Abstract
The soft-sticky dipole-quadrupole-octupole (SSDQO) potential energy function for a coarse-grained single-site water model has Lennard-Jones interactions and an approximate multipole expansion for the electrostatics. Here, the Lennard-Jones parameters and multipole moments of SSDQO were optimized so that the structural, thermodynamic, dynamic, and dielectric properties agreed with experimental values of liquid water at ambient conditions. Using these parameters, the temperature and pressure dependence of various properties were shown to be in good agreement with experiment, including a temperature of maximum density at ∼260 K. This new parametrization, referred to as SSDQO1, is both computationally faster and generally more accurate over a wide range of conditions than traditional three-site water models, which demonstrates that a model with a single dipole, quadrupole, and octupole on each water molecule can reproduce the tetrahedral hydrogen bonded network of water.
INTRODUCTION
Computer simulations are important in understanding the structure and dynamics of water at a molecular level. A number of water models, each with advantages and limitations, has been used,1 most commonly rigid multisite water models with three to five sites. At ambient conditions, of the three-site models, TIP3P (Ref. 2) has reasonable dielectric properties but poor structural and dynamical properties while SPC∕E (Ref. 3) has reasonable structure and dynamics but poor dielectrics.4 The four-site TIP4P (Ref. 5) has even better structure and dynamics but relatively poor dielectrics,6 while TIP4P-Ew (Ref. 7) and TIP4P∕2005 (Ref. 8), which were optimized for use with Ewald summations, give even better properties than the original TIP4P. The five-site TIP5P (Ref. 9) is similar to ST2 (Ref. 10) but was optimized to give good structural, dielectric, and dynamic properties at ambient conditions, and TIP5P-E (Ref. 11) gives similarly good properties but can be used with Ewald summations. However, while the accuracy generally improves as the number of sites increase, the computational time also increases.
A stringent test for a water model is the ability to reproduce the properties of water under a variety of conditions, particularly the anomalous properties. For instance, water exhibits a temperature of maximum density (TMD) at 277 K, which has been attributed to the tetrahedral structure of water.9, 12 Only a few multisite nonpolarizable water models reproduce the TMD within less than 5° of experiment including TIP4P-Ew,7 TIP4P∕2005,8 and TIP5P,9 in contrast with SPC∕E and TIP3P, which have TMD below 238 K.6, 41 In addition, the soft-sticky dipole (SSD) model (Ref. 13), a single-site water model with a soft sphere, a point dipole, and an empirical tetrahedral “sticky” hydrogen bond potential, was found to have a TMD at ∼260 K,12 which is slightly better than the TIP4P TMD at ∼250 K.6 Moreover, over a range of temperatures from about 240 to 400 K, TIP4P-Ew and especially TIP4P∕2005 are in better agreement with experiment than TIP5P, which increases too sharply with temperature below the TMD and decreases too sharply with temperature above the TMD.
The temperature dependence of the diffusion of water at low temperatures is also unusual; it is described by the empirical equation D=Do(T∕Ts−1)γ, indicating a normal component and an anomalous component that diverges at Ts,14, 15 which has been suggested as the mechanical stability limit of the supercooled liquid phase since it is close to the homogeneous nucleation temperature (TH). Water models that reproduce the temperature dependence of liquid water contribute to understanding the underlying molecular behavior near Ts where experiments are difficult. However, only the TIP4P-Ew model has been shown to reproduce the correct temperature dependence of the diffusion constant.7 TIP3P and TIP4P both overestimate the experimental diffusion constant16, 17, 18, 19, 20 at 298 K, and other models that have reasonable diffusion rates at ambient conditions deviate at other temperatures. For instance, although ST2 reproduces the experimental values at higher temperatures, it diffuses very slowly at low temperatures since it is generally overstructured and reaches the low-density, more tetrahedral structure at a relatively high temperature (TMD=310 K) so the onset of jump diffusion may occur because there is still enough thermal energy as the temperature approaches Ts.21 On the other hand, SPC∕E water diffuses too quickly at low temperatures since it is generally understructured and remains in the high-density form until very low temperatures (TMD=235 K) so a kinetic glass transition is observed.22 TIP5P has good structure at 298 K and gives a reasonable temperature dependence23 but still does not reproduce the correct curvature possibly due to structural differences from real water as indicated by deviations of the temperature dependence of the density from experiment. Remarkably, the single-site SSD model had a temperature dependence comparable to five-site TIP5P.24
Overall, TIP5P, TIP4P-Ew, and TIP4P∕2005 generally compare well with experimental results for pure liquid water over a range of conditions. Moreover, the simple SSD model also compares well, indicating that the degree of tetrahedrality is an important factor in reproducing the pure liquid. However, while the above models have similar dipole moments, they differ in their quadrupole moments ranging from 2.3 DÅ for TIP4P∕2005 to 0 for SSD, which may be important for their properties as solvents. In particular, the location of the lone pair charges for TIP5P does not agree with quantum calculations of the electron density25 and may serve to enforce hydrogen bonding through a physically unrealistic charge distribution.
