Abstract
Seeking to unite psychological and biological approaches, this paper links cognitive and cellular hypotheses and data about thought and language abnormalities in schizophrenia. The common thread, it is proposed, is a dysregulated suppression of associations (at the behavioral and functional neural systems level), paralleled by abnormalities of inhibition at the cellular and molecular level, and by an abnormal anatomical substrate (reduced MRI gray matter volume) in areas subserving language.
At the level of behavioral experiments and connectionist modeling, data suggest an abnormal semantic network connectivity (strength of associations) in schizophrenia, but not an abnormality of network size (number of associates). This connectivity abnormality is likely to be a preferential processing of the dominant (strongest) association, with the neglect of preceding contextual information.
At the level of functional neural systems, the N400 event-related potential amplitude is used to index the extent of “search” for a semantic match to a word. In a short stimulus-onset-asynchrony condition, both schizophrenic and schizotypal personality disorder subjects showed, compared with controls, a reduced N400 amplitude to the target words that were related to cues, e.g. cat-dog, a result compatible with behavioral data. Other N400 data strongly and directly suggest that schizophrenics do not efficiently utilize context.
At the level of anatomical system substrates, considerable MRI data indicate abnormalities in the temporal lobe structures that subserve language and verbal associations. Gray matter volume is reduced in the posterior portion of the dominant superior temporal gyrus in both chronic and first episode schizophrenics (but not in manic-depressive psychosis), with the magnitude of reduction correlating with the degree of thought disorder.
At the level of in vitro cellular and molecular analysis, NMDA receptors on inhibitory neurons are much more sensitive to blockade than are excitatory projections. A resulting failure of recurrent inhibition may account for the psychotomimetic effects of such NMDA receptor blockers as ketamine and phencyclidine, and may also be present in schizophrenia, where an endogenous NMDA receptor blocker, NAAG, is increased, and where other abnormalities of recurrent inhibition may be present. A biophysical simulation of this circuit abnormality in a model of learned pattern recognition produced, because of the reduction in recurrent inhibition, aberrant spread of excitation, resulting in confusion of normally distinguishable patterns. We suggest the neural circuit failure of inhibition and consequent aberrant spread of activation may be the substrate for an inability to use context, with the behavioral and functional consequences just described. Furthermore, there is the possibility that the unbalanced excitation might lead to progressive, neurodegenerative changes in gray matter, marked by progressive volume reduction.
Keywords: Language abnormalities, Schizophrenia, Cognitive dysfunction, Thought disorder, Neural circuits
Introduction
A major task in the field of schizophrenia is uniting the biology and psychology of this disorder (McCarley et al. 1993b). This paper focuses on linking cognitive and cellular hypotheses and data as related to thought and language abnormalities in schizophrenia. Definition of a fundamental abnormality on a clinical level as a problem in associations began with the seminal statement of Bleuler (1911/1950, p. 14), “Often ideas are only partially worked out, and fragments of ideas are connected in an illogical way to constitute a new idea… Thus, the process of association often works with mere fragments of ideas and concepts. This results in associations which normal individuals will regard as incorrect, bizarre, and utterly unpredictable… Instead of continuing the thought, new ideas crop up which neither the patient nor the observer can bring into any connection with the previous stream of thought…”
Earlier, Kraepelin (1899) had focused on the possible pathophysiologic and biological underpinnings of the disease, stating in his 1899 Textbook of Psychiatry, “…We thus come to the conclusion that, in dementia praecox, partial damage to, or destruction of, cells of the cerebral cortex must probably occur, which may be compensated for in some cases, but which mostly brings in its wake a singular, permanent impairment of the inner life (page 154)”.
In fact he postulated a tentative cerebral localization, suggesting that frontal lobe dysfunction might cause impairments of reason and volition, and temporal lobe dysfunction might cause hallucinations and delusions.
To understand better how the Bleulerian hypothesis of abnormal associations can be united with the Kraepelinan biological hypothesis about schizophrenia, our laboratory has used a multidimensional approach, looking at measures of symptoms and symptom clusters, of neurocognition, of brain function (high temporal resolution electroencephalography, EEG), of brain structure (high spatial resolution magnetic resonance imaging, MRI), and of in vitro cellular models of schizophrenic circuitry and pharmacology. Table 1 summarizes our model of the common thread weaving through these different levels of description and discussed in this paper: disturbed suppression of associations (at the behavioral and functional neural systems level), MRI gray matter abnormalities in areas subserving language (abnormal anatomical substrate) and abnormalities of inhibition at the cellular and molecular level. This paper follows the “top-down” ordering of topics in the table.
Table 1.
A multilevel view of disturbed associations in schizophrenia: Is there a common theme of failure of suppression/inhibition?
| Clinical level: |
| Presence of thought disorder. |
| Bleuler: “associations which normal individuals will regard as incorrect, bizarre, and utterly unpredictable”. |
| Inference: |
| Loose associations are one of the fundamental disturbances of schizophrenia. |
| Behavioral experimental level: |
| Abnormal connectivity in connectionist network model. |
| High connectivity dominates, context less important. |
| Inference: |
| Failure of suppression of dominant associations = ? failure of context utilization. |
| Functional neural systems level: |
| N400 studies of language and word pairs. |
| Reduced N400 amplitude for word pairs when second word is related to first (short inter-word intervals). |
| Increased N400 to congruent sentence endings. |
| Inferences: |
| Faulty search of lexicon, as indexed by N400. |
| More dominant associations prevail. Failure to use context. Failure to suppress dominant associations. |
| Anatomical systems level: |
| MRI studies: |
| Reduced MRI gray matter volume in language processing areas of the brain (including superior temporal gyrus/Wernicke’s area). |
| Volume reduction correlated with the degree of thought disorder. |
| Inference: |
| Abnormal anatomical substrate for language in schizophrenia. |
| Cellular and molecular level: |
| In vitro model: Exogenous NMDA receptor blockers (Psychotomimetics) – |
| Suppress recurrent inhibition more than feed-forward excitation. |
| Disturbance in a ‘realistic’ neuronal model leads to failure to suppress previously learned Hebbian associative patterns. |
| Post-mortem data: Endogenous NMDA receptor blockers (NAAG) and/or other abnormalities affecting inhibitory neurons may be present in schizophrenia. |
| Inferences: |
| Failure of inhibition at the cellular level. |
| Possible excitotoxic effects of resultant abnormal excitation, and progression of neural tissue damage. Developmental anomalies secondary to NMDA abnormalities. |
At the onset, we should emphasize that our proposal of linkages across different domains is a tentative model designed to provoke experiments that will be able to test it. We recognize very acutely that the concept of inhibition at the neural level has a different meaning than the concept of suppression (inhibition) at the cognitive level. In fact, we ourselves were initially quite skeptical of attempts to link the two. We became persuaded of the – at least heuristic – utility of pursuing connections only after there appeared to be convergence of top-down and bottom-up models generated from experimental data, a convergence we seek to illustrate in this paper.
A connectionist model of disturbed associations in schizophrenia: connectivity is abnormal but network size is not
We recently have described this model and experimental support for it (Nestor et al. 1998). The school of cognitive science known as connectionism (McClelland and Rummelhart 1987) offers a framework to examine the foregoing Bleulerian model of disturbed associations in schizophrenia. In all connectionist models of associative memory, words are represented as networks of interconnected nodes. Early so-called localist connectionist models assumed one-to-one representation between a word and a node (Nelson et al. 1993). By contrast, distributed models of representation are now viewed as more cognitively and biologically plausible: instead of one word being represented by one node, various aspects of words (e.g., orthographic, semantic, phonological) are each represented by corresponding sets of nodes. The key feature of nodes is that they are organized into local networks and activated in parallel such that a word activates or primes a local network of related associates (McClelland and Rummelhart 1987).
Parallel distributed processing (PDP) models of representation have several advantages for understanding schizophrenic cognition. First, they provide a possible link to the biology, since they assume words are organized and stored in a distributed mental lexicon whose location includes zones vulnerable to schizophrenia including the temporal, parietal and frontal lobes (as well as other neocortical regions). Second, in connectionist models of word recall, activation may be modulated by at least two distinct, empirically generated aspects of the to-be-remembered word: (1) number of associates of the word, referred to as network size, and (2) degree of associative strength of the word, referred to as connectivity. That is, English words vary systematically in the number of associates, with some words having relatively few associates and other words having a large number of associates. Likewise, words differ in associative strength, as reflected by the degree of connectivity of a word and its associates, and/or the degree of connectivity among its associates. Thus, connectionist models provide the tools to test whether schizophrenic associative disturbance reflects a failure of connectivity and/or network size to modulate activation within the lexicon (Nelson et al. 1992 and 1993). In so-called “PDP neural models”, the connectivity may be viewed as related to idealized synaptic weights and the network size to the number of idealized neurons activated. While we think the PDP neural models are highly over-simplified, and will be supplanted by more realistic neuronal models such as used in the last section of this paper, it may be a useful introduction for the reader to think in terms of this simple model. Connectivity and network size influence cued recall by governing both the spread and the strength of activation: In normal subjects a highly connected word of a small network is easier to recall than a lowly connected word of a large network because the former produces stronger, more efficient and economical activation.
Nestor et al. (1998) used a cued-recall word paradigm and norms derived from Nelson to attempt to identify the cognitive dynamics underlying schizophrenic associative disturbance. Schizophrenic patients and comparison subjects studied a list of to-be-remembered target words, and then were given a cued recall test in which both word targets and cues were equal in terms of connectivity and network size, as measured by the quantitative normative studies of Nelson et al. (1992, 1993). These studies used the number of associates of a word to determine its network size and the degree of association both among these associates and between the target word and its associates to determine its network connectivity.
