Abstract
High systolic blood pressure (SBP) is a predictor of survival for patients with heart failure (HF). Whether SBP predicts survival in both ischemic and nonischemic HF has not been well examined. We analyzed 2,178 patients with advanced HF (47.3% ischemic etiology, 75.5% men, 93.5% New York Heart Association class III or IV, age 52 ± 13, left ventricular ejection fraction 24 ± 9%) referred to a university center between 1983 and 2006. SBP and invasive hemodynamic variables were recorded after optimization of medical therapy. Patients were divided into SBP quartiles (≤90, 91 to 100, 101 to 112, ≥113 mm Hg) based on SBP frequency. Survival free from death or urgent transplant in ischemic versus nonischemic HF was 53.2% versus 61.1% at 2 years. Higher SBP quartile was associated with increased survival in the total cohort and in subgroups of both nonischemic and ischemic HF. On multivariate analysis adjusting for age, left ventricular ejection fraction, cholesterol, gender, diabetes mellitus, pulmonary capillary wedge pressure, cardiac index, New York Heart Assocation class, β-blocker use, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use, statin use, and smoking history, relative risk (95% confidence interval) of death or urgent transplant at 2 years for quartile 1 compared with quartile 4 was 1.9 (1.4 to 2.6) in the total cohort, 1.6 (1.1 to 2.5) in nonischemic HF, and 2.4 (1.5 to 3.7) in ischemic HF. In conclusion, SBP predicts HF survival in both ischemic and nonischemic HF independent of other risk factors and invasive hemodynamic variables.
The relation between elevated systolic blood pressure (SBP) and adverse cardiovascular outcomes, including myocardial infarction and stroke, has been well documented in the general population.1–5 Recent studies have shown that in populations with heart failure (HF), high SBP is associated with improved, not adverse, outcomes.6–12 Numerous studies found a similar association in both acute6–9 and chronic10,11 HF. The risk factors, natural history, and outcomes of patients with ischemic versus nonischemic etiology of HF are divergent. Thus, this study aimed to investigate whether SBP is an independent predictor of survival in both ischemic and nonischemic chronic HF. In this study, we evaluate the relation between SBP in patients with HF on optimal medical therapy and outcomes in patients with HF of both ischemic and nonischemic etiology. Furthermore, we investigated whether SBP predicts survival in chronic HF independent of invasive measurements of intracardiac filling pressures.
Methods
The initial study population consisted of 2,796 patients referred to a HF center at a university hospital for HF management and/or transplant evaluation from 1983 to 2006; 618 patients were excluded from our study because there was no blood pressure data after optimal HF therapy. The final study cohort consisted of 2,178 patients (1,148 ischemic HF, 1,030 nonischemic HF). Patients with both left ventricular (LV) systolic dysfunction and preserved systolic function were included. Patients with LV ejection fraction (EF) >40% were considered to have preserved systolic function, and those with LVEF ≤40% were considered to have systolic dysfunction. All patients were followed in a comprehensive HF management program as previously described.13 The Medical Institutional Review Board approved the medical record review.
Detailed information on the patients’ baseline characteristics and medications were recorded at their initial visits and each follow-up visit. LVEF was determined by echocardiography obtained at the time of referral. Laboratory testing, echocardiography, and right-sided heart catheterization occurred within 6 weeks of the initial referral date. Hemodynamic variables, including systolic and diastolic blood pressure, used in analyses were those obtained after optimal HF medical therapy had been instituted. Blood pressure values used in our study were obtained via sphygmomanometry. Previous left-sided heart catheterization reports and angiographic films were reviewed. If not done previously, left-sided heart catheterization was performed. Significant coronary artery disease was defined as any single diameter stenosis >70% of the involved artery on angiography. Patients were classified as having HF secondary to nonischemic cardiomyopathy if they had no history of myocardial infarction and cardiac catheterization was without significant coronary artery disease.
The primary end point of the study was a composite of all-cause mortality or urgent transplantation (status 1A). Status 1A transplants were included in the primary end point because these patients were expected to live <1 week without a transplant and are dependent on intravenous medication, ventricular assist device, or mechanical ventilation. Nonurgent transplants (status IB and II) were coded as nonfatal end of follow-up at the time of transplantation. All-cause mortality was analyzed as a secondary end-point. Patients lost to follow-up were censored at the time they were last known to be alive and well.
Death was considered sudden if it was unexpected on the basis of the patient’s clinical status and if it occurred out of the hospital <15 minutes after the onset of unexpected symptoms or during sleep. Death during hospitalization for decompensated HF was considered death due to HF.
Patients were grouped into SBP quartiles (≤90, 91 to 100, 101 to 112, ≥113), diastolic blood pressure quartiles (≤50, 51 to 59, 60 to 67, ≥68), and mean arterial pressure quartiles (≤65, 66 to 72, 73 to 81, ≥82) based on frequency in our cohort. Results are included as mean ± SD for continuous variables and as percentages of the total for categorical variables. The independent samples t test and chi-square test were used for comparison of continuous and categorical variables, respectively. Nonparametric tests were used as appropriate. Survival curves were calculated by the Kaplan-Meier method and differences between the curves were evaluated with the log-rank statistic. Univariate and multivariate Cox regression analyses were employed to calculate the estimated hazard ratio with 95% confidence interval (CI). SPSS for Windows version 15.0 (SPSS, Inc., Chicago, Illinois) was used for all analyses.