Recently, we developed the soft-sticky dipole-quadrupole-octupole (SSDQO) potential energy function, which is simple, accurate, and efficient.26, 27 This function is centered on a single site with orientational dependence and includes a Lennard-Jones interaction to describe a soft repulsion and “sticky” attractive dispersion, and an approximate multipole expansion to describe the electrostatics. The latter is the exact moment expansion with dipole, quadrupole, and octupole moments up to order 1∕r4, contains an approximation for the 1∕r5 term, and neglects the 1∕r6 term. Thus, hydrogen bonds are described solely via the interactions of the electrostatic moments. SSDQO is faster than even three-site models because fewer interatomic distances are calculated and the approximations in the expansion eliminate most of the slow matrix multiplications; SSDQO is about three times faster than three point models such as SPC∕E and TIP3P in Monte Carlo simulations26 and about two times faster in molecular dynamics simulations.27
In previous studies using the Lennard-Jones parameters and multipole moments of TIP3P, SPC∕E, and TIP5P, SSDQO mimics most of the properties of a particular water model, indicating that the approximate moment expansion works well.26 Tellingly, TIP5P required an additional term to reproduce the close interaction between the hydrogen of one molecule and the lone pair of another due to the overly long distance of the latter from the oxygen so that the tetrahedral bonding structure did not come completely from the moments, while that of TIP3P and SPC∕E was reproduced only by the moments. However, using the moments of a model not only mimics the model but also reproduces the limitations of that model. For example, when SPC∕E Lennard-Jones parameters and multipole moments are used in the SSDQO potential energy function (referred to as SSDQO:SPC∕E), it reproduces most of the properties of SPC∕E for pure liquid water, including the rather low dielectric constant of SPC∕E at 298 K.27 In addition, the structure of SSDQO:SPC∕E is very similar to that of SPC∕E in simulations of ions and biomolecules in water.28, 29, 30
However, parameters specifically for the SSDQO potential energy function have not been developed until now and this development provides the opportunity to explore how the electrostatic moments of the water molecule influence the properties of liquid water. SSDQO has four independent parameters for the electrostatic properties (one each for the dipole and quadrupole and two for the octupole) and the moments of SSDQO can be varied independently so as to best describe the charge distribution. On the other hand, denoting qi as the charge on site i and distances between sites i and j as dij, SPC∕E and TIP3P have three independent parameters, qH, dHH, and dOH, TIP4P has four, qH, dHH, dOH, and dMH, where M is the extra charge site, and TIP5P has five, qH, dHH, dOH, dLL, and dOL, where L are the “lone pair” sites, and the moments are coupled based on the geometry of the molecule. Thus, even though SSDQO is faster than the three point models, it has more independent parameters to better model the electrostatic potential of a water molecule.
An additional motivation for exploring the SSDQO potential energy function is that it is an example of a coarse-grained model for water in which the three atoms of a water molecule are replaced by a single site. In particular, the approximate multipole expansion is a means of treating electrostatics in coarse-grained simulations, which we are examining for other chemical species elsewhere.
Here, the Lennard-Jones parameters and multipole moments of the SSDQO potential energy function were optimized in molecular dynamics simulations to reproduce experimental radial distribution functions, density, energy, dielectric constant, and dynamics of pure water. The optimization focused on reproducing properties at ambient condition, and the resulting set of Lennard-Jones parameters and multipole moments is referred to as the SSDQO1 model. In addition, a variety of properties was determined in molecular dynamics simulations of SSDQO1 at temperatures ranging from 238 to 348 K and pressures ranging from 0.1 to 1000 MPa. Comparisons were made to several other models including SCP∕E and various TIPnP (n=3,4,5) models. Overall, the good agreement with experiment indicates that SSDQO1 gives a good description of water not only at ambient conditions but also over a wide range of temperatures and pressures.
METHODS
Detailed descriptions of the SSDQO water-water potential can be found elsewhere26, 27 so only a brief description is given here. The interaction potential, which is centered on the water oxygen, is given by a Lennard-Jones potential and an electrostatic potential that is an exact multipole expansion up to order 1∕r4 with an approximation for the 1∕r5 term,
| (1) |
where r=rn is the internuclear vector from water i to j, ε and σ are Lennard-Jones parameters, m is the power law of the repulsion in the Lennard-Jones interaction, and μ, Θ, and Ω are the dipole, quadrupole, and octupole moment matrices, respectively. The coefficient of the approximate quadrupole-quadrupole term was chosen so that it matches the exact term for a dimer with a linear hydrogen bond, i.e., cQQ=10, and the coefficient of the dipole-octupole term was chosen so that it is halfway between the exact terms for dimer with a linear hydrogen bond and a bifurcated hydrogen bond, cDO=2. In the molecular frame, μz=μ, Θxx=−Θ+Δ, Θyy=Θ+Δ, Θzz=−2Δ, Ωxxz=−Γ+Ω, Ωyyz=Γ+Ω, Ωzzz=−2Ω, and all other unique matrix elements are zero. In previous studies,26, 27, 28m=12 was used but here m=9 was found to improve agreement with experiment (see Sec. 3). Also, dyadic products are denoted by [n(2)]ij=ninj and [n(3)]ijk=ninjnk, and matrix contractions are denoted by A⋅B=ΣiAiBi, A:B=ΣijAijBij, and A∴B=ΣijkAijkBijk.
The molecular dynamics simulations were carried out using the SSDQO potential energy function for a cubic box of 256 water molecules using periodic boundary conditions. The Lennard-Jones, dipole-quadrupole, quadrupole-quadrupole, and dipole-octupole potentials were spherically truncated at 0.5L where L is the box length. The long-range dipole-dipole interactions were treated using the Ewald method with a surrounding dielectric constant ε′=∞ and convergence parameter α=6.4∕L.31 The minimum image convention was used for the real space portion and a cutoff at was used for reciprocal space portion of the Ewald sum. The quaternion formulation of the equations of rotational motion was utilized, with an adapted leap-frog algorithm using a time step of 1 fs.32 The moments of inertia were calculated for an OH bond length of 0.9572 Å and an HOH bond angle of 104.52°; the values were Ixx=1.847 amu Å2, Iyy=0.692 amu Å2, and Izz=1.155 amu Å2.