In the Nestor et al. study, target words (and their cues) were grouped into four classes for the experiment, and the following examples of each class first list the target word (and then the cue word in parentheses and in italics).
high connectivity-small network: e.g., words such as wife (spouse), waves (surf), zoo (monkey). Each of these target words had a limited number of associates but these had a high probability of associations with each other and with the target word.
low connectivity-small network: e.g., clock (time), cap (shower), hive (bee). These target words had a limited number of associates, and the associates had a low probability of associations with each other and with the target word.
high connectivity-large network (e.g., bottle (cork), cloth (fabric), flute (clarinet)).
low connectivity-large network (e.g., party (birthday), blood (cut), rabbit (carrot)).
Schizophrenics showed an overall reduction in recall, but the pattern of the results pointed to a fairly selective connectivity abnormality, as contrasted to the absence of a network abnormality.
Schizophrenics and comparison subjects showed similar effects of network size, recalling more words of small networks than words of large networks. This suggests that, for both groups, the number of associates of a given word had a similar influence on recall, and that decay or loss of associates (i.e. a network size abnormality) did not occur in schizophrenia. In contrast, network connectivity differentially affected results in the comparison and schizophrenic groups. Patients with schizophrenia recalled more words of high connectivity-large network size than words of low connectivity-small network size, exactly the opposite pattern found in comparison subjects. In schizophrenics, unlike comparison subjects, recall improved substantially for words of high connectivity and declined dramatically for words of low connectivity (ANOVA showed a significant interaction between connectivity and group at the p < 0.02 level).
We interpret these findings as suggesting, at the behavioral and cognitive level, an overactivation of strongly connected networks and an underactivation of weakly connected networks in schizophrenic patients. In schizophrenia recall may thus be dominated by semantic network connectivity at the expense of other critical contextual factors. These findings also suggest that a key element of any network, whether it is cognitive or computational (and, even biological, as discussed below) is its ability to maintain stability and to control excitation. In the cognitive level model, a word produces various levels of excitatory buildup, as reflected by competition among simultaneously activated representations (note the similarity to the biological model illustrated in Fig. 4 in the last section of this paper). Schizophrenia may produce a dysregulation of network excitation because of failure of suppression (inhibition) (see, for example, Anderson and Spellman 1995).
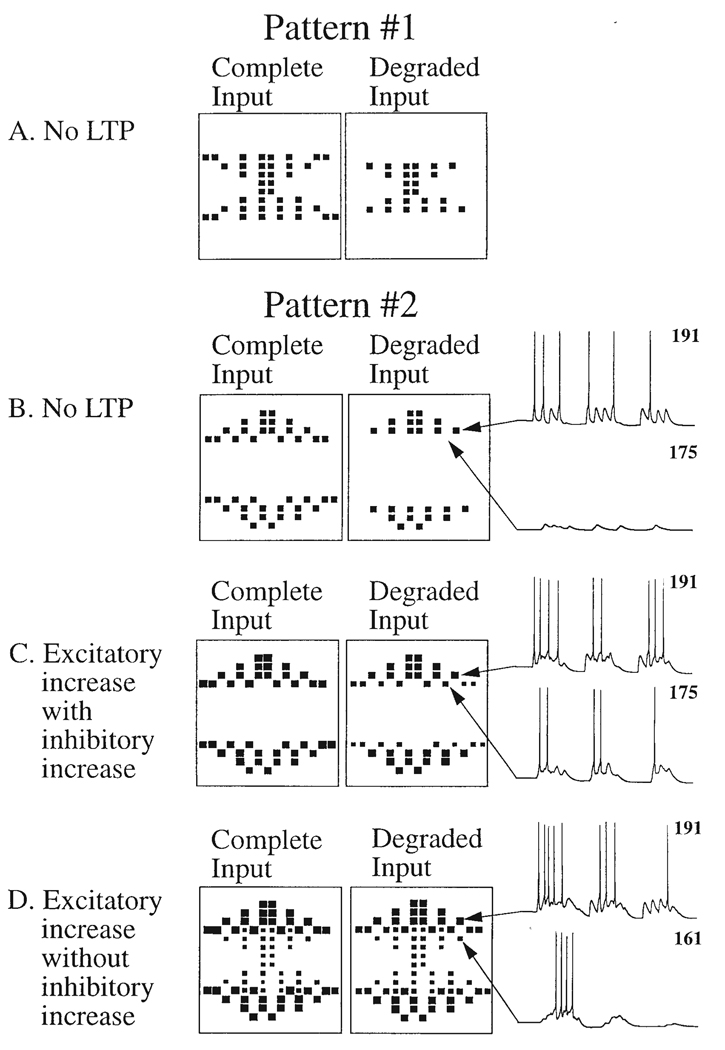
Fig. 4.
Long-term potentiation (LTP) of recurrent inhibition as a mechanism for controlling aberrant spread of lateral excitation. It results in confusion of normally distinguishable patterns of neuronal activity elicited by different inputs. LTP can be thought of as a strengthening of influence of connections by a Hebbian-like learning rule. This is a network biophysical simulation showing activity during recall; activity during learning is not shown.
Parts A and B. Action potential activity induced in a network of 240 pyramidal cells without any strengthening (LTP) of excitatory intrinsic synapses. Each enclosed panel contains 240 neurons plotted in a 15 × 16 matrix. The size of the black squares represents the number of action potentials generated by each pyramidal cell during a 500 ms period of recall, as illustrated in membrane potential traces of neurons 191 and 175 to the right of Pattern #2 (Part B). The black square for 191 represents the six action potentials generated by that neuron, which receives direct afferent input. The absence of a black square for 175 represents the absence of action potentials due to the absence of afferent input to this neuron. For each pattern, the left panel shows the response to the complete input version of the pattern (with 40 neurons active) and the right square shows the response to a degraded input version of the pattern (with 24 neurons activated by afferent input).
Parts C and D. Action potential activity in the network after learning strengthens the excitatory connections between active neurons (Excitatory LTP). The network has been trained on both pattern 1 and pattern 2, but recall is only shown for the complete and degraded version of pattern #2. Activity is shown during a 500 ms recall period.
Part C. Inhibitory LTP present. Part C shows activity with the strengthening of synapses between pyramidal cells and inhibitory interneurons by inhibitory LTP. With both excitatory LTP and inhibitory LTP, the network responds to the complete pattern with no excess spread of activity, and responds to the degraded input with activity spreading only into neurons which were a component of the original learned pattern. For example, on the right the membrane potential traces show neuron 191 responding to afferent input, while neuron 175 responds due to synaptic activity spreading from other neurons.
Part D. Absence of inhibitory LTP. With excitatory LTP but without inhibitory LTP, the network responds to both the complete and degraded versions of pattern #2 with activity which spreads to components of the other stored pattern, pattern #1. This is due to the spread of activity from neurons which were components of both patterns. This additional spread can be prevented by stronger recurrent inhibition. On the right, membrane potential traces show the response of neuron 191 and the excess spread of activity into neuron 161. In this case, differentiation of the two patterns is prevented because input of either pattern recalls elements of both patterns. (Adapted from Grunze et al. 1996)
This is consistent with other experimental findings, most notably those that have demonstrated schizophrenic patients tend to respond to the strongest associate of a word, regardless of context (e.g., “pen” as writing instrument even though the context suggests a fence, see Chapman and Chapman 1973). In addition, the enhanced priming effect demonstrated in several (Manschreck et al. 1988; Kwapil et al. 1990; Spitzer et al. 1994), albeit not all (Barch et al. 1996), word-priming studies of patients with schizophrenia, may also reflect evidence of problems with modulating associative links. Thus, schizophrenic thought processes may be unduly influenced by the activation, or priming, of strong associates, and impervious to the normal constraint of contextual information on the train of thought.
Electrophysiology, functional neural systems, and language in schizophrenia: the N400
The mental, or cognitive, events just described are generated by neurons in the brain, and some of their activity is reflected in the EEG, the electrical activity of the brain recorded from the surface of the scalp. The EEG (and its analog the magnetoencephalogram) are the only functional imaging modalities that operate at the speed of thought and consequently can most sensitively record evidence for altered processing of stimuli in “real time”. (The disadvantage of the EEG is that the scalp recordings do not offer good localization for the brain site(s) of occurrence of the recorded events.) Using averaging techniques, whereby the EEG activity from repeated presentations of a specific stimulus is summed across trials, small potentials related to the specific processing of the target stimulus can be extracted from the EEG. These are referred to as event-related potentials (ERPs). The N400 ERP appears most directly relevant to our discussion of language/thought disorder and to the preceding section on abnormalities of connectivity in schizophrenia. Before proceeding with the N400, we need to comment briefly on subjects used in the N400 and the MRI studies. We have studied abnormalities both in persons with schizotypal personality disorder, a schizophrenia spectrum disorder, as well as in schizophrenic patients, following genetic studies suggesting a common diathesis for these disorders.
Use of schizophrenia spectrum disorder subjects in the study of schizophrenia
Kendler et al. (1993) have demonstrated that schizotypal personality disorder subjects share the same genetic diathesis as subjects with schizophrenia; probands with either disorder have about the same probability of having a sib with schizophrenia (about 6.5%). Schizotypal personality disorder subjects are intrinsically interesting to compare and contrast with schizophrenic subjects as to what features are phenotypically similar and what features are found only in the psychotic subjects. Schizotypal personality disorder subjects also are methodologically advantageous as they, unlike schizophrenic subjects, do not have the potential confounds of chronic illness and of medication – we use DSM-III/IV schizotypal personality disorder subjects who have never been prescribed neuroleptics.
