Abstract
Objective
Myelin-associated inhibitors play a role in limiting axonal growth in the adult central nervous system. Blocking these inhibitors may promote neurological recovery from spinal cord contusion.
Methods
The soluble Nogo-66 receptor (NgR(310)ecto-Fc) protein, which can neutralize three myelin inhibitors, was infused into rats after spinal cord contusion for 28 days. Treatment was initiated intrathecally at the time of injury or 3 days after injury by the intracerebroventricular route at a dose of 0.29mg/kg/day. Recovery of locomotion and of axonal growth was assessed. Some animals received combination therapy with NgR(310)ecto-Fc plus rolipram, a cyclic adenosine monophosphate phosphodiesterase inhibitor.
Results
Seven weeks after spinal injury, the Basso-Beattie Bresnahan locomotor scores were significantly improved in the 3-day delayed NgR(310)ecto-Fc treatment group (9.5 ± 0.7; n = 16) versus the vehicle-treated group, (6.75 ± 0.7; n = 15) (p ≤ 0.01, analysis of variance). The percentage of NgR(310)ecto-Fc–treated animals able to support their weight was twice that in the control group. Delayed therapy was as efficacious as acute therapy. Addition of rolipram did not alter recovery. The beneficial behavioral effects of NgR(310)ecto-Fc correlated with sprouting of raphespinal axons in the caudal spinal cord and of corticospinal axons in the rostral spinal cord.
Interpretation
NgR(310)ecto-Fc treatment improves outcome in a rodent model that closely mimicked human spinal cord injury.
When the adult mammalian central nervous system is injured, severed axons from surviving neurons do not extend and reconnect to their synaptic targets.1–4 The resulting axonal disconnection constitutes a significant cause of persistent neurological deficits in many neurological conditions. Traumatic spinal cord injury (SCI) is perhaps the purest example of a disconnection-based syndrome secondary to failed axonal growth. Although focal neuronal loss contributes to segmental dysfunction at the level of injury after SCI, chronic neurological deficits below the level of injury are principally due to the failure of axonal regrowth.
Recent laboratory studies have identified three myelin-associated proteins, Nogo-A, Myelin-Associated Protein (MAG), and Oligodendrocyte Myelin Glycoprotein (OMgp), which bind to an axonal Nogo-66 receptor (NgR) protein to collapse axonal growth cones and stop axonal extension.1,5–13 The importance of this pathway has been documented in a range of genetic and pharmacological studies in rodent SCI. Pharmacological studies have used peptides that competitively inhibit Nogo-66 interaction with NgR,14,15 anti–Nogo-A antibodies,16,17 and a soluble ectodomain fragment of the NgR.2,18–20 The NgR fragment fused to the constant region of IgG in the NgR(310)ecto-Fc reagent contains the entire ligand-binding site of NgR. Thus, this fragment can sequester all three myelin-associated inhibitors from active endogenous axonal NgR. When infused intrathecally into rats with midthoracic dorsal spinal hemisection injuries, the NgR(310)ecto-Fc protein stimulates corticospinal tract (CST) and raphespinal axonal growth.19 Critically, the recovery of locomotor performance was stimulated by NgR(310)ecto-Fc.
A group of recent studies have generated interest in the notion that intracellular cyclic adenosine mono-phosphate (cAMP) levels play a role in determining the propensity of neurons to extend axons. In particular, elevated cAMP levels reduce inhibition by myelin-associated proteins in vitro.21–25 Several combinatorial therapies to promote axonal growth and functional recovery after for including a method for increasing cAMP.21,22 Ro- lipram is a type VI cyclic nucleotide phosphodiesterase inhibitor most commonly used in axonal regeneration experiments.
An extrapolation of the benefits of blocking myelin-associated inhibitors to human SCI is limited by two factors. First, the experimental studies have focused on simplified transection models of SCI that do not include the complex inflammatory and hemorrhagic tissue reaction that occurs in typical clinical injuries caused by abrupt contusion from displaced vertebral bodies. Second, most previous experiments using simple transection studies have initiated therapy at the time of injury. As is clear from numerous studies of stroke, the benefits of immediate therapy are not always translatable to the clinic.
