Abstract
Aromatase inhibitors (AIs) play a prominent role in the management of postmenopausal women with endocrine sensitive breast cancer, but there is large variability in both efficacy and tolerability. The purpose of our study was to define inter-individual variation in anastrozole metabolism and pharmacodynamics among patients treated with the approved daily dose of one mg in a standard practice setting as adjuvant therapy for resected early breast cancer. This study was performed in 191 women in whom pre-treatment and during anastrozole plasma concentrations of estrone (E1), estradiol (E2), estrone conjugates, androstenedione and testosterone were determined and correlated with plasma concentrations of anastrozole and anastrozole metabolites. There were large inter-individual variations in plasma anastrozole and anastrozole metabolite concentrations as well as pre-treatment and post-drug plasma E1, E2, E1-conjugate and estrogen precursor (androstenedione and testosterone) concentrations. E1 and E2 concentrations were below the lower limit of quantitation (LLQ) in most patients after anastrozole therapy (83% for both), but those with detectable concentrations had a broad range (1.58-45.2 pg/ml and 0.635-97.0 pg/ml, respectively). E1-conjugates after anastrozole therapy were above the LLQ in most patients (93%), with wide interpatient variability (3.50-2990 pg/ml). Two patients appeared to extensively metabolize anastrozole and failed to display substantial decreases in estrogens. Acknowledging the potential factor of variable compliance, our results demonstrated large inter-individual variation in anastrozole metabolism and its effect on circulating estrogens in postmenopausal patients. These findings may have implications in regard to efficacy and adverse events, and may indicate the need to “individualize” therapy with this drug.
Keywords: anastrozole, anastrozole metabolites, estrone, estradiol, estrone conjugates, androstenedione, testosterone, aromatase inhibitors, breast cancer, drug metabolism, pharmacodynamics
Introduction
The third generation aromatase inhibitors (AIs), anastrozole, exemestane and letrozole, have become established therapy for postmenopausal women with breast cancer in the advanced disease (1) and adjuvant (2) settings and are a major focus of research in the prevention setting in women at high risk for developing breast cancer (3). An American Society of Clinical Oncology technology assessment panel concluded that optimal adjuvant therapy for postmenopausal women with receptor positive breast cancer includes an AI, either as initial therapy or after treatment with tamoxifen (4).
Anastrozole is a nonsteroidal AI that was reported to maximally suppress plasma estradiol concentrations at doses of one and 10 mg per day, with both doses suppressing estradiol “to the limits of detection employed (5).” The drug had a plasma β-phase elimination half-life of 38 to 61 hours (5). Geisler et al. (6) reported equipotency of the one and 10 mg dose levels in terms of aromatase inhibition and suppression of plasma estrogen concentrations. Two clinical trials were performed in which the one mg and 10 mg doses were compared with megestrol acetate in patients with advanced breast cancer (7,8), and a joint analysis of these two trials (9) supported equipotency of 1 and 10 mg, resulting in the endorsement of the one mg dose for clinical use. However, in this era of “individualized therapy”, it remains an open question as to whether the single dose currently used is appropriate for all patients. Therefore, in the present study, we set out to determine the nature and extent of anastrozole metabolism and its primary pharmacodynamic effect, i.e., alteration in estrogen precursors and product concentrations, in a large population of postmenopausal breast cancer patients.
Anastrozole was the first AI to receive Food and Drug Administration approval for use in the adjuvant setting to treat women with early stage breast cancer. It has demonstrated value in the initial therapy setting (10), after two to three years of tamoxifen (11-13) and in the extended adjuvant setting after five years of tamoxifen (14). However, adherence to treatment appears to be an important issue with the use of anastrozole. Adherence, to anastrozole therapy, defined as having drug available >80% of days, decreased to 62-79% after three years (15). Thus, it appears that a substantial proportion of women may be suboptimally adherent to anastrozole therapy, a finding that would be expected to be associated with suboptimal efficacy.
Although anastrozole has demonstrated clear efficacy and superiority relative to tamoxifen (10), many patients experience a recurrence of their cancer. In addition, there is substantial inter-individual variability with respect to tolerability; and musculoskeletal complaints can be so severe that some patients withdraw from therapy. This variability is consistent with possible differences among patients in drug pharmacokinetics, especially metabolism, and/or pharmacodynamics, factors that, if understood, would offer the potential for individualizing therapy and ensuring that patients would receive optimal therapy
This study describes changes in plasma concentrations of hormones and of anastrozole and its metabolites in a cohort of 191 patients taking anastrozole one mg per day. We observed striking inter-individual variation in both plasma anastrozole and anastrozole metabolite concentrations with equally striking variation in changes in estrogen and estrogen precursor concentrations after anastrozole therapy.