The density, radial distribution function, and energy were calculated from molecular dynamics simulations in the NPT ensemble using the Berendsen algorithm33 between 238 and 348 K in 10° intervals at 0.1 MPa and also at 0.1, 0.5, 1, 2.5, 5, 7.5, 10, 25, 50, 100, 200, 300, 400, 500, 600, 800, and 1000 MPa at 298 K. A box of water previously equilibrated at 298 K was used for the initial configuration. The system was equilibrated for 9 ns at 238 K, 7 ns at 248 K and 258 K, 5 ns at 268 K, 4 ns at 278 K, and 1 ns at T≥288 K. The production time was 3 ns for all simulations.
The dielectric and dynamical properties were calculated using standard methods34 from simulations in the NVE ensemble between 248 and 348 K in 10° intervals. For each simulation, the initial coordinates and velocities were obtained from previous molecular dynamics simulations in the NPT ensemble and the volume was chosen to correspond to the average density of the corresponding NPT simulation. The simulations were equilibrated for 7 ns at 248 K; 5 ns at 258, 268, and 278 K; and 4 ns at T≥288 K. The production time was 7 ns at 248 and 258 K, 5 ns at 268 and 278 K, and 4 ns at T≥288 K. The translational self-diffusion coefficient D was calculated by integrating the velocity autocorrelation function up to 1.2 ps for T≥288 K and 2.4 ps for T<288 K with the time interval of 0.001 ps. To verify the pressure dependence of the diffusion constant at 298 K, the simulations at 0.1, 1, 2.5, and 25 MPa were simulated for 8 ns. In addition, the translational diffusion coefficient at 298 K was calculated from the long-time limit of the mean-square displacement (MSD) for t=100–300 ps. The orientational correlational times , where l is the rank of the Legendre polynomial and α is a vector in the molecular frame, were obtained by explicit integration of orientational time correlation functions up to 3 ps for T≥288 K and up to 6 ps for T<288 K with the time interval of 0.003 ps and by calculating the integral for the long-time region using an exponential fit.
Many of the properties calculated below are dependent on the position of the hydrogens and since SSDQO is a single-site model, the positions of the two hydrogen atoms do not appear explicitly in the potential. Thus, the hydrogen positions were generated assuming the TIPnP geometry in the molecular coordinate system, given the location and orientation of the molecular coordinate system of each molecule in the laboratory frame.
RESULTS AND DISCUSSION
The Lennard-Jones and moment parameters of the SSDQO potential energy function were optimized at ambient temperature and pressure so that various properties calculated from molecular dynamics simulation agreed with experiment. Although the dipole moment is critical for many properties of liquid water, the importance of the quadrupole as well has been noted for certain properties.11, 35 The moments of SSDQO1 are generally similar to those of multisite models and in particular to TIP4P (Table 1). However, the multisite models have much lower dipole and quadrupole moments compared to the various quantum calculations such as two ab initio MD calculations of liquid water36, 37 and a QM∕MM calculation in which one water calculated at the MP2∕aug-cc-pVQZ with TIP5P geometry was surrounded by 230 classical TIP5P waters at coordinates from Monte Carlo simulations38 (Table 1). Assuming the dipole μ is aligned along the z-axis, Θ measures the planar quadrupole in the x-y plane or dx2−y2 character and Ω measures the linear octupole along the z-axis or fz3 character and are thus related by the molecular geometry to μ; the relationship can be compared relative to a three-site model with a perfect tetrahedral HOH angle such as SPC∕E.26 For a tetrahedral HOH angle of 109.47°, Θ=(√3∕2)bOHμ and , while the smaller HOH angle of 104.52° of TIP3P gives Θ=0.73 μ Å and Ω=0.26 μ Å2, and the larger angle between the negative charge and the two hydrogens by moving the center of the negative charge in the TIP4P and related models gives Θ=0.93 μ Å to 1.0 μ Å and Ω=0.32 μ Å2 to 0.35 μ Å2. Also, the condition Δ=0 is a unique way of defining the zero for the z axis; a tetrahedral HOH angle with the moment expansion centered on the oxygen gives Δ=0 while a sharper HOH angle gives Δ≈0 when centered on the center of mass so these two conventions will be used. Finally, Γ describes the antisymmetry since Γ=(5∕2)Ω for an SPC∕E geometry while Γ=0 if negative charge is moved to two “lone pairs” added at the antisymmetric corners of a tetrahedron formed by the hydrogens. Considering these relationships between the moments, the TIP4P-type models are closest to the quantum calculations while the TIP5P-type models are the furthest.
Table 1.
SSDQO1 parameters compared to the parameters of multisite models and quantum calculations.