Here, we determined whether blocking myelin-associated inhibitors with NgR(310)ecto-Fc is effective under conditions thought to be most similar to those encountered clinically. The recovery of rats from spinal contusion injury was assessed.26 Treatment was initiated by intrathecal spinal catheter at the time of injury or was provided by an intracerebroventricular catheter beginning at 3 days after injury. Furthermore, a combination of rolipram and NgR(310)ecto-Fc was compared with monotherapy. We report that NgR(310)ecto-Fc substantially improves locomotor recovery. This recovery is associated with CST and raphespinal axonal growth. Rolipram is less effective and is not synergistic with NgR(310)ecto-Fc. These findings provide a rationale for investigating the benefit of NgR(310)ecto-Fc therapy in human SCI.
Materials and Methods
Rat Spinal Contusion Injury with Immediate Intrathecal Therapy
At Ohio State University, female Sprague–Dawley rats (11–12 weeks; 250–270gm) were anesthetized with intraperitoneal ketamine (60mg/kg) and xylazine (10mg/kg) for surgeries. Intrathecal catheters of PE60 tubing were heated and stretched to an overall length of approximately 8 to 9cm. The neck of the catheter was about 4.5 cm in length with a pore diameter at the tip of 0.25mm. After a laminectomy, the catheter tip was inserted through the dura between T12 and T13 and threaded rostrally to approximately T10. The catheter was sutured to muscle and ligaments at T13, and a drop of superglue was used to fix the silk suture to the tubing. There was no evidence of spinal trauma during catheter implantation as judged by Basso-Beattie Bresnahan (BBB) scores ≥ 20 in the postcatheter, precontusion period.
In a second surgical procedure 7 days after catheter placement, a laminectomy was created at spinal level T8. Moderate contusion SCI was completed at T8 by rapidly displacing the surface of the cord 1.1mm over a 20-millisecond epoch with the Ohio State University impact device. Osmotic minipumps (Alza Scientific Products, Palo Alto, CA) were connected to the intrathecal cannula immediately after the spinal contusion. The pumps delivered 0.25µl/hour for 28 days and were filled with 1mg NgR(310)ecto-Fc in 0.2ml phosphate-buffered saline (PBS) or PBS alone. The dose of NgR(310)ecto-Fc was 0.14mg/kg/day. Bladder expression, fluid supplementation, and gentomicin antibiotics were administered to all rats after spinal contusion following approved animal care protocols.
Rat Spinal Contusion Injury with Subacute Intracerebroventricular or Subcutaneous Therapy
For delayed intracerebroventricular therapy of spinal contusion, a second group of animals was studied at Yale University. These female Sprague–Dawley rats (11–12 weeks; 250–270gm) were anesthetized with intraperitoneal ketamine (60mg/kg) and xylazine (10mg/kg), and laminectomy was conducted at the caudal portion of T6 and all T7 spinal levels. A T7 moderate contusion injury (10gm, 25mm) was produced by using the Multicenter Animal Spinal Cord Injury Study (MASCIS) impactor.26 Immediately after the contusion injury, rats were placed in a stereotaxic frame. The scalp was opened, a burr hole was drilled through the skull, and a cannula (Alzet brain infusion kit II; Alza Scientific Products) was introduced into the right lateral ventricle at stereotaxic coordinates 0.6mm posterior and 1.2mm lateral to bregma and 4.0mm deep to the pial surface.27,28. The cannula was connected to an osmotic minipump containing PBS placed subcutaneously over the scapulae. The cannula was glued in place with cyanoacrylate, and the skin was sutured.
Three days after contusion injury, the rats were reanesthetized and the minipumps were replaced with new osmotic minipumps (Alza Scientific Products) connected to the same cannula. These pumps delivered 2.5µl/hour for 28 days and were filled with 2.25mg NgR(310)ecto-Fc in 2ml PBS (0.29mg/kg/day) or PBS alone.19,27,28 At 3 days after injury, another Alzet 2ML4 osmotic minipump was implanted subcutaneously without an intracerebroventricular cannula and was filled with either PBS or 8mg rolipram in 16% dimeth-ylsulfoxide/PBS solution (1.2mg/kg/day). In the subacute treatment experiment, there were four treatment groups: intracerebroventricular PBS plus subcutaneous PBS (n = 15), intracerebroventricular NgR(310)ecto-Fc plus subcutaneous PBS (n = 16), intracerebroventricular PBS plus subcutaneous rolipram (n = 10), and intracerebroventricular NgR(310)ecto-Fc plus subcutaneous rolipram (n = 8).