Materials and Methods
Patients studied
This clinical study enrolled postmenopausal women who were to receive anastrozole as adjuvant therapy for resected early stage breast cancer at Mayo Clinic and the M.D. Anderson Cancer Center. Eligibility criteria included age of at least 18 years, postmenopausal status, breast cancer stage I, II, or III according to the American Joint Committee on Cancer (AJCC) Staging Manual (Sixth Edition), a tumor that was estrogen receptor (ER) positive and/or progesterone receptor (PgR) positive, and a planned treatment with anastrozole at the clinically approved dose of one mg per day. Patients could have received prior tamoxifen but other prior endocrine therapy was not permitted. None of the patients were receiving hormone replacement therapy.
Two weeks or less prior to starting anastrozole, a blood sample was obtained for the acquisition of DNA and for pre-treatment hormone measurements. A second blood draw for hormone measurements, anastrozole and anastrozole metabolite concentrations in plasma was scheduled for four weeks to six months after initiation of anastrozole, i.e., long enough to allow steady state of anastrozole to be achieved. Patients were instructed not to take their dose of anastrozole for that day until after the blood was drawn. This trial was performed after approval by local Institutional Review Boards in accordance with assurances filed with and approved by the United States Department of Health and Human Services. Written informed consent was provided by each patient before entry on study.
Anastrozole and metabolite assays
Anastrozole and metabolite assays involved the extraction of plasma, followed by LC/MS/MS assay. To assess the potential role of conjugation in the metabolism of anastrozole and/or its metabolites, total concentrations (free + conjugated) of anastrozole and its hydroxylated metabolite were measured after incubation of plasma samples with β-glucuronidase (β-Glucuronidase Type H-5 from Helix pomata, Sigma-Aldrich, St. Louis, MO). Specifically, 250 μL of plasma was incubated for 18 hours with 20 μL of 1000 units/mL β-glucuronidase, 200 μL of 200 mM acetate buffer and 10 μL of 600 mM sodium azide. Since the β-glucuronidase type H-5 extracted from Helix pomata also contained sulfatase, we could not reliably distinguish between glucuronide and sulfate conjugates. Therefore, we report the data here as “conjugated” anastrozole or “conjugated” hydroxyanastrozole. To obtain the free (unconjugated) concentrations of anastrozole and its hydroxylated metabolite, we used the same incubation approach except the samples were incubated without β-glucuronidase. The difference between the total and free is reported as conjugate drug or metabolite. After incubation, the internal standard, desmethyldiazepam, was added to 250 μL of plasma, and the sample was extracted with ethyl acetate at alkaline pH (0.5 mL of 0.5 M NaOH/ glycine buffer, adjusted to pH 10). The sample was vortex-mixed and centrifuged for 15 minutes at 2500 rpm in a Beckman GS-6R centrifuge. The organic layer was then removed and evaporated to dryness. The residue was reconstituted with 50 μL of 0.1% formic acid in water, and 25 μL was injected onto the LC/MS/MS system.
The LC/MS/MS assay system consisted of an LC-20AB pump with a SIL-20A HT autosampler (Shimadzu Addison, IL) and an API 2000 LC/MS/MS triple quadruple system (Applied Biosystems Foster City, CA) with an electrospray ion source. The separation system consist of a 100×2mm Luna 3 μ C18(2) 100A column (Phenomenex Torrance, CA) with a mobile phase that was de-gassed in a sonicator for 15 minutes. The mobile phase was composed of 50% 0.1% formic acid in water and 50% acetonitrile. The mobile phase flow rate was 0.15 ml/min. The Multiple Reaction Monitoring (MRM) analysis was completed in positive mode for the entire run. The nitrogen nebuliser gas and curtain gas were both set at 20 psi. Dwell time was set at 400 msec and the internal voltage was set at 5200 in positive mode with a temperature of 450°C. Two positive transitions were used for anastrozole (+294/225, +294/115), hydroxy-anastrozole (+310.2/241.5, +310.2/ 214.5), and the internal standard, desmethyldiazepam (+271/140, +271, 208). Anastrozole and hydroxy-anastrozole were measured with the quantifier MRM and confirmed with the qualifier MRM transition.
Plasma concentrations of anastrozole were quantified using the ratio of area under the curve (AUC) of anastrozole to AUC of the internal standard, and calibration curves that were constructed by spiking blank plasma with known amounts of anastrozole. The limit of detection was 50 pg/ml and the limit of quantification was 100 pg/ml. Since an authentic standard of hydroxy-anastrozole was not available, this metabolite was quantified using standard curves generated with anastrozole. The limitation of this approach is that the MS/MS properties of the metabolite and the parent compound may be different as a result of altered chromophore. Therefore, actual concentrations of hydroxy-anastrazole could not be established precisely, and concentrations of that metabolite presented in this paper should be viewed as “apparent” concentrations (arbitrary units per ml plasma). A detailed description of the LC/MS/MS assay and metabolite identification will be published separately.
