Abstract
Aggregation of amyloid-β in the forebrain of Alzheimer's disease subjects may disturb the molecular organization of the extracellular microenvironment that modulates neural and synaptic plasticity. Proteoglycans are major components of this extracellular environment. To test the hypothesis that amyloid-β, or another amyloid precursor protein dependent mechanism modifies the accumulation and/or turnover of extracellular proteoglycans, we examined whether the expression and processing of brevican, an abundant extracellular, chondroitin sulfate-bearing proteoglycan, were altered in brains of amyloid-β-depositing transgenic mice (APPsw) as a model of Alzheimer's disease. The molecular size of chondroitin sulfate chains attached to brevican was smaller in hippocampal tissue from APPsw mice bearing amyloid-β deposits compared to non-transgenic mice, likely due to changes in the chondroitin sulfate chains. Also, the abundance of the major proteolytic fragment of brevican was markedly diminished in extracts from several telencephalic regions of APPsw mice compared to non-transgenic mice, yet these immunoreactive fragments appeared to accumulate adjacent to the plaque edge. These results suggest that amyloid-β or amyloid precursor protein exert inhibitory effects on proteolytic cleavage mechanisms responsible for synthesis and turnover of proteoglycans. Since proteoglycans stabilize synaptic structure and inhibit molecular plasticity, defective brevican processing observed in amyloid-β-bearing mice and potentially end-stage human Alzheimer's disease, may contribute to deficient neural plasticity.
Keywords: amyloid β protein, chondroitin sulfate, proteoglycan, Alzheimer's disease, matrix metalloproteinase (MMP), a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)
INTRODUCTION
Brevican is an aggregating, extracellular matrix (ECM) proteoglycan that is abundantly expressed in the central nervous system. Chondroitin sulfate (CS) chains are covalently linked to the brevican core protein. The presence of brevican in the matrix affects neurite outgrowth and stabilizes synapses (Bandtlow & Zimmermann 2000, Yamaguchi 2000, Hockfield et al. 1990). Data from seizure-induced and perforant-path lesion models indicate that the turnover of brevican contributes to lesion-stimulated sprouting and reinnervation of the dentate gyrus by surviving neurons (Thon et al. 2000, Yuan et al. 2002, Mayer et al. 2005). Brevican is one of four members of the lectican family of aggregating, CS-bearing proteoglycans. Neurons, astrocytes and microglia not only express matrix proteins, but also express members of the MMP (matrix metalloproteinase) and ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) subgroups of metalloproteinases that cleave lecticans, including brevican. Brevican is cleaved by MMPs and ADAMTSs at distinct sites in the protein, allowing neoepitope antibodies raised against the cleavage site sequences to distinguish between MMP-and ADAMTS-derived brevican fragments (Fig. 1), and therefore relative MMP and ADAMTS activity (Gottschall et al. 2005). Lectican deposition in the ECM is thought to be, in part, a sum of synthesis and degradation of the core protein.
Figure 1.
Detection of brevican isoforms and proteolytic degradation by endogenous proteases at specific cleavage sites. Brevican is secreted as a >145 kD protein bearing 1-3 CS chains (A). Brevican is also secreted as the holoprotein without CS chains at 145 kD (B). When probed on western blot with an N-terminal antibody (BD Biosciences, San Jose, CA) three immunoreactive bands appear;: a >145 smear (glycosylated brevican), the 145 kD core protein, and a ~55 kD proteolytic fragment (C, D, E and F). Arrows in (A) and (B) indicate proteolytic cleavage sites. Fragments of brevican are generated by endogenous proteases, the MMPs (D) and ADAMTSs (E). Each has a distinct, specific cleavage site sequence on the brevican protein. Shown here are the specific cleavage sequences for the MMPs and ADAMTSs in mouse, rat and human brevican (based on data from Nakamura et al. 2000). The MMP cleavage-site is 35 amino acid residues upstream from the ADAMTS-specific site (D and E). Distinct “neoepitope” antibodies recognize the MMP- and ADAMTS-derived cleavage fragments of brevican in mouse brain extracts subjected to Western blot on 4-20% gradient SDS-PAGE gels (F). Anti-SAHPSA recognizes the 53 kD, MMP-derived fragment of brevican (F; middle panel) whereas anti-EAMESE detects the 55 kD, ADAMTS-derived form (F; right panel). Mixing the two anti-bodies detects a “thicker” band in this region (F; left panel). “M” indicates molecular weight markers in (F). Antibodies recognize distinct products after proteolytic cleavage with hrADAMTS4 or hrMMP-2 (G). Proteoglycan purified from mouse brain was incubated with 50 nM hrADAMTS-4 (G, lane 1), 50 nM hrADAMTS4 + 5 mM EDTA (G, lane 2), 50 nM hrMMP-2 (G, lane 3) or 50 nM hrMMP-2 + 5 mM EDTA (G, lane 4) and immunoblotted for brevican. Note that the ADAMTS-derived brevican fragment was selectively recognized by anti-EAMESE and the brevican MMP product was recognized by anti-SAHPSA.
Brevican-containing, dense network of ECM in the nervous system, wraps around synapses where irregularly deposited proteins bind to anchored membrane “receptors” to form surface compartments around the synapse (John et al. 2006, Frischknecht et al. 2009). Provocative data has recently demonstrated that these ECM compartments act as a barrier to lateral diffusion of AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate) receptors preventing them from clustering at the active, synaptic site. Removal of ECM using exogenous chondroitinase enhanced the movement of these receptors into the synaptic region, and even elevated paired pulse ratios, possibly by exchanging desensitized receptors for responsive, naïve ones (Frischknecht et al. 2009). In addition, CS-bearing proteoglycans bind low density lipoproteins with high avidity and capacity (Tannock & King 2008), and those lipoprotein particles which contain apolipoprotein E show enhanced proteoglycan binding affinity compared to particles without apo E (Flood et al. 2002). It would not be surprising that changes in CS substitution or proteolytic cleavage of the core protein of proteoglycans would result in subtle alterations in the lateral-medial diffusion of AMPA receptors at excitatory synapses, or might alter the ability of the proteoglycans to bind to apolipoprotein E-containing low density lipoproteins.
One characteristic feature of Alzheimer's disease (AD) is the deposition of insoluble Aβ peptide fibrils into amyloid plaques found in the extracellular space of forebrain (Glenner & Wong 1984, Selkoe 1991). Various aggregate forms of Aβ may induce oxidative stress and a glial-mediated inflammatory response (McGeer et al. 1989, Sastre et al. 2006, Castellani et al. 2008) including the expression of matrix-degrading proteases (Gottschall 1996, Satoh et al. 2000, Deb et al. 2003). A transgenic mouse model that overexpresses the human APP (amyloid precursor protein) gene bearing the Swedish mutation (APPsw) in neurons mimics multiple characteristics of AD such as the deposition of amyloid, chronic neuroinflammation and diminished cognitive function (Hsiao et al. 1996, Terai et al. 2001), but certainly not all aspects, as there is little or no loss of morphologically identifiable synapses or neurons. Although evidence is accumulating around dysfunctional synaptic physiology, overall little is known about Aβ-related molecular mechanisms that may be responsible for cognitive dysfunction in the APPsw mouse model or for dementia in AD subjects. In fact, it has been suggested that AD may be a disease of dysregulated plasticity and synaptic loss (Mucke et al. 2000, Selkoe 2002, Arendt 2009), and aggregated Aβ (oligomers or other aggregates) impacts neuronal and synaptic plasticity (Lacor et al. 2004, Haass & Selkoe 2007). Proteoglycans and their cognate proteases modulate morphological plasticity during neural repair and regeneration (Thon et al. 2000, Yuan et al. 2002, Reeves et al. 2003, Mayer et al. 2005), implying a link between extracellular, deposited Aβ and proteoglycans. Thus, the purpose of this study was to determine whether the presence of aggregate forms of Aβ (or overexpression of APP) in a murine model of AD influences the processing and/or deposition of the CS-bearing proteoglycan, brevican.