Results
The cohort was 75% men and ranged in age from 18 to 87 years (mean age 52 ± 13). New York Heart Association class III or IV was 35.2% and 57.7% of the total cohort, respectively. The etiologies of HF were ischemic (47.3%), idiopathic (34.9%), valvular, toxin-induced, hypertrophic, and peripartum. The mean LVEF was 24 ± 9%. The mean length of follow-up for patients was 1,214 days and the median length of follow-up was 478 days.
The characteristics of the cohort for the SBP quartiles are listed in Table 1. There was an equal distribution of ischemic and nonischemic cardiomyopathy in the 4 SBP quartiles. Patients with higher SBP were more likely to have diabetes mellitus, higher cholesterol, and a history of hypertension. Those with higher SBP had higher cardiac output, higher systemic vascular resistance, lower mean pulmonary arterial pressures, and lower pulmonary capillary wedge pressures. Higher SBP was also associated with higher LVEF and lower LV end-diastolic diameter index. Higher SBP was associated with higher hemoglobin. Patients with higher SBP were more likely to be on β blockers. Higher SBP was also associated with higher body mass index.
Table 1.
Baseline characteristics of SBP quartiles
| n | Total Cohort | SBP (mm Hg) |
p Value | ||||
|---|---|---|---|---|---|---|---|
| ≤90 | 91–100 | 101–112 | ≥113 | ||||
| Ischemic cardiomyopathy | 2,178 | 47.3% | 48.0% | 46.1% | 47.4% | 47.7% | 0.932 |
| Demographics | |||||||
| Age | 2,178 | 52 ± 13 | 53 ± 13 | 51 ± 13 | 53 ± 13 | 53 ± 12 | 0.007 |
| Men | 2,178 | 75.5% | 75.0% | 75.0% | 76.5% | 75.6% | 0.934 |
| Black | 2,178 | 8.0% | 6.4% | 7.9% | 8.5% | 9.4% | 0.315 |
| Body mass index (kg/m2) | 1,849 | 26.1 ± 6.3 | 25.1 ± 8.2 | 25.6 ± 5.4 | 26.2 ± 5.3 | 27.5 ± 5.6 | <0.001 |
| Cardiac risk factors | |||||||
| Diabetes mellitus | 1,824 | 28.3% | 24.8% | 24.9% | 27.9% | 35.3% | 0.001 |
| Hypertension | 1,830 | 41.8% | 31.5% | 34.5% | 45.8% | 54.4% | <0.001 |
| Tobacco use | 2,178 | 67.8% | 68.6% | 71.3% | 67.3% | 63.7% | 0.060 |
| Medications | |||||||
| β-Blocker use | 1,524 | 30.5% | 22.4% | 24.9% | 30.4% | 43.1% | <0.001 |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use | 1,545 | 82.7% | 78.7% | 81.5% | 84.4% | 85.4% | 0.065 |
| Statin use | 2,178 | 39.7% | 38.1% | 37.5% | 37.6% | 46.0% | 0.011 |
| Cardiac characteristics | |||||||
| NYHA class III or IV | 2,178 | 93.5% | 95.8% | 93.9% | 93.1% | 91.0% | 0.015 |
| LVEF (%) | 2,149 | 23.6 ± 9.0 | 21.6 ± 7.7 | 22.5 ± 8.9 | 24.1 ± 8.8 | 26.2 ± 9.8 | <0.001 |
| LVEF ≤40% | 2,149 | 95.9% | 98.5% | 96.2% | 95.8% | 93.0% | <0.001 |
| LV end diastolic diameter index (mm/cm2) | 1,597 | 37.1 ± 6.9 | 38.9 ± 6.9 | 37.5 ± 6.7 | 36.7 ± 6.8 | 35.0 ± 6.8 | <0.001 |
| Peak oxygen consumption (ml/kg/min) | 1,048 | 13.4 ± 4.7 | 12.5 ± 4.2 | 13.3 ± 4.7 | 13.7 ± 5.3 | 13.9 ± 4.3 | 0.008 |
| Hemodynamics | |||||||
| Heart rate (beats/min) | 2,057 | 87 ± 16 | 88 ± 16 | 88 ± 16 | 87 ± 16 | 86 ± 17 | 0.245 |
| SBP (mm Hg) | 2,178 | 103 ± 18 | 84 ± 6 | 97 ±3 | 107 ± 3 | 128 ± 14 | <0.001 |
| Diastolic blood pressure (mm Hg) | 2,178 | 59 ± 12.2 | 50 ± 9 | 56 ±9 | 61 ± 10 | 69 ± 12 | <0.001 |
| Cardiac output (L/min) | 2,019 | 4.7 ± 1.1 | 4.3 ±1.0 | 4.6 ±1.0 | 4.9 ± 1.1 | 5.2 ± 1.3 | <0.001 |
| Cardiac index (L/min/m2) | 1,886 | 2.6 ± 0.8 | 2.4 ± 0.6 | 2.6 ± 1.1 | 2.7 ± 0.6 | 2.8 ± 0.7 | <0.001 |
| Mean pulmonary artery pressure (mm Hg) | 1,902 | 25.9 ± 7.7 | 27.0 ± 7.3 | 26.2 ± 7.9 | 26.0 ± 7.8 | 24.3 ± 7.4 | <0.001 |
| Pulmonary capillary wedge pressure (mm Hg) | 1,948 | 15.5 ± 5.9 | 16.6 ± 6.0 | 15.6 ± 5.7 | 15.6 ± 5.8 | 14.1 ± 5.6 | <0.001 |
| Systolic vascular resistance (dyn*s/cm5) | 1,989 | 1,169 ± 318 | 1,052 ± 293 | 1,127 ± 267 | 1,192 ± 300 | 1,322 ± 354 | <0.001 |