Hormone assays
A validated bioanalytical method using gas chromatography negative ionization tandem mass spectrometry was used to measure physiologically relevant concentrations of the following steroids from 1.0 mL of human serum, with lower limits of quantitation (LLQ): estrone (E1) 1.56 pg/mL, estradiol (E2) 0.625 pg/mL, testosterone 25.0 pg/mL, androstenedione 25.0 pg/mL, and estrone conjugates: 3.13 pg/mL sulfate, 4.15 pg/mL glucuronide. Standards and internal standards used were >98% pure and purchased from Steraloids (Newport, RI), US Pharmacopeia (Rockville, MD), Sigma-Aldrich (St Louis, MO) or CDN Isotopes (Point-Clarie, Quebec). For each batch of samples analyzed, two standard curves for each analyte (front and back, 8 concentration levels) were prepared in water and qualified with quality control samples (2 replicates at low, mid, high levels) prepared in charcoal stripped serum. Analytical runs were accepted when >75% of standards had back-calculated concentrations within ±15% of nominal, except at the LLQ, where ±20% of nominal concentrations was accepted. In addition, at least 67% of the quality control samples met accuracy requirements of being within ±15% of their nominal concentrations. For some results, the LLQ was higher, based on the assay conditions. For this study, a mean LLQ was calculated as 1.64 pg/mL for E1 and 0.66 pg/mL for E2, and 6.04 pg.mL for E1-conjugates.
Briefly, the analytes and their deuterated internal standards were extracted from 1 mL of serum using Bond Elut Certify® (Varian, Harbor City, CA) solid-phase extraction cartridges. Estrone conjugates were eluted from the cartridges with water/acetonitrile (75/25, v/v), dried down and hydrolyzed to estrone using Glusulase® (β-glucuronidase and sulfatase, NEN Research Products Boston, MA). The unconjugated analytes were then eluted with ethyl acetate. Estrogens were derivatized with pentafluorobenzoyl chloride and N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA); the androgens were derivatized with O-(2,3,4,5,6-pentafluorobenzyl)-hydroxylamine and MSTFA. All solvents and reagents were purchased from EMD Science (Gibbstown, NJ) or Sigma-Aldrich (St. Louis, MO). The derivatized analytes were separated on a Varian 3400 gas chromatograph (Walnut Creek, CA) equipped with a DB-17 fused silica capillary column (15 m × 0.025 mm, J&W Scientific Folsom, CA) and quantified using an interfaced Finnigan MAT TSQ-700 mass spectrometer (San Jose, CA) operating in single ion monitoring tandem mass spectrometry negative ion chemical ionization mode.
Statistical methods
To measure correlation of two quantitative variables, we used the Spearman rank correlation coefficient, a method robust to outliers. To evaluate whether clinical variables were statistically associated with baseline hormone concentrations, and to evaluate whether anastrozole or any of its metabolites were statistically associated with changes in hormone concentrations, as well as whether any clinical variables were associated with changes in hormone concentrations, we used linear regression methods. The hormone concentrations (pre-treatment, or change measured as post-treatment minus pre-treatment) were regressed in a step-wise fashion on the following clinical/demographic variables (age at treatment, body mass index [BMI], smoking status, days from initial drug administration to blood draw, Mayo vs. MD Anderson recruitment site, tumor T size, nodal status, ER status, PgR status, HER2 status, prior chemotherapy, and prior tamoxifen). Changes in concentration were also regressed on pre-treatment hormone level and drug levels (anastrazole, anastrozoleconjugates, hydroxyl-anastrozole, and hydroxyl-anastrozole-conjugates). Stepwise selection proceeded in a forward-backward manner, using a p-value of 0.05 to retain a predictor variable in the model. Because some variables were highly skewed with outliers (e.g., hormone concentrations), we used Winsorized variables by replacing extreme values (greater than 3 SD from the mean) with values exactly at 3 SD from the mean. This robust approach uses more information than ranked data, yet is less sensitive to outliers than the original data.
Results
Evaluable patients
Steady state plasma anastrozole and anastrozole metabolite trough concentrations were determined in 196 patients while chronically on a one mg/day dosage. Five patients were excluded from the analyses because one had no detectable plasma anastrozole or anastrozole metabolite (despite reporting that she was taking the drug), three patients had the second blood draw obtained less than four weeks after the initiation of therapy, and one patient was excluded because of technical problems with the comparison hormone assays. Thus, 191 patients were evaluable in these analyses and their characteristics are listed in Table 1.
TABLE 1.
