Abstract
Older adults display positivity preferences in their gaze, consistent with their prioritization of emotion regulation goals. While some research has argued that substantial amounts of cognitive effort are necessary for these information-processing preferences to occur, other work suggests that these attentional patterns unfold with minimal cognitive exertion. The current study used an implicit regulatory context (i.e. viewing facial stimuli of varying emotions) to assess how much cognitive effort was required for positive attentional preferences to occur. Effortful cognitive processing was assessed with a direct measure of change in pupil dilation. Results indicated that minimal cognitive effort was expended when older adults engaged in positive gaze preferences. This finding suggests that gaze acts as a rather effortless and economical regulatory tool for individuals to shape their affective experience.
Keywords: cognitive effort, pupil dilation, emotion regulation, older adults, attention
Emotion regulation abilities seem to be maintained, or even improve, well into old age (Carstensen, Pasupathi, Mayr, & Nesselroade, 2000; Carstensen, Mikels, & Mather, 2006; Gross et al., 1997; Kliegel, Jäger, & Phillips, 2007). Socioemotional selectivity theory (Carstensen, 2006; Carstensen, Isaacowitz, & Charles, 1999) contends that older adults' regulatory competency may be the result of shifts in goal priorities based on an awareness of future time. As we move from a relatively extensive time perspective in young adulthood to a more limited perspective in old age, our focus may shift from future-oriented toward more present-oriented pursuits—such as feeling good and being happy.
A number of studies have suggested that this enhanced regulatory goal focus is reflected in older adults' processing of affective stimuli in the environment (Carstensen, Mikels, & Mather, 2006). For instance, older adults have shown preferences for processing positive and/or ignoring negative information in assessments of episodic memory (Charles, Mather, & Carstensen, 2003; Mather & Carstensen, 2003; Löckenhoff & Carstensen, 2007; Thomas & Hasher, 2006) autobiographical memory (Kennedy, Mather, & Carstensen, 2004) and visual attention (Isaacowitz, Wadlinger, Goren, & Wilson., 2006a, 2006b; Isaacowitz, Toner, Goren, & Wilson, 2008; Mather & Carstensen, 2003); this emotional preference pattern has been termed a “positivity effect” (Carstensen & Mikels, 2005; Mikels, Reuter-Lorenz, Larkin, & Carstensen, 2005). In general, positivity effects in information processing tend to be greater for older as compared to younger adults (e.g., Carstensen & Mikels, 2005; cf. Murphy & Isaacowitz, 2008), and younger adults tend to show greater processing preferences for negative relative to positive material (Baumeister, Bratslavky, Fickenauer, & Vohs, 2001; Rozin & Rozyman, 2001). However, if younger adults are instructed to focus on their emotional state while processing valenced information, positivity effects (or negative avoidance) do emerge (see Kennedy, Mather, & Carstensen, 2004; Xing & Isaacowitz, 2006).
Positivity Effects and Cognitive Control
There have been recent attempts to understand the conditions under which positivity effects emerge for older adults. Some have argued that if these preferences are motivationally based, cognitive control resources should be required for their display (Carstensen, Mikels, & Mather, 2006; Mather & Carstensen, 2005); this is assumed from suggestions that goal processes require overt, conscious processing (e.g. Bandura 1986; Carver & Scheier, 1998). Mather & Knight (2005) found that positivity effects in memory for valenced images only emerged for older adults who demonstrated proficient performance on a series of cognitive control/executive functioning tasks. Similarly, in a recent study in our lab, we examined how individual differences in executive control related to older adults' abilities to use gaze as a regulation tool. We found that more proficient performance on a measure of executive control (the conflict subtest of the Attentional Network Test: Fan, McCandliss, Sommer, Raz, & Posner, 2002), predicted a relationship between older adults' positivity effects in gaze (toward happy and away from angry facial expressions) and their resistance to a declining mood over a 20+ minute testing session (Isaacowitz, Toner, Neupart, 2009). Additional research has suggested that positivity effects do not emerge for older adults when cognitive control resources are constrained by processing distracting information within a divided attention paradigm (Knight, Seymour, Gaunt, Baker, Nesmith, & Mather, 2007; Mather & Knight, 2005). Evidence from a number of neuroimaging studies have found brain regions associated with cognitive control (i.e. prefrontal and parietal areas) to be activated during various emotion regulatory tasks at any age (Ochsner & Gross, 2005; Pessoa 2005, 2008; Pessoa, Kastner, & Ungerleider, 2003; Urry et al., 2006; van Reekum et al., 2007).
A popular method for establishing the neural links between cognitive control and emotion regulation has been to examine participants' explicit use (i.e. through task instructions) of cognitive regulatory strategies. For instance, instructing participants to engage in cognitive reappraisal while viewing negative stimuli has been shown to influence activation in dorsal anterior cingulate and prefrontal cortical regions (Ochsner & Gross, 2005). Additionally, prefrontal activation patterns have been observed in studies assessing the use of cognitive regulatory strategies (i.e. attentional control, reappraisal) in old age. Urry et al. (2006) found increased activation in the superior and inferior frontal gyri, ventromedial prefrontal cortex, and decreased amygdala activation when older adults were instructed to diminish their reaction to a series of negative images. Additionally, van Reekum et al. (2006) observed increased dorsomedial prefrontal cortex, ventrolateral prefrontal cortex, and decreased amygdala activation while older adults used attentional control as a regulatory strategy during negative picture viewing.