| Mean arterial pressure (mm Hg) | 2,178 | 74 ± 13 | 62 ± 7 | 70 ±6 | 76 ±7 | 89 ± 11 | <0.001 |
| Laboratory values | |||||||
| Total cholesterol (mg/dl) | 1,798 | 171 ± 56 | 159 ± 53 | 169 ± 57 | 170 ± 54 | 186 ± 58 | <0.001 |
| B-type natriuretic peptide (pg/ml) | 330 | 1,156 ± 1,109 | 1,299 ± 1,067 | 1,352 ± 1,156 | 1,135 ± 1,121 | 968 ± 1,083 | 0.092 |
| Creatinine (mg/dl) | 1,889 | 1.5 ± 1.0 | 1.5 ± 1.0 | 1.4 ± 0.7 | 1.5 ± 0.9 | 1.5 ± 1.3 | 0.514 |
| Hemoglobin (g/dl) | 1,831 | 13.3 ± 2.0 | 13.0 ± 2.0 | 13.4 ± 1.9 | 13.3 ± 2.0 | 13.5 ± 1.8 | 0.001 |
NYHA = New York Heart Association.
Baseline characteristics for those with ischemic compared to nonischemic HF are listed in Table 2. Patients with ischemic HF were more likely to be men, have diabetes, older, and black. They were also more likely to have a history of hypertension and tobacco use. Patients with ischemic HF were more likely to be on a statin, but slightly less likely to be on an angiotension-converting enzyme inhibitor or angiotensin receptor blocker. Ischemic HF was associated with lower serum hemoglobin levels. There was also an association between ischemic HF and lower heart rate, lower cardiac output, and higher pulmonary capillary wedge pressure.
Table 2.
Baseline characteristics of patients with ischemic versus nonischemic HF
| n | Total Cohort | Ischemic | Nonischemic | p Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 2,178 | 52 ± 13 | 57 ± 9 | 48 ± 14 | <0.001 |
| Men | 2,178 | 75.5% | 85.3% | 66.7% | <0.001 |
| Black | 2,178 | 8.0% | 4.4% | 11.3% | <0.001 |
| Body mass index (kg/m2) | 1,849 | 26.1 ± 6.3 | 25.9 ± 5.1 | 26.3 ± 7.3 | 0.201 |
| Cardiac risk factors | |||||
| Diabetes mellitus | 1,824 | 28.3% | 35.5% | 22.1% | <0.001 |
| Hypertension | 1,830 | 41.8% | 47.3% | 37.1% | <0.001 |
| Tobacco use | 2,178 | 67.8% | 78.6% | 58.0% | <0.001 |
| Medications | |||||
| β-Blocker use | 1,524 | 30.5% | 29.8% | 31.1% | 0.615 |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use | 1,545 | 82.7% | 79.8% | 85.1% | 0.007 |
| Statin use | 2,178 | 39.7% | 60.5% | 21.1% | <0.001 |
| Cardiac characteristics | |||||
| NYHA class III or IV | 2,178 | 93.5% | 94.4% | 92.7% | 0.118 |
| LVEF (%) | 2,149 | 23.6 ± 9.0 | 23.9 ± 8.2 | 23.3 ± 9.6 | 0.164 |
| LVEF ≤40% | 2,149 | 95.9% | 97.3% | 94.6% | 0.001 |
| LV end diastolic diameter index (mm/cm2) | 1,597 | 37.1 ± 6.9 | 36.7 ± 6.3 | 37.4 ± 7.4 | 0.051 |
| Peak oxygen consumption (ml/kg/min) | 1,048 | 13.4 ± 4.7 | 12.7 ± 4.1 | 14.0 ± 5.0 | <0.001 |
| Hemodynamics | |||||
| Heart rate (beats/min) | 2,057 | 87 ± 16 | 84 ± 15 | 90 ± 17 | <0.001 |
| SBP (mm Hg) | 2,178 | 103 ± 18 | 103 ±18 | 103 ±18 | 0.655 |
| Diastolic blood pressure (mm Hg) | 2,178 | 59 ± 12.2 | 59 ± 12 | 59 ± 12 | 0.842 |
| Cardiac output (L/min) | 2,019 | 4.7 ± 1.1 | 4.6 ±1.0 | 4.8 ± 1.2 | 0.034 |
| Cardiac index (L/min/m2) | 1,886 | 2.6 ± 0.8 | 2.5 ± 0.6 | 2.7 ± 0.9 | 0.002 |
| Mean pulmonary artery pressure (mm Hg) | 1,902 | 25.9 ± 7.7 | 26.2 ± 7.7 | 25.6 ± 7.7 | 0.078 |
| Pulmonary capillary wedge pressure (mm Hg) | 1,948 | 15.5 ± 5.9 | 15.8 ± 5.7 | 15.2 ± 6.0 | 0.016 |
| Systolic vascular resistance (dyn*s/cm5) | 1,989 | 1,169 ± 318 | 1,170 ± 310 | 1,167 ± 326 | 0.834 |
| Mean arterial pressure (mm Hg) | 2,178 | 74 ± 13 | 74 ± 12 | 74 ± 13 | 0.735 |
| Laboratory values | |||||
| Total cholesterol (mg/dl) | 1,798 | 171 ± 56 | 172 ± 56 | 171 ± 57 | 0.688 |
| B-type natriuretic peptide (pg/ml) | 330 | 1,156 ± 1,109 | 1,240 ± 1,149 | 1,079 ± 1,069 | 0.187 |
| Creatinine (mg/dl) | 1,889 | 1.5 ± 1.0 | 1.5 ± 0.9 | 1.4 ± 1.0 | 0.208 |
| Hemoglobin (g/dl) | 1,831 | 13.3 ± 2.0 | 13.2 ± 1.9 | 13.4 ± 2.0 | 0.003 |
NYHA = New York Heart Association.