Patient Characteristics (n=191)
| Age, years | |
| Median | 60 |
| Range | 39-82 |
| Race | |
| American Indian or Alaska Native | 1 (1%) |
| Asian | 5 (3 %) |
| Black or African American | 11 (6%) |
| Native Hawaiian or other Pacific Islander | 0 |
| White | 162 (85%) |
| Unknown | 12 (6%) |
| Ethnicity | |
| Hispanic or Latino | 15 (8%) |
| Not Hispanic or Latino | 160 (84%) |
| Unknown | 16 (8%) |
| Body mass index | |
| Median | 27.5 |
| Range | 17.7--45.1 |
| AJCC tumor stage* | |
| I | 102 (54%) |
| II | 60 (32%) |
| III | 28 (15%) |
| ER/PgR status | |
| Positive/positive | 158 (83%) |
| Positive/negative | 30 (16%) |
| Negative/positive | 3 (2%) |
| HER2 status | |
| Negative | 149 (78%) |
| Positive | 36 (19%) |
| Unknown | 6 (3%) |
| Smoking status | |
| Never smoked | 116 (61%) |
| Ever smoked | 68 (36%) |
| Current smoker | 14 (7%) |
| Missing data | 7 (4%) |
| Prior chemotherapy | |
| Yes | 81 (42%) |
| No | 110 (58%) |
| Prior tamoxifen | |
| Yes | 21 (11%) |
| No | 170 (89%) |
| Time from starting anastrozole to second blood draw (weeks) | |
| 4-6 | 9 (5%) |
| >6-9 | 25 (13%) |
| >9-12 | 30 (16%) |
| >12-18 | 87 (46%) |
| >18-24 | 20 (10%) |
| >24 | 20 (10%) |
AJCC Sixth Edition, One patient was T1NxM0
Plasma anastrozole and anastrozole metabolite concentrations
The three major metabolites detected in plasma were anastrozole conjugates, hydroxy-anastrozole and hydroxy-anastrozole conjugates (Figure 1A). The median plasma concentration of free anastrozole was 32.2 ng/ml, with a range from 0.0 to 98.8 ng/ml. Two patients without detectable anastrozole were included in the analysis because they had measurable hydroxy-anastrozole and hydroxy-anastrozole-conjugates. These patients will be discussed in more detail subsequently. The frequency distribution for anastrozole concentrations shown in Figure 1B demonstrates the wide variation among patients.
Figure 1.
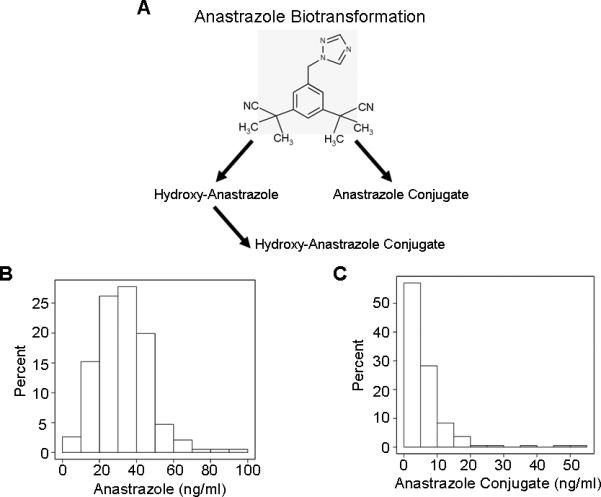
Steady state plasma anastrozole and anastrazole conjugate concentrations in breast cancer patients treated with one mg/day oral dose of anastrozole. Observed anastrozole metabolism (A); frequency distribution histograms for levels of anastrozole (B) and anastrozole conjugates (C).
The median plasma concentration of anastrozole conjugates was 4.2 ng/ml (range: 0.0-54.4 ng/ml) and the frequency distribution shown in Figure 1C demonstrates wide variation in their plasma concentrations. Anastrozole and anastrozole conjugate concentrations were not statistically correlated (Spearman correlation = 0.10, p-value = 0.18).
The majority (over 80%) of the hydroxy-anastrozole was recovered as conjugates. As noted previously, a lack of internal standards prevented absolute quantitation of these two metabolites, but there was a 29-fold range in hydroxy-anastrozole conjugate concentrations. Hydroxy-anastrozole and hydroxy-anastrozole conjugates were positively correlated (Spearman correlation =0.63, p-value < 0.001), implying that subjects with higher hydroxylated metabolites formed more conjugates. However, two outliers had high hydroxy-anastrozole but low hydroxy-anastrozole-conjugates, indicating a possible deficiency in their ability to catalyze the conjugation reaction for the hydroxylated metabolite. Conversely – and more important clinically – two different patients had undetectable anastrozole concentrations, very high anastrozole conjugate concentrations and very little drug response in terms of change in their estrogen hormone levels. These observations raised the possibility that these latter two patients might represent “ultrarapid” conjugators of the drug, and, as a result, might fail to have the desired therapeutic response. These latter two patients will be discussed in greater detail subsequently.
There was no statistical association between time to second blood draw and anastrozole concentration (Spearman correlation = -0.004, p-value = 0.95), or for anastrazole conjugate concentration (Spearman correlation = 0.07, p-value = 0.36). However, there were significant correlations between time to second blood draw and hydroxy-anastrozole (Spearman correlation = 0.18, p-value = 0.008) and hydroxyanastrozole-conjugate (Spearman correlation = -.20, p-value = 0.005) concentrations.
Estrone, estradiol and estrone-conjugate concentrations pre-treatment and after anastrozole therapy
Pre- and post-treatment plasma levels of E1, E2, E1-conjugates, androstenedione and testosterone are listed in Table 2. Pre-treatment levels for all of these hormones demonstrated substantial variability. Among patients considered to be clinically postmenopausal by their oncologist, 28 (15%) had E2 levels greater than10 pg/ml, the conventional concentration separating premenopausal from postmenopausal women, with a range of 10.2-40.3 pg/ml (median: 13.55 pg/ml). Sixteen of these patients had been entered from Mayo and 12 from MD Anderson. The median age of these 28 patients was 58.5 years, with a range from 47 to 80 years. Only one patient in this group had received prior tamoxifen, nine (32%) had received prior chemotherapy, and 15 (54%) were active smokers. The median BMI for these 28 patients was 36.3 (range: 19.9-45.0), and nine (32%) had a BMI greater than 40.0. All but one of these patients had a decrease in their E2 levels after anastrozole therapy, with 18 (64%) dropping to undetectable levels.