Apart from investigating the role of cognitive control effort in older adults' use of deliberate emotion regulation strategies (e.g. Urry et al., 2006; van Reekum et al., 2007), little has been done to assess these links when older adults are left to their own devices. For instance, how much cognitive effort is needed when older adults utilize unguided emotion regulation strategies when faced with an affective state in need of regulating? We recently conducted a study examining selective gaze as a potential regulatory strategy when younger and older adults were induced into various mood states: positive, negative, and neutral. After the mood induction procedure, participants' eyes were tracked while viewing a series of emotional-neutral image pairs depicting facial expressions of happiness, sadness, anger and fear. While younger adults showed mood-congruent gaze patterns (i.e. young adults in a positive mood showed a preference toward positive faces; young adults in a negative mood showed a preference toward negative faces), older adults in a negative mood showed a regulatory gaze pattern. Specifically, older adults showed fixation preferences toward happy and away from angry and sad faces (Isaacowitz et al., 2008).
It is possible that older adults demonstrated these positivity effects in their gaze because they were motivated by a desire to improve their bad mood. Thus, if these fixation patterns reflected older adults' emotion regulation goals, a substantial level of cognitive control may have been necessary for their emergence. However, there has been some work that has called into question whether full cognitive effort is necessary to generate older adults' positive emotional preferences. Thomas & Hasher (2006) assessed younger and older adults' memory for valenced words that were encoded while performing an additional numerical decision task and found that older adults remembered a higher proportion of positive relative to negative words. In another study, Rösler and colleagues (Rösler, Ulrich, Billino, Sterzer, Weidauer, Bernhardt, et al., 2005) measured fixation preferences to emotional-neutral and emotional-emotional image pairs in a group of healthy younger and older adults, as well as a group of older adults suffering from mild cognitive impairment as a result of early onset subcortical vascular dementia (SVD). Both healthy older adults and older adults with SVD demonstrated fixation preferences away from negative and toward neutral images as compared to younger adults. Interestingly, a number of the older adults with SVD suffered from lesions to a number of white matter pathways located within the prefrontal cortex. Thus, despite structural decline to certain cognitive control centers, gaze preferences consistent with their emotion regulation goals (selective avoidance of negative stimuli) were adequately maintained.
One recent study in our lab also examined inconsistencies in determining the role of full cognitive control in the emergence of age-related positivity effects. We examined younger and older adults' fixation preferences to a series of emotional images in conditions of full attention and when processing resources were constrained by a secondary task when attention was divided. Regardless of whether images were viewed in full or divided attention, older adults demonstrated a fixation preference for positive over negative images (Allard & Isaacowitz, 2008). Thus, in spite of certain forms of cognitive control distraction, older adults seem to be able maintain greater preferences in their gaze for positive relative to negative emotional stimuli.
Overall, there appear to be inconsistencies as to whether a full cognitive control effort is necessary for successful emotion regulation in old age. While some work seems to indicate that a full level of cognitive control functioning is necessary (e.g. Knight et al., 2007; Mather & Knight, 2005), there is other work suggesting that minimal amounts are required (e.g. Allard & Isaacowitz, 2008; Rösler et al., 2005; Thomas & Hasher, 2006), or that situational demands (i.e. short-term versus long-term regulation) may determine the influence of cognitive control processing on emotion regulation (e.g. Isaacowitz et al., 2009). We believe that a more direct measure of cognitive exertion during emotional stimulus processing would be useful in addressing these discrepant findings. Therefore, we chose to use a physiological measure of cognitive exertion—pupil dilation—to assess the degree of effort expended older adults' regulatory gaze deployment.
Pupil Dilation and Cognitive/Regulatory Effort
Increased pupil dilation in response to an incrementally-demanding cognitive task has been shown to be a reliable measure of effortful processing (Kahneman & Beatty, 1966; Peavler, 1974; Steinhauer, Siegle, Condray, & Pless, 2004). Furthermore, pupillary changes have also been observed in response to the use of effortful, cognitive emotion regulation strategies. In a recent study from van Reekum and colleagues (2007), a group of older adults were asked to utilize a reappraisal strategy while viewing a series of negative images (i.e. envision that the image you are viewing is not real). Participants viewed these images both while in an fMRI scanner and while their eyes were being tracked. In addition to examining fixation preferences toward the images, changes in pupil dilation in response to the regulation instructions were recorded. When participants were asked to reappraise the negative images, they tended to spend less time looking at the negative portions of the image; pupil dilation also increased as compared to when they were instructed to just attend to the image. Thus, increased pupil dilation may have been indicative of enhanced cognitive control effort while engaging in this emotion regulation strategy. Given these results, we sought to examine the pupillary responses of younger and older adults (experiencing a variety of mood states) during emotional image viewing. Our goal was to use this pupillary measure to determine the level of cognitive exertion necessary during the use of gaze as an implicit and unguided emotion regulation strategy.
