Abstract
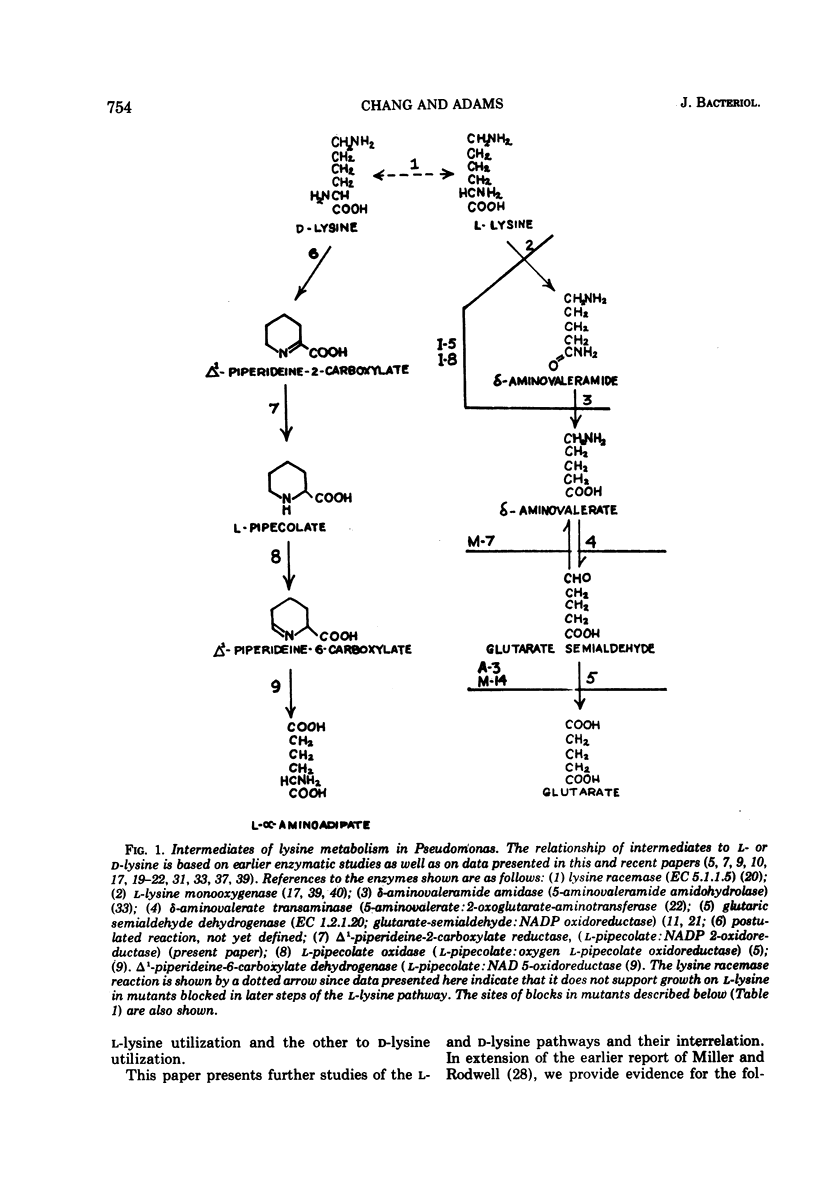
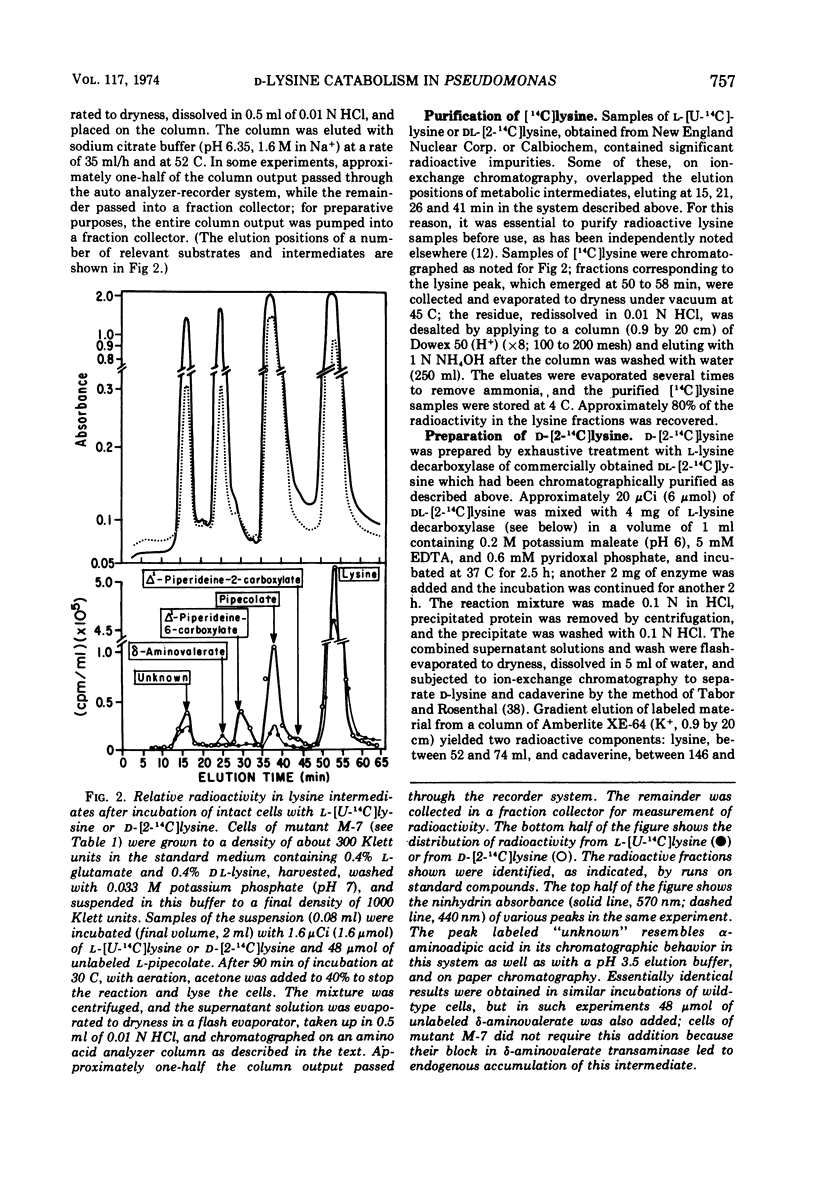
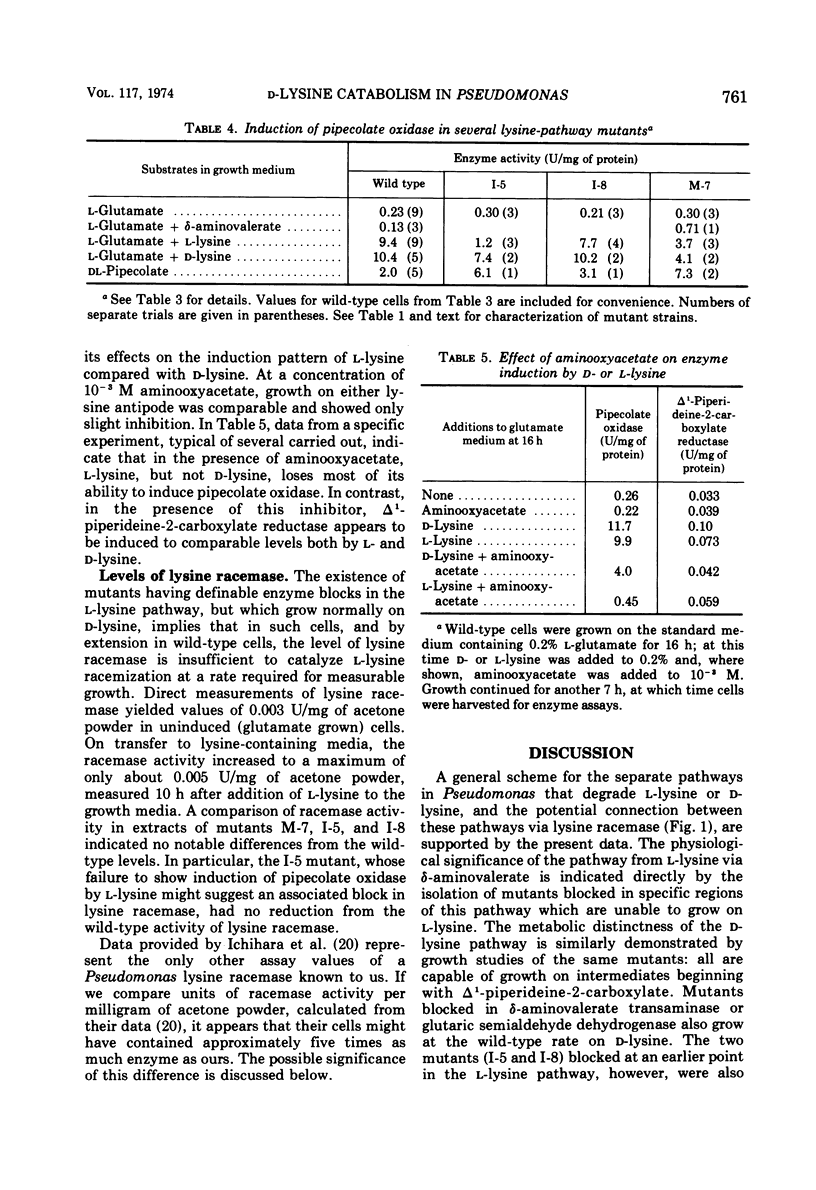
The isolation of several mutant strains blocked in l-lysine degradation has permitted an assessment of the physiological significance of enzymatic reactions related to lysine metabolism in Pseudomonas putida. Additional studies with intact cells involved labeling of metabolic intermediates from radioactive l- or d-lysine, and patterns of enzyme induction in both wild-type and mutant strains. These studies lead to the conclusions that from l-lysine, the obligatory pathway is via δ-aminovaleramide, δ-aminovalerate, glutaric semialdehyde, and glutarate, and that no alternative pathways from l-lysine exist in our strain. A distinct pathway from d-lysine proceeds via Δ1-piperideine-2-carboxylate, l-pipecolate, and Δ1-piperideine-6-carboxylate (α-aminoadipic semialdehyde). The two pathways are independent in the sense that certain mutants, unable to grow on l-lysine, grow at wild-type rates of d-lysine, utilizing the same intermediates as the wild type, as inferred from labeling studies. This finding implies that lysine racemase in our strain, while detectable in cell extracts, is not physiologically functional in intact cells at a rate that would permit growth of mutants blocked in the l-lysine pathway. Pipecolate oxidase, a d-lysine-related enzyme, is induced by d-lysine and less efficiently by l-lysine. Aminooxyacetate virtually abolishes the inducing activity of l-lysine for this enzyme, suggesting that lysine racemase, although functionally inactive for growth purposes, may still have regulatory significance in permitting cross-induction of d-lysine-related enzymes by l-lysine, and vice versa. This finding suggests a mechanism in bacteria for maintaining regulatory patterns in pathways that may have lost their capacity to support growth. In addition, enzymatic studies are reported which implicate Δ1-piperideine-2-carboxylate reductase as an early step in the d-lysine pathway.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS E. Hydroxyproline metabolism. I. Conversion to alpha-ketoglutarate by extracts of Pseudomonas. J Biol Chem. 1959 Aug;234(8):2073–2084. [PubMed] [Google Scholar]