Rat Corticospinal Tract Tracing
To trace CST axons in the intracerebroventricular treatment groups, we made a burr hole in the skull overlying the left sensorimotor cortex after removal of the minipump and cannula at 36 days after contusion injury.15,19,27 Biotin dextran amine (BDA; molecular weight 10,000; 10% in PBS; Molecular Probes, Eugene, OR) was applied at seven injection sites at a depth of 1.5mm from the cortical surface.
Behavioral Testing
The BBB locomotor scale was used for rat behavioral testing.29,30 All behavioral tests were performed by a researcher who was blinded to the identity of the compound in the minipump.
Histology and Analysis
Two weeks after BDA injection, animals were perfused transcardially with PBS, followed by 4% paraformaldehyde/PBS solution. The spinal cord 10mm rostral to and 10mm caudal to the lesion center was embedded in a glutaraldehyde-polymerized albumin matrix and cut parasagittally in the thickness of 40µm on a vibratome. Transverse sections (40µm) were collected from the spinal cord 11 to 16mm rostral to and 11 to16mm caudal to the lesion center. The sections were incubated with avidin-biotin-peroxidase complex plus anti–5-hydroxytryptamine (anti–5-HT) antibody (1:10,000; Immunostar, Hudson, WI) to detect corticospinal and raphespinal fibers. BDA-labeled CST axons were visualized with nickel-enhanced diaminobenzidine horseradish peroxidase reaction or while serotonergic fibers were detected with Alexa568-labeled secondary antibody.15,19,27,31
Image analysis was accomplished with National Institutes of Health (NIH) image version 1.62, as described previously.15,19,27,31 For analysis of serotonin innervation, immunoreactive serotonin fibers in the ventral horn of transverse sections rostral or caudal to the lesion center were selected by thresholding; then the length of serotonin fiber per area was measured after using the skeletonize function. Three longitudinal sections with largest cystic cavity from each animal were selected for analysis of tissue damage. Lesion cavity areas and the widths of spared tissue at the lesion center were measured, and the values from these sections were averaged. For analysis of CST sprouting, three longitudinal and transverse sections 11 to 15mm rostral to the lesion center were selected. The CST fiber number at various distances rostral to the lesion center of the injury site was measured as described earlier. The optical density of dorsal CST and ipsilateral dorsal area were measured. The value was obtained by using the value of ipsilateral upper quarter dorsal area divided by the density of dorsal CST. For camera Lucida-style tracing of BDA-labeled CST fibers, serial longitudinal sections were photographed digitally, and fibers were traced on a computer using Adobe Photoshop 7.0 software (Adobe Systems, Mountain View, CA). For camera Lucida tracing of 5-HT–immunoreactive fibers, 10 serial sections at 200µm intervals from each animal were traced using the same method as for CST axons.