The N400 is a negative deflection in the ERP which is inversely proportional to the predictability of a word by a preceding word or sentence fragment, and therefore appears to reflect the degree to which prior semantic context constrains word selection (Kutas & Hillyard 1980). For example the magnitude of N400 is inversely proportional to the cloze probability of the cue and the target word, and so N400 amplitude may index the extent of a “search” for a semantic match. In an associational semantic network, activation of any representation is thought to prime, or pre-activate, any semantically related node to an extent proportional to the semantic distance (Collins and Loftus 1975), which can be thought of as the “degree of connectivity” as discussed in the preceding section. Subjects are able to make judgements about semantically related words more quickly than about unrelated words (e.g., Meyer and Schvaneveldt 1973). Congruent with its indexing of contextual constraint, N400 amplitude is larger to unrelated than to related words (e.g., Kutas & Hillyard 1989). N400 latency reflects the speed of linguistic operations related to semantic search (Van-Petten and Kutas 1990).
The most direct use of the N400 as an index of processes discussed in the preceding section has been by Niznikiewicz et al. (1997) in our laboratory. These experiments involved N400 measurements during word-pair lexical decision paradigms in which subjects were asked simply to judge whether the second word was a word or non-word. We used a short (250 ms) stimulus-onset-asynchrony (the interval from the onset of one word to the on-set of the next) to tap lexical processes related to semantic activation (as contrasted with a longer stimulus-onset-asynchrony, perhaps more related to working memory/ context utilization). In the short stimulus-onset-asynchrony condition, when compared with control subjects, both schizophrenic and schizotypal personality disorder subjects showed reduced N400 amplitude to the target words that were related – i.e. were associatively linked to the first words. Such a result appears to be compatible with the Nestor et al. study (1998), in the sense that related words fall into a category of associated words which are easier to process for the schizophrenic and schizophrenia spectrum subjects than for control subjects. The superior performance of clinical subjects on this task is associated, at the electrophysiological level, with a reduced N400 amplitude. The obvious next step is to use the N400 paradigm to measure directly the ERP to the same word pairs as used by Nestor et al., so as to obtain direct electrophysiological correlates of experimentally manipulated connectivity and associative network size.
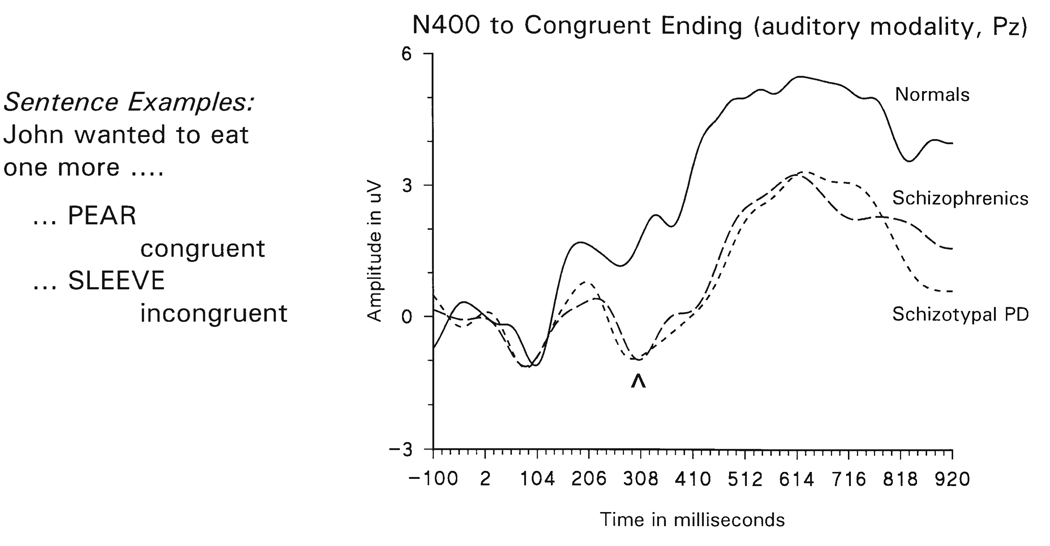
While the use of word pairs and N400 to discriminate connectivity and network size abnormalities is just beginning, there is now a considerable N400 literature using sentence terminal words suggesting that schizophrenic subjects do not use the context of the preceding portion of the sentence. These results are compatible with both the Nestor et al. data and reports that schizophrenic subjects select fill-in responses to phrases based on the immediately preceding word rather than the whole sentence or passage (Maher 1972). Both the N400 literature (Andrews et al. 1993; Grillon et al. 1991; Hokama et al. 1990) and our work (Adams et al. 1993; Nestor et al. 1997; Niznikiewicz et al. 1997) document N400 evidence supporting contextual abnormalities in schizophrenia. Studies from our laboratory found more negative (larger) N400 amplitude to both congruent and incongruent sentence endings in schizophrenia, suggesting an insensitivity to context and a possible dysfunction of early semantic activation. As illustrated in Fig. 1, both schizophrenic and schizotypal subjects (Niznikiewicz et al. 1997, and Niznikiewicz et al. 1998) have a more negative (larger) N400 amplitude to congruent sentence endings, compatible with a decreased use of context. In terms of our model of failure of suppression/inhibition in schizophrenia (and, at least to some extent, in spectrum disorders), the larger N400 represents the more extensive search in the lexicon due to the failure to use the context provided by the preceding words to narrow the search to the most probable sentence endings.
Fig. 1.
N400 in schizophrenia and schizotypal personality disorder. Left panel gives examples of congruent and incongruent sentence endings. The right panel shows grand average waveforms from normal control subjects, schizophrenic subjects, and schizotypal personality disorder subjects to congruent sentence endings. Note that the schizophrenic and schizotypal personality disorder subjects both show an N400 (carat) to congruent sentence endings, a response found in controls to incongruent endings only. (“N400” reflects the latency (400 ms) of visually presented stimuli; auditory presentation, done in this experiment, produces a ≈ 100 ms shorter latency “N400”.)
Evidence for abnormalities in temporal lobe anatomical substrates of language and other clinical/ functional disturbances in schizophrenia
This section focuses on the possible anatomical substrates of language and other clinical abnormalities. It begins by discussing the MRI findings and then concludes with a discussion of correlations.
Overview of structural imaging studies in schizophrenia
Despite the conviction of many theoreticians, researchers, and clinicians, including Bleuler and Kraepelin, that schizophrenia had an underlying anatomic pathology, several generations of neuropathologists were unable to discern the nature of the brain disturbances in schizophrenia. Early evidence that at first seemed to support pathology came from qualitative studies of post-mortem brains (e.g., Jacobi and Winkler 1927). However, more careful methodologically controlled studies often led to negative findings (e.g., Dunlap 1924), and results from these early studies thus became more suspect. This disparaging of earlier studies led Plum to assert that “schizophrenia is the graveyard of neuropathologists” (1972). The belief that schizophrenia involved organic changes survived nonethe-less although as recently as 1987 Roberts and coworkers (1987) described this belief as “an article of faith rather than a demonstrable fact.” In retrospect, the failure of pathologists to document changes can be attributed to their reliance on direct visual assessment instead of using quantitative measurement techniques. Other factors leading to confusion were the use of methods of analysis and of staining and fixation that were not standardized, as well as the lack of uniform diagnostic criteria.
Although a small number of researchers continued to theorize about the role of brain dysfunction in schizophrenia, general interest was not rekindled until the landmark study done by Johnstone and coworkers in 1976, which used the emerging technology of computed tomography (CT) to demonstrate an increase in lateral ventricular size in schizophrenic patients. This study, in fact, confirmed earlier pneumoencephalographic studies (e.g., Jacobi and Winkler 1927), which were ignored in the Zeitgeist of skepticism about brain changes in schizophrenia.
The advent of magnetic resonance (MR) imaging has added a new dimension to our view of the brain in schizophrenia. This technique, which allows gray-white matter differentiation, was first applied to schizophrenic patients by Smith and coworkers in 1984. These newer techniques have led to a series of studies that have convincingly demonstrated that brain abnormalities can be clearly delineated in at least some subgroups of schizophrenics, as documented in our recent review of the 118 MRI studies published from 1988 through May 1998 (McCarley et al. 1999, and also see our earlier review, Shenton et al. 1997). McCarley et al. (1999) is the source of the percentages of studies with abnormalities quoted here. In the present paper we will concentrate on the temporal lobe MRI alterations, as these have been most strongly related to the thought disorder of schizophrenia. It is first useful to describe the main anatomical and functional divisions of the temporal lobe (see McCarley et al. 1993 b). These are illustrated in Fig. 2.
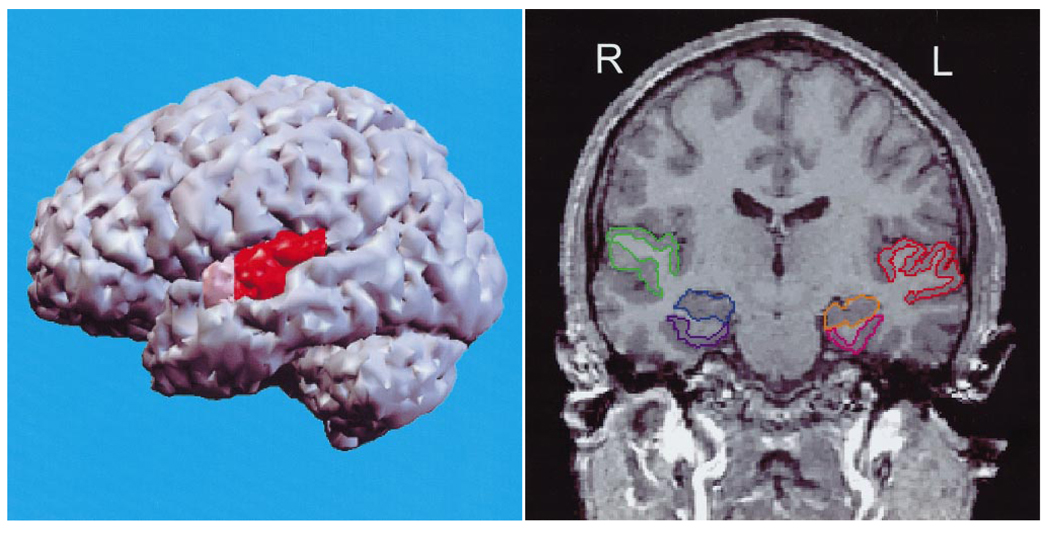
Fig. 2.