- BASSO L. V., RAO D. R., RODWELL V. W. Metabolism of pipecolic acid in a Pseudomonas species. II. delta1-Piperideine-6-carboxylic acid and alpha-aminoadipic acid-delta-semial-dehyde. J Biol Chem. 1962 Jul;237:2239–2245. [PubMed] [Google Scholar]
- Baginsky M. L., Rodwell V. W. Metabolism of Pipecolic Acid in a Pseudomonas Species IV. Electron Transport Particle of Pseudomonas putida. J Bacteriol. 1966 Aug;92(2):424–432. doi: 10.1128/jb.92.2.424-432.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baginsky M. L., Rodwell V. W. Metabolism of pipecolic acid in a Pseudomonas species. V. Pipecolate oxidase and dehydrogenase. J Bacteriol. 1967 Oct;94(4):1034–1039. doi: 10.1128/jb.94.4.1034-1039.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert A. F., Rodwell V. W. Metabolism of pipecolic acid in a Pseudomonas species. 3. L-alpha-aminoadipate delta-semialdehyde:nicotinamide adenine dinucleotide oxidoreductase. J Biol Chem. 1966 Jan 25;241(2):409–414. [PubMed] [Google Scholar]
- Chang Y. F., Adams E. Induction of separate catabolic pathways for L- and D-lysine in Pseudomonas putida. Biochem Biophys Res Commun. 1971 Nov 5;45(3):570–577. doi: 10.1016/0006-291x(71)90455-4. [DOI] [PubMed] [Google Scholar]
- Chou W. S., Kesner L., Ghadimi H. Purity and purification of 14C-lysine. Anal Biochem. 1970 Oct;37(2):276–281. doi: 10.1016/0003-2697(70)90048-5. [DOI] [PubMed] [Google Scholar]
- Free C. A., Julius M., Arnow P., Barry G. T. Inhibition of alanine racemase by aminoxyacetic acid. Biochim Biophys Acta. 1967;146(2):608–610. doi: 10.1016/0005-2744(67)90252-5. [DOI] [PubMed] [Google Scholar]
