdala and control-related PFC regions during the perceptual processing of negative emotional facial expressions. We employed a large healthy sample to avoid confounds with mental illness. We limited analyses to female participants to maximize homogeneity of emotional responses (28,29). We used a well-characterized perceptual face processing task (30,31) to ensure robust activation of the amygdala and interconnected PFC regions. We assessed typical reappraisal use by administering the ERQ, which assesses individual differences in everyday use of reappraisal. We controlled for individual differences in emotion reactivity by assessing neuroticism and trait anxiety and controlled for the use of another common strategy to downregulate emotion by assessing suppression. We hypothesized that participants with higher reported use of reappraisal would be more likely to engage in spontaneous reappraisal and would thus show: 1) decreased amygdala activity, and 2) increased activity in control-related PFC regions. We also hypothesized that these effects would not be due to individual differences in reactivity or the use of suppression.
Methods and Materials
Participants
Fifty-six right-handed female volunteers (mean age 44.0 ± 6.7 years) participated after providing informed consent according to the guidelines of the University of Pittsburgh Institutional Review Board. All participants were recruited from a larger parent study, the AHAB (Adult Health and Behavior) project, which assesses behavioral and biological traits among a community sample of nonpatient, middle-age volunteers. Participants were in good general health and did not exhibit conditions affecting cerebral blood flow and metabolism or current Axis I disorders as diagnosed by the Structured Clinical Interview for DSM-IV.
Experimental Task
The experimental fMRI paradigm (30,31) consisted of four blocks of a perceptual face processing task interleaved with five blocks of a sensorimotor control task. During the face processing blocks, subjects viewed a trio of faces of the same gender, all of which expressed either anger or fear. Subjects selected one of two different faces (presented on the bottom half of the screen) that matched an identical target face (presented on the top half of the screen). Each face processing block consisted of six face trios balanced for gender and emotion (angry or fearful), all derived from a standard set of pictures of facial affect (32). During the sensorimotor control blocks, subjects viewed a trio of simple geometric shapes (circles, vertical, and horizontal ellipses) and selected one of two shapes (bottom) as matching an identical target shape (top). Each image (faces or shapes) was presented for 4 sec with a variable interstimulus interval (2–6 sec, mean 4 sec), resulting in a total of 9 blocks lasting 48 sec each. Because we were interested in eliciting a maximal amygdala response across all subjects that we could then interrogate for relationships with reappraisal, we chose to not use neutral faces as control stimuli, because neutral faces can be subjectively experienced as affectively laden or ambiguous and thus engage the amygdala (33,34).
Individual Difference Measures
Individual difference measures were administered before fMRI scanning. The primary measure of interest was the reappraisal scale of the ERQ (18). This scale consists of six items designed to assess individual differences in reappraisal use (e.g., “I control my emotions by changing the way I think about the situation I’m in”). This scale previously has been shown to have good internal consistency and test-retest reliability and to be independent from intelligence (18). To ensure independence of ERQ reappraisal from both intelligence and socioeconomic status in our sample, we obtained two markers of intelligence (highest level of education and IQ score from the Wechsler Adult Intelligence Scale) as well as two markers of socioeconomic status (median household income from the 1999 census and current self-reported family income). Consistent with prior reports, ERQ reappraisal was not correlated with any of these indices (all p values >.38). In addition, we administered control measures, including: 1) the neuroticism scale (N) of the Neuroticism Extroversion Openness Personality Inventory—Revised (NEO-PIR) (35), which assesses an individual’s tendency to experience psychological distress; 2) the trait version of the State Trait Anxiety Inventory (STAI trait version) (36), which assesses relatively stable individual differences in anxiety proneness; and 3) the suppression scale of the ERQ, which assesses use of expressive suppression in everyday life.