Left Panel. Left lateral view of a three dimensional reconstruction of the cortex, with pink indicating the anterior portion and red the posterior portion of the superior temporal gyrus (STG). Right Panel. SPGR coronal image (1.5 mm thick, 0.9375 × 0.9375 mm in plane voxels), showing the manually drawn outlines of regions of interest. L = left and R = right side for subject. The anterior-posterior location of this coronal slice is just posterior to the onset of the posterior portion of the STG in the left panel and is from the same subject. The regions of interest outlined are: the gray matter of the superior temporal gyrus (STG; subject left, red; subject right, green); more medially, the amygdala-hippocampal complex is shown (left, orange; right, blue) with the parahippocampal gyrus underneath (left, pink; right, purple). Adapted from Hirayasu et al. (1998)
Superior Temporal Gyrus: 1) Primary and secondary (association) auditory cortex. Initial analysis of auditory signals. 2) Language-related area in the posterior superior temporal gyrus, the planum temporale. Language function is usually left-lateralized in right-handed individuals.
Middle and Inferior Temporal Gyri: Linked to further processing of sensory stimuli, both auditory and visual. Some neurons in this area may be selectively responsive to complex and species-specific features, such as faces.
Medial Temporal Lobe Structures: 1) Hippocampus and adjacent (parahippocampal and entorhinal) cortex are involved in laying down and retrieval of long-term memory through interconnections with neocortical areas. 2) Amygdala. Its function is less well defined, but it is thought to be involved in emotion-related aspects of behavior, memory and learning. It may provide the emotional valence associated with memories and facilitate coding.
Overview of studies of the temporal lobe
Whole temporal lobe
Of the 37 studies of whole temporal lobe (i.e., all structures lumped together) 62% showed positive findings of volume reduction and/or abnormal asymmetry. The higher percentage of abnormalities in specifically defined regions of interest of medial temporal lobe and superior temporal gyrus suggests a non-diffuse distribution of temporal lobe structural changes.
Medial temporal lobe
Of the 31 studies evaluating one or more of these structures (hippocampus, amygdala, parahippocampal gyrus, entorhinal cortex), there were positive findings in 77%, one of the higher percentages of abnormalities in all regions of interest throughout the brain.
Superior temporal gyrus (STG)
The neocortical structure of superior temporal gyrus has recently been the subject of a number of studies, driven by the report of Barta et al. (1990) that a reduction in volume in the anterior region was associated with hallucinations and our report (Shenton et al. 1992) that the gray matter volume reduction in posterior STG was associated with thought disorder. Of the 15 studies surveyed, 80% showed abnormalities, the highest percentage of any cortical ROI.
Examination of the studies that did and did not separate gray from white matter is instructive, since all 7 of the studies evaluating gray matter found gray matter volume reduction, while a smaller percentage, 67%, of the 9 studies lumping gray and white matter found abnormalities in schizophrenia. It is of note that comparisons of STG white matter alone have indicated no differences, although the same studies reported gray matter abnormalities (Menon et al. 1995, Hajek et al. 1997). Thus, it appears useful and important that studies of cortical gyri should separately evaluate gray and white matter. Studies subdividing the STG into anterior and posterior regions have somewhat less inter-study agreement on which portion(s) are abnormal, likely due, at least in part, to differing definitions of subregions.
The Planum Temporale
This means literally the “temporal plain” and was originally defined on the basis of its surface appearance in whole brain specimens. It includes the posterior portion of the STG. Most (N = 5) of the 8 studies in the literature report abnormalities in schizophrenia. Barta et al. (1997) review in some detail methodological and definitional differences between studies, which may account for differences in findings. Because most of the studies focus on reversal of asymmetry in schizophrenics from the usual L > R pattern, the abnormalities of Planum Temporale consist, in large measure, of asymmetry differences.
Description of the temporal lobe structural MRI abnormalities in schizophrenia, and the relationship with clinical and functional abnormalities in schizophrenia
Our group’s focus on the temporal lobe in schizophrenia was based on our early P300 evoked potential data (Morstyn et al. 1982) and a subsequent CT study, which showed an association between left sylvian fissure enlargement and positive symptoms (McCarley et al. 1989). We followed up on this CT study with an MRI investigation of temporal lobe structural abnormalities and their association with thought disorder and evoked potential abnormalities. Subjects for the MR study (Shenton et al. 1992) were 15 male, right-handed, chronic (16 yr mean duration of illness) schizophrenic patients recruited from the Brockton Veterans Affairs Medical Center and 15 age-, sex-, and handedness-matched normal controls with no major mental disorder in themselves or first degree relatives. Based on the Scales for the Assessment of Positive and Negative Symptoms (Andreasen 1981, 1984) our patient subjects had predominantly positive symptoms (11 of 15 subjects). To assess thought disorder, we used the Thought Disorder Index (TDI, Johnston and Holzman 1979), which uses Rorschach cards to elicit speech samples that can be reliably scored for numerous categories of thought disorder, including bizarre word usage, neologisms, looseness, and fragmentation. The schizophrenic patients in our study had a mean total TDI score of 60.4, whereas normal subjects score less than 5. All subjects were screened for factors that could affect brain MRI, including substance abuse and neurological illness.
There were no significant mean differences between the two groups on any of the whole brain measures (white matter, gray matter, or CSF). Schizophrenics showed a significant increase in size in the temporal horn subdivisions of both lateral ventricles, with left temporal horn enlargement (180%) being greater than right (74%).
We next examined local, smaller regions of interest in the temporal lobe. These showed quite remarkable tissue volume reductions in schizophrenic patients compared to normal controls. While no statistically significant differences were observed in the overall volume of the temporal lobe, we observed statistically significant reductions in gray matter regions of interest (ROI) of the left anterior hippocampus-amygdala (19% decrease), parahippocampal gyrus (13% on the left and 8% on the right), and left superior temporal gyrus (STG, 15%).
An important clinico-pathological correlation was observed between left posterior STG gray matter volume and total TDI-rated thought disorder (r = −0.81). As gray matter decreased in the left posterior STG there was a concomitant increase in thought disorder. We found this correlation especially intriguing because the posterior portion of STG includes Heschl’s gyrus (primary auditory cortex) and a large portion of the planum temporale and Wernicke’s area, which have long been implicated as a neuroanatomical substrate of language (see references to the literature and discussion in Kwon et al. 1999). Furthermore, the extent of reductions in volume of the amygdala-hippocampal complex and the parahippocampal gyrus, regions important in verbal memory, correlated with the extent of STG volume reduction, suggesting to us that abnormalities in an interconnected neural network might be the substrate for schizophrenic loosening of associations, as discussed below.
First episode schizophrenics
In any study of chronic, medicated patients there is the possibility that the findings might be related to chronic illness or chronic medication, rather than to the disorder itself. We thus recently studied patients from McLean Hospital during their first hospitalization for psychosis, including patients with diagnoses of schizophrenia (n = 16) and affective psychosis (N = 18, N = 16 being manic-depressive subjects in a manic phase) and age-matched controls (Hirayasu et al. 1998). The results confirmed our major temporal lobe findings in chronic schizophrenic patients, while offering MRI evidence that affective and schizophrenic psychosis had different “endophenotypes”. The first episode schizophrenia patients had significantly smaller gray matter volume in left posterior superior temporal gyrus with a significant left < right asymmetry compared with first episode affective psychosis patients and comparison subjects. First episode schizophrenia patients also showed smaller gray matter volume of the left posterior amygdala-hippocampal complex than comparison subjects. Both patients with schizophrenia and affective psychosis had significant left < right asymmetry of the posterior amygdala-hippocampal complex. These findings suggest that temporal lobe abnormalities are present at the first hospitalization for schizophrenia and that reductions of left posterior superior temporal gyrus gray matter are specific to schizophrenia compared with affective disorder.
Schizotypal personality disorder subjects
Data from DSM-III schizotypal subjects with no history of neuroleptic medication and age-matched controls have indicated that this spectrum disorder also has a reduction of gray matter volume in the left superior temporal gyrus, but has less pronounced medial temporal lobe changes than in schizophrenia (Dickey et al. 1999).
We have recently examined a larger extent of posterior superior temporal gyrus than that evaluated in Shenton et al. (1992) and Hirayasu et al. (1998), the planum temporale (Kwon et al. 1999, which should be consulted for literature references for this paragraph). This brain region, originally defined as a surface in postmortem work, includes the posterior extension of the superior temporal gyrus, not included in our previous work. The planum temporale is of particular interest because it evinces the most left-right asymmetry in the human brain (left > right in two-thirds of all brains). The left PT is partially co-extensive with Wernicke’s area, critical for language and speech, and its size is linked to handedness and, among musicians, to perfect pitch perception. Developmentally, PT asymmetry is apparent by the 29th–31st weeks of gestation and thus abnormalities in this brain region may suggest a disruption of neurodevelopmental processes involved in hemispheric lateralization. Phylogenetically, PT asymmetry first appears in higher nonhuman primates (chimpanzees) and increases in the human brain, suggesting a possible link with the evolution of language.
We found gray matter volume underneath the left planum temporale was significantly reduced (28.2%) in chronic schizophrenic patients compared with normal controls (different chronic sample than in Shenton et al. 1992). Schizophrenic patients also showed a reversal of the left > right planum temporale asymmetry found in the normal controls. Heschl’s gyrus, primary auditory cortex, showed no differences between the left and right sides in either group. Although TDI thought disorder was not measured in this sample, the suspiciousness/persecution subscale score of the Positive and Negative Syndrome Scale (PANSS) was associated with a reduced left planum temporale volume in schizophrenic patients.