| Model | σ (Å) | ε (kcal∕mol) | μ (D) | Θ (DÅ) | Δ (DÅ) | Ω (DÅ2) | Γ (DÅ2) |
|---|---|---|---|---|---|---|---|
| SSDQO1a | 3.433 | 0.089 | 2.12 | 2.13 | 0.00 | 0.67 | 1.15 |
| SPC∕Ea | 3.166 | 0.155 | 2.35 | 2.04 | 0.00 | 0.78 | 1.96 |
| TIP3P | 3.151 | 0.152 | 2.35 | 1.72 | 0.04 | 0.61 | 1.49 |
| TIP4P | 3.154 | 0.155 | 2.18 | 2.15 | 0.06 | 0.77 | 1.86 |
| TIP4P-Ew | 3.164 | 0.163 | 2.32 | 2.16 | 0.05 | 0.77 | 1.88 |
| TIP4P∕2005 | 3.159 | 0.185 | 2.31 | 2.30 | 0.06 | 0.82 | 1.99 |
| TIP5P | 3.120 | 0.160 | 2.29 | 1.56 | 0.08 | 0.50 | 0.42 |
| TIP5P-E | 3.097 | 0.178 | 2.29 | 1.56 | 0.08 | 0.50 | 0.42 |
| Ab initio MDb | NA | NA | 2.95 | 3.27 | 0.11 | NA | NA |
| Ab initio MDa c, c | NA | NA | 2.43 | 2.72 | 0.05 | NA | NA |
| 1+230 TIP5P MP2∕aug-cc-pVQZd | NA | NA | 2.55 | 2.81 | 0.20 | 0.74 | 1.50 |
| Exp. (gas phase)e | NA | NA | 1.86 | 2.57 | 0.07 | NA | NA |
A major difference between SSDQO1 and the multisite models (and the previous results for SSDQO:SPC∕E) is the use of a 6–9 instead of a 6–12 Lennard-Jones potential. Neutron diffraction studies indicated that a softer repulsive wall than a 6–12 potential may fit experimental data better.39 Also, the O–O radial distribution for SSDQO:SPC∕E exhibited a higher first peak than SPC∕E when the same Lennard-Jones potential was used for both,26 further indicating the repulsive wall in the 6–12 potential was too steep especially when used with a moment expansion. Thus, the SPC∕E Lennard-Jones parameters give a too high density at 0.1 MPa for SSDQO:SPC∕E and if the Lennard-Jones parameters are altered to reproduce a density close to experiment at 0.1 MPa, the second “tetrahedral” peak disappears. The Lennard-Jones parameters for SSDQO1 (Table 1) were chosen such that the potential is approximately equal to that of the multisite models at the first peak in the radial distribution function and the long-range dependence is similar. Moreover, when the SSDQO1 moments are used with a 6–12 potential with σ=3.197 Å, ε=0.15 kcal∕mol, which gives the same density as SSDQO1 at 0.1 MPa, the first peak shifts outward and the second peak almost disappears, indicating the loss of tetrahedrality (Fig. S1),40 and the pressure dependence of the density does not agree as well with experiment (Fig. 1).
Figure 1.
The pressure dependence of the density for SSDQO1 with a Lennard-Jones 6–9 potential in the NPT ensemble (red) compared to an SSDQO model with the standard Lennard-Jones 6–12 potential (light red) and experiment (Ref. 58) (black).
SSDQO1 at ambient conditions
At 298 K, the average pressure of SSDQO1 was −3.24 MPa compared to the experimental value of 0.1 MPa in the NVE simulations at the experimental density of 0.997 g∕cm3, while the density of SSDQO1 was 0.999 g∕cm3 in the NPT simulations at 0.1 MPa. The O–O radial distribution (gOO) function at ambient conditions of SSDQO1 was in good agreement with results from neutron diffraction39 (Fig. 2). The first peak in both SSDQO1 and experiment was at 2.73 Å with a coordination number of 4.3 (calculated by integrating the first peak to 3.3 Å), although the height of the peak was slightly lower for SSDQO1 than experiment. The well-defined second peak near 4.5 Å for SSDQO1 indicated the tetrahedral nature of water was reproduced well and the H–H and O–H radial distributions (gHH and gOH) of SSDQO1 were close to experiment (Fig. 2), indicating that the tetrahedral hydrogen bond network is reproduced well using only the electrostatic moments to describe the hydrogen bonds. SSDQO1 is also similar to TIP5P (Fig. 2) and also to TIP4P (Ref. 6) (not shown).
Figure 2.
Comparison of the O–O, H–H (shifted by 2), and O–H (shifted by 4) radial distribution functions of SSDQO1 (red), TIP5P (Ref. 9) (blue), and experiment (Ref. 39) (black) at 298 K and 0.1 MPa.
As a whole, thermodynamic, dielectric, and dynamic properties of SSDQO1 at 298 K and 0.1 MPa agreed well with experiment (Table 2). In particular, SSDQO1 had a better dielectric constant ε than the other models except for TIP5P and it had the best diffusion constants D and rotational correlation times τ, even better than TIP5P. Interestingly, SSDQO1 was much better than TIP4P for both ε and D despite the similarity in moments and generally the models with Ω<0.33 μ Å2 had better ε. Also, the dynamics of SSDQO1 was relatively insensitive to the internal geometry since using an SPC∕E geometry rather than a TIPnP geometry to define the moments of inertia lead to only a 2% difference in D and τ. The major problem with SSDQO1 seems to be a too low heat of vaporization ΔHvap. The reported value does not include a self-polarization correction;3 however, because of the small dipole moment, the correction is only −0.35 kcal∕mol or −0.42 kcal∕mol including additional corrections for vibration and nonideal behavior.7
Table 2.
Properties of SSDQO1 compared to site models and experiment at 298 K and 0.1 MPa.
| Model | ρ (g∕cm3) | ΔHvapa (kcal∕mol) | ε | 109D (m2∕s) | (ps) | (ps) | (ps) | (ps) |
|---|---|---|---|---|---|---|---|---|
| SSDQO1 | 0.999 | 9.82 | 75 | 2.22 | 4.30 | 1.64 | 2.12 | 2.51 |
| SSDQO:SPC∕Ec | 0.997b | 67 | 2.21 | 4.15 | 1.52 | 1.98 | 2.35 | |
| SPC∕Ec | 0.997b | 11.8 (10.5) | 68 | 2.43 | 3.71 | 1.33 | 1.53 | 1.66 |
| TIP3Pd | 0.997b | 10.26 | 95 | 5.45 | 1.98 | 0.66 | 0.70 | 0.67 |
| TIP4Pe | 1.001 | 10.65 | 52 | 3.6 | 1.4 | |||
| TIP4P-Ewf | 0.995 | 11.76 (10.58) | 63 | 2.4 | ||||
| TIP4P∕2005g | 0.998 | 11.99 (10.89) | 60 | 2.08 | ||||
| TIP5Ph | 0.999 | 10.46 | 82 | 2.6 | 1.58 | |||
| TIP5P-Ei | 1.000 | 10.38 | 92 | 2.8 | 1.55 | |||
| Exp. | 0.997 | 10.51 | 78.3j | 2.3k | 4.76l | 1.92m | 1.95n | 2.46o |
Temperature dependence of SSDQO1 at 0.1 MPa
The radial distribution functions of SSDQO at 278, 298, and 318 K at 0.1 MPa indicate the liquid structure is reasonable, with the decrease in structure with increasing temperature apparent from the decrease in peak height and increase in peak width (Fig. 3). The temperature dependence of the density at 0.1 MPa of SSDQO1 was reasonably good in comparison to experiment (Fig. 4), which is important because it affects the temperature dependence of other properties. In particular, SSDQO1 exhibited a TMD near 260 K (Table 3), which is remarkably close to the experimental value of 277 K given the simplicity of the model; the TMD is an important indicator of the balance of interactions since it marks the transition as the temperature decreases from increasing density due to smaller fluctuations to decreasing density due to more open tetrahedral structure. In comparison to the multisite models, the TMD of SSDQO1 is better than SPC∕E although not quite as good as TIP4P-Ew, TIP5P, and TIP5P-E. Interestingly, SSDQO1 has a very similar temperature dependence including the TMD to TIP4P (Fig. 4), perhaps due to the similarity of their dipole and quadrupole moments.