MATERIALS & METHODS
Animals
All animal procedures described here were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Florida (USF) and every effort was made to minimize pain and discomfort and limit the number of animals used in this study. Animals expressing human APPsw (Tg2576) mice were bred and raised in a colony maintained at USF in rooms with a 12 hour light cycle and regulated temperature and humidity. Mice were housed 3 to 4 per cage and had free access to food and water. APPsw and their non-transgenic littermates were genotyped as described previously (Hsiao et al. 1995, Morgan et al. 2000). Mice between 15-16 months of age were euthanized with an overdose of pentobarbital, cardiac-perfused with ice-cold phosphate-buffered saline (pH 7.4), decapitated and the brain removed from the skull for biochemical and immunohistochemical analysis. Each brain was cut sagitally at the midline, separating the hemispheres. One hemisphere was dissected into regions and frozen (−80°C) for later biochemical analyses and the other hemisphere was fixed in fresh 4% paraformaldehyde in 0.1M phosphate buffer (PB; pH 7.4) overnight at 4°C; (n=6, APPsw(+) and n=4, APPsw(−) non-transgenic littermates). Unless otherwise stated, all chemicals were purchased from Sigma Aldrich, St. Louis, MO.
Proteoglycan extraction and purification, and brevican degradation assay
Soluble extracts were prepared from whole mouse brain. Proteins bound to DEAE Sepharose Fast Flow (GE Healthcare Life Sciences, Piscataway, NJ) were sequentially eluted; weakly bound proteins eluted with a step-wise gradient of increasing concentrations of NaCl (0.15M to 0.5M), and highly, negatively charged proteoglycans eluted with 1M NaCl as previously described (Yamada et al. 1994, Hamel et al. 2008). This preparation was used as a brevican substrate to conduct cleavage assays where novel antibodies were used to detect the selective MMP-derived or ADAMTS-derived proteolytic fragments of brevican (Hamel et al. 2008). Human recombinant ADAMTS4 was a gift of Roche Biosciences Palo Alto, CA, and human recombinant MMP-2 was purchased from R & D Systems (Minneapolis, MN). Proteases (50 nM) were mixed with proteoglycan substrate in 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM CaCl2, 0.01% Brij-35 and incubated at 37 C for 3 h. Samples were diluted with SDS-PAGE sample buffer and subjected to Western blot.
Western Blotting
Brain tissue was extracted using a teflon-glass homogenizer in 10 volumes (10 ml/g) of Triton-X-100-containing buffer (20 mM Tris-HCl at pH 7.4, 5 mM EDTA, 1% Triton-X-100 and 1:100 protease inhibitor cocktail, Calbiochem type III, LaJolla, CA) for the data in Figs. 1 and 2 and in RIPA buffer (50 mM Tris-HCl at pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% Triton-X-100, 1% sodium deoxycholate and 0.1% SDS) for Figs. 3 and 7. The homogenate was centrifuged in a microcentrifuge at 12,800 × g for 15 minutes. The supernatant was collected and stored at −80°C.
Figure 2.
Immunoreactivity of Aβ and brevican isoforms in APPsw (+) transgenic mice compared to littermate non-transgenic (−) mice. Low power micrograph of immunoreactive Aβ burden in forebrain of 15-16 month old non-transgenic and APPsw mice (A). Hippocampal proteins from 15-16 month old non-transgenic (−)(n=3) and APPsw (+)(n=3) mice were separated on 4-20% SDS-PAGE, transferred to PVDF membrane and probed with anti-Aβ 95-2-5 (B). Note high and low molecular weight Aβ immunoreactivity (arrows). Lane “m” indicates molecular weight markers, lanes “h” are human frontal cortex tissue samples from patients diagnosed with AD, lanes Aβ 10 and Aβ 1 are synthetic Aβ(1-42) at 10 ng and 1 ng per lane respectively (a). Same blot was probed for anti-GAPDH immunoreactivity to normalize for protein loading (b). Identical hippocampal extracts were subjected to Western blot on 4-20% SDS-PAGE gels and probed for anti-brevican, anti-EAMESE and anti-SAHPSA (C). Densitometric semi-quantitative analysis of the western blots in (C) as expressed in arbitrary densitometric units (D). There was no change in abundance of >145 kD brevican protein in hippocampus, although an an identifiable molecular weight shift was apparent in APPsw extract. An increase in the abundance of the core 145 kD brevican (p ≤ 0.05) was accompanied by a decrease in the generalized N-terminal fragment of brevican in APPsw extracts (p ≤ 0.05). A marked decrease in the abundance of the brevican fragment generated by MMP-mediated proteolytic cleavage (p ≤ 0.05) was observed, denoted by anti-SAHPSA immunoreactivity, in hippocampal samples of APPsw mice.
Figure 3.
Cha'se, N-glycanase and O-glycanase (+ sialidase) treatment of hippocampal extract derived from 15-16 month old non-transgenic and APPsw mice (A). Enzyme-treated-hippocampal proteins were separated on 6% Tris-glycine SDS-PAGE gels, blotted to PVDF and probed with an anti-brevican antibody. In samples from both genotypes, glycosidase treatments released the brevican core proteins to migrate to lower molecular weights indicating the presence N-and O-linked oligosaccharides and CS chains (A). The lower block of the image designated by the “star and brackets” indicates that the portion of the blot was exposed for a longer period of time compared to the upper region. (B) Hippocampal samples from three non-transgenic and three APPsw animals, with (+) and without (−) treatment with Ch'ase, subjected to SDS-PAGE, blotted, and probed with anti-brevican. Note the consistent migration difference in CS-bearing brevican, and the difference in the abundance of the core protein between the genotypes both before Ch'ase treatment but the little difference in abundance after Ch'ase treatment. Representative blots are shown from experiments that were repeated once.
Tissue extracts were loaded (equal amounts of total protein, 15 μg for brevican, 2x reducing Laemmli sample buffer, heated 95 C for 5 min) onto pre-cast 4-20% gradient or 6% SDS-PAGE gels (Tris-glycine, Invitrogen, Carlsbad, CA). Protein was electrophoretically transferred to a polyvinylidine difluoride membrane (PVDF, Immobilon, Millipore, Billerica, MA) and the membranes were probed with various antibodies. For immunoblotting, the membranes were washed with buffer B (10 mM phosphate buffered saline, pH 7.4 containing 0.05% Tween 20) for 5 minutes, blocked in buffer B containing 5% dry milk for 1 hour and then incubated overnight at 4°C with primary antibody: mouse anti-brevican (1:1000, BD Biosciences, San Jose, CA), rabbit anti-EAMESE (1:1000) (Mayer et al. 2005), rabbit anti-SAHPSA (1:500, see description of this antibody below and in Results from Fig. 1). Primary antibodies were detected with corresponding secondary antibodies of anti-mouse or anti-rabbit IgG conjugated to horseradish peroxidase (Millipore, Temecula, CA) and antigens visualized using the Supersignal chemiluminescence substrate (Pierce, Rockford, IL). The neoepitope antibody, anti-SAHPSA, was custom-raised and used for the first time in this study. Antigen conjugation, injections and bleeding were conducted by Sigma-Genosys (see below). The SAHPSA sequence corresponds to the C-terminal epitope of the N-terminal fragment resulting from MMP cleavage of brevican (Nakamura et al. 2000), and a product is predicted to be slightly smaller (35 amino acid residues) than the ADAMTS-derived brevican fragment. After Western blotting, PVDF membranes were stained with Coomassie blue and normalization bands quantified (see Data and Statistical Analysis).