Table 2.
Pre-treatment hormone levels (pg/ml) and during anastrozole therapy
| Pre-treatment (n=191) | During Anastrozole (n=191) | |
|---|---|---|
| Estrone | ||
| No. patients | 189 | 191 |
| Missing | 2 | 0 |
| Mean (SD) | 23.2 (15.64) | 1.0 (4.28) |
| Median | 19.9 | 0.0 |
| Range | 0.0-111.0 | 0.0-45.2 |
| Estradiol | ||
| N. patients | 188 | 189 |
| Missing | 3 | 2 |
| Mean (SD) | 5.8 (5.41) | 1.1 (7.75) |
| Median | 3.9 | 0.0 |
| Range | 0.0-40.3 | 0.0-97.0 |
| Estrone conjugates | ||
| No. patients | 189 | 190 |
| Missing | 2 | 1 |
| Mean (SD) | 340.5 (386.78) | 53.4 (239.15) |
| Median | 226.0 | 12.1 |
| Range | 7.7-3320.0 | 0.0-2990.0 |
| Androstenedione | ||
| No. patients | 189 | 191 |
| Missing | 2 | 0 |
| Mean (SD) | 515.8 (310.85) | 573.3 (303.73) |
| Median | 451.0 | 486.0 |
| Range | 0.0-2470.0 | 0.0-1560.0 |
| Testosterone | ||
| No. patients | 187 | 191 |
| Missing | 4 | 0 |
| Mean (SD) | 183.4 (134.53) | 183.1 (116.92) |
| Median | 151.0 | 160.0 |
| Range | 0.0-1120.0 | 0.0-678.0 |
Relationship of anastrozole concentrations to estrone, estradiol and estrone-conjugate concentrations
Figure 2 (panels A-C) displays changes in E1, E2, and E1-conjugate concentrations, color-coded for quartile of anastrozole level. Figure 2A demonstrates that only a small proportion of the patients (17%) had E1 concentrations above the LLQ while on anastrozole, with a median of 2.87 pg/ml but a very wide range (1.58-45.2 pg/ml). Likewise for E2, Figure 2B shows that only a small proportion of patients (17%) had concentrations above the LLQ on anastrozole, with a median of 1.26 pg/ml but once again with a very wide range (0.65-97.0 pg/ml). The findings with respect to E1-conjugates were quite different, with the vast majority of patients (93%) having levels above the LLQ with a median of 12.95 pg/ml but an exceedingly wide range (3.50-2990 pg/ml).
Figure 2.
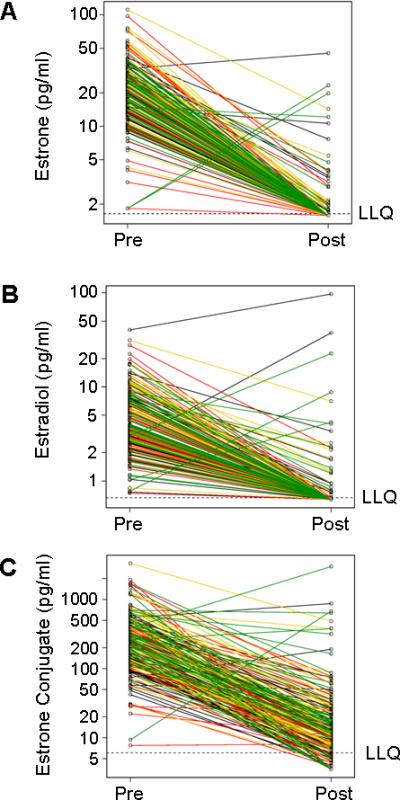
Plasma concentrations of estrone (A), estradiol (B), and estrone conjugates (C) according to quartile of anastrozole concentration in breast cancer patients before and after treatment with one mg/day oral dose of anastrozole. Key for line color: black, lowest quartile; red, second quartile; yellow, third quartile; green highest quartile.
As anticipated, the majority of patients experienced a drop in E1, E2 and E1-conjugate concentrations after anastrozole therapy, but unexpected rises were identified in three (2%) patients for E1, in five (3%) patients for E2, and in six (3%) patients for E1-conjugates (Figure 2, panels A, B, C). Eight patients (4%) had a rise in at least one of the estrogenic compounds (E1, E2, E1-conjugates), and the ages of those patients were 47, 50, 52, 52, 57, 57, 58 and 66 years, indicating that the rises did not occur only in the younger postmenopausal women. All eight patients had FSH and LH levels in the postmenopausal range at the time of the rise in one of the estrogenic compounds. The median BMI in these eight patients was 25.2 with a range of 18.3 to 38.7. The BMIs for these eight patients was not significantly different from the other 183 patients in this study (Wilcoxon p=0.20). As can be seen in Figure 2, the majority of patients with a rise in the level of at least one of these estrogenic compounds fell within the highest quartile for anastrozole concentrations.