Image Acquisition
Each subject was scanned with a Siemens 3T Allegra scanner. Blood oxygenation-level dependent (BOLD) functional images were acquired with a gradient echo planar imaging sequence and covered 34 axial slices (3-mm thick) beginning at the cerebral vertex and encompassing the entire cerebrum and the majority of the cerebellum (repetition time/echo time = 2000/25 msec, FOV = 20 cm, matrix = 64 × 64). Before the collection of fMRI data for each subject a reference echo planar imaging scan was acquired and visually inspected for artifacts (e.g., ghosting) as well as good signal across the entire volume of acquisition, including the medial temporal lobes and ventromedial PFC. Data from all 56 participants were free from artifacts and exhibited good signal in our regions of interest.
Functional MRI Statistical Analysis
Whole brain image analysis was completed with SPM2 (http://www.fil.ion.ucl.ac.uk/spm). Images for each subject were realigned to the first volume in the time series to correct for head motion, spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) with a 12 parameter affine model, and smoothed with a Gaussian filter, set at 6 mm full-width at half-maximum. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean. Condition effects at each voxel were calculated with a t statistic, and regionally specific effects were compared with linear contrasts.
We conducted group level, random-effects analyses to investigate the relationship between individual differences in reappraisal and brain activation during face processing. The ERQ reappraisal scores were entered into a correlation analysis (simple regression) examining the contrast between face processing and sensorimotor control blocks. Unless indicated otherwise, whole brain regression analyses employed a significance threshold of p < .001 (uncorrected) with a five voxel extent threshold (37).
Given our a priori hypotheses regarding the relationship between reappraisal scores and amygdala activation, an anatomical mask of the amygdala (dilated by a factor of 1) (38) was applied bilaterally. A significance threshold of p <.01 (uncorrected) with a five voxel spatial extent threshold was applied in this a priori region of interest. We then defined a functional region of interest (ROI) within this anatomical ROI, which encompassed the amygdala voxels above our significance threshold at the group level.
The average contrast values (faces > shapes) for the amygdala voxels in the functional ROI were extracted for each individual with MarsBaR (39). Average values were also extracted from functional clusters in prefrontal and parietal regions shown to be positively correlated with reappraisal. Within each region, contrast values > ± 2.5 SDs from the mean were excluded from subsequent analyses. To determine the correct voxel-level threshold and cluster extent for amygdala activation, AlphaSim, a Monte Carlo simulation bootstrapping program in the Analysis of Functional NeuroImage (AFNI) library (Medical College of Wisconsin), was employed to identify a joint probability consisting of a voxel-wise threshold and a minimum cluster volume threshold to establish a cluster-wise p value that protects against false-positives. At a voxel-wise threshold of p <.01, AlphaSim determined that a cluster size of 12 voxels is required to produce an overall p <.05.
Selective between-subjects functional connectivity analyses were conducted with a simple regression model in SPSS. Average contrast values were extracted from the amygdala functional ROI in the procedure described in the preceding text. These values were correlated with average extracted values from the prefrontal and parietal regions identified in the group level whole-brain analyses as significantly positively correlated with reappraisal.
A final set of regression analyses were conducted to assess whether self-reported reappraisal use predicted brain activation over and above emotional reactivity (neuroticism and anxiety) and another form of regulation (suppression).
Results
We hypothesized that self-reported reappraisal use would be negatively correlated with activity in the amygdala and positively correlated with activity in control-related prefrontal regions. We did not expect that these associations would be attributable to individual differences in emotional reactivity or in the use of another common regulation strategy, namely suppression.