Antibody generation
A rabbit antibody raised against the neoepitope C-terminal sequence of the N-terminal, 53 kD brevican fragment formed by MMP cleavage was generated by Sigma-Genosys (St. Louis, MO) and purified in our laboratory. The novel C-terminal sequence “SAHPSA” from the mouse was the neoepitope, and the peptide used for antibody generation contained a glycine spacer and a Cys0 for conjugation resulting in the following peptide, “CGGGQSAHPSA”. Synthesized by Sigma-Genosys, this peptide was conjugated to keyhole limpet hemocyanin and rabbits were subjected to standard immunization protocols. Serum collected after the fifth booster was titered against the peptide using a solid-phase detection system and specific antibody was purified using immobilized peptide affinity chromatography (Sulfo-Link, Thermo Pierce). Incubation of the antibody with excess SAHPSA peptide (10 μM), prior to using the antibody for western blot or immunohistochemistry eliminated immunoreactivity.
Proteoglycan deglycosylation
Deglycosylation of proteins was carried out similar to the method of Viapiano (Viapiano et al. 2005). Buffer (20 mM Tris-HCl, 20 mM sodium acetate, 25 mM NaCl, pH 7.0) was added to RIPA buffer-extracted hippocampal brain samples to make a protein concentration of about 1 μg/μl. Then glycosidases were added in different combinations at the following concentrations: chondroitinase (Ch'ase) ABC (Sigma Chemical Company, St. Louis, MO) 0.5 U/ml, O-glycosidase (endo-α-N-acetylgalactosaminidase, Prozyme Glyko, San Leandro, CA) 90 mU/ml together with sialidase A 400 mU/ml (recombinant from Arthrobacter ureafaciens expressed in E. coli, Glyko), and/or N-glycanase 180 mU/ml (peptide-N-glycosidase F, Glyko). Incubations were carried out at 37°C for 4 hours. Reducing SDS-PAGE sample buffer was added and the samples were subjected to western blot for brevican or versican.
quantitative RT-PCR
Total RNA was isolated from frontal cortex of APPsw mice (n = 8) and non-transgenic littermates (n = 6) according to the manufacturers instructions included with the SV Total RNA Isolation System (Promega, Madison, WI) that included a step to remove genomic DNA. RNA was quantified using a Nanodrop 1000 (Thermo Scientific ,Waltham, MA). One μg of RNA was reverse transcribed (Qiagen, Valencia, CA) and 50 ng cDNA product was subjected to PCR using TaqMan technology (ABI, Foster City, CA) with ABI mastermix and primer-probe sets in an ABI 7900 real time PCR system. Samples were assayed in triplicate and all samples had a standard deviation of < 0.5 Ct units. The logarithmic average Ct value for each gene was converted to a linear value using the conversion 2−Ct (Nairn et al. 2007, Mori et al. 2008). GAPDH was used as the normalization gene and values for GAPDH were not different across groups. Converted values were normalized to GAPDH by dividing the individual gene value by the value for GAPDH.
Immunohistochemistry
One hemisphere of mouse brain was fixed in fresh 4% paraformaldehyde (diluted in 0.1 M phosphate buffer, PB; pH 7.4) overnight and cryoprotected with 15% and 30% sucrose (in PBS) for 24 hours each at 4°C. Individual brains were mounted on a cryostat chuck at −20°C and sectioned at 30 μm. Sections were stored freely floating in antifreeze solution at −20°C.
Selected sections to be used for immunohistochemistry were washed in PBS 3 times for 5 minutes each, blocked and permeabilized in 10% normal goat serum, 3% 1 M lysine and 3% Triton-X-100 for 1 hour, and incubated overnight in primary antibodies against: C-terminal (G3) anti-brevican rb18 (1:100; a generous gift of Yu Yamaguchi, Burnham Institute, La Jolla, CA, (Hagihara et al. 1999)), anti-EAMESE (1:1000), anti-SAHPSA (1:500), 4G8 anti-Aβ (1:250, AbD Serotec, Raleigh, NC), and anti-Aβ-95-2-5 (raised against Aβ1-40 and recognizes both Aβ1-42 and Aβ 1-40; for characterization see (Morgan et al. 2000, Wilcock et al. 2001, Gordon et al. 2001) at 4°C. Doubly probed sections were washed and incubated in anti-rabbit IgG conjugated to Alexa-Fluor 488 (Molecular Probes, Eugene, OR) and anti-mouse IgG conjugated to Alexa-Fluor 594 (Molecular Probes) or vice versa for 1 hour at room temperature. The sections were washed for 15 minutes, wet mounted on glass slides and coverslipped with VectaShield mounting medium (Vector Labs, Burlingame, CA). Other sections were stained for anti-Aβ with Vector Labs nickel intensified ABC system (Yuan et al. 2002).
Microscopy and image acquisition
Multi-labeled, epifluorescent photomicrographs were acquired using a Zeiss Axioskop microscope, interfaced with an Axiocam and images acquired with Openlab software. Controls for each immunomarker included secondary antibody in the absence of a primary antibody in which the staining was minimal to absent. Exposure times and aperture opening were constant for each magnification and antibody used. All pictures were minimally and equally (control and treatment group) modified using Adobe Photoshop.
Brevican cleavage assay in BV2 cell culture
BV2 cells were seeded in 24-well plates at 100,000 cells per well in Dulbecco's Modified Eagles Media (DMEM) containing 10% fetal bovine serum (MediaTech, Manassas, VA) and 1% antibiotic-antimycotic. After three days, growth medium was changed to serum-free medium (high glucose DMEM with 25 mM HEPES) for 2 h and then BV2 cells were treated with 2 μM Aβ(1-42) (Bachem, Torrance, CA) or 2 μg/ml LPS (O55:B5, Sigma) in the absence and the presence of the MMP inhibitor AG3340 (selective for MMP-2 and MMP-9). Two h later, 6 μg/well of a DEAE-purified preparation of proteoglycan (see above) was added for an additional 6 h and 20 h. Supernatant was collected and subjected to Western blot for anti-brevican, anti-EAMESE and anti-SAHPSA.
Data and statistical analysis
Quantitative analysis was conducted on images scanned (BioRad Personal Densitometer, Hercules, CA) from exposed film or images acquired from a Kodak 4000MM (Carestream Health, Toronto, Canada) from the western blots in order to measure optical density of the signals. Samples for a single antigen from an individual brain region were analyzed together on a single blot, ie. all frontal cortex samples for brevican were separated on a single SDS-PAGE gel. Exposures were minimized to keep the bands in a linear range for density measurements. Rectangular sample areas were drawn around the immunoreactive bands, the integrated densities (U/mm2) calculated using Molecular Imaging Software, and the background density subtracted from each measurement. The mean densities ± SEM, expressed as arbitrary densitometric units, were calculated for each group and differences between non-transgenic and APPsw transgenic mice determined by using Student's t-test. A p ≤ 0.05 was considered a significant difference between groups. Completed immunoblots were stained with Coomassie blue (Bio-Rad), the membranes scanned and the abundant, β-actin-containing band at about 42kD was measured for optical density. On a single blot, the difference between the high and the low density for actin was less than 15% indicating equal protein loading of the blot. Individual brevican densities were not corrected for these differences (Aldridge et al. 2008).
For the qRT-PCR, data for each gene was analyzed using Student's t-test and a p < 0.05 was considered a significant difference between the two groups.