These MRI findings confirmed the postmortem study by Falkai et al. (1995), which reported reduced cortical volume under the left planum temporale, as well as abnormal volume asymmetry, in schizophrenia patients compared with controls. Overall, 5 of the 8 MRI studies of the planum temporale in schizophrenia report abnormalities (see review in McCarley et al. 1999).
Comment on anatomical substrate in schizophrenia for disturbances in association and language and on anatomical-functional correlates
A growing body of evidence suggests that medial temporal lobe structures (amygdala-hippocampal complex, parahippocampal gyrus) and STG should be viewed as part of an interconnected neural network that is functionally important for associative links in memory (see McCarley et al. 1993 b). Specifically, evidence now suggests that the hippocampus (and linked parahippocampal gyrus and entorhinal cortex) is important in gating memory storage and retrieval, with memory storage likely occurring at neocortical sites in response to input arising from hippocampus (Squire and Zola-Morgan 1991), and with STG being important for auditory associative memory (Ojemann 1991; Penfield and Perot 1963). More ventral temporal lobe cortex may be involved in N400 abnormalities (see below). We speculate that abnormalities in this interconnected network may result in both physiological and clinical (thought disorder) disturbances. We have elsewhere extensively reviewed abnormalities in schizophrenia of the P300 ERP, an ERP that has been postulated to index processing and assimilation of infrequently occurring new information (see Salisbury et al. 1999; McCarley et al. 1997; O’Donnell et al. 1999). Our laboratory was alerted to possible temporal lobe abnormalities by a left temporal scalp region topographic deficit of the auditory P300. This left temporal deficit is not only present in chronic schizophrenics, but also is present at the first psychotic episode, and appears to be specific to schizophrenic as contrasted with affective (manic-depressive) psychosis, both at the first episode (Salisbury et al. 1998) and in chronic patients (Salisbury et al. 1999). It is similarly present in schizotypal personality disorder (Salisbury et al. 1996), and thus may reflect a common characteristic of spectrum disorders. In terms of the anatomical substrate of the auditory P300 abnormality, the left temporal scalp P300 amplitude is positively correlated with the gray matter volume of the underlying left superior temporal gyrus both in chronic schizophrenic patients (McCarley et al. 1993 a) and at the first psychotic episode (Hirayasu et al. 1997). Clinically, the left temporal P300 amplitude is inversely correlated with the extent of SAPS delusions and TDI thought disorder (McCarley et al. 1993a). In terms of our preceding discussion, we suggest the left temporal P300 can be viewed as an index of the incorporation of new information that contradicts current expectations (the “surprise” in the P300). Failure to incorporate such information would logically be expected in delusions (by definition, delusions are a belief not sustained by external events). The correlation with thought disorder might reflect the laying down of abnormal associative links and a common disordered anatomical substrate, the left superior temporal gyrus, for both the left temporal P300 generator(s) and for disordered thinking.
We emphasize that our concentration, in this paper, on certain regions of the temporal lobe, should not be taken to suggest that other regions are not also involved. The parietal lobe, especially the angular gyrus, is also likely to be an important substrate for language (Mesulam 1990), as is the frontal lobe, especially Brodmann’s area and areas important in verbal working memory. Nor, as discussed next, have we evaluated all important regions in the temporal lobe, such as those likely to be implicated in N400 generation.
N400
Intracranial recordings suggest the N400 component is associated with activity in many brain structures including the amygdala and hippocampus, the cingulate, ventral neocortex (inferior temporal, lingual and fusiform gyri), dorsolateral and ventrolateral prefrontal cortex, superior lateral temporal cortex, the supramarginal gyrus and the inferior parietal lobule (Halgren et al. 1998), some of which have been implicated in schizophrenia (McCarley et al. 1999, Shenton et al. 1997). No MRI study has yet looked at the association between ventral neocortex volume (the inferior temporal, lingual and fusiform gyri) and scalp-recorded N400 in schizophrenia. Our group’s failure to find correlations with the more dorsal temporal lobe regions of interest described above does suggest these do not play a prominent role in the scalp-recorded N400. The negative scalp polarity of the N400 also suggests that ventral temporal lobe structures may be critical in its generation.
Finally we note that the great majority of findings of MRI volume reduction have been in gray matter and not in white matter (McCarley et al. 1999). This, together with neuropathological data suggesting a reduction in neuropil but not in cell number (e.g. Selemon et al. 1995), suggests a correspondence between the anatomical substrate data in this section and connectionist model data in the first part of this paper. They both suggest the main neural abnormality in schizophrenia is an abnormality of neural connectivity (dendrite/neuropil/gray matter changes) rather than the number of neurons (network size).
Cellular circuit abnormalities in schizophrenia: a failure of inhibition
One of the most challenging but unfinished projects in schizophrenia is to link clinical and behavioral abnormalities and the MRI abnormalities to abnormalities at the cellular circuit level. Together with other laboratories (see review in McCarley et al. 1996), we have been very involved in such an effort, especially as related to abnormalities of association and language. We will suggest that a “failure of inhibition” on the cellular level is present in schizophrenia and may be linked to a “failure of inhibition” at the cognitive level. We will preface this discussion with a presentation of information on the circuitry, neurotransmitters and receptor types that we postulate to be involved.
Several research groups have proposed that glutamate, the major excitatory neurotransmitter in the central nervous system, plays a central role in the pathogenesis of schizophrenia (Coyle 1996; McCarley et al. 1991; Olney 1990; Olney and Farber 1995; Robinson and Coyle 1987). More specifically, abnormal Excitatory-Amino-Acid (EAA) neurotransmission may be a mechanism for anomalies in neural development secondary to disruption of EAA-mediated neural guide mechanisms, and may also result in ongoing, use-dependent excitotoxic damage to cells (likely primarily confined to the dendritic tree rather than cellular loss) (Coyle 1996; Coyle and Puttfarcken 1993; McCarley et al. 1991; Olney 1990). Moreover, both developmental and excitotoxic aspects of EAA neurotransmission abnormalities could be explanatory mechanisms for the marked gray matter volume reductions observed in MRI and postmortem studies of schizophrenia.
Briefly, the supporting evidence at the clinical level for glutamatergic abnormalities comes from several sources. NMDA antagonists, such as ketamine (“Special K”) or PCP (“angel dust”), cause symptoms that are so similar to schizophrenia that many abusers are admitted to psychiatric hospitals. The psychosis produced by PCP includes auditory hallucinations, thought disorganization, and negative symptoms. This constellation of symptoms has been noted to more accurately mimic the symptoms observed in schizophrenia than do dopamine agonists, such as amphetamine or serotonergic agents, such as LSD, which tend to produce visual hallucinations without marked formal thought disorder and negative symptoms (see Javitt and Zukin 1991; Krystal et al. 1994; Malhotra et al. 1996). Also, as Farber et al. (1999) have recently reviewed, administration of PCP to stabilized patients with chronic schizophrenia can trigger a recrudescence of acute psychotic symptoms lasting for up to several months (Luby et al. 1959; Ban et al. 1961) whereas, in contrast, LSD causes only a brief hallucinogenic state that does not last longer in schizophrenic patients than in healthy subjects (Domino and Luby 1981).
Age-dependent findings for activity of NMDA receptors also are consistent with the time course of schizophrenia. Olney and coworkers have reported that the neurotoxic effects of N-methyl-D-aspartate (NMDA) antagonist MK801 are low in rats that are young (but past the pre- and peri-natal stage), and maximize at about 90 days, roughly early adulthood, perhaps analogous to the age of onset of schizophrenia (Olney and Farber 1995). Similarly, human children are not susceptible to the toxic effects of NMDA antagonists (ketamine, PCP) until after puberty (Karp et al. 1980; Welch and Correa 1980). Also relevant is the observation that a high percentage of adults display psychotic symptoms (called “emergence” reactions) upon awakening from ketamine anesthesia, whereas pediatric patients at any age prior to adolescence show little or no susceptibility (e.g. Reich and Silvay 1989). Of great interest in terms of neurodevelopment are data that NMDA receptors are important for certain aspects of neuronal development, including axonal guide pathways, migration, and differentiation, with the mediating factor thought to be Ca2+ influx (Komuro and Rakic 1993, 1998). An important addition to this literature was the recent report by Ikonomidou et al. (1999), demonstrating that apoptotic neuronal death in the pre- and perinatal period occurs with the application of NMDA channel blockers (e.g. MK801, PCP, ketamine, and carboxypiperazin-4-yl-propyl-1-phosphonic acid (CPP)). This supports a role for NMDA abnormalities in neurodevelopmental abnormalities.