Figure 3.
The temperature dependence of the O–O radial distribution function of SSDQO1 water at 278 K (light red), 298 K (solid red), and 318 K (dashed red).
Figure 4.
The temperature dependence of the density for SSDQO1 (red), TIP5P (Ref. 9) (solid blue), SPC/E (Ref. 41) (dashed blue), TIP4P (Ref. 6) (dotted green), TIP4P-Ew (Ref. 7) (solid green), TIP4P/2005 (Ref. 8) (dashed green), and experiment (Ref. 54) (black).
Table 3.
Temperature dependency at 0.1 MPa.
The temperature dependence of other static properties for SSDQO1 was reasonable, although not better as a whole than the multisite models. The temperature dependence of ΔHvap (Fig. 5) shows that compared to experiment, SSDQO1 generally underestimates the energy and as a result ΔHvap, but the slope is good. SSDQO1 performs worse than TIP5P, which fits experiment better at 298 K, but better than SPC∕E, which is too high compared to experiment at 298 K. However, if a self-polarization term is added,3 the SPC∕E results are quite good. The dielectric constant ε as a function of temperature shows that although SSDQO1 agreed with experiment at 298 K, the slope was larger for SSDQO1 (Fig. 6). Although TIP5P is somewhat high at 298 K and SPC∕E is somewhat low, they both have a better slope. Interestingly, the polarizable AMOEBA model42, 43 has a good slope while the polarizable TIP4P-FQ model44, 45 has a slope similar to SSDQO1. Although the average quadrupole moments are not reported for the AMOEBA model, it is suggestive that Θ≈bOHμ for both TIP4P-FQ and SSDQO1 whereas Θ<bOHμ for the rigid models that have better slopes.
Figure 5.
Temperature dependence of the heat of vaporization for SSDQO1 (red), TIP5P (Ref. 9) (solid blue), SPC/E (Ref. 55) (dashed blue), and experiment (Ref. 56) (black).
Figure 6.
Temperature dependence of the dielectric constant of SSDQO1 (red), TIP5P (Ref. 9) (blue), SPC/E (Ref. 55) (dashed blue), TIP4P-FQ (Ref. 45) (dashed orange) and AMOEBA (Ref. 43) (solid orange) water, and experiment (Ref. 57) (black).
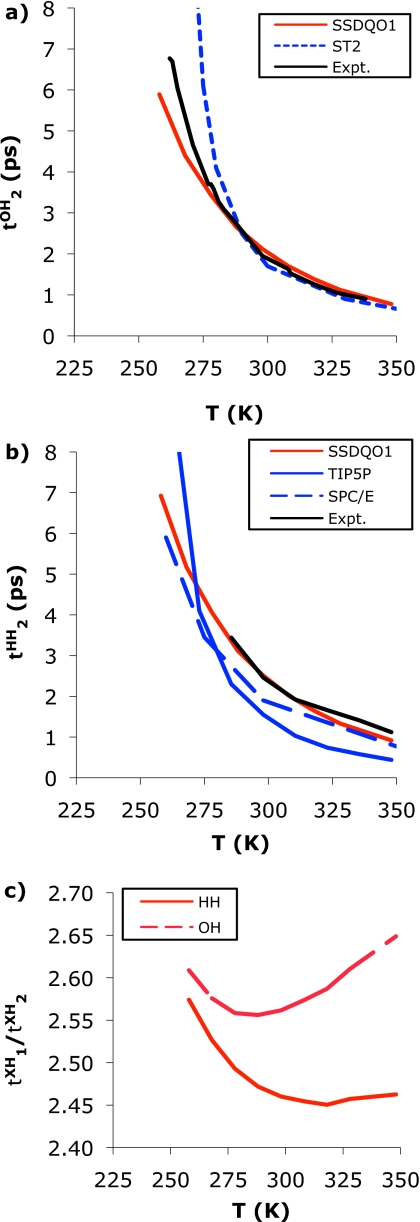
The temperature dependence of the dynamics of SSDQO1 was quite good in comparison to experiment. The temperature dependence of D (Fig. 7), and and (Fig. 8) indicate that SSDQO1 translates and rotates close to experiment above its TMD=260 K but somewhat faster at low temperatures presumably because the transition to the low density form occurs at a somewhat lower temperature than experiment. Moreover, SSDQO1 agrees well with experiment for the parameters of the empirical equation D=Do(T∕Ts−1)γ (Table 3). The ratio indicates changes in the rotational motion as the TMD [Fig. 8c] is approached since a rotational motion consisting of small angular steps has , while larger angular jumps have a smaller ratio.4 In comparison with the other multisite models, SSDQO1 performs as well as TIP4P-Ew and TIP4P∕2005, while TIP5P is somewhat worse and ST2 and SPC∕E are even further from experiment, most likely because the good performers have a reasonable TMD and generally good water structure. The temperature dependence of D (Fig. 7) and [Fig. 8a] for ST2 also agrees with experiment above its TMD=310 K but much slower than experiment at low temperatures because its TMD is too high and so presumably is too structured. The temperature dependence of D (Fig. 7) and [Fig. 8b] for SPC∕E indicates it translates and rotates slower than experiment at high temperatures and faster than experiment at low temperatures while that of TIP5P water indicates the reverse.