RESULTS
Processing of brevican core protein
Brevican consists of a core protein with up to three, negatively-charged, sulfated, glycosaminoglycan, chondroitin sulfate chains covalently bound to the central domain (Fig. 1A). When proteins from mouse brain tissue extract were separated on a 4-20% gradient SDS-PAGE gel, these isoforms of brevican appeared on western blot as a smear at >145 kD (Fig. 1C). A proportion of brevican immunoreactivity is found without glycosaminoglycan chains (Fig. 1B) identified as a sharp band at 145 kD (Fig. 1C). The core protein also bears N- and O- linked oligosacharrides (not shown in Fig. 1) (Viapiano et al. 2003, Viapiano et al. 2005). An abundance of a 53-55 kD immunoreactive band, the N-terminal proteolytic fragment of brevican, is observed on western blot when using a G1-domain antibody that detects all N-terminal region isoforms (Fig. 1C). In the extracellular microenvironment, brevican and other lecticans are selectively cleaved by endogenous proteases, the ADAMTSs and MMPs, at specific sites toward the N-terminus, revealing novel sequences of amino acids at their C-termini (Fig. 1D & 1E) (Matthews et al. 2000). In the mouse brevican sequence, the ADAMTS cleavage site (residues 395-396) is 35 amino acids downstream from the MMP site (residues 360-361), resulting in an N-terminal, ADAMTS-derived fragment that is slightly larger than the MMP fragment (Nakamura et al. 2000, Matthews et al. 2000). Antibodies raised against the newly exposed C-termini amino acid sequences distinguish between the protease-specific (ADAMTS-vs. MMP-derived), N-terminal fragments of brevican. The ability to detect proteolytic degradation of brevican implies the presence of active proteases and is functionally important since proteolytic cleavage may modulate structural synaptic plasticity (Thon et al. 2000, Yuan et al. 2002, Mayer et al. 2005). The characterization of an antibody that detects the murine ADAMTS-derived fragment, anti-EAMESE (Fig. 1F, left panel), has been reported previously (Mayer et al. 2005, Ajmo et al. 2008) and here we make use of a novel antibody raised against the neoepitope derived from the cleavage of brevican by members of the MMP family. MMP cleavage forms an N-terminal fragment of murine brevican with the C-terminal sequence, SAHPSA (Fig. 1D, 1F). When probed on a western blot, the anti-SAHPSA antibody detects a single immunoreactive band at ~53 kD (Fig. 1F, middle panel), on a 4-20% gradient SDS-PAGE gel, compared to the ~55 kD band recognized by anti-EAMESE. When proteoglycan purified from mouse brain was incubated with human recombinant ADAMTS4 (Fig. 1G, lane 1) or MMP-2 (Fig. 1G, lane 3), the antibodies selectively detect the appropriate size fragment of brevican that was produced by the appropriate protease. Addition of EDTA (lanes 2 and 4) blocked both respective cleavage activities. Thus, the two antibodies selectively and specifically recognize the ADAMTS-derived (anti-EAMESE) and the MMP-derived (anti-SAHPSA) cleavage fragments of brevican. Changes in the abundance of these fragments as detected by the antibodies may indicate altered ADAMTS or MMP activity.
Decreased size of CS-bearing brevican in APPsw mice
Aβ is deposited into the extracellular mileu as plaques in both AD forebrain and in older APPsw mouse brain (Fig. 2A), where RIPA buffer soluble Aβ is found as a monomer/oligomer and an SDS-stable high molecular weight aggregate (arrows, Fig. 2B). Since brevican is a major component of the ECM, we examined whether the presence of aggregating/fibrillar Aβ would affect the deposition and disposition of brevican in transgenic mice overexpressing APPsw and bearing numerous Aβ deposits (Fig. 2A). Hippocampal tissue samples from 15-16 month old APPsw mice and 15-16 month old non-transgenic littermates were probed with anti-brevican antibody that detects an N-terminal region epitope. Three immunoreactive isoforms of brevican were identified; the >145 kD brevican that bears CS chains, the 145 kD core protein, which does not contain CS chains, and a non-selective, general 53-55 kD N-terminal fragment (Fig. 2C). Densitometric analysis was used to quantitate the relative levels of brevican isoform immunoreactivity in hippocampus. There was no significant change in the abundance of CS-containing brevican at >145 kD (Fig. 2D). However, there was an apparent decrease in the molecular weight of the molecules that compose this isoform in APPsw mice, such that the CS-containing brevican “smear” migrated faster in hippocampal extracts from APPsw mice compared to their non-transgenic counterparts (Fig. 2C). Extract from APPsw hippocampus also showed a significant increase (about 3-fold) in the amount of core protein that did not bear CS chains (145 kD band), and a marked decrease (about 65%) in the abundance of the 53-55 kD, generalized N-terminal fragment (Fig. 2D). To delineate the family of proteases which may be responsible for the decrease in the 53-55 kD generalized fragment, these samples were probed with the ADAMTS- and MMP-specific neoepitope antibodies. No changes were seen in the amount of ADAMTS-derived fragment (EAMESE), but interestingly, a significant decrease in the abundance of the MMP-derived fragment (SAHPSA) was seen in the hippocampus of APPsw mice compared to non-transgenic littermates (Fig. 2C, middle and lower panels and 2D). None of the observed changes in brevican molecular weight or processing appeared to be due to Aβ binding to brevican or its fragments, since the molecular size of Aβ immunoreactivity on Western blot was clearly different from the molecular weights of the brevican isoforms.
Several brain regions of APPsw and non-transgenic littermates were probed for the brevican isoforms using generalized and protease-specific antibodies. The results are shown in Table 1. The shift in the molecular weight of the >145 kD isoform was observed consistently, and in several regions the abundance of the CS-bearing form of brevican was significantly reduced compared to non-transgenic animals. Furthermore, an increase in the amount of the 145 kD core protein was found in frontal cortex and temporal lobe, together with a decrease in the abundance of the 55 kD fragment in the temporal lobe similar to effects observed in hippocampus. Hippocampus and cerebral cortex are brain regions burdened with Aβ plaques (Fig. 2A). However, even in a brain region that does not bear Aβ-containing plaques, cerebellum, there was an increase in core protein and a significant decline in the MMP-derived brevican fragment (Table 1). Brain stem, also lacking in plaques, revealed no changes in the generalized fragment of brevican even though an increase in the core protein was apparent in that region.
Table 1.
Quantitation of immunoreactivity of brevican isoforms in brain regions of APPsw transgenic (+) mice and non- transgenic littermates (expressed as arbitrary densitometric units).