The statistical association of clinical variables with pre-treatment concentrations of E1, E2 and E1-conjugate, as evaluated by step-wise regression, showed that BMI was positively correlated with all three hormone concentrations (in addition, smoking status was associated with E1, and stage and age were associated with E1-conjugate). Hence, it was critical to adjust for clinical variables when evaluating the association of anastrozole concentrations with changes in E1, E2 and E1-conjugate concentrations. In addition to clinical variables, pre-treatment hormone levels and anastozole concentration were evaluated in step-wise regression. This allowed us to evaluate the contribution of each variable, adjusted for the others, in case variables might be correlated (such as pre-treatment hormone level and BMI). In no instance was the level of anastrozole statistically associated with change in E1, E2 or E1-conjugates, although changes were often associated with BMI and pre-treatment hormone concentrations. However, whereas this was true of the entire group, there were two outliers in whom the lack of detectable anastrozole was associated with lack of change in E1-conjugates (see the subsequent section).
Similar to the regression analyses for change in hormone concentrations, we also evaluated actual post-treatment concentrations. In no instance was the concentration of anastrozole statistically associated with the final concentrations of E1, E2 or E1-conjugates.
Relationships among anastrozole, anastrozole conjugate and estrone conjugate concentrations
Figure 3A displays the relationship among anastrozole, anastrozole conjugate and E1-conjugate concentrations. Two patients (red open circles) had extremely low concentrations of anastrozole but very high levels of anastrozole conjugates. Those same two patients showed a relatively small change in plasma estrone conjugate levels after receiving anastrozole, i.e., a decrease by 58 pg/ml and 124 pg/ml, which are substantially less than the median decrease of 208 pg/ml. These observations raise the possibility that these two patients might represent “ultra-rapid” conjugators of anastrozole, accounting for the low parent drug concentrations and relatively small changes in hormone levels after drug. Neither of these patients was a current smoker and neither were being treated with drugs known to induce microsomal drug-metabolizing enzymes.
Figure 3.
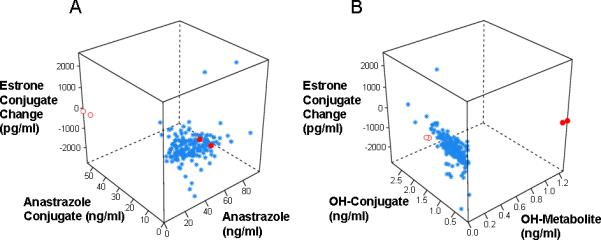
Correlation of anastrozole, anastrozole conjugates and changes in estrone conjugates (A) and of hydroxyl-anastrozole, hydroxyl-anastrozole-conjugates and changes in estrone conjugates (B) in breast cancer patients treated with one mg/day oral dose of anastrozole. Red open and closed circles described in text.
Figure 3B displays the relationship among hydroxy-anastrozole, hydroxyanastrozole conjugate and E1-conjugate concentrations. Two different patients (red closed circles) displayed high levels of hydroxy-anastrozole, but low hydroxy-anastrozole conjugates, which raises the possibility of a relative decrease in the ability to conjugate the hydroxy metabolite. The E1-conjugates in these two patients decreased by 234 pg/ml and 214 pg/ml, which are near the median change of -208 pg/ml, as anticipated since they had measurable concentrations of the parent drug.
Androstenedione and testosterone concentrations pre-treatment and after anastrozole therapy
Figure 4A displays the relationship between pre- and post-treatment androstenedione concentrations. Substantial variability is evident in pre-treatment androstenedione concentrations but with no consistent change in androstenedione concentrations after treatment with anastrozole. In 187 patients, 55% showed a rise and 43% showed a drop in androstenedione after treatment with anastrozole
Figure 4.
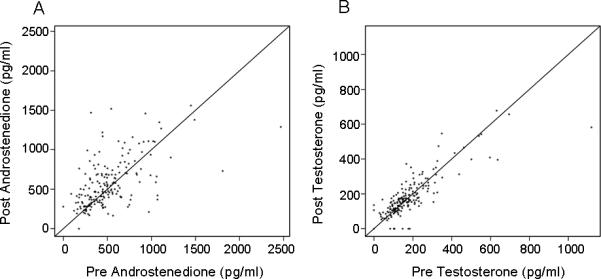
Concentrations of androstenedione (A) and testosterone (B) in breast cancer patients before and after treatment with one mg/day oral dose of anastrozole.
Figure 4B displays the relationship between pre- and post-treatment testosterone concentrations. Again, there was substantial variability in pre-treatment testosterone concentrations. In 189 patients, 61% showed a rise and 38% showed a drop in testosterone after treatment with anastrozole. Finally, there was not a consistent relationship between the changes in androstenedione and testosterone concentrations after anastrozole therapy (data not shown).