During our follow-up period, there were 457 deaths and 298 urgent transplants at 1 year, and 583 deaths and 340 urgent heart transplants at 2 years. Causes of death included 19.7% sudden death, 27.7% worsening HF, and 13.1% other causes at 1 year and 19.7% sudden death, 27.4% worsening HF, and 16.0% other causes at 2 years. Two patients were lost to follow-up over the study period.
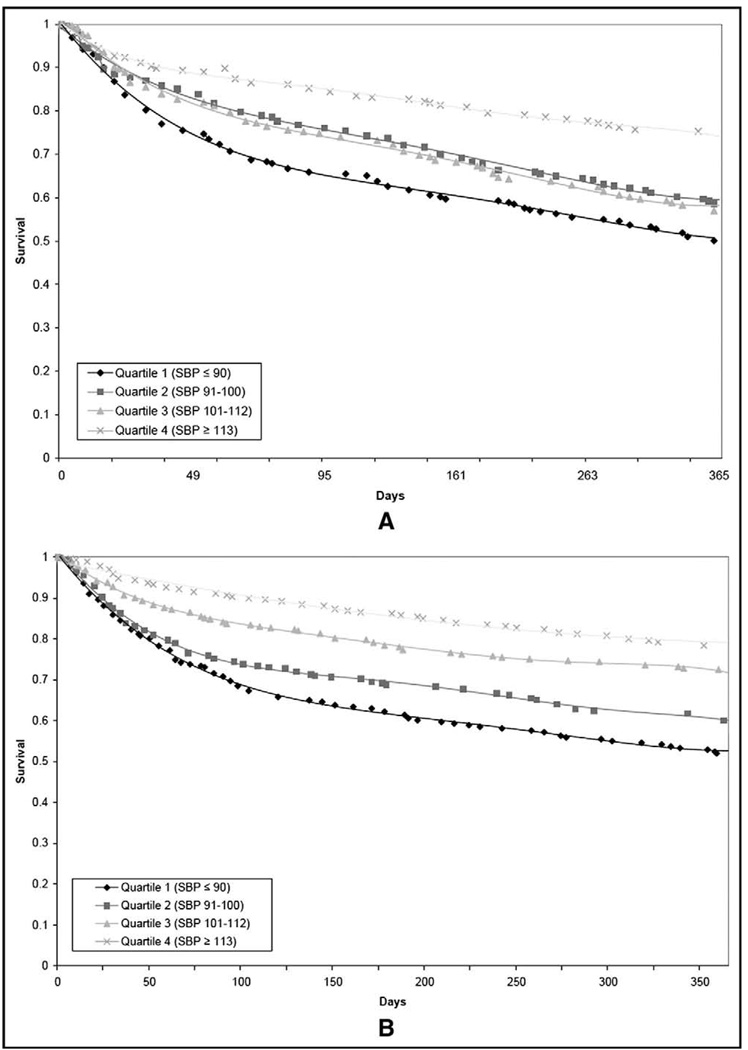
Higher SBP quartiles were associated with significantly improved survival free from all-cause mortality or urgent transplantation. In the total cohort, urgent transplant-free survival at 1 year were 55%, 63%, 73%, and 79%, for quartiles 1 (SBP ≤90), 2 (SBP 91 to 100), 3 (SBP 101 to 112), and 4 (SBP ≥ 113), respectively (p <0.001). Survival without urgent transplantation at 2 years was 49%, 57%, 66%, and 73%, respectively (p <0.001). Patients with either ischemic or nonischemic HF in the lowest SBP quartile were found to have significantly impaired survival free from urgent heart transplant compared with patients in the highest quartile (Figure 1). Higher diastolic blood pressure and mean arterial pressure were also associated with improved survival in the total cohort and in subgroups with nonischemic HF and ischemic HF (data not shown).
Figure 1.
(A) Kaplan-Meier survival estimates for SBP quartiles in patients with ischemic HF during a 1-year follow-up period. (B) Kaplan-Meier survival estimates for SBP quartiles in patients with nonischemic HF over a 1-year follow-up period.