Results
Effectiveness of NgR(310)ecto-Fc in Recovery from Spinal Contusion
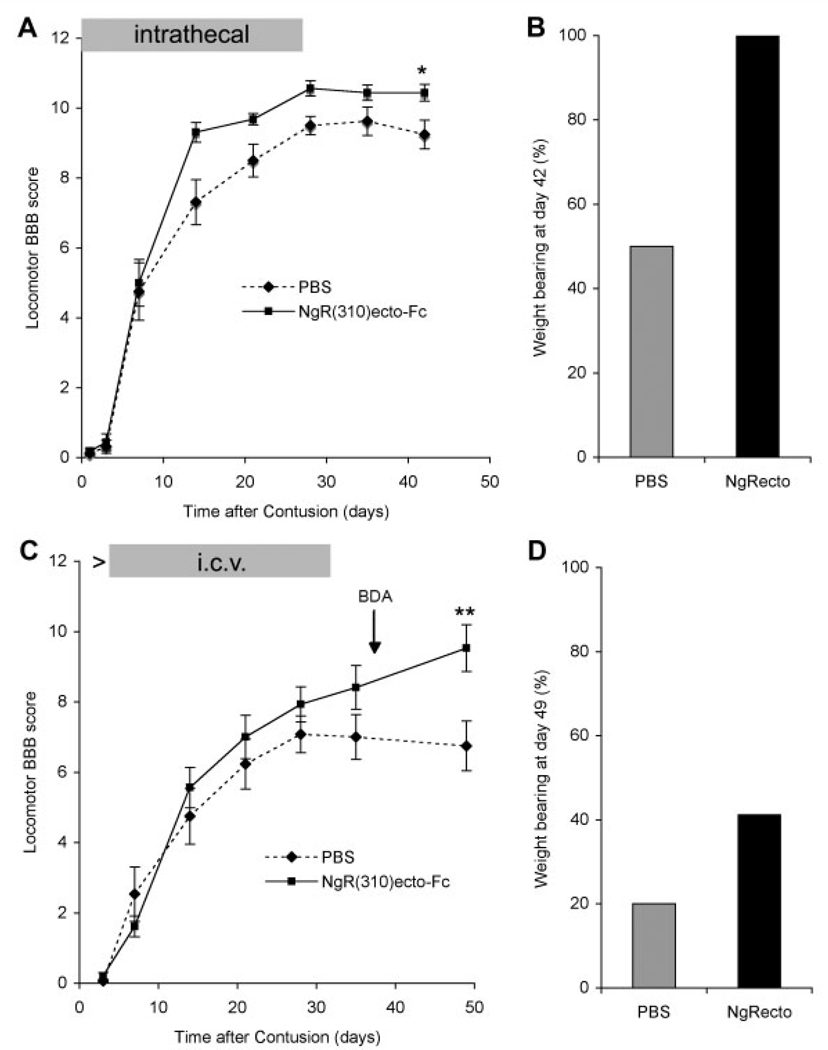
We administered NgR(310)ecto-Fc to rats with moderate spinal contusion injuries. In a first round of experimentation, a preimplanted intrathecal spinal catheter was attached to a minipump providing NgR(310)ecto-Fc (0.14mg/kg/day) or vehicle for 28 days. Behavioral recovery was assessed by the BBB locomotor score (Fig 1A). Using repeated-measures analysis of variance (ANOVA), we found significant between-group differences (p = 0.054) across time (p < 0.001). Recovery was greater at postinjury day 42 in the NgR(310)ecto-Fc–treated group (10.44 ± 0.24) compared with the vehicle group (9.25 ± 0.41) (p ≤ 0.03, post hoc one-way ANOVA). A significantly greater fraction of rats achieved the ability to bear weight on at least one hind limb after NgR(310)ecto-Fc treatment (8/8) than with PBS (4/8) (see Fig 1B).
Fig 1.
NgR(310)ecto-Fc treatment improves functional recovery after rat spinal contusion injury. (A) The locomotor Basso-Beattie Bresnahan (BBB) score is reported as a function of time after contusion injury at T8 spinal level in the acute intrathecal vehicle-(dashed line) or NgR(310)ecto-Fc–treated (solid line) rats. Treatment duration is indicated by the gray bar. Data are mean ± standard error of the mean (SEM); n = 8. Repeated-measures analysis of variance (ANOVA) for treatment group, time, and their interaction indicated an effect of NgR(310)ecto-Fc treatment with p = 0.054. Post hoc one-way ANOVA at 42 days after contusion demonstrated a significant (*p ≤ 0.03) improvement of locomotion at day 42 after contusion. (B) The percentage of the animals that support their body weight with at least one hind limb is greater in the NgR(310)ecto-Fc–treated animals than that in phosphate-buffered saline (PBS)–treated rats at day 42 after injury. (C) Delayed subacute intracerebroventricular (i.c.v.) treatment with PBS or NgR(310)ecto-Fc was provided to rats after the moderate contusion injury at T7 spinal level from days 3 to 31 (gray bar). The BBB score is reported as a function of time. The timing of biotin dextran amine (BDA) injection for axonal tracing is indicated. Repeated-measures ANOVA analysis for treatment group, time, and their interaction indicated an effect of NgR(310)ecto-Fc treatment with p ≤ 0.05. One-way ANOVA at 49 days after contusion demonstrated a significant (**p ≤ 0.01) improvement of locomotion at day 49 after contusion. Data are mean ± SEM; n = 15 to 16 animals per group. (D) The percentage of the animals with BBB score ≥ 9 (supporting their body with hind limbs) is reported for the subacute delayed i.c.v. treatment rats at 7 weeks after contusion.