Current Study
The current study builds upon findings from a recent eye tracking study demonstrating that older adults in a negative mood show positivity effects in their gaze consistent with an emotion regulation goal (see Isaacowitz et al., 2008). While a number of studies have made connections between cognitive control and the use of explicit emotion regulation strategies in old age (see Urry et al., 2006; van Reekum et al., 2007), we attempted to examine whether gaze is utilized as an effortful regulatory strategy for older adults even in the absence of explicit instructions to do so. Age-related changes in pupil dilation for fixation patterns in line with emotion regulation goals could follow one of two hypothesized directions, and these predictions are dependent on the amount of cognitive effort necessary for regulatory gaze deployment. If these gaze preferences require a significant amount of cognitive effort, increases in pupil dilation should be observed when older adults' demonstrate positivity effects in their gaze. This would be indicated by increases in pupil dilation when older adults in a negative mood are fixated toward happy and away from neutral facial expressions; and toward neutral and away from sad and angry faces. Conversely, if we find that pupillary responses for older adults in a negative mood are equivalent to younger adults in a negative mood or older adults in a positive or neutral mood, this would demonstrate that the use of gaze as a regulation strategy by older adults may only require minimal cognitive exertion.
Method
Participants
Data for the current analyses come from a larger study on the effects of mood on gaze preferences toward emotional-neutral images (Isaacowitz, Toner, Goren, & Wilson, 2008). Participants were 85 young adults (49 women) aged 18-25 (M = 19.72, SD = 1.82) recruited via an introductory psychology course and advertisements around campus and 106 community-dwelling older adults (76 women) aged 58-89 (M = 72.39, SD = 7.23) through a lifelong learning initiative and community advertisements. Participants were either given course credit or a monetary honorarium for their participation. Participants were excluded from the current analyses if their eyes could not be successfully tracked for at least 68 (25%) of the experimental trials. This left 80 (94%) young and 59 (55%) older adults available for analysis1. Further information regarding this participant sample, including comparisons between trackable and untrackable participants can be found in Isaacowitz et al. (2008).
Stimuli and Recording
Stimuli for the current analyses consisted of 38 male and 38 female computer-generated synthetic faces (Goren & Wilson, 2006). Each male and female face consisted of an emotional (happy, sad, angry, fear) and neutral counterpart. Emotional and neutral face pairs were constructed for presentation during the eye tracking task. These face stimuli were utilized in order to control for perceptual characteristics (i.e. luminance, wrinkles, skin and hair color/texture) that could bias attention toward/away from a particular face outside of the intended valence being portrayed (Wilson, Loffler, & Wilkinson, 2002). Additional information on the creation and validation of these stimuli can be found in Isaacowitz et al. (2006b). An emotional face and its neutral counterpart (same individual), against a gray background screen, comprised a presentation slide. Three variables were counterbalanced within each slide presentation: side of the screen (left or right) in which the emotional or neutral face appeared, sex of the face (136 male, 136 female), and emotion type (happy, sad, angry, fear).
GazeTracker software (Eye Response Technologies, Inc., Charlottesville, VA) was used for stimulus presentation. Stimuli were presented on a 17′ computer monitor at a distance of 35″ from the participant's left eye. Eye movements, fixation, and pupil dilation were recorded via an Applied Science Laboratories Model 504 Eye Tracker with Magnetic Head Transmitter at 60 Hz. Fixations (and pupil dilation for a particular fixation) were defined as an interval in which gaze was focused within 1° visual angle within a particular, predetermined Area of Interest (AOI) for at least 100 ms (Manor & Gordon, 2003). Gaze fixations were also recorded for areas of the screen outside of the emotional and neutral face; these fixations were coded as “off.”
Mood Recording
Prior to the eye tracking session, participants were asked to make a rating of their current mood state. A potentiometer slider (Empirisoft Coroporation, New York, NY) was used whereby participants moved a slider bar to indicate their mood on a range from 0 (worst possible mood) to 100 (best possible mood). While participants provided slider ratings throughout the eye tracking session, only the initial slider rating was used to separate participants into various mood conditions for the current analyses. Based on this rating, 23 young and 19 older adults reported being in a positive mood; 26 young and 23 older adults reported being in a negative mood; and 29 young and 15 older adults reported being in a neutral mood. One older adult participant did not give an initial rating.