Discussion
This study examined the metabolism and pharmacodynamics of anastrozole when administered at the approved one mg daily dose as adjuvant endocrine therapy in a standard practice setting in two large oncology centers. The most striking observations were the degree of variation of pre-treatment hormone levels, of change in concentrations of E1, E2 and E1-conjugates after anastrozole therapy and of anastrozole and anastrozole metabolite concentrations in these women. To our knowledge, this is the largest study of this type, and it provides a “real life” view into the use of anastrozole in women with early stage breast cancer.
Anastrozole and anastrozole metabolite concentrations, like hormone concentrations, also revealed substantial variability, with steady state concentrations of anastrozole ranging from zero in two patients with detectable anastrozole metabolites, to 98.8 ng/ml. Three major metabolites were detected, with wide variations in anastrozole conjugate, hydroxy-anastrozole and hydroxy-anastrozole conjugate concentrations. The 29-fold range in the concentrations of hydroxy-anastrozole conjugates illustrates this wide variability. It is assumed that it is the parent drug, anastrozole, that has activity as an inhibitor of aromatase, but to our knowledge there are no data regarding anastrozole metabolites. The patterns of the histograms for the steady state free anastrozole and conjugated anastrozle plasma concentrations (Figure 1) are very different suggesting marked variation in metabolism of anastrozole to its conjugates. The marked variability in anastrozole levels clearly indicates that the current “one size fits all” approach to anastrozole dosing may need to be re-evaluated.
Pharmacodynamic studies also showed large variation. Most striking was the fact that eight patients (4%) had a rise in at least one of the estrogenic compounds (E1, E2, E1-conjugates) after drug exposure. Although all of the patients in this group had detectable anastrozole concentrations, the majority of the patients with a rise in estrogen concentrations were in the highest quartile for anastrozole concentration. The explanation for this observation is unclear. The FSH and LH levels were in the postmenopausal range in all eight patients at the time of the rise in one of the estrogenic compounds and the ages of four of the patients were 57 to 66 years of age. Specimen miss-labeling must always be considered a possibility, but the specimens were collected concurrently. It is of note that a recent report (16) found that four of 66 women treated with anastrozole, the AI utilized in our study, had decreased E2 levels at three months but an increase in E2 at six months, and two additional patients with decreased E2 levels at three and six months had an increased E2 level at nine months while receiving anastrozole.
The other finding of note with respect to the pharmacodynamic effects of anastrozole was the variability observed in decreases of the E1, E2 and E1-conjugates. Patients varied from those having profound reductions from relatively high pre-treatment levels to undetectable concentrations to those who displayed more modest decreases, with post-anastrozole levels remaining in the detectable range. Given the increased appreciation of variation in the clinical tolerability of aromatase inhibitors, in general, and anastrozole, in particular, these observations raise the possibility that the degree of change in estrogen concentrations, rather than the final concentrations may be related to a woman's tolerance and adherence to the drug and to toxicity such as musculoskeletal adverse events (15, 17).
Examination of the relationship among anastrozole, anastrozole metabolites and change in estrone conjugates revealed two patients with very low anastrozole and very high anastrozole conjugates but relatively small changes in estrone conjugates after drug treatment (Figure 3A, red open circles). The relationship between low anastrozole with small decreases in estrone conjugates is consistent with the fact that anastrozole is the active inhibitor of aromatase. However, the very high levels of anastrozole conjugates in these patients raise the possibility that these two patients may have had elevated activity of phase II enzymes that conjugate, thereby inactivating anastrozole. If this possibility can be confirmed, it would indicate that the metabolism of anastrozole in some patients results in their being denied optimal therapy. These findings indicate that future studies should determine the drug metabolizing enzymes that catalyze anastrozole hydroxylation and conjugation. Two other patients displayed high hydroxy-anastrozole concentrations, low hydroxy-anastrozole conjugates, and above average anastrozole levels (Figure 3B and 3A, red closed circles). These two patients, as expected for subjects with adequate parent drug, displayed decreases in plasma estrogen, but they may have a relative deficiency in their ability to conjugate hydroxy-anastrozole. This latter observation would not be expected to have clinical management implications as these metabolites are assumed to be pharmacologically inactive.
The sample of patients studied here showed variability in terms of age (range 39-82 years), BMI (range: 17.7-45.1), smoking status, prior chemotherapy and to a minor extent, prior tamoxifen and ethnicity/race. Fifteen percent of the women had E2 levels greater than 10 pg/ml, whereas a level of less than 10 pg/ml is generally considered characteristic of postmenopausal women. The median age of these women was 58.5 years (range 47-80) and, as a group, they were more overweight (median BMI 36.3) than the remainder of the patients, and slightly over half were active smokers. Despite having E2 concentrations above the conventional postmenopausal level, all but one patient had a drop in E2 concentrations after anastrozole, with almost two-thirds dropping to undetectable levels. These results suggest that the 10 pg/ml level may not be a definitive cutoff for defining postmenopausal women, especially since the age of these patients was up to 80 years. It can be speculated that the high levels of obesity seen in this group, with one-third having a BMI greater than 40, may have contributed to these observations.