A second group of spinal contused rats received NgR(310)ecto-Fc treatment only in a subacute mode, with therapy beginning 3 days after contusion injury. In this experiment, the severity of contusion created with a different apparatus at a different institution was slightly greater than in the first experiment, as evidenced by the control group recovery. Because treatment was initiated after injury, we chose to avoid any manipulation of the inflamed injury site by infusing protein into one lateral ventricle, physically distinct from the contusion itself. To further simplify surgical methods, we inserted intracerebroventricular catheters into all animals during the same operative procedure as for the contusion, but a minipump with PBS was connected to the catheter for the first 3 days. After 3 days, the pump was exchanged for a second pump containing either NgR(310)ecto-Fc or vehicle. The intracerebroventricular infusion continued from days 3 to 31 after injury at the dose of 0.29mg/kg/day, with behavioral observation over 49 days.
BBB locomotor scores showed a significant improvement in locomotion for the NgR(310)ecto-Fc–treated group (see Fig 1C; repeated-measures ANOVA, difference between groups p ≤ 0.05 and across time p ≤ 0.001). At the day 49 end point, scores in the control group (6.75 ± 0.7; n = 15) were lower than in the NgR(310)ecto-Fc group (9.5 ± 0.7; n = 16) by oneway ANOVA (p ≤ 0.01). Notably, the benefit of NgR(310)ecto-Fc increased with time, even beyond the end of treatment at 31 days. The percentage of animals reaching a weight-bearing status (BBB ≥ 9) was 42% in the NgR(310)ecto-Fc group versus 20% in the control group (see Fig 1D).
Histological Analysis for NgR(310)ecto-Fc Treatment
The molecular rationale behind NgR(310)ecto-Fc therapy is the stimulation of axonal sprouting and repair rather than a neuroprotective effect.2,19 Consistent with this hypothesis, histological studies demonstrate that the lesion size, cavitation, and tissue sparing were unaffected by the subacute NgR(310)ecto-Fc treatment (Fig 2).
Fig 2.
Intracerebroventricular NgR(310)ecto-Fc treatment does not alter cavitation or spared tissue at the site of a contusion injury. (A, B) Parasagittal sections of the lesion segment of spinal cord from both the phosphate-buffered saline (PBS)– (A) and NgR(310)ecto-Fc–treated (B) animals show a large cystic cavity within the lesion center. The area of the cavity is outlined in black, and the spared tissue is indicated by the vertical bars. Rostral is to the left, and dorsal is up. Horizontal scale bar = 500µm. (C, D) There is no difference between the extent of spared tissue (C) or the area of cystic cavity (D) between two treatment groups. Data are mean ± standard error of the mean; n = 15 to 16 animals per group.
Two axonal fiber systems were examined in the subacute treatment groups. Raphespinal serotonergic axons were assessed by immunohistology in transverse sections of the lumbar spinal cord more caudal to the lesion and in thoracic sections rostral to the contusion (Fig 3). In rostral sections, intact 5-HT fiber ramification is extensive in the ventral horn and is unaltered by NgR(310)ecto-Fc treatment (see Figs 3A, B, E). Caudal to the contusion site, nearly all 5-HT fibers are lost in control spinal contused animals, but 5-HT fiber length is significantly increased in the NgR(310)ecto-Fc–treated animals (see Figs 3C, D, F). To explore the basis of increased caudal raphespinal innervation in the NgR(310)ecto-Fc–treated animals, we examined longitudinal sections that spanned the injury (see Fig 3G–N). Camera Lucida drawings and fiber counts indicate that NgR(310)ecto-Fc increases the number of 5-HT fibers in spared tissue bridges at the contusion site and further increases fiber density in caudal spinal cord. Thus, the delayed NgR(310)ecto-Fc treatment is likely to enhance both caudal sprouting from spared fibers and long-distance regenerative growth across the lesion.
Fig 3.