On multivariate analysis adjusting for age, LVEF, cholesterol, gender, diabetes, pulmonary capillary wedge pressure, cardiac index, New York Heart Association class, β-blocker use, angiotension-converting enzyme inhibitor or angiotensin receptor blocker use, statin use, and tobacco use, relative risk (95% CI) of death or urgent transplant at 2 years for quartile 1 compared with quartile 4 was 1.9 (95% CI 1.4 to 2.6) in the total cohort, 1.6 (95% CI 1.1 to 2.5) in nonischemic HF, and 2.4 (95% CI 1.5 to 3.7) in ischemic HF. The hazard ratios for the endpoints—death from any cause or urgent transplantation, death from any cause (all cause mortality), death from HF, sudden death, and need for urgent transplantation—were similar to the endpoint of death or urgent transplant (Table 3).
Table 3.
Hazard ratios (95% CI) for survival at 1 and 2 years in the SBP quartiles
| Outcomes | SBP (mm Hg) |
|||
|---|---|---|---|---|
| ≤90 (n = 539) |
91–100 (n = 551) |
101–112 (n = 553) |
≥113 (n = 518) |
|
| Total cohort | ||||
| 1 year | ||||
| Death or urgent transplant | 2.59 (2.08–3.23) | 1.95 (1.55–2.45) | 1.60 (1.27–2.02) | 1.00 |
| All-cause mortality | 2.41 (1.83–3.17) | 1.59 (1.19–2.13) | 1.49 (1.11–1.99) | 1.00 |
| Sudden death | 1.49 (0.92–2.41) | 1.52 (0.95–2.43) | 1.37 (0.85–2.19) | 1.00 |
| HF death | 3.99 (2.55–6.23) | 2.31 (1.44–3.71) | 1.78 (1.09–2.91) | 1.00 |
| Urgent transplant | 2.95 (2.03–4.30) | 2.66 (1.83–3.87) | 1.83 (1.23–2.73) | 1.00 |
| Multivariate analysis (death or urgent transplant)* | 2.02 (1.42–2.88) | 1.39 (0.97–1.99) | 1.37 (0.96–1.96) | 1.00 |
| 2 years | ||||
| Death or urgent transplant | 2.48 (2.03–3.02) | 1.94 (1.58–2.37) | 1.60 (1.30–1.97) | 1.00 |
| All-cause mortality | 2.38 (1.87–3.03) | 1.69 (1.31–2.17) | 1.54 (1.20–1.99) | 1.00 |
| Sudden death | 1.83 (1.17–2.86) | 1.75 (1.13–2.71) | 1.62 (1.05–2.51) | 1.00 |
| HF death | 3.67 (2.47–5.45) | 2.27 (1.50–3.45) | 1.76 (1.15–2.71) | 1.00 |
| Urgent transplant | 2.68 (1.91–3.78) | 2.45 (1.74–3.44) | 1.72 (1.20–2.45) | 1.00 |
| Multivariate analysis (death or urgent transplant)* | 1.87 (1.36–2.56) | 1.37 (1.01–1.88) | 1.33 (0.97–1.81) | 1.00 |
| Ischemic HF | (n = 259) | (n = 253) | (n = 262) | (n = 247) |
| 1 year | ||||
| Death or urgent transplant | 2.41 (1.77–3.30) | 1.82 (1.31–2.51) | 1.86 (1.35–2.56) | 1.00 |
| All-cause mortality | 2.94 (1.97–4.40) | 1.97 (1.29–3.01) | 2.30 (1.52–3.48) | 1.00 |
| Sudden death | 1.76 (0.88–3.54) | 1.66 (0.83–3.34) | 1.86 (0.94–3.68) | 1.00 |
| HF death | 5.34 (2.70–10.56) | 3.10 (1.51–6.35) | 2.90 (1.41–5.96) | 1.00 |
| Urgent transplant | 1.72 (1.04–2.85) | 1.61 (0.97–2.66) | 1.27 (0.75–2.16) | 1.00 |
| Multivariate analysis (death or urgent transplant)* | 2.73 (1.64–4.55) | 1.30 (0.73–2.31) | 1.85 (1.09–3.14) | 1.00 |
| 2 years | ||||
| Death or urgent transplant | 2.26 (1.71–2.98) | 1.89 (1.42–2.50) | 1.82 (1.37–2.41) | 1.00 |
| All-cause mortality | 2.63 (1.86–3.71) | 2.03 (1.42–2.90) | 2.12 (1.49–3.02) | 1.00 |