| G1 brevican isoforms |
ADAMTS-derived fragment |
MMP-derived fragment |
||||
|---|---|---|---|---|---|---|
| brain region | Tg | > 145 kD | 145 kD | 55 kD | EAMESE | SAHPSA |
| Frontal cortex | nt | 28.25 ± 3.20 | 5.93 ± 0.43 | 1.87 ± 0.62 | 0.85 ± 0.16 | 0.18 ± 0.13 |
| APPsw | 17.39 ± 2.21 * | 7.85 ± 0.86* | 1.62 ± 0.55 | 1 06 ± 0.14 | 0.08 ± 0.05 | |
|
| ||||||
| Temporal lobe | nt | 25.05 ± 2.13 | 2.50 ± 0.03 | 3 18 ± 0.56 | 0.93 ± 0.28 | 0.59 ± 0.15 |
| APPsw | 17.16 ± 2.40* | 6.31 ± 0.44* | 1.35 ± 0.26* | 1.17 ± 0.39 | 0.33 ± 0.12 | |
|
| ||||||
| Cerebellum | nt | 11.29 ± 1.08 | 2.98 ± 0.23 | 4.53 ± 0.44 | 1.22 ± 0.03 | 0.93 ± 0.05 |
| APPsw | 8.54 ± 0.72* | 6.85 ± 0.28* | 2.37 ± 0.23* | 1.41 ± 0.20 | 0.43 ± 0.12* | |
|
| ||||||
| Hypothalamus | nt | 11.90 ± 1.19 | 2.37 ± 0.56 | 1.35 ± 0.19 | 3.67 ± 0.31 | 0.68 ± 0.21 |
| APPsw | 7.99 ± 1.36* | 4.98 ± 0.74* | 0.79 ± 0.11 * | 3.18 ± 0.23 | 0.83 ± 0.30 | |
|
| ||||||
| Brain stem | nt | 9.64 ± 2.25 | 1.90 ± 0.51 | 5.28 ± 0.72 | 2.31 ± 0.31 | 0.79 ± 0.26 |
| APPsw | 7.31 ± 1.60 | 4.73 ± 0.83* | 5.72 ± 0.81 | 2.29 ± 0.24 | 0.73 ± 0.26 | |
indicates significantly different from non-transgenic
Ch'ase and N- and O-glycanase treatment
Extracts from non-transgenic and APPsw hippocampus were treated with Ch'ase, N-glycanase and O-glycanase (+ sialidase A) and combinations to identify possible changes in the glycoforms of brevican in APPsw hippocampus. These brevican isoforms were separated on 6% acrylamide gels, in an effort to better distinguish the higher molecular weight isoforms. Similar to Fig. 2, brevican isoforms bearing CS chains migrated markedly faster in extracts from APPsw hippocampus compared to non-transgenic extract (Fig. 3A lane 6 compared to lane 12 and Fig. 3B). The brevican core protein without CS migrated at 115 kD on 6% gels, rather than the more commonly stated 145 kD. Removing CS chains with Ch'ase resulted in identical molecular weight core proteins between non-transgenic and APPsw extracts (Fig. 3A lanes 5 and 6 compared with lanes 11 and 12). There were no qualitative differences in brevican isoforms between non-transgenic or APPsw when extracts were treated with Ch'ase, N-glycanase, and/or O-glycanase or combinations thereof (Fig. 3A). As found by others (Viapiano et al. 2003), treatment with each glycanase, reduced the molecular weight of the brevican core protein and the N-terminal brevican fragment, indicating that the core protein and the fragment bear N- and O-linked oligosacharrides. Interestingly, Ch'ase treatment alone resulted in the appearance of a lower molecular weight 90 kD core protein potentially representing the truncated, GPI-anchored, isoform of brevican (Fig. 3A 90 kD band in lane 5 compared to lane 6). This species bears N- and O-linked oligosaccharides as well.
There were, however, clear quantitative differences in the isoforms of brevican between the two genotypes. In untreated extract, the 115 kD core brevican protein that does not bear CS chains is markedly more abundant in APPsw samples compared to non-transgenic ones (Fig. 3B lanes 1, 5 and 9 compared to lanes 3, 7 and 11). In contrast, after Ch'ase treatment, the total core protein was modestly reduced in the APPSw compared to non-transgenic (Fig. 3B lanes 2, 6 and 10 compared to lanes 4, 8 and 12).
Quantification of chondroitin sulfate synthesizing enzymes
Since there was a molecular weight shift in the CS-bearing form of brevican, appearing at a lower molecular weight) in APPsw mice, we postulated that this shift may be due to changes in the expression of the enzymes responsible for the synthesis of CS. Thus, qRT-PCR was conducted using RNA isolated form frontal cortex of non-transgenic (n = 6) and APPsw mice (n = 8) (Fig. 4). We demonstrated that the same molecular weight shift in brevican immunoreactivity occurred in these frontal cortex APPsw samples compared to non-transgenic tissue that looked identical to hippocampus (data not shown). There were no significant differences in the expression of Csgalnact1, Csgalnact2 (chondroitin sulfate N-acetyl-galactosaminyltransferase-1 and -2); Chsy1, Chsy2 (chondroitin sulfate synathase-1 and -2); Chpf (chon-droitin polymerizing factor) transcripts between both groups of mice (Fig. 4).
Figure 4.
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) for chondroition synthase synthesizing enzymes using RNA isolated from frontal cortex of non-transgenic (n = 6) and APPsw mice (n = 8). Threshold cycle number (2−Ct) for the gene of interest was divided by the threshold for the GAPDH gene (2−Ct) for each sample. There were no differences in expression for any of the five transcripts. Csgalnact1, Csgalnact2 (chondroitin sulfate N-acetyl-galactosaminyltransferase-1 and -2); Chsy1, Chsy2 (chondroitin sulfate synathase-1 and -2); Chpf (chon-droitin polymerizing factor).
Localization of brevican and its proteolytic fragments relative to plaques in APPsw brain
Immunohistochemistry revealed distinct patterns of immunoreactivity for brevican and its protease-selective fragments around Aβ plaques in APPsw mouse brain. Brevican immunoreactivity was found adjacent to Aβ immunoreactivity, even partially in the central core of the plaque, but there appeared to be little co-localization (Fig. 5A-C) between the two antigens. In plaques with obvious cores, anti-EAMESE immunoreactivity was found mainly surrounding the plaque periphery (Fig. 5 D compared with F and G compared with I) in regions where dystrophic neuritic growth is often located. In some plaques, a slightly different pattern was apparent when staining with anti-SAHPSA. Anti-SAHPSA immunoreactivity is found closer to the core as compared to anti-EAMESE staining, and brevican immunoreactivity was located more to the periphery (Fig. 5J-L) as well. Overall, in cortex and hippocampus, anti-SAHPSA the abundance of immunoreactivity was lower in APPsw tissue, except in regions of plaques (not shown).
Figure 5.
Immunohistochemical localization of brevican (rb18 monoclonal), the proteolytically cleaved fragments of brevican (rabbit polyclonal against the ADAMTS-derived fragment, anti-EAMESE; MMP-derived fragment, anti-SAHPSA; and Aβ plaques (95-2-5 rabbit polyclonal) in APPsw transgenic mice (representative from n = 6 APPsw mice). Epifluorescent micrographs of brevican immunoreactivity (A, H, K), anti-EAMESE immunoreactivity (D, G), anti-SAHPSA immunoreactivity (J) and Aβ (B, E) in fixed frontal/parietal cortical sections. Merged composites (C, F, I & L). All images captured at 200x magnification.
Brevican cleavage after Aβ-stimulated protease release in BV2 cells
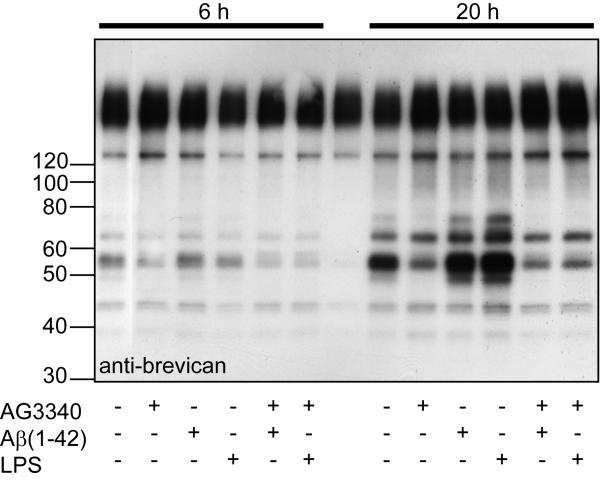
Since the deposition of Aβ-containing amyloid plaques is an obvious phenotype of this transgenic animal, we wanted to determine whether Aβ-treatment of the BV2 cell line, a microglial cell line, would stimulate, inhibit or not change protease cleavage of brevican. For this experiment, BV2 cells were treated with Aβ or lipopolysaccharide (LPS) in the presence or absence of an MMP inhibitor AG3340 (Fig. 6). After a 2 h pre-treatment period, DEAE-purified proteoglycan was added to the culture for a period of 6 h or 20 h, and supernatant was then collected and subjected to Western blot for brevican. It is evident that LPS, and to a lesser extent, Aβ(1-42) stimulated the cleavage of brevican at both 6 and 24 h. Addition of AG3340 (1 μM) inhibited the predominant proteolytic activity responsible for increased abundance of the brevican fragment (Fig. 6). These results indicate that as opposed to in vivo where proteolytic activity was inhibited in APPsw brain extracts, Aβ appears to stimulate activity from BV2 cells in vitro.