Raphespinal fiber growth is increased by NgR(310)ecto-Fc treatment after spinal contusion injury. (A, B) Transverse sections of spinal cord stained with anti-serotonin antibodies 11 to 15mm rostral to the lesion center from phosphate-buffered saline (PBS)–(A) and NgR(310)ecto-Fc–treated (B) animals show similar numbers of serotonergic fibers in the ventral horn. (C, D) Transverse sections of spinal cord stained with anti-serotonin antibodies 11 to 15mm caudal to the lesion center show a significantly greater number of serotonergic fibers in the ventral horn of NgR(310)ecto-Fc–treated (D) animals compared with the PBS-treated (C) animals. (E, F) Measurements of serotonergic fiber length per 100µm2 in the ventral horn rostral and caudal to the contusion indicate that there is a significant increase of serotonergic fibers in the ventral horn caudal to the lesion in the NgR(310)ecto-Fc–treated animals, but no difference above the lesion. Data are mean ± standard error of the mean; n = 15 to 16 animals per group. **p < 0.01, Student’s t test. (G, H) Low-magnification view of serotonin-stained parasagittal section from PBS- (G) and NgR(310)ecto-Fc–treated (H) animals. Rostral is to the left, and dorsal is up. (M) High-magnification view of squared area in (H) demonstrates the serotonin-positive stained fibers several millimeters caudal to the lesion. (N) Serotonergic fiber number at various distances rostral and caudal to the lesion center from NgR(310)ecto-Fc–treated (black bars) and control animals (gray bars denote PBS treatment) is reported. **p < 0.01, Student’s t test. For the x-axis, a positive value is rostral to the center of the contusion, and a negative value is caudal to the center of the lesion. (I–L) Camera Lucida drawings of 5-hydroxytryptamine (5-HT) fibers from four separate rats. Each drawing is a composite assembled from a set of 10 parasagittal sections spaced at intervals of 200µm across the spinal cord. The contusion cavity is encircled near the center of each image. Increased numbers of serotonergic fibers are observed in the caudal spinal cord in the NgR(310)ecto-Fc–treated (K, L) animals compared with the PBS-treated (I, J) animals. Scale bars = 25µm (A); 1,000µm (G, I).
Descending CST fibers were traced from cortical BDA tracer injections in these animals. In control animals, all dorsal column CST fibers were severed by the contusion, and most retract rostrally from the lesion center by 3mm (Fig 4). In NgR(310)ecto-Fc–treated animals, spinal cross sections at 10mm rostral to the contusion showed increased numbers of fibers that sprouted from the dorsal CST, similar to the pattern observed in our previously published dorsal hemisection injury studies.19 In longitudinal sections, containing the injury site, many fibers from the NgR(310)ecto-Fc–treated rats extend close to the injury cavity, and significantly greater numbers of CST fibers were present at 2 to 4mm rostral to the injury. No CST fibers were observed caudal to the lesion sites in any animals.
Fig 4.
Intracerebroventricular NgR(310)ecto-Fc treatment increases the sprouting of corticospinal tract (CST) fibers rostral to the injury. (A, B) Transverse sections at 11 to 15mm rostral to the lesion center from phosphate-buffered saline (PBS)– (n = 7) and NgR(310)ecto-Fc–treated (n = 9) rats show a greater number of biotin dextran amine (BDA)–labeled CST fibers outside of the dorsal column in NgR(310)ecto-Fc–treated animals (arrow). (inset) Schematic illustrates the orientation of the section. Dorsal is up. (C) Ratio of optical density of sprouting BDA-labeled fibers in the ipsilateral upper quarter outside of the dorsal column based on the density of BDA staining in the dorsal column was measured. There is no statistical difference of the value between two groups (Student’s t test). Data are mean ± standard error of the mean (SEM); n = 15 to 16 animals per group. (D, E) Parasagittal sections illustrate the distance of BDA-labeled descending corticospinal tract (dCST) fibers to the lesion center (D: PBS-treated animal; E: NgR(310)ecto-Fc– treated animal; C indicates lesion cystic cavity). Rostral is to the left, and dorsal is up. (F) Counts of CST fibers show a great number of BDA-labeled dCST fibers in the NgR(310)ecto-Fc–treated group than in control group from 2.0 to 4.0mm rostral to the lesion center. Data are mean ± SEM; n = 15 to 16 animals per group. *p < 0.05; **p < 0.01, Student’s t test. (G–J) Camera Lucida drawings of CST fibers from four spinal-contused animals (G, H: PBS-treated group; I, J: NgR(310)ecto-Fc–treated group). Images are generated from all serial parasagittal sections for each rat. Rostral is to the left, and dorsal is up. The contusion cavity is at the right. Scale bars = 100µm (B); 500µm (D, J).