Figure 7.
Temperature dependence of the diffusion constant for SSDQO1 (red), TIP5P (Ref. 23) (solid blue), SPC/E (Ref. 41) (dashed blue), TIP4P-Ew (Ref. 7) (solid green), TIP4P/2005 (Ref. 46) (dashed green), and experiment (Refs. 19, 20) (black).
Figure 8.
(a) The temperature dependence of for SSDQO1 (red), ST2 (Ref. 21) (dotted blue), and experiment (Ref. 53) (black). (b) The temperature dependence of for SSDQO1 (red), TIP5P-E (Ref. 11) (blue), SPC/E (Ref. 55) (dashed blue), and experiment (Ref. 47) (black). (c) The ratio of SSDQO1, where X is H (solid lines) or O (dashed lines).
Pressure dependence of SSDQO1 at 298 K
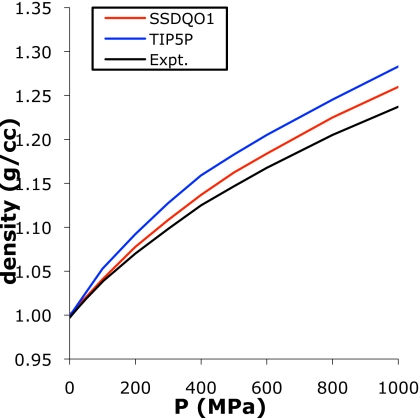
The O–O radial distributions as a function of pressure for SSDQO1 showed the pressure-induced disruption of the hydrogen-bonded tetrahedral network also seen in experiment39 (Fig. S2).40 The second peak, which is distinctive for a tetrahedral network, decreased with increasing pressure while the third peak around 6 Å, which corresponds to the second peak of simple liquids, increased with pressure. The pressure dependence of the density at 298 K of SSDQO1 agreed well with experiment (Fig. 9). While at higher pressure the density of SSDQO1 overestimated the real density, the density profile of SSDQO1 was better than TIP5P relative to experiment, indicating that TIP5P is more compressible than real water.
Figure 9.
The pressure dependence of the density at 298 K for SSDQO1 (red), TIP5P (Ref. 9) (blue), and experiment (Ref. 58) (black).
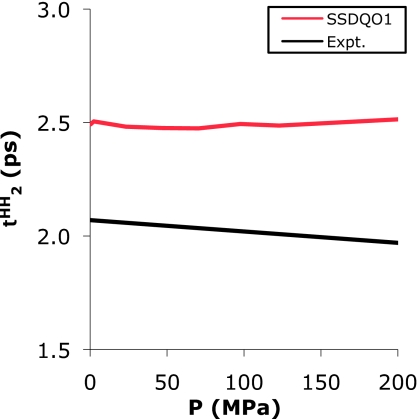
The pressure dependence of the diffusion constant at 298 K of SSDQO1 showed no initial increase in D (Fig. S3) (Ref. 40) or decrease in (Fig. 10) with increasing pressure as seen in the experiment data, which have been attributed to disruption of the hydrogen bond network so that water can diffuse faster. SPC∕E shows a similar pressure dependence while TIP5P shows a better pressure dependence. Experiments47 also show that the pressure dependence of D and remain virtually flat at 363 K because the motion of the water molecules is faster and the H-bond network is not well defined and the initial drop becomes more prominent as the temperature decreases. This indicates that at 298 K SSDQO1 is still further from its transition to the low density form compared to real water, which is consistent with its density profile as a function of temperature.
Figure 10.
The pressure dependence of the for SSDQO1 (red) at 298 K and experiment (Ref. 47) (black) at 303 K.
CONCLUSIONS
The SSDQO model of water has been optimized to mimic properties of water at 298 K and 0.1 MPa. The new parameters, referred to as SSDQO1, gave properties that were in good agreement with experiment at ambient conditions. Interestingly, the moments are remarkably similar to TIP4P and many properties were similar except SSDQO1 had a better dielectric constant, perhaps due to the lower octupole moment.
The temperature and pressure dependence of properties of SSDQO1 were also generally in good agreement with experiment. In particular, the temperature of maximum density for SSDQO1 is ∼260 K, which is impressive for a single-site model. SSDQO1 also has a better description of the temperature dependence of translation and rotation compared to most multisite models. In addition, the pressure dependence of SSDQO1 was able to qualitatively reproduce the density and somewhat the dynamic properties. In some cases, SSDQO1 improved on the properties of TIP5P, which is considered by some as one of the best water models.
Overall, these results indicate that SSDQO1 using only electrostatic point moments gives a good description of the hydrogen bond interaction and the tetrahedral structure of water at a variety of temperatures and pressures. The SSDQO1 moments are reasonable in comparison with quantum calculations, and simulations currently underway indicate that the solvation properties of SSDQO1 are also excellent. Moreover, these results are encouraging for using the approximate multipole expansion to treat electrostatics in coarse-grained potentials.
ACKNOWLEDGMENTS
The authors are grateful to the National Science Foundation for the support of this work through Grant No. MCB-0544629. The calculations were performed on facilities provided by Georgetown University and administered by the division of Advanced Research Computing (ARC). Support was also provided by the William G. McGowan Foundation. In addition, this research was supported in part by the Intramural Research Program of the National Institutes of Health, National Heart, Lung, and Blood Institute (Laboratory of Computational Biology).