- Gryder R. M., Adams E. Inducible degradation of hydroxyproline in Pseudomonas putida: pathway regulation and hydroxyproline uptake. J Bacteriol. 1969 Jan;97(1):292–306. doi: 10.1128/jb.97.1.292-306.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryder R. M., Adams E. Properties of the inducible hydroxyproline transport system of Pseudomonas putida. J Bacteriol. 1970 Mar;101(3):948–958. doi: 10.1128/jb.101.3.948-958.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline R. A., Rodwell V. W. Metabolism of pipecolic acid in a Pseudomonas species. VI. Precursors of glutamate. Arch Biochem Biophys. 1971 Jan;142(1):32–39. doi: 10.1016/0003-9861(71)90256-6. [DOI] [PubMed] [Google Scholar]
- Hutzler J., Odievre M., Dancis J. Analysis for lysine, arginine, histidine, and tyrosine in biological fluids. Anal Biochem. 1967 Jun;19(3):529–541. doi: 10.1016/0003-2697(67)90243-6. [DOI] [PubMed] [Google Scholar]
- ICHIHARA A., ICHIHARA E. A. Metabolism of L-lysine by bacterial enzymes. V. Glutaric semialdehyde dehydrogenase. J Biochem. 1961 Feb;49:154–157. doi: 10.1093/oxfordjournals.jbchem.a127272. [DOI] [PubMed] [Google Scholar]
- IRREVERRE F., PIEZ K. A., WOLFF H. L. The separation and determination of cyclic imino acids. J Biol Chem. 1956 Dec;223(2):687–697. [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J., JACOBY G. A. INDUCTION AND MULTI-SENSITIVE END-PRODUCT REPRESSION IN THE ENZYMIC PATHWAY DEGRADING MANDELATE IN PSEUDOMONAS FLUORESCENS. Biochem J. 1965 Mar;94:569–577. doi: 10.1042/bj0940569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEISTER A., RADHAKRISHNAN A. N., BUCKLEY S. D. Enzymatic synthesis of L-pipecolic acid and L-proline. J Biol Chem. 1957 Dec;229(2):789–800. [PubMed] [Google Scholar]
- Miller D. L., Rodwell V. W. Metabolism of basic amino acids in Pseudomonas putida. Catabolism of lysine by cyclic and acyclic intermediates. J Biol Chem. 1971 May 10;246(9):2758–2764. [PubMed] [Google Scholar]
- Miller D. L., Rodwell V. W. Metabolism of basic amino acids in Pseudomonas putida. Properties of the inducible lysine transport system. J Biol Chem. 1971 Mar 25;246(6):1765–1771. [PubMed] [Google Scholar]