Effectiveness of Subacute Rolipram in Spinal Contusion
Another cohort of spinal contused animals was treated with subcutaneous rolipram from 3 to 31 days after contusion with the MASCIS device. The rats received a total of 8mg rolipram over 28 days per 275gm rat, corresponding to 1mg/kg/day, similar to that in published studies.21,22 There was a trend toward improved performance as measured by the BBB score, but this did not reach statistical significance (Fig 5A). Day 49 BBB score in the control group was 6.75 ± 0.7 and in the rolipram group 7.8 ± 0.3 (n = 10). There was also a trend for rolipram to improve early postinjury performance to a greater extent than later performance.
Fig 5.
Combined treatment of NgR(310)ecto-Fc and rolipram does not yield additional benefit for locomotor recovery after contusion injury. (A) Basso-Beattie-Bresnahan (BBB) scores as a function of time in rolipram- (n = 10) versus vehicle-treated (n = 15) rats after spinal injury. Data are mean ± standard error of the mean (SEM). Solid line indicates PBS treatment; dotted line indicates rolipram. (B) BBB scores are reported for the rolipram plus NgR(310)ecto-Fc combined treatment group (n = 8) relative to the vehicle- and NgR(310)ecto-Fc–treated groups from Figure 1C. One-way analysis of variance at 49 days after contusion demonstrated a significant (*p ≤ 0.05) improvement of locomotion in both the Nogo-66 receptor (NgR; dashed line) alone and the NgR+ rolipram groups (dotted line) relative to the phosphate-buffered saline (PBS; solid line) group at day 49 after contusion. Data are mean ± SEM. BDA = biotin dextran amine.
Lack of Synergy for NgR(310)ecto-Fc/Rolipram Dual Therapy in Spinal Contusion
Rolipram and NgR(310)ecto-Fc treatments were combined in a set of eight animals with spinal contusions created by the MASCIS device. The doses, routes, and timing of administration were identical to the monotherapy trials described earlier. The NgR+rolipram group showed a degree of recovery from spinal contusion that was indistinguishable from the NgR-only group and was significantly greater than the PBS group (see Fig 5B; p ≤ 0.05, ANOVA at day 49). There was no evidence for a synergistic benefit for rolipram therapy under these conditions despite the trend toward rolipram efficacy as monotherapy (see Fig 5A).
Discussion
The principal finding of this work is that NgR(310)ecto-Fc therapy increases the recovery of spinal-injured rats under conditions most likely to mimic clinical SCI. The percentage of rats achieving a weight-bearing status was doubled by NgR(310)ecto-Fc treatment. The contusion lesion has been recognized as a faithful reproduction of the histological and neurological pattern of human SCI.26 Although the acute and subacute experimental groups are not identical, NgR(310)ecto-Fc therapy appears equally effective when initiated at the time of injury or 3 days afterward. The 3-day delay for NgR(310)ecto-Fc therapy fits well within a time frame when acutely injured patients may be stabilized and therapy initiated.
As predicted from its mechanism of action in vitro, NgR(310)ecto-Fc promoted axonal growth but not tissue sparing from contusion damage.18–20,27 Axonal growth was observed in both the corticospinal system and the raphespinal system. Raphespinal fibers were not completely severed by spinal contusion in control animals. In the NgR(310)ecto-Fc–treated animals, increased numbers of fibers were observed in remaining tissue bridges, and increased fibers were detected within caudal spinal cord. Presumably, the increased axons in NgR(310)ecto-Fc rats sprouted at some time more than 3 days after injury. Extensive time course studies might be used to define the window for such fiber growth. Notably, CST growth was confined to the region rostral to the injury and did not reach the distal cord; full regenerative growth was not observed. The findings differ from the result in simple hemisection19 and are likely to be due to the paucity in astroglial scar-free tissue bridges bypassing the injury site after contusion injury. These scars contain chondroitin sulfate proteoglycan inhibitors of axonal growth that are not sensitive to NgR(310)ecto-Fc treatment. It remains likely that the rostral CST axon sprouts induced by NgR(310)ecto-Fc may contribute to recovery through an enhancement of polysynaptic connections to the caudal spinal cord.32
Rolipram increases intracellular cAMP by inhibiting its breakdown by phosphodiesterase. After central nervous system injury, elevated cAMP levels have been shown to contribute to enhanced axonal growth in several treatment paradigms combining pharmacological and cellular therapies.21–23 In one study, a subgroup of rats received a 2-week course of rolipram alone beginning at the time of a contusion.21 Histological and behavioral evidence supported a neuroprotective benefit of this immediate therapy. In this study, we demonstrate a short-term benefit for rolipram therapy when begun with a clinically feasible delay of 3 days after contusion. This effect was most pronounced early and was not significant at later time points, consistent with a mechanism of action that is neuroprotective or anti-inflammatory.21 In vitro studies are consistent with the hypothesis that altering cAMP levels in astroglial or microglial cells may be an additional site of action beyond changes in axonal regeneration.