Main Effect of Task
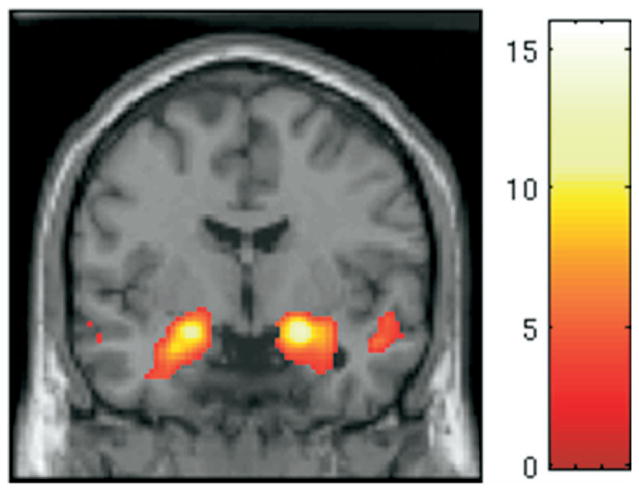
As expected, we replicated previous findings, showing that this task robustly activates the amygdala. For the contrast faces > shapes, bilateral amygdala activation can be seen in Figure 1.
Figure 1.
Main effect of task: faces > shapes. From the one-sample t test across all 56 subjects for the contrast faces > shapes. The display threshold was p <.001 with an extent of five voxels. Peak of activation was centered at (x = 22, y = −4, z = −14) in the right amygdala and (x = −20, y = −6, z = −14) in the left amygdala.
BOLD Responses in Emotion-Generative Regions
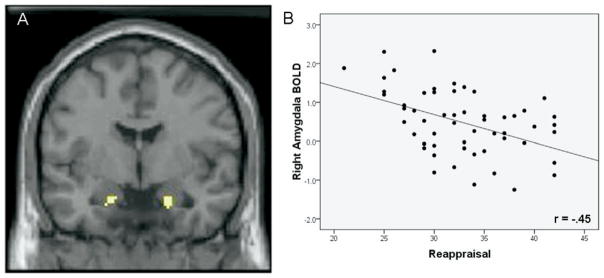
As hypothesized, we observed a negative correlation between self-reported reappraisal use and BOLD responses within the right (T = 2.40, p <.01, uncorrected) and left (T = 2.40, p <.01, uncorrected) anatomical amygdalae. Follow-up analyses confirmed that individuals who reported using reappraisal more frequently in everyday life showed lesser activation in both the right (r = −45, p <.001) and left (r =−.37, p <.006) amygdala during face processing. Activation extent and scatterplot for this negative correlation is reported in Figure 2.
Figure 2.
Negative correlation between reappraisal and amygdala activation. (A) From the simple regression (correlation) between Emotion Regulation Questionnaire (ERQ) reappraisal and the contrast faces > shapes. The display threshold was p <.01 with an extent of five voxels. Peak of activation was centered at (x = 20, y = −3, z = −20; 32 voxels; mean contrast value =.49, SEM =.11) in the right amygdala and (x = −18, y = −5, z = −20; 12 voxels; mean contrast value =.41, SEM =.10) in the left amygdala. (B) Scatter plot of ERQ reappraisal and average contrast values extracted from voxels identified in panel A. BOLD, blood oxygenation-level dependent.
BOLD Responses in Control-Related Prefrontal Regions
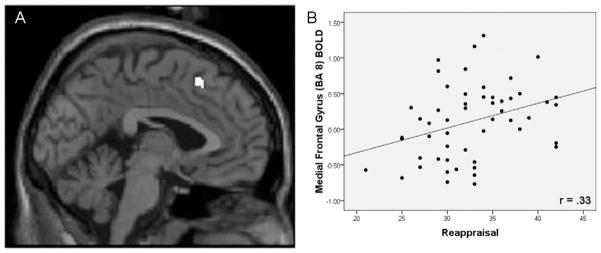
As hypothesized, we observed a positive correlation between self-reported reappraisal use and BOLD responses in a subset of regions previously identified in studies of instructed reappraisal, including dorsal medial prefrontal cortex (dmPFC) (Brodmann area [BA] 8; r =.33, p =.016), lateral OFC (BA 10; r =.42, p =.002), and dlPFC (BA 9; r =.39, p =.004), with a whole-brain approach. Selected activation extent and scatterplot for dmPFC is reported in Figure 3, and all activations are reported in Table 1. In addition to these control-related PFC effects, whole-brain analyses also revealed a positive correlation between reappraisal use and BOLD responses in both inferior (BA 40; r =.34, p <.012) and superior parietal lobule (BA 7; r =.31, p =.025).