Mechanism of NMDA action: decrease in recurrent inhibition
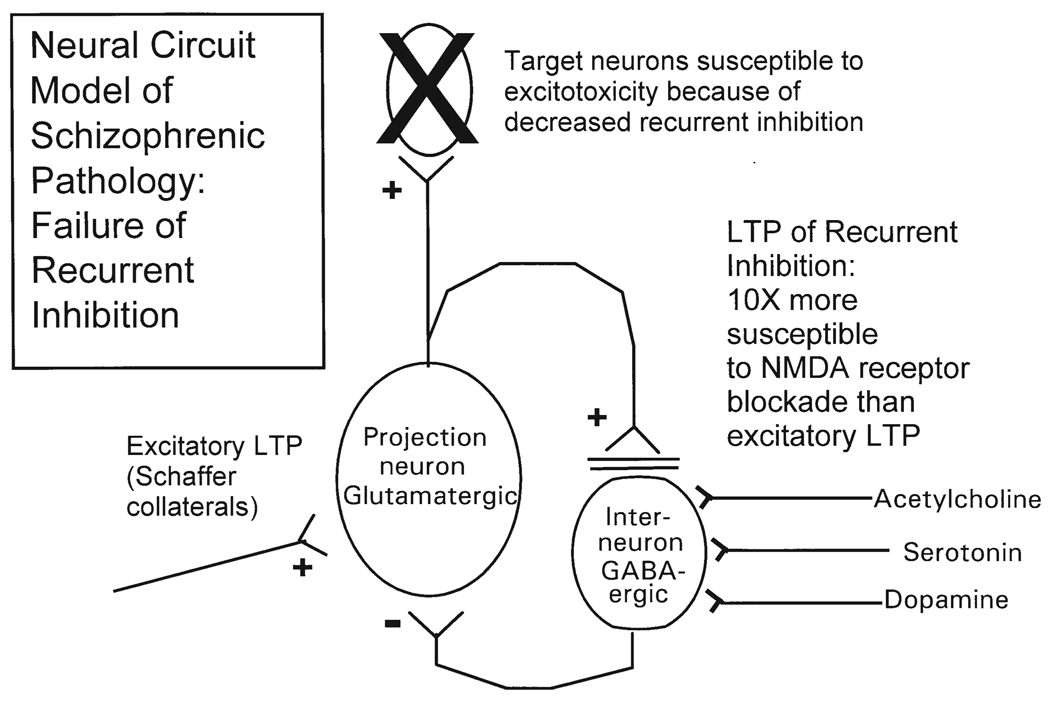
It may seem paradoxical that application of blockers of excitatory NMDA neurotransmission has the capability of causing excitotoxic effects (Ikonomidou et al. 1999). However, studies by Grunze et al. (1996) in our laboratory indicate this paradox may be resolved by the differential sensitivity of NMDA receptors on inhibitory interneurons and those involved in feed-forward excitation. Whole cell and extracellular recording techniques were used to examine local circuit inhibition in the CA1 region of the rat hippocampus in vitro (see schematic in Fig. 3). Activation of the recurrent inhibitory circuit elicited an inhibitory post-synaptic potential (IPSP) in pyramidal neurons that was dependent on NMDA receptor activation. Application of a tetanizing stimulus evoked long-term potentiation (LTP) of the intracellularly recorded recurrent IPSPs. This LTP was also NMDA-dependent and was more than 10-fold more sensitive to blockade by the NMDA antagonist APV than the excitatory LTP produced by stimulation of excitatory input (Schaffer collateral). (The non-potentiated IPSP was similarly more sensitive to blockade than the excitatory PSP.) The endogenous substance N-acetyl-aspartyl-glutamate (NAAG) had similar effects as APV. It is noteworthy that Tsai et al. (1995) found evidence for increased NAAG, and decreased levels of its degradative enzyme, in schizophrenia in postmortem tissue.
Fig. 3.
Cellular model of failure of recurrent inhibition. + is excitatory (glutamatergic) and – is inhibitory (GABA-ergic) synapse. The two horizontal lines on the projection neuron’s synapse with the GABA-ergic neuron represent NMDA receptor blockade. Note that inhibitory interneuron also has cholinergic, serotonergic, and dopaminergic modulatory synapses. These are also possible sites of abnormal receptors and/or abnormal inputs. See text for further discussion
In summary, in this circuit recurrent inhibition is more susceptible to NMDA blockade than excitation, suggesting that NMDA receptor antagonists, such as phencyclidine (PCP), ketamine, or NAAG differentially reduce recurrent inhibition, allowing overactivation of projection circuits, with possible excitotoxic damage to neurons to which they project (note: non-NMDA receptors are not blocked). Note that Fig. 3 shows cholinergic, serotonergic and dopaminergic inputs to the interneuron. These and/or their receptors may be abnormal, leading functionally to the same decreased recurrent inhibition as the NMDA receptor abnormality just discussed. For example, Freed-man and colleagues (Frazier et al. 1998) have provided evidence for an abnormality in the alpha-bungarotoxin sensitive nicotinic receptor on interneurons, which is associated with decreased recurrent inhibition and decreased P50 gating, and is prevalent in schizophrenic probands and their families.
Decreased recurrent inhibition and abnormalities of association
The consequences of this decreased recurrent inhibition are of interest in terms of abnormalities of association. Grunze et al. (1996) used a biophysical simulation of hippocampal CA1 circuitry in a model of learned pattern recognition that included LTP in both excitatory and inhibitory recurrent circuits. As illustrated in Fig. 4, selective blockade of inhibitory LTP in this model produced, because of the reduction in recurrent inhibition, aberrant spread of lateral excitation, resulting in confusion of normally distinguishable patterns of neuronal activity. We think it possible that some schizophrenic abnormalities, such as those in association learning, may stem from a failure of this inhibitory circuit or its cortical analogs.
We see a remarkable similarity between the results of the biophysical model of absence of recurrent inhibition presented in Fig. 4 and the cognitive model described in the first section of this paper (quoted here for the convenience of the reader): “In the cognitive level model, a word produces various levels of excitatory buildup, as reflected by competition among simultaneously activated representations … Schizophrenia may produce a dysregulation of network excitation because of failure of suppression (inhibition) (see, for example, Anderson and Spell-man 1995).” There are two aspects to the neuronal failure of inhibition. First, there should be difficulty in learning new verbal associations (corresponding to the LTP illustration in Fig. 4). Indeed, schizophrenics do show a deficit on the Verbal Paired Associate Learning Test, a deficit that is correlated with reduced volume in the putative anatomical substrate of left posterior superior temporal gyrus (Nestor et al. 1993). Second, there should be abnormalities in retrieval and sequencing of previously learned verbal associations, due to two factors: a) because they were originally stored with aberrant connectivity weighting at the time of learning (due to abnormalities of inhibitory LTP) and also b) because in “real time” sequencing of words in sentences, the acute abnormality of inhibition (i.e. that not involving LTP) prevents a channeling of activation toward the most probable sentence ending. Both factors may contribute to the greater “search activity” to congruent sentence endings seen in the N400 studies (Fig. 1).
The biophysically realistic neuronal model of Fig. 4 is also formally consistent with the connectionist model of the first section of this paper in that the abnormalities are not in network size but in connectivity. Low connectivity words would elicit many competing patterns of activity, with resulting difficulty in recall, as in the biophysical simulation of Fig. 4. In contrast high connectivity words would have less competition and thus less interference.
Gamma frequency band abnormalities in schizophrenia
Can one obtain more direct clinical evidence consistent with our proposed circuit abnormalities in schizophrenia? Gamma range (30 to 50 Hz) neural synchronization may be a key mechanism of information processing in neural networks, underlying the binding of both sensory and temporal features of objects. Furthermore gamma range synchronization is thought to depend on the glutamatergically mediated interplay between excitatory projection neurons and GABAergic circuits (see Traub et al. 1996), which we have postulated to be abnormal. We thus have obtained preliminary data on synchronization in response to auditory input (McCarley et al., in press). Schizophrenic patients showed reduced EEG power at 40 Hz, but not at lower frequencies of stimulation. Control subjects showed early onset of entrainment to 40 Hz stimuli, while schizophrenics showed delayed onset of entrainment, poorer synchronization, and a longer persistence of entrainment after the end of the 40 Hz stimulus train, findings compatible with our model.
Therapeutic implications
If there is an intrinsic blockade of NMDA receptors, then agents which modulate NMDA receptors may have beneficial effects. The NMDA receptor (NMDAR) has a strychnine-insensitive binding site where glycine acts to allosterically facilitate NMDAR function. In fact, there are now reports that D-cycloserine, a partial agonist at the glycine modulatory site reduces negative symptoms (Goff et al. 1999) and that large doses of glycine itself have a similar effect (Heresco-Levy et al. 1999). These effects have been puzzling, since the concentration of glycine in the cerebrospinal fluid is about 6 uM, a concentration level that should saturate the NMDAR glycine site. There is, however, the interesting possibility of a microregulation of glycine near the receptor by glycine transporters (GLYT). Bergeron and colleagues (1998) have recently reported that a type 1 GLYT inhibitor, NFPS, N[3-(49-fluorophenyl)-3-(49-phenylphenoxy)propyl]sarcosine, significantly modulates NMDA neurotransmission in vitro, indicating a lack of saturation of the receptor and a role of this transporter in regulation of glycine concentration. It is also obvious that drugs affecting GLYT potentially furnish another method for regulation of glycine and hence of NMDAR activity, and that they might be useful in the treatment of schizophrenia.
Progressive neurodegeneration in schizophrenia?
This is a hotly debated current issue. Our P300 latency data, which show a greater age-related increase in latency in schizophrenia than in controls (O’Donnell et al. 1995), offer positive evidence as do clinical course studies (see review in Anderson et al. 1998). MRI data, recently reviewed by us, also appear to offer supporting evidence, but are not yet conclusive (McCarley et al. 1999). Prospective studies of MRI gray matter volume change over time that is greater in schizophrenia than in controls appear best suited to settle this issue. Our laboratory has preliminary data over a mean 1.5 year MRI scan-rescan interval supporting a progressive gray matter volume reduction in the posterior superior temporal gyrus in schizophrenia but not in controls or in affective (manic-depressive) psychosis (McCarley et al. 1998). This is an area in which further data are both needed and also likely to appear in the next few years. Our wager is that Kraepelin will be proven correct about progressive neurodegeneration, at least in some patients.
Overall, we are heartened by the promise of new approaches linking the biology and psychology of schizophrenia, which we think will follow the successful path of studies linking psychology and biology in the study of normal cognition and brain function.
Acknowledgements
This work was supported by NIMH 40977 and 52 807, and by MERIT and Schizophrenia Center Awards from the Department of Veterans Affairs (RWM); NIMH 01110 and 50 747 (MES).