Pupil Dilation
We extracted changes in pupil dilation toward emotional-neutral face pairs for each trial by emotion type. Horizontal pupil diameter was used as the main variable of interest. To account for individual and age differences in pupil diameter (i.e. younger adults tend to have larger pupil dilation in response to ambient light), a transformation procedure was used to determine average rate of change in pupil dilation during fixations for younger and older adults (see Piquado, Isaacowitz, & Wingfield, in press; Urry et al., 2006). This transformation procedure uses individual differences in the range of pupil diameters to calculate a ratio percentage in pupil dilation for a particular trial. The transformation is as follows: ([minimum overall pupil diameter – current pupil diameter] / [maximum overall pupil diameter – minimum overall pupil diameter]). Higher ratio percentage scores indicated a greater range of change in pupil dilation (larger pupil diameter, thus increased deployment of cognitive effort) while viewing a particular image. For the present analyses, we extracted ratio percentage scores into early (first 2 s) and late (last 2 s) time windows; a similar aggregation procedure was used in van Reekum et al. (2007).
Procedure
Prior to the eye tracking session, participants first performed a 17-point eye calibration procedure to ensure that the eye tracker was accurately recording the position of the participant's left eye toward various areas on the screen. Participants were next instructed that they would be viewing a slide show consisting of a series of face pairs; they were to view the images “naturally, as if at home watching television” (see Isaacowitz, Wadlinger, Goren, & Wilson, 2006b). Participants were also told that they would be using the potentiometer slider to rate their mood throughout the eye tracking session. However, as mentioned above, only the initial slider rating obtained prior to the eye tracking presentation was used to assign participants to a particular mood condition (for complete rationale for this procedure, see Issacowitz et al., 2008).
The eye tracking presentation consisted of 272 emotional-neutral face pair trials that were presented for 4 s each. A 0.5 s ISI slide was used in order to realign gaze to a center fixation cross prior to the start of each subsequent trial. Two separate criteria were used in order to eliminate trials where unreliable recording could skew our results (i.e. as a result of pupil obfuscation, excessive head movement, blinks, etc.): trials where there were only fixations to “off” regions (no fixations to either face), or trials where there was < 900ms of recording time anywhere on the screen (see Isaacowitz et al., 2008). Following the eye tracking presentation, participants induced into a negative mood were shown a series of positively-valenced images. Upon completing one final cognitive measure, participants were fully debriefed as to the nature of their participation in the study. Each experimental session ranged from 90 minutes to 2 hours.
Results
Emotional Images
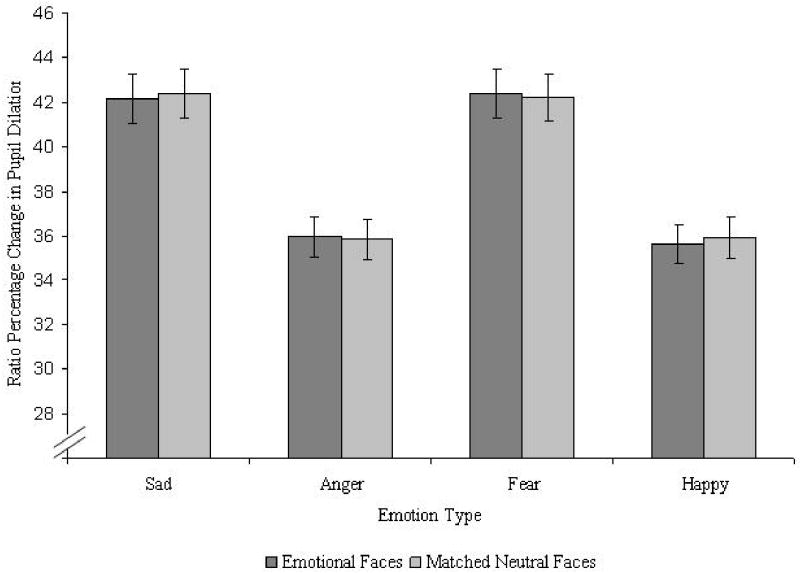
We first present results of pupil ratio percentages toward emotional images across age group. A 2 (Age; young, old) × 2 (Time; early, late) × 3 (Mood; positive, negative, neutral) × 4 (Emotion Type; happy, sad, angry, fear) mixed model ANOVA was conducted. A main effect of Age emerged, F(1, 999.68) = 131.50, p < .001, η2 = .12, indicating that younger adults showed a larger range of change in pupil dilation (M = 44.79) as compared to older adults (M = 33.30). A main effect of Mood, F(2, 999.68) = 6.75, p < .01, η2 = .01, was also observed. Pairwise comparisons indicated that participants in the neutral mood group had the largest range of change in pupil dilation (M = 41.68) as compared to participants in both the positive (M = 37.31; p < .01) and negative (M = 38.16; p < .01) mood group. Additionally, a main effect of Emotion (Figure 1), F(3, 465) = 13.91, p < .001, η2 = .08, indicated that pupil dilations were larger when fixated on sad (M = 42.16) and fear (M = 42.40) faces as compared to happy (M = 35.64) and angry (M = 35.99) faces: sad-happy (p < .001); sad-angry (p < .001); fear-happy (p < .001); fear-angry (p < .001). Finally, there was a significant Age × Mood interaction, F(2, 999.68) = 4.64, p < .05, η2 = .01. Within-group t-tests revealed that this interaction was driven mainly by older adults' range of change in pupil dilation across the separate mood conditions: older adults in a neutral mood showed greater pupil dilation than those in either a positive, t(57) = 3.77, p < .001, r2 = .20, or negative mood, t(57) = 3.71, p < .001, r2 = .19. Finally, we did not observe a significant Age × Mood × Emotion interaction. Thus, we did not find that the fixation preferences toward happy faces for older adults in a negative mood (as was found in Isaacowitz et al., 2008) corresponded to increases in pupil dilation in the current analyses.