In summary, our study of anastrozole therapy at the approved daily dose of one mg has revealed substantial variability in both drug metabolism and drug effect in a large sample of women with early breast cancer. We acknowledge that variable compliance must be considered a potential factor but it is clear that variability exists in both drug metabolism and drug effect. The variability observed suggests that this commonly employed agent for the treatment of breast cancer is a prime candidate for pharmacogenomic studies aimed at identifying genetic variation in drug metabolism. The results of those studies might help to make it possible to move toward the goal of truly “individualized” anastrozole therapy.
Financial support
Funded in part by National Institutes of Health grants U01 GM61388 and U01 GM061373 (Pharmacogenetics Research Network), K24RR020815, P50 CA166201 (Mayo Clinic Breast Cancer Specialized Program of Research Excellence), R01 GM28157 and a PhRMA Foundation “Center of Excellence in Clinical Pharmacology” Award. ClinicalTrials.gov study number NCT00283608.
Footnotes
Conflict of interest: Dr. Ingle has received honoraria for consulting with Pfizer, Novartis and AstraZeneca. Dr. Flockhart receives research funding from Pfizer and Novartis.
References
- 1.Ingle JN, Suman VJ. Aromatase inhibitors for therapy of advanced breast cancer. J Steroid Biochem Molec Biol. 2005;95:113–9. doi: 10.1016/j.jsbmb.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Ingle JN. Adjuvant endocrine therapy for postmenopausal women with early breast cancer. Clin Cancer Res. 2006;12(3 Part 2 Suppl S):1031S–6S. doi: 10.1158/1078-0432.CCR-05-2122. [DOI] [PubMed] [Google Scholar]
- 3.Ingle JN. Endocrine therapy trials of aromatase inhibitors for breast cancer in the adjuvant and prevention settings. Clin Cancer Res. 2005;11(2 Part 2 Suppl S):900S–5S. [PubMed] [Google Scholar]
- 4.Winer EP, Hudis C, Burnstein HJ, et al. American Society of Clinical Oncology Technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2007. J Clin Oncol. 2005;23:619–29. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 5.Plourde PV, Dyroff M, Dukes M. Arimidex®: a potent and selective fourth-generation aromatase inhibitor. Breast Cancer Res Treat. 1994;30:103–11. doi: 10.1007/BF00682745. [DOI] [PubMed] [Google Scholar]
- 6.Geisler J, King N, Dowsett M, et al. Influence of anastrozole (Arimidex), a selective, non-steroidal aromatase inhibitor, on in vivo aromatisation and plasma oestrogen levels in postmenopausal women with breast cancer. Br J Cancer. 1996;74:1286–91. doi: 10.1038/bjc.1996.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonat W, Howell A, Blomqvist C, et al. A randomized trial comparing two doses of the new selective aromatase inhibitor anastrozole (Arimidex) with megestrol acetate in postmenopausal patients with advanced breast cancer. Eur J Cancer. 1996;32:404–12. doi: 10.1016/0959-8049(95)00014-3. [DOI] [PubMed] [Google Scholar]
- 8.Buzdar AU, Jones SE, Vogel CL, et al. A phase III trial comparing anastrozole (1 and 10 milligrams), a potent and selective aromatase inhibitor, with megestrol acetate in postmenopausal women with advance breast carcinoma. Cancer. 1997;79:730–9. [PubMed] [Google Scholar]
- 9.Buzdar A, Jonat W, Howell A, et al. Anastrozole versus megestrol acetate in the treatment of postmenopausal women with advanced breast carcinoma. Cancer. 1998;83:1142–52. [PubMed] [Google Scholar]
- 10.The Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann M, Jonat W, Hilfrich J, et al. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: The ARNO 95 study. Clin Oncol. 2007;25:2664–70. doi: 10.1200/JCO.2006.08.8054. [DOI] [PubMed] [Google Scholar]
- 12.Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455–62. doi: 10.1016/S0140-6736(05)67059-6. [DOI] [PubMed] [Google Scholar]
- 13.Boccardo F, Rubagotti A, Puntoni M, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J Clin Oncol. 2005;23:5138–5147. doi: 10.1200/JCO.2005.04.120. [DOI] [PubMed] [Google Scholar]
- 14.Jakesz R, Greil R, Gnant M, et al. Extended adjuvant treatment with anastrozole: Results from the Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst. 2007;99:1845–53. doi: 10.1093/jnci/djm246. [DOI] [PubMed] [Google Scholar]
- 15.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26(4):556–62. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 16.Nagao T, Kira M, Takahashi M, et al. Serum estradiol should be monitored not only during the peri-menopausal period but also the psot-menopausal period at the time of aromatase inhibitor administration. World J Surg Oncol. doi: 10.1186/1477-7819-7-88. Published online Nov 12, 2009; doi:10.1186/1477-7819-7.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast. 2007;16:223–34. doi: 10.1016/j.breast.2007.01.011. [DOI] [PubMed] [Google Scholar]