Figure 6.
Growth medium was changed to serum-free medium for 2 h and then cultured BV2 cells were treated with 2 μM Aβ(1-42) or 2 μg/ml LPS in the absence and the presence of the MMP inhibitor AG3340 (selective for MMP-2 and MMP-9). Two h later, 6 ug/well of a DEAE-purified preparation of proteoglycan was added for an additional 6 h (left panel) and 20 h (right panel). Supernatant was collected and subjected to Western blot for anti-brevican, anti-EAMESE and anti-SAHPSA.
DISCUSSION
The formation of Aβ peptides by the processing of the APP protein appears critical in the pathogenesis of AD, yet the functional relationship of Aβ to neuronal pathophysiology and synaptic modulation in AD remain remarkably unclear (Palop et al. 2006). As secreted Aβ forms extracellular oligomers which aggregate to form plaques within the extracellular space, there may be a disruption of extracellular molecular complexes that interact with neurons which could contribute to AD-associated, dysfunctional neural plasticity. Several ECM-related components, such as laminin-1, agrin, glypican-1, thrombospondin and integrins, bind Aβ in vitro and modulate Aβ aggregation and neurotoxicity (Cotman et al. 2000, Drouet et al. 1999, Kowalska & Badellino 1994, Watanabe et al. 2004), suggesting that ECM molecules interact with Aβ in a manner that could contribute to AD pathogenesis. Here, we demonstrate that the processing and extracellular proteolytic cleavage of brevican is altered in brain from a mouse model of AD bearing Aβ deposits. Thus, it is possible that amyloid-induced inhibition of extracellular brevican processing may be associated with an environment less permissive to neural plasticity or one that exhibits “abnormal” neural plasticity, particularly in regions bearing potent oligomers Aβ. These changes may also be related to the area of irregular (dystrophic) neuritic growth that typically surrounds many plaques. In fact, synapses appear to be lost in these concentric regions surrounding plaques even in Aβ-depositing animal models (Dong et al. 2007).
In APPsw mice, there was a change in the abundance of all three isoforms of brevican. The immunoreactive smear that was >145 kD on western blot (which represents CS-containing isoforms of brevican) was strikingly more condensed and appeared at a lower molecular weight compared to a higher molecular weight smear, that extended over a larger span of molecular weights, that was found in non-transgenic animals. It may be that extracts from APPsw mice bear shorter chains or fewer numbers of CS chains bound to the core protein, ie. one or two in APPsw mice instead of two or three, shifting the smear in APPsw mice to a lower molecular weight. Alternatively, the CS chains may be shorter in APPsw extract. The reason less CS may be bound to the core protein of brevican in APPsw mice brain is undetermined but we did demonstrate that there are no significant differences in the expression of the major CS synthesizing enzymes between genotypes. This could mean that potential alterations in CS substitution of the brevican core protein must be post-translational in origin. One possibility may involve decreased glycosyltransferase activity or changes in the catabolism of these forms after interaction with Aß. These molecular alterations in CS chains may be linked to plaque-associated astrogliosis, especially since a significant portion of brevican issynthesized by astrocytes. In accordance with the concept of diminished CS linkage in APPsw tissue, the abundance of the brevican isoform containing no CS chains was increased in APPsw mice. Interestingly, ADAMTSs have a higher activity and binding affinity for lecticans bearing CS chains compared to holoprotein without CS (Flannery et al. 2002, Tortorella et al. 2000, Flannery 2006). Thus, diminished cleavage of the holoprotein that lacks CS chains likely accounts for the greater abundance of this isoform. In addition, reduced levels of the proteolytic fragment are consistent with the hypothesis that the absence of CS on the brevican substrate diminishes proteolytic cleavage.
The abundance of the N-terminal, G1 generalized fragment of brevican is attenuated in APPsw mice brain, leading to the question of the identity of the protease responsible for the diminished fragment. When mouse brain extracts were probed with an ADAMTS-specific neoepitope antibody (anti-EAMESE), no difference in fragment abundance was found between the APPsw mice and non-transgenic littermates. This indicates that ADAMTSs are not prevalent in their capacity to cleave brevican in the APPsw mouse, at least not in whole tissue (rather than local effects). However, the amount of the MMP-derived fragment (anti-SAHPSA) was clearly reduced in several brain regions in APPsw mice compared to non-transgenic control mice, indicating that the decline in the generalized fragment was due to a loss of MMP activity in APPsw mice.
Although expression is relatively low in unperturbed brain tissue, MMP protein levels have been shown to be increased in AD brain (Asahina et al. 2001, Backstrom et al. 1996, Leake et al. 2000, Yoshiyama et al. 2000) and these proteases were capable of degrading ECM and Aβ (Backstrom et al. 1996, Lorenzl et al. 2003, Lee et al. 2005, Yan et al. 2006). In three month old APPsw mice, before the accumulation of plaques, MMP-9 immunoreactivity was reported to be absent (Lee et al. 2003), however levels were elevated in astrocytes surrounding plaques in older, plaque-bearing mice (Lee et al. 2005, Yan et al. 2006). MMP-1, 2, 3, 7 and 10 (but not MMP-9) cleave CS-containing brevican in normal mouse brain (Okada 2000), but this activity appears to be reduced in plaque-bearing mice, as evidenced by reduced levels of the MMP-cleaved fragment of brevican. The production of active MMPs has been shown to be induced by Aß in cultured rat astrocytes (Deb et al. 2003) and recent data indicates that MMPs can degrade fibrillar Aβ and may aid to the ongoing clearance of plaques from amyloid laden brains (Yin et al. 2006). It may be that the high amyloid load occupied most of the active MMP activity, thereby leaving little to act on the endogenous lectican substrate. This could explain the confound of high MMP levels of protein and diminished brevican proteolytic activity in vivo. On the other hand, we demonstrated that treatment of BV2 cells with Aβ with resulted in a stimulation of MMP and ADAMTS activity capable of cleaving exogenous brevican.
A distinct staining pattern was observed surrounding the core of Aβ plaques using antibodies against brevican (rb18) and the neoepitope sequences for the ADAMTS- and MMP-derived fragments of brevican. Elevated immunoreactivity adjacent to and surrounding the plaque indicates a local increase of protease activity in the region of abnormal neurite growth, but often diminished activity within the plaque as denoted by the absence of protease-specific staining. These results, along with data showing a diminished content of MMP-derived proteolytic fragment of brevican in whole tissue, indicates how important local (plaque-associated) or cell-specific subpopulations may be affected by disease, ie. the presence of plaques. Immunoreactivity for several MMPs has been reported in the proximity of extracellular plaques (Backstrom et al. 1996) and in some cases immunoreactivity for the MMP-derived fragment of brevican is found near the plaque core (Fig. 5). MMPs and ADAMTSs may also play a role in dysfunctional neural plasticity by their involvement in the formation of dystrophic neurites surrounding the plaque. These proteases may promote neurite outgrowth and synaptogenesis after injury (Malemud 2006, Pizzi & Crowe 2006, Hamel et al. 2008) by creating a more permissive ECM. Change in the activity of the protease, due to interaction with Aβ, could disturb this recovery mechanism and may ultimately affect neural plasticity.