Figure 1.
Change in pupil dilation as a function of emotional valence type across age while viewing emotional-neutral face pairs.
Note. Error bars reflect standard error.
Neutral Images
A separate 2 × 2 × 3 × 4 mixed model ANOVA assessed changes in pupil dilation while participants were fixated on neutral images. Similar to results for the emotional images, a main effect of Age, F(1, 1004.89) = 130.77, p < .001, η2 = .12, indicated that younger adults demonstrated a greater range of change in pupil dilation (M = 44.84) as compared to older adults (M = 33.37). A main effect of Mood was also obtained, F(2, 1004.90) = 6.30, p < .01, η2 = .01. Pairwise comparisons revealed that participants in the neutral mood group showed a larger range of change in pupil dilation (M = 41.65) as compared to participants in both the positive (M = 37.44; p < .01) and negative (M = 38.23; p < .01) mood groups. Additionally, a main effect of Emotion (Figure 1), F(3, 469.37) = 13.58, p < .001, η2 = .08, demonstrated that range of change in pupil dilation was greater when participants fixated on neutral images paired with sad (M = 42.39) and fear (M = 42.22) as compared to happy (M = 35.94) and angry (M = 35.86) faces: sad-happy (p < .001); sad-angry (p < .001); fear-happy (p < .001); fear-angry (p < .001). As with our analysis of pupil dilation during fixations toward emotional images, a significant Age × Mood interaction emerged, F(2, 1004.90) = 4.720, p < .01, η2 = .01. Within-group t-tests revealed that, again, this interaction was mainly driven by older adults' range of change in dilation amongst the three mood groups: older adults in a neutral mood (M = 38.13) showed larger dilations as compared to older adults in both a positive (M = 30.67), t(57) = 3.79, p < .001, r2 = .20, and negative (M = 31.30), t(57) = 3.61, p < .001, r2 = .19. We did not observe a significant Age × Mood × Emotion interaction. Therefore, we did not find support for the prediction that the avoidance of sad and angry faces for older adults in a negative mood required a substantial amount of cognitive effort.
Discussion
Older adults in bad moods demonstrate positivity preferences in their gaze suggestive of emotion regulatory goals (e.g. Isaacowitz et al., 2008). However, inconsistencies exist in the literature as to whether or not these positivity preferences require a considerable investment of cognitive resources to emerge. Some researchers argue that these regulatory preferences in older adults' information processing require a substantial amount of cognitive effort, predominantly involving executive control (Knight et al., 2007; Mather & Knight, 2005). Conversely, other researchers have found that these attentional preferences unfold with minimal cognitive exertion (Allard & Isaacowitz, 2008; Rösler et al., 2005; Thomas & Hasher, 2006). The current study used a naturally implicit regulatory context (i.e. viewing facial stimuli of varying emotions) to test whether these positive information-processing preferences, as a regulatory strategy, required significant cognitive effort. Changes in pupil dilation were assessed as a direct measure of effortful cognitive processing (Kahneman & Beatty, 1966).
Older and younger adult participants were first divided into three mood groups (positive, negative, or neutral) based on initial mood ratings made on a potentiometer slider at the beginning of the eye tracking session. Participants were then instructed to “look naturally” while viewing a series of several hundred pairs of synthetic face images (emotional face matched with its neutral counterpart) that varied on emotion type displayed (happy, anger, sad, or fear). Several significant main effects were observed.
Younger adults demonstrated a greater change in pupil dilation in response to all stimuli types across mood condition as compared to older adults. This difference may have been an artifact of younger adults possibly experiencing more emotional arousal overall during the stimuli presentation, although the current study did not specifically measure this dimension of the participants' emotional experience (Lawton, Kleban, Rajagopal, & Dean, 1992; Levenson, Carstensen, Friesen, & Ekman, 1991; Mather, Canli, English, Whitfield, Wais, Ochsner, et al., 2004). Another explanation may be that older adults physiologically possess a lower sensitivity of the pupil, as compared to younger adults, when cognitive load only varies to a small extent (Van Gerven et al., 2004). Even though we controlled for individual differences in the range of pupillary responses with our transformation, it is possible that older adults' smaller overall changes in pupillary response may reflect reduced fluctuations in dilation from their baseline. Another main effect was observed where participants in neutral moods showed the largest range of change in pupil dilation as compared to participants in negative and positive mood groups.
These main effects were qualified by a significant Age × Mood interaction where older adults in a neutral mood showed the largest range of change in pupil dilation as compared to older adults in either a positive or negative mood. Younger adults showed no differences in their pupil dilation across the three mood conditions. One possible explanation for this effect could be that older adults in a neutral mood may have been experiencing minimal baseline arousal as compared to participants in positive or negative moods. Therefore, larger changes in pupillary responses in the neutral group may indicate general heightened arousal that may have accompanied the appearance of any emotional stimulus.