| Sudden death | 1.94 (1.01–3.69) | 1.98 (1.05–3.74) | 2.03 (1.08–3.80) | 1.00 |
| HF death | 4.25 (2.40–7.53) | 2.64 (1.44–4.83) | 2.50 (1.36–4.58) | 1.00 |
| Urgent transplant | 1.70 (1.07–2.71) | 1.66 (1.05–2.64) | 1.34 (0.83–2.16) | 1.00 |
| Multivariate analysis (death or urgent transplant)* | 2.37 (1.52–3.69) | 1.37 (0.85–2.20) | 1.69 (1.07–2.67) | 1.00 |
| Nonischemic HF | (n = 280) | (n = 298) | (n = 291) | (n = 271) |
| 1 year | ||||
| Death or urgent transplant | 2.76 (2.02–3.78) | 2.09 (1.52–2.87) | 1.36 (0.97–1.92) | 1.00 |
| All-cause mortality | 1.99 (1.36–2.90) | 1.31 (0.88–1.96) | 0.91 (0.59–1.40) | 1.00 |
| Sudden death | 1.27 (0.65–2.49) | 1.41 (0.75–2.66) | 1.00 (0.51–1.96) | 1.00 |
| HF death | 3.05 (1.67–5.57) | 1.79 (0.94–3.39) | 1.06 (0.52–2.14) | 1.00 |
| Urgent transplant | 5.39 (2.95–9.85) | 4.70 (2.57–8.58) | 2.90 (1.54–5.44) | 1.00 |
| Multivariate analysis (death or urgent transplant)* | 1.66 (1.02–2.69) | 1.49 (0.93–2.37) | 1.00 (0.61–1.65) | 1.00 |
| 2 years | ||||
| Death or urgent transplant | 2.68 (2.02–3.56) | 2.00 (1.50–2.67) | 1.40 (1.03–1.89) | 1.00 |
| All-cause mortality | 2.12 (1.50–2.99) | 1.41 (0.98–2.01) | 1.08 (0.74–1.57) | 1.00 |
| Sudden death | 1.74 (0.94–3.22) | 1.56 (0.85–2.87) | 1.30 (0.70–2.41) | 1.00 |
| HF death | 3.13 (1.80–5.44) | 1.98 (1.11–3.53) | 1.18 (0.63–2.22) | 1.00 |
| Urgent transplant | 4.36 (2.57–7.38) | 3.77 (2.23–6.38) | 2.34 (1.35–4.07) | 1.00 |
| Multivariate analysis (death or urgent transplant)* | 1.62 (1.05–2.50) | 1.39 (0.91–2.11) | 1.04 (0.68–1.61) | 1.00 |
Multivariate analysis (adjusted for age, LVEF, total cholesterol, gender, diabetes, pulmonary capillary wedge pressure, cardiac index, New York Heart Association class, β-blocker use, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use, statin use, smoking history).
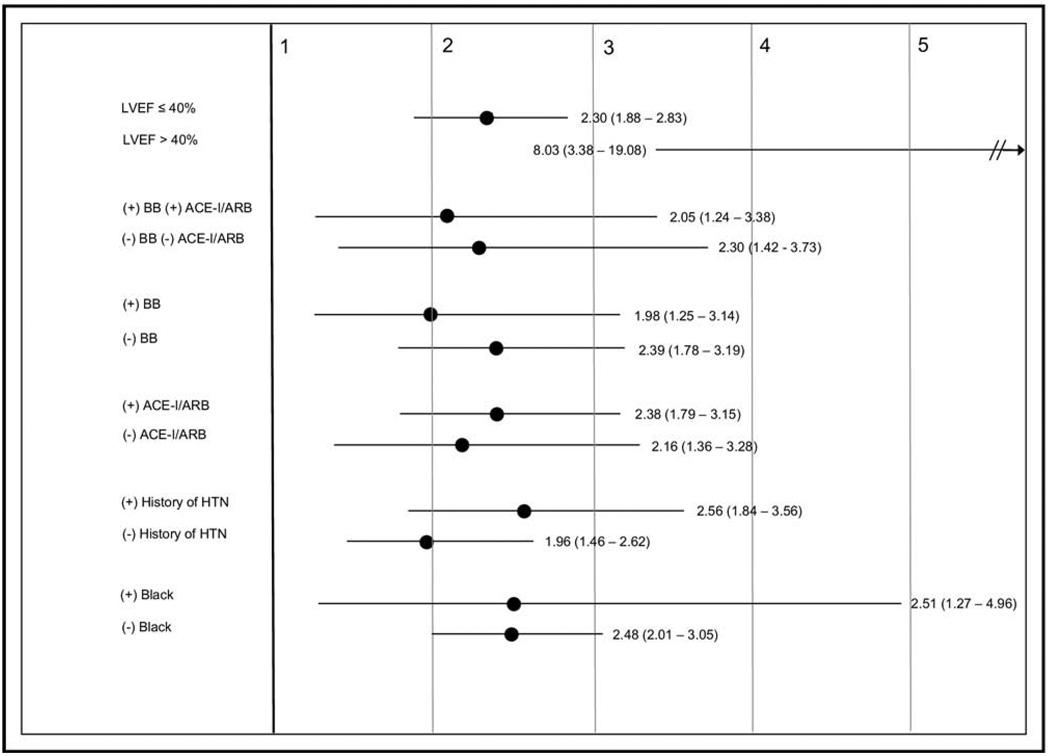
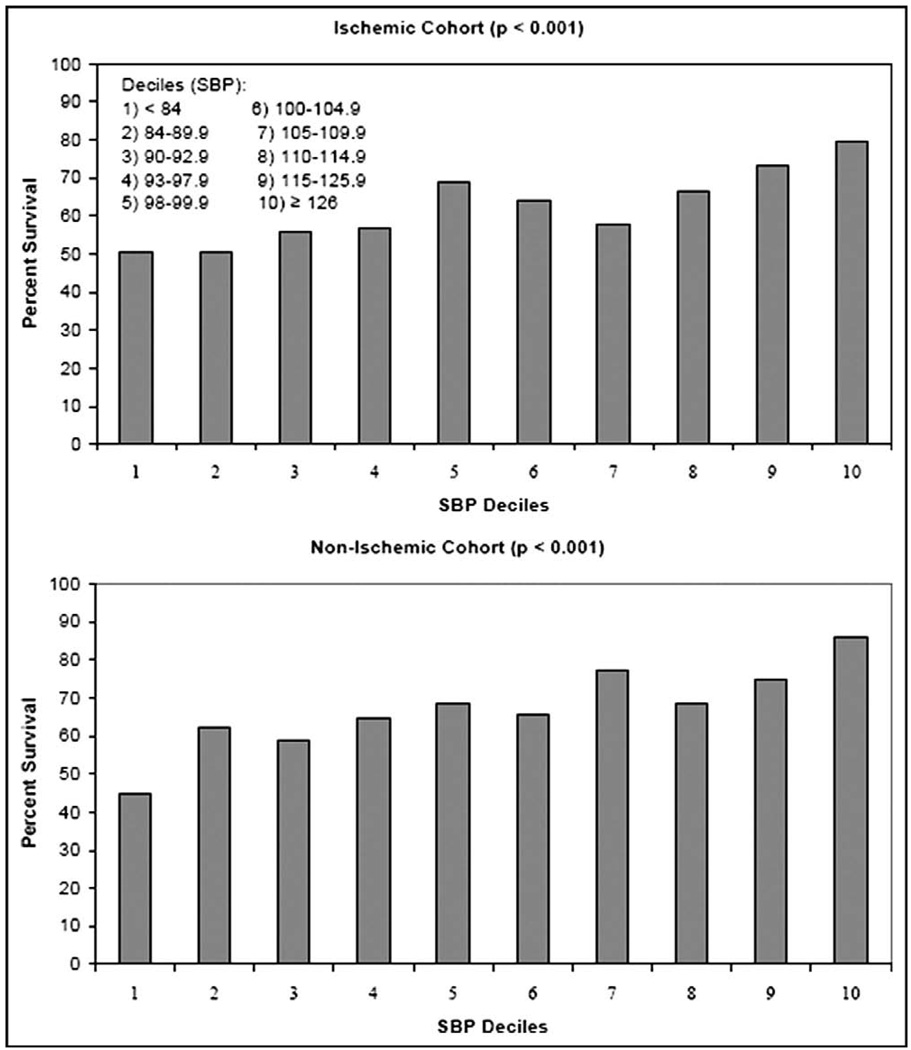
Subgroup analyses for impaired systolic function (LVEF ≤40), medications, history of hypertension, and black descent showed improved survival free from death or urgent transplant for patients in the higher SBP quartiles at both 1 and 2 years. The hazard ratios for quartile 1 compared with quartile 4 is shown in Figure 2 for these subgroups. When the cohort was divided into SBP deciles, there was no J-shaped or U-shaped relation between SBP and survival but rather improved survival even in the highest SBP deciles (Figure 3).
Figure 2.
Two-year hazard ratios and 95% CI for death or urgent transplant for subgroups based on EF, HF medications, and history of hypertension. ACE-I/ARB = angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker; BB =β blocker; HTN = hypertension.
Figure 3.
Survival free from death or urgent transplant at 1 year for patients with ischemic HF and nonischemic HF based on SBP deciles.
Discussion