Overall, the deposition of Aβ into the ECM may disrupt matrix processing and alter extracellular molecular events that modulate neural plasticity. Evidence is presented suggesting that Aβ disrupts the action of MMPs on the proteolytic cleavage of brevican. Of course, how these changes more broadly relate to the total course of the pathology in AD, or to pathology of the APPsw mouse, remain to be examined.
ACKNOWLEDGEMENTS
This work was supported in part by the National Institutes of Health (AG022101, AG018478 and AG015490) and Alzheimer's Association (grant # IIRG-02-3758). The authors would like to acknowledge Dr. Yu Yamaguchi for his gift of the brevican antibody RB18. Autumn K Eakin (USF) is recognized for her technical assistance.
REFERENCES
- Ajmo JM, Eakin AK, Hamel MG, Gottschall PE. Discordant localization of WFA reactivity and brevican/ADAMTS-derived fragment in rodent brain. BMC Neurosci. 2008;9:14. doi: 10.1186/1471-2202-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods. 2008;172:250–254. doi: 10.1016/j.jneumeth.2008.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt T. Synaptic degeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:167–179. doi: 10.1007/s00401-009-0536-x. [DOI] [PubMed] [Google Scholar]
- Asahina M, Yoshiyama Y, Hattori T. Expression of matrix metalloproteinase-9 and urinary-type plasminogen activator in Alzheimer's disease brain. Clin Neuropathol. 2001;20:60–63. [PubMed] [Google Scholar]
- Backstrom JR, Lim GP, Cullen MJ, Tokes ZA. Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1-40) J Neurosci. 1996;16:7910–7919. doi: 10.1523/JNEUROSCI.16-24-07910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev. 2000;80:1267–1290. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Lee HG, Zhu X, Perry G, Smith MA. Alzheimer disease pathology as a host response. J Neuropathol Exp Neurol. 2008;67:523–531. doi: 10.1097/NEN.0b013e318177eaf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman SL, Halfter W, Cole GJ. Agrin binds to beta-amyloid (Abeta), accelerates abeta fibril formation, and is localized to Abeta deposits in Alzheimer's disease brain. Mol Cell Neurosci. 2000;15:183–198. doi: 10.1006/mcne.1999.0816. [DOI] [PubMed] [Google Scholar]
- Deb S, Wenjun Zhang J, Gottschall PE. Beta-amyloid induces the production of active, matrix-degrading proteases in cultured rat astrocytes. Brain Res. 2003;970:205–213. doi: 10.1016/s0006-8993(03)02344-8. [DOI] [PubMed] [Google Scholar]
- Dong H, Martin MV, Chambers S, Csernansky JG. Spatial relationship between synapse loss and beta-amyloid deposition in Tg2576 mice. J Comp Neurol. 2007;500:311–321. doi: 10.1002/cne.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouet B, Pincon-Raymond M, Chambaz J, Pillot T. Laminin 1 attenuates beta-amyloid peptide Abeta(1-40) neurotoxicity of cultured fetal rat cortical neurons. J Neurochem. 1999;73:742–749. doi: 10.1046/j.1471-4159.1999.0730742.x. [DOI] [PubMed] [Google Scholar]
- Flannery CR. MMPs and ADAMTSs: functional studies. Front Biosci. 2006;11:544–569. doi: 10.2741/1818. [DOI] [PubMed] [Google Scholar]
- Flannery CR, Zeng W, Corcoran C, et al. Autocatalytic cleavage of ADAMTS-4 (Aggrecanase-1) reveals multiple glycosaminoglycan-binding sites. J Biol Chem. 2002;277:42775–42780. doi: 10.1074/jbc.M205309200. [DOI] [PubMed] [Google Scholar]
- Flood C, Gustafsson M, Richardson PE, Harvey SC, Segrest JP, Boren J. Identification of the proteoglycan binding site in apolipoprotein B48. J Biol Chem. 2002;277:32228–32233. doi: 10.1074/jbc.M204053200. [DOI] [PubMed] [Google Scholar]
- Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12:897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Gordon MN, King DL, Diamond DM, et al. Correlation between cognitive deficits and Abeta deposits in transgenic APP+PS1 mice. Neurobiol Aging. 2001;22:377–385. doi: 10.1016/s0197-4580(00)00249-9. [DOI] [PubMed] [Google Scholar]
- Gottschall PE. beta-Amyloid induction of gelatinase B secretion in cultured microglia: inhibition by dexamethasone and indomethacin. Neuroreport. 1996;7:3077–3080. doi: 10.1097/00001756-199611250-00057. [DOI] [PubMed] [Google Scholar]
- Gottschall PE, Sandy JD, Zimmermann DR. Substrates for metalloendopeptidases in the central nervous system. In: Conant K, Gottschall PE, editors. Matrix metalloproteinases in the central nervous system. Imperial College Press; London: 2005. pp. 87–118. [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hagihara K, Miura R, Kosaki R, Berglund E, Ranscht B, Yamaguchi Y. Immunohistochemical evidence for the brevican-tenascin-R interaction: colocalization in perineuronal nets suggests a physiological role for the interaction in the adult rat brain. J Comp Neurol. 1999;410:256–264. [PubMed] [Google Scholar]
- Hamel MG, Ajmo JM, Leonardo CC, Zuo F, Sandy JD, Gottschall PE. Multimodal signaling by the ADAMTSs (a disintegrin and metalloproteinase with thrombospondin motifs) promotes neurite extension. Exp Neurol. 2008 doi: 10.1016/j.expneurol.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockfield S, Kalb RG, Zaremba S, Fryer H. Expression of neural proteoglycans correlates with the acquisition of mature neuronal properties in the mammalian brain. Cold Spring Harb Symp Quant Biol. 1990;55:505–514. doi: 10.1101/sqb.1990.055.01.049. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Hsiao KK, Borchelt DR, Olson K, et al. Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron. 1995;15:1203–1218. doi: 10.1016/0896-6273(95)90107-8. [DOI] [PubMed] [Google Scholar]
- John N, Krugel H, Frischknecht R, Smalla KH, Schultz C, Kreutz MR, Gundelfinger ED, Seidenbecher CI. Brevican-containing perineuronal nets of extracellular matrix in dissociated hippocampal primary cultures. Mol Cell Neurosci. 2006;31:774–784. doi: 10.1016/j.mcn.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Kowalska MA, Badellino K. beta-Amyloid protein induces platelet aggregation and supports platelet adhesion. Biochem Biophys Res Commun. 1994;205:1829–1835. doi: 10.1006/bbrc.1994.2883. [DOI] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, et al. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake A, Morris CM, Whateley J. Brain matrix metalloproteinase 1 levels are elevated in Alzheimer's disease. Neurosci Lett. 2000;291:201–203. doi: 10.1016/s0304-3940(00)01418-x. [DOI] [PubMed] [Google Scholar]
- Lee JM, Yin K, Hsin I, Chen S, Fryer JD, Holtzman DM, Hsu CY, Xu J. Matrix metalloproteinase-9 in cerebral-amyloid-angiopathy-related hemorrhage. J Neurol Sci. 2005;229-230:249–254. doi: 10.1016/j.jns.2004.11.041. [DOI] [PubMed] [Google Scholar]
- Lee JM, Yin KJ, Hsin I, Chen S, Fryer JD, Holtzman DM, Hsu CY, Xu J. Matrix metalloproteinase-9 and spontaneous hemorrhage in an animal model of cerebral amyloid angiopathy. Ann Neurol. 2003;54:379–382. doi: 10.1002/ana.10671. [DOI] [PubMed] [Google Scholar]