Significant differences were observed in emotional stimuli type where greater changes in pupil dilations from baseline were recorded for sad and fearful stimuli as compared to happy and angry stimuli. This pattern of findings was also identical in the neutral counterpart stimuli: larger pupillary change was recorded while participants viewed neutral faces that were paired with sad and fearful faces, as opposed to neutral faces that were paired with happy and angry faces. These identical patterns of pupillary dilation observed across each trial (e.g. for both images—neutral and emotional—on each slide) may suggest that possible carryover effects may have occurred for each emotion type.
We were surprised to observe this pattern of discrepant emotion effects in pupil dilation for happy/angry and sad/fear faces. One possibility for this discrepancy could be the amount of regulatory effort necessary to process sad and fear faces versus happy and angry faces. Perhaps anger and happy expressions send a fairly clear message: anger is arguably the most intense negative emotion, and happiness is easily detected as the only positive emotion presented (Aronoff, Woike, & Hyman, 1992; Lundqvist & Öhman, 2005). Therefore, the clarity of the intended emotion being displayed may take less effort to “digest.” On the other hand, sad and fear faces may send mixed signals. In other words, there may be some ambiguity in the display of these expressions. For example, fearful expressions can sometimes be misconstrued as surprise for both younger and older adults (Adolphs, 2002; Isaacowitz et al., 2007; Ruffman, Henry, Livingstone, & Phillips, 2008). Thus, the subsequent necessity to attend to environmental cues (both from the emotional and neutral face in the pair) while processing these expressions may have required a greater amount of cognitive exertion.
Ultimately, we did not find evidence that older adults experiencing a negative mood showed a greater range of change in pupil dilation when fixated towards positive faces and away from negative faces, as compared to younger adults in a negative mood or older adults in a positive or neutral mood. This finding suggests that a significant amount of effortful cognitive processing may not be necessary for older adults to display positivity preferences in gaze fixations while experiencing a negative mood.
Gaze as a Less Effortful Regulatory Strategy
Isaacowitz and colleagues (2009) found that substantial cognitive resources, related to executive control, were not required for older adults to demonstrate positive, regulatory gaze patterns over short time intervals. Combined with the outcome of the current study, these results suggest that using gaze, as a simple regulatory tool to manage affective experience, requires little cognitive exertion in certain situations (Isaacowitz et al., 2009). Specifically, gaze may serve as a relatively expeditious strategy to effortlessly manage overt selective attention.
Importantly, extensive cognitive resources such as executive control may not be necessary to enlist regulatory strategies when faced with stressful emotional contexts. Evidence from the current study suggests that some antecedent-focused emotion regulatory strategies, like certain types of attentional deployment, may not require much cognitive effort to utilize. Because attentional deployment takes place early in the development of an affective response, regulatory interception at this phase may require fewer cognitive resources due to smaller levels of occurring affect (Gross, 1998). For example, attentional distraction may require less cognitive control, but this may come at the cost of only selectively encoding certain aspects of one's environment that would be later inaccessible to memory (Sheppes & Meiran, 2008). Despite this limitation, these more cognitively economical strategies may be particularly effective for certain populations like older adults who generally have more limited cognitive resources (Gross, 1998; Salthouse, 1988). Specficially, older adults with low internal alerting abilities were found to benefit the most from using gaze to regulate their mood (Isaacowitz et al., 2009).
Limitations and Future Directions
One possible explanation as to why individuals in a negative mood did not demonstrate larger changes in pupil dilation, indicative of increased cognitive effort, may be that the stimuli used in the study were uniform grayscale images, varying only on the valence dimension. While these stimuli controlled for many psychophysiological properties, they may have been less arousing than real photographic images. Although the emotional nature of these stimuli would require at least a minimal amount of regulatory processing, the stimuli may not have elicited especially strong emotional reactions from participants that would demand large regulatory responses. In particular, the fact that no differences were observed between images within a trial (between an emotional face and its neutral counterpart), lends support to this speculation.
In addition, the aim of the current study was to observe whether significant cognitive effort was required for a non-deliberate regulatory process (i.e. passive viewing). If we had employed a cognitively effortful regulatory task (e.g. cognitive reappraisal or suppression) or had deliberately instructed participants to regulate their emotions, then a larger range of change in pupillary responses for participants in negative mood states may have been observed. Finally, one potential confound may be that across all experimental conditions participants' moods tended to decrease over the course of the eye tracking session, perhaps due to fatigue effects (see Isaacowitz et al., 2008; cf. Stanley & Isaacowitz, 2009). This attenuation of participants' initial mood, that had initially differentiated the three mood conditions, may have created less distinct boundaries between the three conditions later in the stimulus presentation.