In patients with established, chronic HF, higher SBP is associated with improved survival.10,11 A study utilizing the Digitalis Investigation Group database, studying 5,174 patients with stable HF New York Heart Association class II or III and LVEF ≤45%, found that patients with the lowest SBP (<100 mm Hg) had significantly higher all-cause mortality than the reference group of patients with SBP of 130 to 139 mm Hg.10 A retrospective study examining 2,289 patients from the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial by Rouleau JL et al14, looking at the influence of pretreatment SBP on the efficacy and safety of carvedilol in patients with HF, found that the lower the pretreatment SBP of patients in the cohort, the higher the risk of a major clinical event. They showed that for each 10-mm Hg decrease in the pretreatment SBP, there was an 18% increase in the risk of death, 11% increase in the combined risk of death or hospitalization for HF, and 9% increase in the combined risk of death or hospitalization for any reason.14
Furthermore, multiple studies have found that higher admission SBP is an independent predictor of decreased mortality in acute decompensated HF.6–9 The Acute Decompensated Heart Failure National Registry (ADHERE) of patients developed a risk-stratification model for acute decompensated HF, using the first 33,046 hospitalizations as the derivation cohort and 32,229 subsequent hospitalizations as the validation cohort. They found that 1 of the better predictors for mortality was low admission SBP (<115 mm Hg).8 More recently, a cohort study examined the association between SBP at admission, clinical characteristics, and outcomes in 48,612 patients hospitalized with HF using data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) registry. Admission SBP was a significant predictor of the odds of in-hospital mortality, with an increase of 21% for each 10-mm Hg decrease in SBP <160 mm Hg and no change for SBP >160 mm Hg. SBP was also a significant predictor for postdischarge mortality.9
Our study is unique in that we have shown that low SBP is a risk factor for mortality in both ischemic and nonischemic chronic HF independent of invasive hemodynamic variables including pulmonary capillary wedge pressure and cardiac index. Further, this is the first study to our knowledge to assess whether this relation between higher SBP and improved survival is consistent in both patients with ischemic and nonischemic HF. After adjustment for multiple risk factors including age, LVEF, cholesterol, gender, diabetes mellitus, pulmonary capillary wedge pressure, cardiac index, New York Heart Association class, β-blocker use, angiotension-converting enzyme inhibitor or angiotensin receptor blocker use, statin use, and tobacco use, higher SBP was independently associated with improved outcomes in both ischemic and nonischemic HF. For patients with ischemic and nonischemic HF, the highest mortality was observed in the lowest SBP quartile and the best survival in the highest quartile of SBP.