Footnotes
This paper incorporates some elements of earlier reviews, McCarley RW, Shenton ME, O’Donnell BF, Nestor PG (1993) The psychology of schizophrenia and the biology of temporal lobe abnormalities. Harvard Rev. Psychiatry. 1:36–57 and McCarley RW, Wible C, Frumin M, Hirayasu Y, Levitt JJ, Fischer I, Shenton ME. (1999) MRI Anatomy of Schizophrenia. Biological Psychiatry 45: 1099–1119
References
- Adams J, Faux SF, Nestor PG, Shenton ME, Marcy B, Smith S, McCarley RW. ERP abnormalities during semantic processing in schizophrenia. Schiz Research. 1993;10:247–257. doi: 10.1016/0920-9964(93)90059-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JE, O’Donnell BF, McCarley RW, Shenton ME. Progressive changes in schizophrenia: do they exist and what do they mean? Restor Neurol Neurosci. 1998;12:1–10. [PubMed] [Google Scholar]
- Anderson MC, Spellman BA. On the status of inhibitory mechanisms in cognition: memory retrieval as a model case. Psychol Rev. 1995;102:68–100. doi: 10.1037/0033-295x.102.1.68. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the assessment of negative symptoms (SAPS) Iowa City: Department of Psychiatry, University of Iowa College of Medicine; 1981. [Google Scholar]
- Andreasen NC. Scale for the assessment of positive symptoms (SAPS) Iowa City: Department of Psychiatry, University of Iowa College of Medicine; 1984. [Google Scholar]
- Andrews S, Shelly AM, Ward PB, Fox A, Catts SV, McConaghy N. Event-related potential indices of semantic processing in schizophrenia. Biol Psychiatry. 1993;34:443–458. doi: 10.1016/0006-3223(93)90235-6. [DOI] [PubMed] [Google Scholar]
- Ban TA, Lohrena JJ, Legmann HE. Observations on the action of Sernyl – a new psychotropic drug. Can Psychiatric Assoc J. 1961;6:150–157. doi: 10.1177/070674376100600307. [DOI] [PubMed] [Google Scholar]
- Barch DM, Cohen JD, Servan-Schreiber D, Steingard S, Cohen JD, Steinhauer SS, Van Kammen DP. Semantic priming in schizophrenia: an examination of spreading activation using word pronunciation and multiple SOAs. Journal of Abnormal Psychology. 1996;105(4):592–601. doi: 10.1037//0021-843x.105.4.592. [DOI] [PubMed] [Google Scholar]
- Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry. 1990;147:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Barta PE, Pearlson GD, Brill LB, 2nd, Royall R, McGilchrist IK, Pulver AE, Powers RE, Casanova MF, Tien AY, Frangou S, Petty RG. Planum temporale asymmetry reversal in schizophrenia: replication and relationship to gray matter abnormalities. Am J Psychiatry. 1997;154:661–667. doi: 10.1176/ajp.154.5.661. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Torsten M, Coyle JT, Greene RW. Proc Natl Acad Sci USA. 1998;Vol. 95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler E. In: Dementia Praecox or the Group of Schizophrenias. Zinkin H, translator. New York: International Universities Press; 1911/1950. [Google Scholar]
- Chapman LJ, Chapman JP. Disordered thought in schizophrenia. Englewood Cliffs, NJ: Prentice-Hall; 1973. [Google Scholar]
- Collins AM, Loftus EF. A spreading-activation theory of semantic processing. Psychological Review. 1975;82:407–428. [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harvard Review of Psychiatry. 1996;3(5):241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, Fischer A, The EK, Van Roades R, Jakab M, Kikinis R, Jolesz FA, Shenton ME. Schizotypal personality disorder and MRI abnormalities of temporal lobe gray matter. Biol Psychiatry. 1999 doi: 10.1016/s0006-3223(99)00030-x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF, Luby ED. Abnormal mental states induced by phencyclidine as a model of schizophrenia. In: Domino EF, editor. Historical and Current Perspectives. Ann Arbor, Michigan: NPP Books; 1981. pp. 401–418. [Google Scholar]
- Dunlap CB. Dementia praecox: some preliminary observations on brains from carefully selected cases and a consideration of certain sources of error. Am J Psychiatry. 1924;80(3):403–421. [Google Scholar]
- Falkai P, Bogerts B, Schneider T, Greve B, Pfeiffer U, Pilz K, Gonsiorzcyk C, Majtenyi C, Ovary I. Disturbed planum temporale asymmetry in schizophrenia: a quantitative postmortem study. Schizophrenia Research. 1995;14:161–176. doi: 10.1016/0920-9964(94)00035-7. [DOI] [PubMed] [Google Scholar]
- Farber NB, John W, Newcomer JW, Olney JW. Glycine agonists: what can they teach us about schizophrenia? Arch Gen Psychiatry. 1999;56:13–17. doi: 10.1001/archpsyc.56.1.13. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillon C, Ameli R, Glazer WM. N400 and semantic categorization in schizophrenia. Biological Psychiatry. 1991;29(5):467–480. doi: 10.1016/0006-3223(91)90269-r. [DOI] [PubMed] [Google Scholar]
- Goff DC, Tsai G, Levitt J, Amico E, Manoach D, Schoenfeld DA, Hayden DL, McCarley R, Coyle JT. A placebo-controlled trial of D-cycloserine added to conventional neuroleptics in patients with schizophrenia. Arch Gen Psychiatry. 1999;56:21–27. doi: 10.1001/archpsyc.56.1.21. [DOI] [PubMed] [Google Scholar]
- Grunze HCR, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J Neuroscience. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek M, Huonker R, Boehle C, Volz HP, Nowak H, Sauer H. Abnormalities of auditory evoked magnetic fields and structural changes in the left hemisphere of male schizophrenics – a magnetoencephalographic-magnetic resonance imaging study. Biol Psychiatry. 1997;42:609–616. doi: 10.1016/s0006-3223(96)00428-3. [DOI] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalography and Clinical Neurophysiology. 1998;106(2):156–164. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry. 1999;56:29–36. doi: 10.1001/archpsyc.56.1.29. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, Shenton ME, Salisbury DF, Dickey CD, Fischer IA, Mazzoni P, Kissler T, Arakaki H, Kwon JS, Anderson JA, Yurgelun-Todd D, Tohen M, McCarley RW. Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry. 1998;155:1384–1391. doi: 10.1176/ajp.155.10.1384. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, Shenton ME, Salisbury DF, Fischer IA, Dickey CC, Kisler T, Arakaki H, Yurgelun-Todd DA, Tohen M, McCarley RW. MRI and ERP abnormalities in first episode psychosis. Biol Psychiatry. 1997;41 doi: 10.1176/ajp.155.10.1384. 60s. Abstract. [DOI] [PubMed] [Google Scholar]
- Hokama H, Koyama S, Miyatani M, Miyazato Y, Ogura C, Nageishi Y, Shimodochi M. Abnormality of contextual effects on ERPs in schizophrenia and N400 topography. In: Brunia CHM, Gaillard AWK, Kok A, editors. Psychophysiological Brain Research. Vol 2. Tilburg University Press; 1990. [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA Receptors and Apoptotic Neurodegeneration in the Developing Brain. Science. 1999 Jan;:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Jacobi W, Winkler H. Encephalographische studien an chronisch schizophrenen. Arch Psychiatrie Nervenkrankheiten. 1927;81:299–332. [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J of Psychiatry. 1991;148(10):1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Crow TJ, Frith CD, Husband J, Kreel L. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet. 1976;2:924–926. doi: 10.1016/s0140-6736(76)90890-4. [DOI] [PubMed] [Google Scholar]
- Johnston MH, Holzman PS. Assessing Schizophrenic Thinking. San Francisco: Jossey-Bass Inc.; 1979. Publishers. [Google Scholar]
- Karp HN, Kaufman ND, Anand SK. Phencyclidine poisoning in young children. J Pediatr (Phila) 1980;19:510–514. doi: 10.1016/s0022-3476(80)80447-1. [DOI] [PubMed] [Google Scholar]
- Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D. The Roscommon family study III. Schizophrenia-related personality disorders in relatives. Arch Gen Psychiatry. 1993;50:781–788. doi: 10.1001/archpsyc.1993.01820220033004. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993;260(5104):95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intra-cellular Ca2+ fluctuations. Neurobiol. 1998;37:110–130. [PubMed] [Google Scholar]
- Kraepelin E. Psychiatry: A Textbook for Students and Physicians. Vol.2. New Delhi: Amerind Publishing Co; 1899/1989. (A translation of Psychiatrie, 1899 by Ayed S) [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: brain potentials reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. An electrophysiological probe of incidental semantic association. Journal of Cognitive Science. 1989;1:38–49. doi: 10.1162/jocn.1989.1.1.38. [DOI] [PubMed] [Google Scholar]
- Kwapil TR, Hegley DC, Chapman LJ, Chapman JP. Facilitation of word recognition by semantic priming in schizophrenia. J of Abnormal Psychology. 1990;99(3):215–221. doi: 10.1037//0021-843x.99.3.215. [DOI] [PubMed] [Google Scholar]
- Kwon JS, McCarley RW, Hirayasu Y, Anderson JE, Fischer IA, Kikinis R, Jolesz FA, Shenton ME. Left planum temporale volume reduction in schizophrenia. Arch Gen Psychiatry. 1999 doi: 10.1001/archpsyc.56.2.142. In Press. [DOI] [PubMed] [Google Scholar]