Future work is needed to further clarify to what extent cognitive resources are necessary to utilize other attentional substrates in the service of emotion regulation. For example, future studies might assess the degree of cognitive effort needed to initiate and maintain several different emotion regulatory strategies. Researchers could assess the amount of cognitive effort required when using a cognitive reappraisal condition as compared with a no instruction condition, both unfolding within a context that would require regulation. In addition, future work may also use other physiological recording methods (e.g. heart rate, galvanic skin response) beyond pupillary response as a proxy for exertion during emotion regulatory tasks. However, these studies would have to be mindful of disentangling and attributing effects of cognitive effort versus arousal. Overall, the current study represents an important first step in determining how older adults rather effortlessly regulate their emotions through positive gaze preferences that require little cognitive capital to initiate.
Acknowledgments
This work was supported by NIH Grant R01AG026323 to Derek Isaacowitz. The authors wish to acknowledge Deborah Goren and Hugh Wilson for creating the synthetic faces used in the study.
Footnotes
In contrast to the participant Ns used in our previous work (see Isaacowitz et al., 2008; 2009), we used a slightly larger sample. Extra exclusionary criterion was used in our previous studies based on participant's whose fixation ratio scores fell beyond +/- 3 SDs from the group means. Given that the current study focused on range of change in pupil dilation as a dependent variable apart from overall fixation metrics, we only excluded participants based on overall trackability and not based on the nature of the recorded fixations.
References
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Allard ES, Isaacowitz DM. Are preferences in emotional processing affected by distraction? Examining the age-related positivity effect in visual fixation within a dual-task paradigm. Aging, Neuropsychology, and Cognition. 2008;15:725–743. doi: 10.1080/13825580802348562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff J, Woike BA, Hyman LM. Which are the stimuli in facial displays of anger and happiness? Configurational biases of emotion recognition. Journal of Personality and Social Psychology. 1992;62:1050–1066. [Google Scholar]
- Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall, Inc.; 1986. [Google Scholar]
- Baumeister RF, Bratslavsky E, Fickenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5:323–370. [Google Scholar]
- Carstensen LL. The influence of a sense of time on human development. Science. 2006;312:1913–1915. doi: 10.1126/science.1127488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: A theory of socioemotional selectivity. American Psychologist. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA. At the intersection of emotion and cognition. Current Directions in Psychological Science. 2005;14:117–121. [Google Scholar]
- Carstensen LL, Mikels JA, Mather M. Aging and the intersection of cognition, motivation, and emotion. In: Birren JE, Schaire WK, editors. Handbook of the psychology of aging. 6th. Amsterdam, Netherlands: Elsevier; 2006. pp. 343–362. [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology. 2000;79:644–655. [PubMed] [Google Scholar]
- Carver CS, Scheier MF. On the self-regulation of behavior. New York, NY: Cambridge University Press; 1998. [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and interdependence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Goren D, Wilson HR. Quantifying facial expression recognition across viewing conditions. Vision Research. 2006;46:1253–1262. doi: 10.1016/j.visres.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998;2:271–299. [Google Scholar]
- Gross JJ, Carstensen LL, Pasupathi M, Tsai J, Götestam Skorpen C, Hsu AYC. Emotion and aging: Experience, expression, and control. Psychology and Aging. 1997;12:590–599. doi: 10.1037//0882-7974.12.4.590. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Löckenhoff CE, Lane RD, Wright R, Sechrest L, Riedel R, et al. Age differences in recognition of emotion in lexical stimuli and facial expressions. Psychology and Aging. 2007;22:147–159. doi: 10.1037/0882-7974.22.1.147. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Toner K, Goren D, Wilson HR. Looking while unhappy: Mood congruent gaze in young adults, positive gaze in older adults. Psychological Science. 2008;19:848–853. doi: 10.1111/j.1467-9280.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Toner K, Neupert S. Use of gaze for real-time mood regulation: Effects of age and attentional functioning. 2009 doi: 10.1037/a0017706. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye tracking study. Psychology and Aging. 2006a;21:40–48. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Is there an age-related positivity effect in visual attention? A comparison of two methodologies. Emotion. 2006b;6:511–516. doi: 10.1037/1528-3542.6.3.511. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Beatty J. Pupil diameter and load on memory. Science. 1966;154:1583–1585. doi: 10.1126/science.154.3756.1583. [DOI] [PubMed] [Google Scholar]
- Kennedy Q, Mather M, Carstensen LL. The role of motivation in the age-related positivity effect in autobiographical memory. Psychological Science. 2004;15:208–214. doi: 10.1111/j.0956-7976.2004.01503011.x. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Jäger T, Phillips LH. Emotional development across adulthood: Differential age-related emotional reactivity and emotion regulation in a negative mood induction. International Journal of Aging and Human Development. 2007;64:217–244. doi: 10.2190/U48Q-0063-3318-1175. [DOI] [PubMed] [Google Scholar]