- Lorenzl S, Albers DS, Relkin N, Ngyuen T, Hilgenberg SL, Chirichigno J, Cudkowicz ME, Beal MF. Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer's disease. Neurochem Int. 2003;43:191–196. doi: 10.1016/s0197-0186(03)00004-4. [DOI] [PubMed] [Google Scholar]
- Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–1701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Gary SC, Zerillo C, Pratta M, Solomon K, Arner EC, Hockfield S. Brain-enriched hyaluronan binding (BEHAB)/brevican cleavage in a glioma cell line is mediated by a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family member. J Biol Chem. 2000;275:22695–22703. doi: 10.1074/jbc.M909764199. [DOI] [PubMed] [Google Scholar]
- Mayer J, Hamel MG, Gottschall PE. Evidence for proteolytic cleavage of brevican by the ADAMTSs in the dentate gyrus after excitotoxic lesion of the mouse entorhinal cortex. BMC Neurosci. 2005;6:52. doi: 10.1186/1471-2202-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Akiyama H, Itagaki S, McGeer EG. Immune system response in Alzheimer's disease. Can J Neurol Sci. 1989;16:516–527. doi: 10.1017/s0317167100029863. [DOI] [PubMed] [Google Scholar]
- Morgan D, Diamond DM, Gottschall PE, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Mori R, Wang Q, Danenberg KD, Pinski JK, Danenberg PV. Both beta-actin and GAPDH are useful reference genes for normalization of quantitative RT-PCR in human FFPE tissue samples of prostate cancer. Prostate. 2008;68:1555–1560. doi: 10.1002/pros.20815. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, et al. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn AV, Kinoshita-Toyoda A, Toyoda H, et al. Glycomics of proteoglycan biosynthesis in murine embryonic stem cell differentiation. J Proteome Res. 2007;6:4374–4387. doi: 10.1021/pr070446f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Fujii Y, Inoki I, Sugimoto K, Tanzawa K, Matsuki H, Miura R, Yamaguchi Y, Okada Y. Brevican is degraded by matrix metalloproteinases and aggrecanase-1 (ADAMTS4) at different sites. J Biol Chem. 2000;275:38885–38890. doi: 10.1074/jbc.M003875200. [DOI] [PubMed] [Google Scholar]
- Okada Y. Tumor cell-matrix interaction: pericellular matrix degradation and metastasis. Verh Dtsch Ges Pathol. 2000;84:33–42. [PubMed] [Google Scholar]
- Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–773. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- Pizzi MA, Crowe MJ. Matrix metalloproteinases and proteoglycans in axonal regeneration. Exp Neurol. 2006 doi: 10.1016/j.expneurol.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Reeves TM, Prins ML, Zhu J, Povlishock JT, Phillips LL. Matrix metalloproteinase inhibition alters functional and structural correlates of deafferentation-induced sprouting in the dentate gyrus. J Neurosci. 2003;23:10182–10189. doi: 10.1523/JNEUROSCI.23-32-10182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer's disease: molecular mechanisms. Int J Dev Neurosci. 2006;24:167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Satoh K, Suzuki N, Yokota H. ADAMTS-4 (a disintegrin and metalloproteinase with thrombospondin motifs) is transcriptionally induced in beta-amyloid treated rat astrocytes. Neurosci Lett. 2000;289:177–180. doi: 10.1016/s0304-3940(00)01285-4. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease. In the beginning. Nature. 1991;354:432–433. doi: 10.1038/354432a0. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Tannock LR, King VL. Proteoglycan mediated lipoprotein retention: a mechanism of diabetic atherosclerosis. Rev Endocr Metab Disord. 2008;9:289–300. doi: 10.1007/s11154-008-9078-0. [DOI] [PubMed] [Google Scholar]
- Terai K, Iwai A, Kawabata S, Tasaki Y, Watanabe T, Miyata K, Yamaguchi T. beta-amyloid deposits in transgenic mice expressing human beta-amyloid precursor protein have the same characteristics as those in Alzheimer's disease. Neuroscience. 2001;104:299–310. doi: 10.1016/s0306-4522(01)00095-1. [DOI] [PubMed] [Google Scholar]
- Thon N, Haas CA, Rauch U, Merten T, Fassler R, Frotscher M, Deller T. The chondroitin sulphate proteoglycan brevican is upregulated by astrocytes after entorhinal cortex lesions in adult rats. Eur J Neurosci. 2000;12:2547–2558. doi: 10.1046/j.1460-9568.2000.00109.x. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Pratta M, Liu RQ, Austin J, Ross OH, Abbaszade I, Burn T, Arner E. Sites of aggrecan cleavage by recombinant human aggrecanase-1 (ADAMTS-4) J Biol Chem. 2000;275:18566–18573. doi: 10.1074/jbc.M909383199. [DOI] [PubMed] [Google Scholar]
- Viapiano MS, Bi WL, Piepmeier J, Hockfield S, Matthews RT. Novel tumor-specific isoforms of BEHAB/brevican identified in human malignant gliomas. Cancer Res. 2005;65:6726–6733. doi: 10.1158/0008-5472.CAN-05-0585. [DOI] [PubMed] [Google Scholar]
- Viapiano MS, Matthews RT, Hockfield S. A novel membrane-associated glycovariant of BEHAB/brevican is up-regulated during rat brain development and in a rat model of invasive glioma. J Biol Chem. 2003;278:33239–33247. doi: 10.1074/jbc.M303480200. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Araki W, Chui DH, Makifuchi T, Ihara Y, Tabira T. Glypican-1 as an Abeta binding HSPG in the human brain: its localization in DIG domains and possible roles in the pathogenesis of Alzheimer's disease. Faseb J. 2004;18:1013–1015. doi: 10.1096/fj.03-1040fje. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Gordon MN, Ugen KE, et al. Number of Abeta inoculations in APP+PS1 transgenic mice influences antibody titers, microglial activation, and congophilic plaque levels. DNA Cell Biol. 2001;20:731–736. doi: 10.1089/10445490152717596. [DOI] [PubMed] [Google Scholar]
- Yamada H, Watanabe K, Shimonaka M, Yamaguchi Y. Molecular cloning of brevican, a novel brain proteoglycan of the aggrecan/versican family. J Biol Chem. 1994;269:10119–10126. [PubMed] [Google Scholar]
- Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P, Hu X, Song H, et al. Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. J Biol Chem. 2006;281:24566–24574. doi: 10.1074/jbc.M602440200. [DOI] [PubMed] [Google Scholar]
- Yin KJ, Cirrito JR, Yan P, et al. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J Neurosci. 2006;26:10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y, Asahina M, Hattori T. Selective distribution of matrix metalloproteinase-3 (MMP-3) in Alzheimer's disease brain. Acta Neuropathol. 2000;99:91–95. doi: 10.1007/pl00007428. [DOI] [PubMed] [Google Scholar]
- Yuan W, Matthews RT, Sandy JD, Gottschall PE. Association between protease-specific proteolytic cleavage of brevican and synaptic loss in the dentate gyrus of kainate-treated rats. Neuroscience. 2002;114:1091–1101. doi: 10.1016/s0306-4522(02)00347-0. [DOI] [PubMed] [Google Scholar]