Figure 3.
Positive correlation between reappraisal and prefrontal activations. (A) From the simple regression (correlation) between Emotion Regulation Questionnaire (ERQ) reappraisal score and the contrast faces > shapes. The display threshold was p <.001 with an extent of five voxels. (A) Activation in the medial frontal gyrus (Brodmann area [BA] 8; x = −2, y = −22, z = −47; 20 voxels) identified within the whole brain analysis (other activations reported in Table 1). (B) Scatter plot of ERQ reappraisal score and average contrast values extracted from voxels identified in panel A. BOLD, blood oxygenation-level dependent.
Table 1.
Regions Showing a Positive Correlation with Reappraisal
| Region | BA | Cluster Size | T | z | x | y | z | r | p |
|---|---|---|---|---|---|---|---|---|---|
| Inferior Parietal Lobule | BA 40 | 18 | 3.79 | 3.55 | 50 | −45 | 41 | .34 | .012 |
| Medial Frontal Gyrus (Dorsal Medial PFC) | BA 8 | 20 | 3.75 | 3.52 | −2 | 22 | 47 | .33 | .016 |
| Middle Frontal Gyrus (Lateral OFC) | BA 10 | 7 | 3.72 | 3.49 | 40 | 52 | −6 | .42 | .002 |
| Middle Frontal Gyrus (Dorsal Lateral PFC) | BA 9 | 16 | 3.56 | 3.36 | −36 | 15 | 38 | .39 | .004 |
| Superior Parietal Lobule | BA 7 | 9 | 3.43 | 3.25 | −38 | −62 | 51 | .31 | .025 |
Activations for the contrast faces > shapes for whole brain analysis. The display threshold was p <.001 with an extent threshold of five voxels. Coordinates are listed in Talairach space. The r and p values represent correlation between extracted values and reappraisal. BA, Brodmann area; PFC, prefrontal cortex; OFC, orbitofrontal cortex.
Between-Subjects Functional Connectivity Between Amygdala and Prefrontal Regions
Correlational functional connectivity analyses revealed that activation in the right amygdala was negatively correlated with dmPFC (BA 8; r =−.28, p =.041) and lateral OFC (BA 10; r =−.27, p =.045). Activation in the left amygdala was negatively correlated with dlPFC (BA 9; r =−.25, p =.049).
Specificity of Findings
Are Findings Independent of Emotional Reactivity?
To address this question, we used a stepwise linear regression to test 1) whether N and STAIi scores were related to BOLD responses in brain regions associated with reappraisal (left amygdala, right amygdala, BA 7, BA 8, BA 9, BA 10, BA 40), and 2) whether the relationship between reappraisal and these regions withstood correction for N and STAI. In the right amygdala, N predicted neural response at a trend level (r =.23, p =.083). The addition of STAI did not change the model (p =.683). However, the addition of ERQ reappraisal did change the model (r =.46, p =.002). In BA 40, N predicted neural response (r =−.27, p =.046). The addition of STAI did not change the model (p =.263). However, the addition of ERQ reappraisal scores did change the model (r =.49, p =.003). For all other regions, neither N (all p >.19) nor the addition of STAI (all p >.13) predicted neural response, and the addition of ERQ reappraisal scores significantly increased the proportion of the variance explained by the model (all p <.005). These findings indicate that our primary results were not due to individual differences in emotional reactivity.
Are Findings Specific to Reappraisal?