- PETRAKIS P. L., GREENBERG D. M. STUDIES ON L-PROLINE:NAD(P)+2-OXIDOREDUCTASE OF HOG KIDNEY. Biochim Biophys Acta. 1965 Apr 26;99:78–95. doi: 10.1016/s0926-6593(65)80009-1. [DOI] [PubMed] [Google Scholar]
- RAO D. R., RODWELL V. W. Metabolism of pipecolic acid in a Pseudomonas species. I. alpha-Aminoadipic and glutamic acids. J Biol Chem. 1962 Jul;237:2232–2238. [PubMed] [Google Scholar]
- Reitz M. S., Miller D. L., Rodwell V. W. Synthesis of delta-aminovaleramide. Anal Biochem. 1969 Apr 4;28(1):269–272. doi: 10.1016/0003-2697(69)90178-x. [DOI] [PubMed] [Google Scholar]
- Reitz M. S., Rodwell V. W. Delta-aminovaleramidase of Pseudomonas putida. J Biol Chem. 1970 Jun;245(12):3091–3096. [PubMed] [Google Scholar]
- STADTMAN E. R., NOVELLI G. D., LIPMANN F. Coenzyme A function in and acetyl transfer by the phosphotransacetylase system. J Biol Chem. 1951 Jul;191(1):365–376. [PubMed] [Google Scholar]
- Soda K., Misono H., Yamamoto T. L-Lysine:alpha-ketoglutarate aminotransferase. I. Identification of a product, delta-1-piperideine-6-carboxylic acid. Biochemistry. 1968 Nov;7(11):4102–4109. doi: 10.1021/bi00851a045. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Takeda H., Hayaishi O. Crystalline L-lysine oxygenase. J Biol Chem. 1966 Jun 10;241(11):2733–2736. [PubMed] [Google Scholar]
- Takeda H., Yamamoto S., Kojima Y., Hayaishi O. Studies on monooxygenases. I. General properties of crystalline L-lysine monooxygenase. J Biol Chem. 1969 Jun 10;244(11):2935–2941. [PubMed] [Google Scholar]
- Vandecasteele J. P., Hermann M. Regulation of a catabolic pathway. Lysine degradation in Pseudomonas putida. Eur J Biochem. 1972 Nov 21;31(1):80–85. doi: 10.1111/j.1432-1033.1972.tb02503.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Nakazawa T., Hayaishi O. Studies on monooxygenases. IV. Anaerobic formation of an -keto acid by L-lysine monooxygenase. J Biol Chem. 1972 Jun 10;247(11):3434–3438. [PubMed] [Google Scholar]