There are several potential mechanisms explaining the association between higher SBP and improved outcomes in chronic HF. It has been suggested that higher blood pressures in patients with HF may be due to intact neurohormonal pathways with activation of the adrenergic nervous system, the renin-angiotensin-aldosterone system, and increased production of antidiuretic hormone to increase systemic vascular resistance.12 With this model, patients in the lower quartiles of SBP may be unable to mount an appropriate physiological response if they have more advanced disease. Patients with higher SBP in this cohort were more likely to be treated with standard, life-prolonging HF medications such as β blockers and angiotension-converting enzyme inhibitors or angiotensin receptor blockers; it is possible this more comprehensive treatment contributed to the improved survival in those with higher blood pressure. The decreased treatment with β blockers in those with low SBP may represent low patient tolerability of β blockers or a physician unwillingness to add a medication with potential blood-pressure lowering properties. However, a subanalysis of the COPERNICUS trial found that carvedilol was well-tolerated and associated with decreased mortality at all levels of SBP, including those with low pretreatment SBP, ranging from 85 to 95 mm Hg.14
We acknowledge limitations to our study. The cohort is a select population of patients with advanced systolic HF referred to a single university center. Our study is retrospective in nature. Beta-blocker use was different in the SBP quartiles. This study spans a period of time during which HF therapies were changing; for example, the use of β blockers became standardized only during the latter years of the study time period. Although we performed multivariate adjusted analyses, there may be unmeasured confounding factors not accounted for in the current analysis. The subgroup of patients with preserved LV systolic function is small, and further studies to confirm the relation between SBP and outcomes in this population is warranted.
Acknowledgments
This research was supported in part by the Ahmanson Foundation, Los Angeles, California. Dr. Horwich was supported by National Heart, Lung, and Blood Institute, Bethesda, Maryland, grant 1K23HL085097. Dr. Fonarow holds the Eliot Corday Chair in Cardiovascular Medicine and Science.
References
- 1.Chobanian AV, Barkris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 3.Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet. 2001;358:1305–1315. doi: 10.1016/S0140-6736(01)06411-X. [DOI] [PubMed] [Google Scholar]
- 4.Miura K, Daviglus ML, Dyer AR, Liu K, Garside DB, Stamler J, Greenland P. Relationship of blood pressure to 25-year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men: the Chicago Heart Association Detection Project in Industry. Arch Intern Med. 2001;161:1501–1508. doi: 10.1001/archinte.161.12.1501. [DOI] [PubMed] [Google Scholar]
- 5.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 6.Adamopoulos C, Zannad F, Fay R, Mebazaa A, Cohen-Solal A, Guize L, Juilliere Y, Alla F. Ejection fraction and blood pressure are important and interactive predictors of 4-week mortality in severe acute heart failure. Eur J Heart Fail. 2007;9:935–941. doi: 10.1016/j.ejheart.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 8.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 9.Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O’Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–2226. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 10.Lee TT, Chen J, Cohen DJ, Tsao L. The association between blood pressure and mortality in patients with heart failure. Am Heart J. 2006;151:76–83. doi: 10.1016/j.ahj.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Pulignano G, Del Sindaco D, Tavazzi L, Lucci D, Gorini M, Leggio F, Porcu M, Scherillo M, Opasich C, Di Lenarda A, Senni M, Maggioni AP. Clinical features and outcomes of elderly outpatients with heart failure followed up in hospital cardiology units: data from a large nationwide cardiology database (in-CHF registry) Am Heart J. 2002;143:45–55. doi: 10.1067/mhj.2002.119608. [DOI] [PubMed] [Google Scholar]
- 12.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 13.Fonarow GC, Stevenson LW, Walden JA, Livingston NA, Steimle AE, Hamilton MA, Moriguchi J, Tillisch JH, Woo MA. Impact of a comprehensive heart failure management program on hospital read-mission and functional status of patients with advanced heart failure. J Am Coll Cardiol. 1997;30:725–732. doi: 10.1016/s0735-1097(97)00208-8. [DOI] [PubMed] [Google Scholar]
- 14.Rouleau JL, Roecker EB, Tendera M, Mohacsi P, Krum H, Katus HA, Fowler MB, Coats AJ, Castaigne A, Scherhag A, Holcslaw TL, Packer M. Influence of pretreatment systolic blood pressure on the effect of carvedilol in patients with severe chronic heart failure: the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. J Am Coll Cardiol. 2004;43:1423–1429. doi: 10.1016/j.jacc.2003.11.037. [DOI] [PubMed] [Google Scholar]