- Knight M, Seymour TL, Gaunt JT, Baker C, Nesmith K, Mather M. Aging and goal-directed emotional attention: Distraction reverses emotional biases. Emotion. 2007;7:705–714. doi: 10.1037/1528-3542.7.4.705. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Kleban MH, Rajagopal D, Dean J. Dimensions of affective experience in three age groups. Psychology and Aging. 1992;7:171–184. doi: 10.1037//0882-7974.7.2.171. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Friesen WV, Ekman P. Emotion, physiology, and expression in old age. Psychology and Aging. 1991;6:28–35. doi: 10.1037//0882-7974.6.1.28. [DOI] [PubMed] [Google Scholar]
- Löckenhoff CE, Carstensen LL. Aging, emotion, and health-related decision strategies: Motivational manipulations can reduce age differences. Psychology and Aging. 2007;22:134–146. doi: 10.1037/0882-7974.22.1.134. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Öhman A. Caught by the evil eye: Nonconscious information processing, emotion, and attention to facial stimuli. In: Barrett LF, Niedenthal PM, Winkielman P, editors. Emotion and Consciousness. New York, NY: Guilford Press; 2005. pp. 97–122. [Google Scholar]
- Manor BR, Gordon E. Defining the temporal threshold for ocular fixation in free-viewing visuocognitive tasks. Journal of Neuroscience Methods. 2003;128:85–93. doi: 10.1016/s0165-0270(03)00151-1. [DOI] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, et al. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15:259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychological Science. 2003;14:409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults' emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mikels JA, Larkin GR, Reuter-Lorenz PA, Carstensen LL. Divergent trajectories in the aging mind: Changes in working memory for affective versus visual information with age. Psychology and Aging. 2005;20:542–553. doi: 10.1037/0882-7974.20.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy NA, Isaacowitz DM. Preferences for emotional information for younger and older adults: A meta-analysis of memory and attention tasks. Psychology and Aging. 2008;23:263–286. doi: 10.1037/0882-7974.23.2.263. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Peavler WS. Pupil size, information overload, and performance differences. Psychophysiology. 1974;11:59–566. doi: 10.1111/j.1469-8986.1974.tb01114.x. [DOI] [PubMed] [Google Scholar]
- Pessoa L. To what extent are emotional visual stimuli processed without attention and awareness? Current Opinion in Neurobiology. 2005;15:188–196. doi: 10.1016/j.conb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews in Neuroscience. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerledier LG. Neuroimaging studies of attention: From modulation of sensory processing to top-down control. Journal of Neuroscience. 2003;23:3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piquado T, Isaacowitz DM, Wingfield A. Pupillometry as a measure of cognitive effort in younger and older adults. Psychophysiology. doi: 10.1111/j.1469-8986.2009.00947.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler A, Ulrich C, Billino J, Sterzer P, Weidauer S, Bernhardt T, et al. Effects of arousing emotional scenes of the distribution of visuospatial attention: Changes with aging and early subcortical vascular dementia. Journal of the Neurological Sciences. 2005;229-230:109–116. doi: 10.1016/j.jns.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Rozin P, Rozyman EB. Negativity bias, negativity dominance, and contagion. Personality and Social Psychology Review. 2001;5:296–320. [Google Scholar]
- Ruffman T, Henry JD, Livingstone V, Phillips LH. A meta-analytic review of emotion recognition and aging: Implications for neuropsychological models of aging. Neuroscience and Biobehavioral Reviews. 2008;32:863–881. doi: 10.1016/j.neubiorev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Resource-reduction interpretations of cognitive aging. Developmental Review. 1988;8:138–272. [Google Scholar]
- Sheppes G, Meiran N. Divergent cognitive costs for online forms of reappraisal and distraction. Emotion. 2008;8:870–874. doi: 10.1037/a0013711. [DOI] [PubMed] [Google Scholar]
- Stanley JT, Isaacowitz DM. Age-related differences in profiles of mood-change trajectories. 2009 doi: 10.1037/a0021023. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer SR, Siegle GJ, Condray R, Pless M. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. International Journal of Psychophysiology. 2004;52:77–86. doi: 10.1016/j.ijpsycho.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Thomas RC, Hasher L. The influence of emotional valence on age differences in early processing and memory. Psychology and Aging. 2006;21:821–825. doi: 10.1037/0882-7974.21.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation and negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gerven PWM, Pass F, Van Merriënboer JJG, Schmidt HG. Memory load and the cognitive pupillary response in aging. Psychophysiology. 2004;41:167–174. doi: 10.1111/j.1469-8986.2003.00148.x. [DOI] [PubMed] [Google Scholar]
- van Reekum CM, Urry HL, Johnstone T, Thurow ME, Frye CJ, Jackson CA, Schaefer HS, Alexander AL, Davidson RJ. Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. Journal of Cognitive Neuroscience. 2007;19:237–248. doi: 10.1162/jocn.2007.19.2.237. [DOI] [PubMed] [Google Scholar]
- Wilson HR, Loffler G, Wilkinson F. Synthetic faces, face cubs, and the geometry of face space. Vision Research. 2002;42:2909–2923. doi: 10.1016/s0042-6989(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Xing C, Isaacowitz DM. Aiming at happiness: how motivation affects attention to and memory for emotional images. Motivation and Emotion. 2006;30:249–256. [Google Scholar]