To address this question, we examined whether the use of suppression was associated with BOLD responses in any of the regions that significantly correlated with reappraisal (left amygdala, right amygdala, BA 7, BA 8, BA 9, BA 10, BA 40). With one exception, no significant relationships emerged (all p >.18). In one area, BA 8, there was a significant relationship with suppression (r =−.27 p =.047). It bears noting that this relationship is in the opposite direction to that of reappraisal, and we found that the relationship between BA 8 and reappraisal remained significant even when accounting for suppression use (r2 change =.256, p <.001).
Discussion
A growing literature demonstrates that instructed reappraisal is associated with decreased activity in the amygdala and increased activity in a prefrontal cognitive control network. In the current study, we examined whether individual differences in everyday reappraisal use would predict activity in the same neural systems identified in studies of instructed regulation. Our findings revealed that self-reported reappraisal use predicted lesser amygdala activation and greater activity in prefrontal and parietal regions, and activity in these regions was inversely correlated across subjects. Importantly, these effects withstood controls for emotional reactivity (as assessed by neuroticism and trait anxiety) and for another common form of emotion regulation (suppression).
Amygdala Activation and Individual Differences in Reappraisal Use
Studies of instructed reappraisal have shown reliable down-regulation of amygdala activation in response to negative stimuli (11–15). In the current study, we found that greater everyday reappraisal use also predicted decreased amygdala activation. Given that amygdala responses are one important central index of emotional responding, as demonstrated by the amygdala’s role in animal and human fear conditioning and coordinating downstream fear responses (see [40] for review), these results suggest an important role for individual differences in reappraisal in underlying emotional behavior.
Control-Related Activations in Instructed Versus Spontaneous Emotion Regulation
We found that, consistent with studies of instructed reappraisal, greater everyday reappraisal use predicted increased activation in regions associated with deploying a cognitive strategy (dlPFC) (41), appraising emotional states of self and others (dmPFC) (42), evaluating emotional value on the basis of context (lateral OFC) (43), and directing spatial attention (parietal cortex) (44).
Given that the regions of neural activation observed here closely resemble those found in instructed reappraisal (see [7] for a review) and that these activations are specifically correlated with self-reported reappraisal use, one interpretation is that these activations result from spontaneous use of reappraisal during the experimental task. It bears noting, however, that whereas the current study reveals activation in many of the same regions previously associated with instructed reappraisal, the spatial extents of these activations are dramatically smaller than the typical activations seen in studies of instructed reappraisal, particularly in dlPFC. Why might this be?
One possibility is that, although initial use of reappraisal requires some effort, with frequent use it might become less effortful and thus require fewer cortical resources to achieve the same reduction in amygdala activation. This idea is consistent with research showing that frequent use of a working memory strategy results in decreased activation in dlPFC during subsequent memory trials. This decrease in neural activation in dlPFC is accompanied by an increase in working memory performance (45), suggesting that frequent use of the strategy results in greater neural efficiency. In the current study, it is possible that the reduced activation seen in the cognitive control network indicates that spontaneous reappraisal is more automatic and neurally efficient.
Methodological Implications
The current findings have important implications for the interpretation of results from simple tasks thought to index “pure” emotional reactivity. Our task robustly engages the amygdala and is therefore thought to be an excellent probe of the neural basis of emotional reactivity. Indeed, the task employed here has become a staple in affective neuroimaging in recent years as an assay of emotional reactivity in a wide array of populations. However, the current findings suggest that even “pure” reactivity tasks such as the one used here might actually be intertwined with emotion regulation, which is dissociable from emotional reactivity to the stimuli. This should be taken into account when interpreting findings from these types of tasks.
For example, several researchers have used this task to examine the differences in a putative emotion regulation circuit between amygdala and PFC on the basis of genetic background (e.g., [46]), personality (47), and clinical diagnosis (48). Findings from these studies have been interpreted as between-group differences in emotion regulation ability, given the association between amygdala and PFC regions in studies of instructed regulation. The present results buttress such accounts and provide the first empirical evidence for the claim that individual differences in emotion regulation might mediate the decreases in amygdala activity and increases in PFC activity observed during this task.