References
- Guillot B., J. Mol. Liq. 101, 219 (2002). 10.1016/S0167-7322(02)00094-6 [DOI] [Google Scholar]
- Jorgensen W. L., J. Am. Chem. Soc. 103, 335 (1981). 10.1021/ja00392a016 [DOI] [Google Scholar]
- Berendsen H. J. C., Grigera J. R., and Straatsma T. P., J. Phys. Chem. 91, 6269 (1987). 10.1021/j100308a038 [DOI] [Google Scholar]
- van der Spoel D., van Maaren P. J., and Berendsen H. J. C., J. Chem. Phys. 108, 10220 (1998). 10.1063/1.476482 [DOI] [Google Scholar]
- Jorgensen W. L., Chandrasekhar J., Madura J. D., Impey R. W., and Klein M. L., J. Chem. Phys. 79, 926 (1983). 10.1063/1.445869 [DOI] [Google Scholar]
- Jorgensen W. L. and Jenson C., J. Comput. Chem. 19, 1179 (1998). [DOI] [Google Scholar]
- Horn H. W., Swope W. C., Pitera J. W., Madura J. D., Dick T. J., Hura G. L., and Head-Gordon T., J. Chem. Phys. 120, 9665 (2004). 10.1063/1.1683075 [DOI] [PubMed] [Google Scholar]
- Abascal J. L. F. and Vega C., J. Chem. Phys. 123, 234505 (2005). 10.1063/1.2121687 [DOI] [PubMed] [Google Scholar]
- Mahoney M. W. and Jorgensen W. L., J. Chem. Phys. 112, 8910 (2000). 10.1063/1.481505 [DOI] [Google Scholar]
- Stillinger F. H. and Rahman A., J. Chem. Phys. 68, 666 (1978). 10.1063/1.435738 [DOI] [Google Scholar]
- Rick S. W., J. Chem. Phys. 120, 6085 (2004). 10.1063/1.1652434 [DOI] [PubMed] [Google Scholar]
- Tan M. L., Fischer J. T., Chandra A., Brooks B. R., and Ichiye T., Chem. Phys. Lett. 376, 646 (2003). 10.1016/S0009-2614(03)01044-3 [DOI] [Google Scholar]
- Liu Y. and Ichiye T., J. Phys. Chem. 100, 2723 (1996). 10.1021/jp952324t [DOI] [Google Scholar]
- Speedy R. J. and Angell C. A., J. Chem. Phys. 65, 851 (1976). 10.1063/1.433153 [DOI] [Google Scholar]
- Prielmeier F. X., Lang E. W., Speedy R. J., and Ludemann H., Phys. Rev. Lett. 59, 1128 (1987). 10.1103/PhysRevLett.59.1128 [DOI] [PubMed] [Google Scholar]
- Chandra A. and Ichiye T., J. Chem. Phys. 111, 2701 (1999). 10.1063/1.479546 [DOI] [Google Scholar]
- Watanabe K. and Klein M. L., J. Chem. Phys. 131, 157 (1989). 10.1016/0301-0104(89)80166-1 [DOI] [Google Scholar]
- Frattini R., Ricci M. A., Ruocco G., and Sampoli M., J. Chem. Phys. 92, 2540 (1990). 10.1063/1.457946 [DOI] [Google Scholar]
- Prielmeier F. X., Lang E. W., Speedy R. J., and Ludemann H. D., Ber. Bunsenges. Phys. Chem. 92, 1111 (1988). [Google Scholar]
- Holz M., Heil S. R., and Sacco A., Phys. Chem. Chem. Phys. 2, 4740 (2000). 10.1039/b005319h [DOI] [Google Scholar]
- Paschek D. and Geiger A., J. Phys. Chem. B 103, 4139 (1999). 10.1021/jp984075p [DOI] [Google Scholar]
- Sciortino F., Gallo P., Tartaglia P., and Chen S. H., Phys. Rev. E 54, 6331 (1996). 10.1103/PhysRevE.54.6331 [DOI] [PubMed] [Google Scholar]
- Mahoney M. W. and Jorgenson W. L., J. Chem. Phys. 114, 363 (2001). 10.1063/1.1329346 [DOI] [Google Scholar]
- Tan M. L., Brooks B. R., and Ichiye T., Chem. Phys. Lett. 421, 166 (2006). 10.1016/j.cplett.2006.01.048 [DOI] [Google Scholar]
- Partridge H. and Schwenke D. W., J. Chem. Phys. 106, 4618 (1997) 10.1063/1.473987 [DOI] [Google Scholar]; Bader R. F. W. and Jones G. A., Can. J. Chem. 41, 586 (1963). 10.1139/v63-084 [DOI] [Google Scholar]
- Ichiye T. and Tan M. L., J. Chem. Phys. 124, 134504 (2006). 10.1063/1.2161201 [DOI] [PubMed] [Google Scholar]
- Chowdhuri S., Tan M. L., and Ichiye T., J. Chem. Phys. 125, 144513 (2006). 10.1063/1.2357117 [DOI] [PubMed] [Google Scholar]
- Tan M. L., Lucan L., and Ichiye T., J. Chem. Phys. 124, 174505 (2006). 10.1063/1.2177240 [DOI] [PubMed] [Google Scholar]
- Te J. A., Tan M. L., and Ichiye T., Chem. Phys. Lett. 486, 70 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te J. A., Tan M. L., and Ichiye T., “Solvation of glucose, trehalose, and sucrose by the soft-sticky dipole-quadrupole-octupole water model,” Chem. Phys. Lett. (in press). [DOI] [PMC free article] [PubMed]