- Krystal JS, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug-Sernyl. Arch Neurol Psychiatry. 1959;81:363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- Maher B. The language of schizophrenia: a review and interpretation. Brit J of Psychiatry. 1972;120(554):3–17. doi: 10.1192/bjp.120.554.3. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14:301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- Manschreck TC, Maher BA, Milavetz JJ, Ames D, Weisstein CC, Schneyer ML. Semantic priming in thought disordered schizophrenic patients. Schizophrenia Research. 1988;1(1):61–66. doi: 10.1016/0920-9964(88)90041-2. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Faux SF, Shenton ME, LeMay M, Cane M, Ballinger R, Duffy FH. CT abnormalities in schizophrenia: a preliminary study of their correlations with P300/P200 electrophysiological features and positive/negative symptoms. Arch Gen Psychiatry. 1989;46:698–708. doi: 10.1001/archpsyc.1989.01810080028004. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Faux SF, Shenton ME, Nestor PG, Adams J. Event-related potentials in schizophrenia: their biological and clinical correlates and a new model of schizophrenic pathophysiology. Schiz Res. 1991;4:209–231. doi: 10.1016/0920-9964(91)90034-o. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Shenton ME, O’Donnell BF, Faux SF, Kikinis R, Nestor PG, Jolesz FA. Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiatry. 1993 a;50:190–197. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Shenton ME, O’Donnell BF, Nestor PG. The psychology of schizophrenia and the biology of temporal lobe abnormalities. Harvard Rev Psychiatry. 1993 b;1:36–57. doi: 10.3109/10673229309017055. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Hsiao J, Freedman R, Pfefferbaum A, Donchin E. Neuroimaging and the cognitive neuroscience of schizophrenia. Schiz Bulletin. 1996;22:703–726. doi: 10.1093/schbul/22.4.703. [DOI] [PubMed] [Google Scholar]
- McCarley RW, O’Donnell BF, Niznikiewicz MA, Salisbury DF, Potts GF, Hirayasu Y, Nestor PG, Shenton ME. Update on electrophysiology in schizophrenia. Int Rev Psychiatry. 1997;9:373–386. [Google Scholar]
- McCarley RW, Hirayasu Y, Salisbury DF, Fischer IA, Yurgelun-Todd DA, Tohen M, Shenton ME. Progressive gray matter volume reduction in left posterior superior temporal gyrus in first episode schizophrenia, but not in first episode affective psychosis or controls, or in other temporal lobe ROI; Los Croabas, Puerto Rico. Paper presented at the 1998 American College of Neuropsychopharmacology Meeting.1998. [Google Scholar]
- McCarley RW, Wible C, Frumin M, Hirayasu Y, Levitt JJ, Fischer I, Shenton ME. MRI anatomy of schizophrenia. Biological Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW, Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME. Gamma frequency range abnormalities to auditory stimulation in schizophrenia; Santa Fe, New Mexico. Paper accepted for presentation, International Congress of Schizophrenia; April 1999; (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, Rummelhart DE. A distributed model of human learning and memory. In: McClelland JL, Rummelhart DE, editors. Parallel Distributed Processing: Explorations in the Microstructure of Cognition. Vol. 2. Cambridge, Mass: MIT Press; 1987. pp. 170–215. [Google Scholar]
- Menon RR, Barta PE, Aylward EH, Richards SS, Vaughn DD, Tien AY, Harris GJ, Pearlson GD. Posterior superior temporal gyrus in schizophrenia: grey matter changes and clinical correlates. Schizophr Research. 1995;16:127–135. doi: 10.1016/0920-9964(94)00067-i. [DOI] [PubMed] [Google Scholar]
- Mesulam M. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28(5):597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Schvaneveldt RW. Facilitation in recognizing pairs of words: Evidence of a dependence between retrieval operations. J Experimental Psychology. 1971;90(2):227–234. doi: 10.1037/h0031564. [DOI] [PubMed] [Google Scholar]
- Morstyn RM, Duffy FH, McCarley RW. Altered P300 topography in schizophrenia. Arch Gen Psychiatry. 1983;40:729–734. doi: 10.1001/archpsyc.1983.01790060027003. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Schrieber TA, Mevoy CL. Processing implicit and explicit representations. Psychol Review. 1992;99:322–348. doi: 10.1037/0033-295x.99.2.322. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Bennett DJ, Gee NR, Schreiber TA, McKinney VM. Implicit memory: effects of network size and interconnectivity on cued recall. J Exp Psychol Learn Mem Cogn. 1993;19:747–764. doi: 10.1037//0278-7393.19.4.747. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Shenton ME, McCarley RW, Haimson J, Smith RS, O’Donnell BF. Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia. Amer J Psychiatry. 1993;150:1849–1855. doi: 10.1176/ajp.150.12.1849. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kimble MO, O’Donnell BF, Smith L, Niznikiewicz MA, Shenton ME, McCarley RW. Aberrant semantic activation in schizophrenia: a neurophysiological study. Amer J Psychiatry. 1997;157:640–646. doi: 10.1176/ajp.154.5.640. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Akdag SJ, O’Donnell BF, Niznikiewicz M, Law S, Shenton ME, McCarley RW. Word recall in schizophrenia: A connectionist model. Amer J Psychiatry. 1998;155:1685–1690. doi: 10.1176/ajp.155.12.1685. [DOI] [PubMed] [Google Scholar]
- Niznikiewicz MA, O’Donnell BF, Nestor PG, Smith L, Law S, Karapelou M, Shenton ME, McCarley RW. ERP assessment of visual and auditory language processing in schizophrenia. J Abnormal Psychology. 1997;106(1):85–94. doi: 10.1037//0021-843x.106.1.85. [DOI] [PubMed] [Google Scholar]
- Niznikiewicz MA, Shenton M, Voglmaier M, Seidman L, Dickey C, The E, Delong S, McCarley RW. N400 amplitude indexes language abnormality in female schizotypy; The XIIth International Conference on Event-Related Potentials of the Brain; July 1998; 1998. (abstract) [Google Scholar]
- O’Donnell BF, Faux SF, McCarley RW, Kimble MO, Salisbury DF, Nestor PG, Kikinis R, Jolesz FA, Shenton ME. Increased rate of P300 latency prolongation with age in schizophrenia. Arch Gen Psychiatry. 1995;52:544–549. doi: 10.1001/archpsyc.1995.03950190026004. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, McCarley RW, Potts GF, Salisbury DF, Nestor PG, Hirayasu Y, Niznikiewicz MA, Barnard J, Shen ZJ, Weinstein DM, Bookstein FL, Shenton ME. Identification of neural circuits underlying P300 abnormalities in schizophrenia. Psychophysiology. 1999 doi: 10.1017/s0048577299971688. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann GA. Cortical organization of language. J Neuro-science. 1991;11(8):2281–2287. doi: 10.1523/JNEUROSCI.11-08-02281.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW. Excitotoxic amino acids and neuropsychiatric disorders. Ann Rev Pharmacol Toxicol. 1990;30:47–71. doi: 10.1146/annurev.pa.30.040190.000403. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Penfield W, Perot P. The brain’s record of auditory and visual experience: a final summary and discussion. Brain. 1963;86:596–695. doi: 10.1093/brain/86.4.595. [DOI] [PubMed] [Google Scholar]
- Plum F. Prospects for research on schizophrenia. 3. Neuropsychology. Neuropathological Findings. Neurosciences Research Program, Bulletin. 1972;10:384–388. [PubMed] [Google Scholar]
- Reich DL, Silvay G. Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth. 1989;36:186–197. doi: 10.1007/BF03011442. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Colter N, Lofthouse R, Johnstone EC, Crow TJ. Is there gliosis in schizophrenia? Investigation of the temporal lobe. Biol Psychiatry. 1987;22:1459–1468. doi: 10.1016/0006-3223(87)90104-1. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Coyle JT. Glutamate and related acidic excitatory neurotransmitters: from basic science to clinical application. FASEB Journal. 1987;1(6):446–455. doi: 10.1096/fasebj.1.6.2890549. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Voglmaier MM, Seidman LJ, McCarley RW. Topographic abnormalities of P3 in schizotypal personality disorder. Biol Psychiatry. 1996;40:165–172. doi: 10.1016/0006-3223(95)00373-8. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, Sherwood AR, Fischer IA, Yurgelun-Todd DA, Tohen M, McCarley RW. First-episode schizophrenic psychosis and controls in P300 amplitude over left temporal lobe. Arch Gen Psychiatry. 1998;55:173–180. doi: 10.1001/archpsyc.55.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, McCarley RW. P300 topography differs in schizophrenia and manic psychosis. Society of Biol Psychiatry. 1999;45:98–106. doi: 10.1016/s0006-3223(98)00208-x. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally High Neuronal Density in the Schizophrenic Cortex A Morphometric Analysis of Prefrontal Area 9 and Occipital Area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW. Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Eng J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Wible CG, McCarley RW. A review of magnetic resonance imaging studies of brain abnormalities in schizophrenia. In: Krishnan KRR, Doraiswamy PM, editors. Brain Imaging in Clinical Psychiatry. New York: Marcel Dekker, Inc; 1997. [Google Scholar]
- Smith RC, Calderon M, Ravichandran GK, Largen J, Vroulis G, Shvartsburd A, Gordon J, Schoolar JC. Nuclear magnetic resonance in schizophrenia: a preliminary study. Psychiatry Research. 1984;12:137–147. doi: 10.1016/0165-1781(84)90013-1. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Weisker I, Winter M, Maier S, Hermle L, Maher BA. Semantic and phonological priming in schizophrenia. J of Abnormal Psychology. 1994;103(3):485–494. doi: 10.1037//0021-843x.103.3.485. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Stanford IM, Jeffreys JGR. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996;383:621–624. doi: 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- Tsai GC, Coyle JT. N-Acetylaspartate in neuropsychiatric disorders. Prog Neurobiol. 1995;46:531–540. doi: 10.1016/0301-0082(95)00014-m. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Kutas M. Interactions between sentence context and word frequency in event-related brain potentials. Mem Cognit. 1990;18:380–393. doi: 10.3758/bf03197127. [DOI] [PubMed] [Google Scholar]
- Welch MJ, Correa GA. PCP intoxication in young children and infants. Clin Pediatr (Phila) 1980;19:510–514. doi: 10.1177/000992288001900801. [DOI] [PubMed] [Google Scholar]