Clinical Implications
There is an increasing appreciation that emotion dysregulation underlies a variety of psychiatric illnesses, including mood and anxiety disorders (49). Empirical studies that aim to characterize mood and anxiety disorders have begun to focus on how individual differences function as risk factors for such disorders. Decreased use of reappraisal in particular, as indexed by the ERQ, is associated with increased depressive symptoms (18). In addition, neuroimaging research has shown that increased amygdala activation in response to negative stimuli is associated with depression (50). Our results represent a crucial link between increased amygdala activity and the decreased use of reappraisal during basic emotion processing. The present study, therefore, offers a potential neural mechanism by which individual differences in emotion regulation result in greater neural responses to emotional stimuli, which might characterize both risk for (30) and pathophysiology of (51) depression and related affective disorders.
One of the most widely used treatment interventions for mood and anxiety disorders is cognitive behavioral therapy (CBT) (52,53). One feature of CBT is that relatively brief interventions (e.g., 10 weeks) can have long-lasting effects (54). A key element to understanding the effects of CBT involves characterizing the transition from therapist-guided reinterpretations of negative cognitions to automatic reinterpretations of negative cognitions. Given the relationship between instructed reappraisal and the therapist-guided reinterpretations that are part of CBT, the present finding highlights a potential mechanism by which the reframing taught in CBT becomes more self-generated and automatic. Future work should track the neural changes associated with reappraisal use in relation to progress made over the course of CBT.
Limitations and Future Directions
The present study has several important limitations. First, only healthy female participants were studied, and it is therefore unknown whether our results are generalizable to clinical samples or to men. Because gender differences are often observed in studies of emotional reactivity (28,29) and sometimes observed in studies of emotion regulation (55), it will be important to examine the neural correlates of emotion regulation use in men as well as women. Given the relationship between emotion dysregulation and mood and anxiety disorders, studying emotion regulation in various clinical populations is necessary to improve our understanding of emotion regulation deficits and offer insight into treatment implications.
Second, we examined only one task context—namely, simple perceptual face processing. This task robustly activates corticolimbic circuitry, providing a strong target for downregulation without specific instructions to regulate, and thus was an ideal choice for this investigation of the relationship between everyday reappraisal use and spontaneous neural responses to negative emotions. However, it is important to go beyond basic processing to better characterize the interplay between emotion generative and emotion regulatory brain systems across different contexts. Subsequent studies could examine emotion regulation in relation to positive as well as negative faces, subliminal face presentation, or a direct comparison of instructed verses uninstructed contexts.
Third, reappraisal was assessed with one self-report measure—namely the ERQ. Previous research has shown that the ERQ predicts important real-world outcomes such as well-being and depressive symptomatology (18), and there is ample precedent for using single self-report measures to examine relations between individual differences in emotional reactivity and brain activity (23–26). In future work, however, it will be useful to also employ non–self-report measures of emotion regulation when examining brain responses to look for convergence across diverse measures.
Fourth, we did not directly assess which emotion regulation strategies participants were using, if any, during the task. We felt it was important to avoid calling attention to any potential regulation processes by having participants indicate which strategy they were using. However, future studies might ask individuals to retrospectively report what strategies (if any) they used to impact their emotional responses to these faces. This might further clarify the differences in task processing associated with self-reported reappraisal use. Other types of processing could result in similar activation patterns, such as distraction (56), decreasing attention to the affectively salient features of the stimuli (15), or simply labeling the affective expressions depicted (57–60). However, it is noteworthy that in the present study these effects were not explained by individual differences in suppression.
Acknowledgments
This work was supported by National Institutes of Health Grants HL040962 to SBM and MH072837 to ARH as well as a National Alliance for Research on Schizophrenia And Depression Young Investigator Award to ARH. We thank Sarah M. Brown for assistance with fMRI and ERQ data collection and fMRI analyses.
Footnotes
The authors reported no biomedical financial interests or potential conflicts of interest.