- De Leeuw S. W., Perram J. W., and Smith E. R., Annu. Rev. Phys. Chem. 37, 245 (1986) 10.1146/annurev.pc.37.100186.001333 [DOI] [PubMed] [Google Scholar]; De Leeuw S. W., Perram J. W., and Smith E. R., Proc. R. Soc. London, Ser. A 373, 27 (1980) 10.1098/rspa.1980.0135 [DOI] [Google Scholar]; De Leeuw S. W., Perram J. W., and Smith E. R., Proc. R. Soc. London, Ser. A 388, 177 (1983). 10.1098/rspa.1983.0077 [DOI] [Google Scholar]
- Allen M. P. and Tildesley D. J., Computer Simulations of Liquids, 1st ed. (Clarendon, Oxford, 1987). [Google Scholar]
- Berendsen H. J. C., Postma J. P. M., van Gunsteren W. F., Dinola A., and Haak J. R., J. Chem. Phys. 81, 3684 (1984). 10.1063/1.448118 [DOI] [Google Scholar]
- Hansen J. P. and McDonald I. R., Theory of Simple Liquids (Academic, New York, 2006). [Google Scholar]
- Abascal J. L. F. and Vega C., Phys. Rev. Lett. 98, 237801 (2007) 10.1103/PhysRevLett.98.237801 [DOI] [PubMed] [Google Scholar]; Abascal J. L. F. and Vega C., J. Phys. Chem. C 111, 15811 (2007) 10.1021/jp074418w [DOI] [Google Scholar]; Carnie S. L. and Patey G. N., Mol. Phys. 47, 1129 (1982). 10.1080/00268978200100822 [DOI] [Google Scholar]
- Silvestrelli P. L. and Parrinello M., J. Chem. Phys. 111, 3572 (1999). 10.1063/1.479638 [DOI] [Google Scholar]
- Site L. D., Alavi A., and Lynden-Bell R. M., Mol. Phys. 96, 1683 (1999). 10.1080/00268979909483112 [DOI] [Google Scholar]
- Coutinho K., Guedes R. C., Costa Cabral B. J., and Canuto S., Chem. Phys. Lett. 369, 345 (2003). 10.1016/S0009-2614(02)02026-2 [DOI] [Google Scholar]
- Soper A. K., J. Chem. Phys. 258, 121 (2000). 10.1016/S0301-0104(00)00179-8 [DOI] [Google Scholar]
- See supplementary material at 10.1063/1.3359432 for the 6–9 versus 6–12 Lennard-Jones parameters for the SSDQO1 moments and the pressure dependence of the O–O radial distribution function and the diffusion coefficients.
- Baez L. A. and Clancy P., J. Chem. Phys. 101, 9837 (1994). 10.1063/1.467949 [DOI] [Google Scholar]
- Ren P. Y. and Ponder J. W., J. Phys. Chem. B 107, 5933 (2003). 10.1021/jp027815+ [DOI] [Google Scholar]
- Ren P. Y. and Ponder J. W., J. Phys. Chem. B 108, 13427 (2004). 10.1021/jp0484332 [DOI] [Google Scholar]
- Rick S. W., Stuart S. J., and Berne B. J., J. Chem. Phys. 101, 6141 (1994). 10.1063/1.468398 [DOI] [Google Scholar]
- Rick S. W., J. Chem. Phys. 114, 2276 (2001) 10.1063/1.1336805 [DOI] [Google Scholar]; Medeiros M. and Costas M. E., J. Chem. Phys. 107, 2012 (1997). 10.1063/1.474552 [DOI] [Google Scholar]
- Vega C., Abascal J. L. F., Conde M. M., and Aragones J. L., Faraday Discuss. 141, 251 (2009). 10.1039/b805531a [DOI] [PubMed] [Google Scholar]
- Jonas J., DeFries T., and Wilbur D. J., J. Chem. Phys. 65, 582 (1976). 10.1063/1.433113 [DOI] [Google Scholar]
- Clough S. A., Beers Y., Klein G. P., and Rothman L. S., J. Chem. Phys. 59, 2254 (1973). 10.1063/1.1680328 [DOI] [Google Scholar]
- Eisenberg D. and Kauzmann W., The Structure and Properties of Water (Oxford University Press, New York, 1969). [Google Scholar]
- Krynicki K., Green C. D., and Sawyer D. W., Faraday Discuss. Chem. Soc. 66, 199 (1978). 10.1039/dc9786600199 [DOI] [Google Scholar]
- Sansom M. S., Kerr I. D., Breed J., and Sankararamakrishnan R., Biophys. J. 70, 693 (1996). 10.1016/S0006-3495(96)79609-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynicki K., Physica 32, 167 (1966). 10.1016/0031-8914(66)90113-3 [DOI] [Google Scholar]
- Ludwig R., Weinhold F., and Farrar T. C., J. Chem. Phys. 103, 6941 (1995) 10.1063/1.470371 [DOI] [Google Scholar]; Lang E. W. and Ludemann H. D., Ber. Bunsenges. Phys. Chem. 85, 603 (1981). [Google Scholar]
- Lide D. R., ed., CRC Handbook of Chemistry and Physics (CRC, Boca Raton, FL, 2004). [Google Scholar]
- English N. J., Mol. Phys. 103, 1945 (2005). 10.1080/00268970500105003 [DOI] [Google Scholar]
- Dorsey N. E., Properties of Ordinary Water Substance (Reinhold, New York, 1940). [Google Scholar]
- Malmberg C. G. and Maryott A. A., J. Res. Natl. Bur. Stand. 56, 1 (1956). [Google Scholar]
- Kell G. S., J. Chem. Eng. Data 12, 66 (1967). 10.1021/je60032a018 [DOI] [Google Scholar]
- Starr F. W., Sciortino F., and Stanley H. E., Phys. Rev. E 60, 6757 (1999). 10.1103/PhysRevE.60.6757 [DOI] [PubMed] [Google Scholar]