Regardless of the in vivo site(s) of action, the addition of rolipram to the NgR(310)ecto-Fc treatment regimen did not result in further benefit. The lack of further improvement might be explained by shared redundant actions on axonal growth regulating pathways, by a “ceiling effect” for maximal benefit that was reached by NgR(310)ecto-Fc alone, or by the minimal benefit afforded by delayed rolipram therapy. In addition, although this dose of rolipram has been associated with elevated cAMP levels in vivo,21 it remains a possibility that cAMP levels could have been either too low or too high in our treatment group to see an effect with rolipram.
We conclude that NgR(310)ecto-Fc therapy can promote axonal growth and functional recovery in preclinical rodent SCI studies designed to mimic most closely human SCI. Thus, this work provides an indication that clinical use of human NgR(310)ecto-Fc protein may reduce the neurological deficits associated with traumatic SCI.
Acknowledgments
This work was supported by the Christopher Reeve Paralysis Foundation, (S.M.S.), the Falk Medical Research Trust, (S.M.S.), the NIH, (R01NS39962, S.M.S.) and BiogenIdec (S.M.S, D.M.B.). The NgR(310)ecto-Fc protein was provided by BiogenIdec.
References
- 1.McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- 2.Lee DH, Strittmatter SM, Sah DW. Targeting the Nogo receptor to treat central nervous system injuries. Nat Rev Drug Discov. 2003;2:872–878. doi: 10.1038/nrd1228. [DOI] [PubMed] [Google Scholar]
- 3.Grandpre T, Strittmatter SM. Nogo: a molecular determinant of axonal growth and regeneration. Neuroscientist. 2001;7:377–386. doi: 10.1177/107385840100700507. [DOI] [PubMed] [Google Scholar]
- 4.Fournier AE, Strittmatter SM. Repulsive factors and axon regeneration in the CNS. Curr Opin Neurobiol. 2001;11:89–94. doi: 10.1016/s0959-4388(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 5.Wang KC, Koprivica V, Kim JA, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 6.Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 7.Domeniconi M, Cao Z, Spencer T, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- 8.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 9.Prinjha R, Moore SE, Vinson M, et al. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- 10.GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 11.Chen MS, Huber AB, van der Haar ME, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 12.Mukhopadhyay G, Doherty P, Walsh FS, et al. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 13.McKerracher L, David S, Jackson DL, et al. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- 16.Liebscher T, Schnell L, Schnell D, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- 17.Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Kim JE, Budel S, et al. Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury. Mol Cell Neurosci. 2005;29:26–39. doi: 10.1016/j.mcn.2004.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Liu BP, Budel S, et al. Blockade of nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fournier AE, Gould GC, Liu BP, Strittmatter SM. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci. 2002;22:8876–8883. doi: 10.1523/JNEUROSCI.22-20-08876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearse DD, Pereira FC, Marcillo AE, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 22.Nikulina E, Tidwell JL, Dai HN, et al. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu P, Yang H, Jones LL, et al. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu J, Cai D, Dai H, et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- 25.Cai D, Qiu J, Cao Z, et al. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young W. Spinal cord contusion models. Prog Brain Res. 2002;137:231–255. doi: 10.1016/s0079-6123(02)37019-5. [DOI] [PubMed] [Google Scholar]
- 27.Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JH, Gimbel DA, GrandPre T, et al. Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition. J Neurosci. 2006;26:1386–1395. doi: 10.1523/JNEUROSCI.3291-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basso DM, Beattie MS, Bresnahan JC, et al. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- 30.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neuro-trauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 31.Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Bareyre FM, Kerschensteiner M, Raineteau O, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]