We also tested a model in which STAI was entered as the first step and N was entered as the second step. Only BA 8 (r = −.27, p =.047) and BA 40 (r =−.29, p =.029) showed significant relationship with STAI. The addition of N to the model did not significantly change the model’s explanatory power (p =.94; p =.49). However, the addition of ERQ reappraisal scores to the model significantly increased the proportion of variance explained by the model (r =.50, p =.001; r =.49; p =.003). In every other case, coding STAI as the first step and N as second steps provided less explanatory power when compared with coding N as the first step and STAI as the second step (all p values > .18).
References
- 1.Lazarus RS. Cognition and motivation in emotion. Am Psychol. 1991;46:352–367. doi: 10.1037//0003-066x.46.4.352. [DOI] [PubMed] [Google Scholar]
- 2.Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 3.Baumeister RF, Vohs KD, DeWall CN, Zhang L. How emotion shapes behavior: Feedback, anticipation, and reflection, rather than direct causation. Pers Soc Psychol Rev. 2007;11:167–203. doi: 10.1177/1088868307301033. [DOI] [PubMed] [Google Scholar]
- 4.Gross JJ. Handbook of Emotion Regulation. New York: Guilford Press; 2007. [Google Scholar]
- 5.Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- 6.Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- 7.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Kubzansky LD, Thurston RC. Emotional vitality and incident coronary heart disease: Benefits of healthy psychological functioning. Arch Gen Psychiatry. 2007;64:1393–1401. doi: 10.1001/archpsyc.64.12.1393. [DOI] [PubMed] [Google Scholar]
- 9.Campbell-Sills L, Barlow DH. Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. In: Gross J, editor. Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 542–559. [Google Scholar]
- 10.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J Cogn Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- 13.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 15.van Reekum CM, Johnstone T, Urry HL, Thurow ME, Schaefer HS, Alexander AL, et al. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage. 2007;36:1041–1055. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 16.Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John OP, Gross JJ. Individual differences in emotion regulation strategies: Links to global trait, dynamic, and social cognitive constructs. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 351–372. [Google Scholar]
- 18.Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto D, Yoo SH, Nakagawa S. Culture, emotion regulation, and adjustment. J Pers Soc Psychol. 2008;94:925–937. doi: 10.1037/0022-3514.94.6.925. [DOI] [PubMed] [Google Scholar]
- 20.Jackson DC, Mueller CJ, Dolski I, Dalton KM, Nitschke JB, Urry HL, et al. Now you feel it, now you don‘t: Frontal brain electrical asymmetry and individual differences in emotion regulation. Psychol Sci. 2003;14:612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- 21.Haas BW, Omura K, Constable RT, Canli T. Is automatic emotion regulation associated with agreeableness? A perspective using a social neuroscience approach. Psychol Sci. 2007;18:130–132. doi: 10.1111/j.1467-9280.2007.01861.x. [DOI] [PubMed] [Google Scholar]
- 22.Abler B, Erk S, Herwig U, Walter H. Anticipation of aversive stimuli activates extended amygdala in unipolar depression. J Psychiatr Res. 2007;41:511–522. doi: 10.1016/j.jpsychires.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 24.Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Canli T. Functional brain mapping of extraversion and neuroticism: learning from individual differences in emotion processing. J Pers. 2004;72:1105–1132. doi: 10.1111/j.1467-6494.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- 26.Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: Personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav Neurosci. 2007;121:249–256. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- 27.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1:300–319. [PubMed] [Google Scholar]
- 29.Fischer AH. Gender and emotion: Social psychological perspectives. New York: Cambridge University Press; 2000. [Google Scholar]
- 30.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 31.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: A comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 32.Ekman P, Friesen WV, Scherer KR. Body movement and voice pitch in deceptive interaction. Semiotica. 1976;16:23–27. [Google Scholar]
- 33.Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, et al. Differential amygdalar response to novel versus newly familiar neutral faces: A functional MRI probe developed for studying inhibited temperament. Biol Psychiatry. 2003;53:854–862. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- 34.Wright CI, Martis B, Schwartz CE, Shin LM, Fischer HH, McMullin K, et al. Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. Neuroimage. 2003;18:660–669. doi: 10.1016/s1053-8119(02)00037-x. [DOI] [PubMed] [Google Scholar]
- 35.Costa PT, Jr, McCrae RR. Normal personality assessment in clinical practice: The NEO Personality Inventory. Psychological Assessment. 1992;4:5–13. [Google Scholar]
- 36.Spielberger CD, Gorsuch RL, Lushene RE. The State-Trait Anxiety Inventory: Test Manual for Form X. Palo Alto, California: Consulting Psychologists Press; 1970. [Google Scholar]
- 37.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 38.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 39.Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox, 8th International Conference on Functional Mapping of the Human Brain. Vol. 16. Sendai, Japan: Neuroimage; 2002. [Google Scholar]
- 40.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 41.Lungu OV, Binenstock MM, Pline MA, Yeaton JR, Carey JR. Neural changes in control implementation of a continuous task. J Neurosci. 2007;27:3010–3016. doi: 10.1523/JNEUROSCI.5051-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, et al. Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- 43.Boettiger CA, D’Esposito M. Frontal networks for learning and executing arbitrary stimulus-response associations. J Neurosci. 2005;25:2723–2732. doi: 10.1523/JNEUROSCI.3697-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayer JS, Bittner RA, Nikolic D, Bledowski C, Goebel R, Linden DE. Common neural substrates for visual working memory and attention. Neuroimage. 2007;36:441–453. doi: 10.1016/j.neuroimage.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Koch K, Wagner G, von Consbruch K, Nenadic I, Schultz C, Ehle C, et al. Temporal changes in neural activation during practice of information retrieval from short-term memory: An fMRI study. Brain Res. 2006;1107:140–150. doi: 10.1016/j.brainres.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 47.Rubino V, Blasi G, Latorre V, Fazio L, d’Errico I, Mazzola V, et al. Activity in medial prefrontal cortex during cognitive evaluation of threatening stimuli as a function of personality style. Brain Res Bull. 2007;74:250–257. doi: 10.1016/j.brainresbull.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 48.Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, et al. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005;8:991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- 49.Taylor SF, Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends Cogn Sci. 2007;11:413–418. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Brody AL, Barsom MW, Bota RG, Saxena S. Prefrontal-subcortical and limbic circuit mediation of major depressive disorder. Semin Clin Neuropsychiatry. 2001;6:102–112. doi: 10.1053/scnp.2001.21837. [DOI] [PubMed] [Google Scholar]
- 51.Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 52.Beck AT, Rush S, Shaw P, Emery N. Cognitive Therapy and Depression. New York: Guilford Press; 1979. [Google Scholar]
- 53.Bedrosian RC, Beck AT. Principles of Cognitive therapy. New York: Plenum Press; 1980. [Google Scholar]
- 54.Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al. Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland. Health Technol Assess. 2005;9:1–174. doi: 10.3310/hta9420. [DOI] [PubMed] [Google Scholar]
- 55.McRae K, Ochsner KN, Mauss IB, Gabrieli JD, Gross J. Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Group Processes & Intergroup Relations. 2008;11:143–162. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erk S, Abler B, Walter H. Cognitive modulation of emotion anticipation. Eur J Neurosci. 2006;24:1227–1236. doi: 10.1111/j.1460-9568.2006.04976.x. [DOI] [PubMed] [Google Scholar]
- 57.Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- 58.Lieberman MD, Jarcho JM, Obayashi J. Attributional inference across cultures: Similar automatic attributions and different controlled corrections. Pers Soc Psychol Bull. 2005;31:889–901. doi: 10.1177/0146167204274094. [DOI] [PubMed] [Google Scholar]
- 59.Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: Affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 60.Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: Effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]