Summary
Purpose
Determination of the origin of extra-temporal neocortical onset seizures is often challenging due to the rapid speed in which they propagate throughout the cortex. Typically, these patients are poor surgical candidates and many times experience recurrences of seizure activity following resection of the assumed seizure focus.
Methods
We applied a causal measurement technique – the directed transfer function (DTF) – in an effort to determine the cortical location responsible for the propagation of the seizure actvity. Intracranial seizure recordings were obtained from a group of eleven pediatric patients with medically intractable neocortical-onset epilepsy. Time windows were selected from the recordings following onset of the ictal activity. The DTF was applied to the selected time windows and the frequency-specific statistically significant source activity arising from each cortical recording site was quantified. The DTF-estimated source activity was then compared with the seizure onset zone(s) identified by the epileptologists.
Results
In an analysis of the eleven pediatric patients, the DTF was shown to identify estimated ictal sources which were highly correlated with the clinically-identified foci. Additionally, it was observed that in the patients with multiple ictal foci, the topography of the casual source activity from the analyzed seizures was associated with the separate clinically-identified seizure onset zones.
Discussion
Although localization of neocortical-onset seizures is typically challenging, the causal measures employed in this study – namely the directed transfer function – identified generators of the ictal activity which were highly correlated with the cortical regions identified as the seizure onset zones by the epileptologists. This technique could prove useful in the identification of seizure-specific propagation pathways in the presurgical evaluation of patients with epilepsy.
Keywords: Seizure localization, epilepsy, functional connectivity, directed transfer function
I. Introduction
Neocortical onset seizures are often characterized by the rapid propagation of ictal activity generated by the epileptogenic foci (Spencer, 1988, Schiller et al., 1998, Lee et al., 2000, Timofeev and Steriade, 2004, Worrell et al., 2004). The explosive manner in which these seizures spread throughout the cortex poses a significant hurdle in the localization and subsequent surgical resection of the seizure-generating regions. Often, the precise location of the seizure onset zone (SOZ) responsible for the generation of the ictal activity is difficult to discern and following surgical excision, many patients may continue to experience seizures if removal of these primary ictal generators is incomplete (Engel et al., 1993, Zentner et al., 1996, Semah et al., 1998, Hong et al., 2002). In animal models of epilepsy, examination of neocortical slices obtained from rats has demonstrated that the activation site of these seizure-generating regions is fairly focally confined (Tsau et al., 1999). However, less is know regarding the extent of the networks involved in the generation of ictal activity in humans with neocortical epilepsy.
Currently, patients with non-lesional neocortical onset epilepsy undergo long-term invasive monitoring in order to determine the source and extent of the ictal generators. This is performed through surgical placement of intracranial electrodes and the subsequent recording of several independent seizures, typically over the course of several days. In these situations, determination of the SOZ is made through visual inspection of the electrographic recordings. This technique, though, does not take full advantage of the intrinsic properties of these epileptogenic networks. Tools to aid in the identification of these networks could prove useful in the treatment of patients with neocortical epilepsy (Blumenfeld et al., 2003, Gotman, 2003, Michel et al., 2004, Tao et al., 2007, Gotman, 2008).
Connectivity analysis techniques are particularly well-suited for this application due to the manner in which neuronal activity propagates throughout the cortex (Wu et al., 1999). These measures have been developed and utilized to quantify the interactions between selected brain regions (Friston et al., 1993a, Friston et al., 1993b, Horwitz, 2003, Lee et al., 2003). Most often, brain regions are determined to be functionally coupled based upon bivariate measures, such as coherence (Thatcher et al., 1986, Towle et al., 1998, Towle et al., 1999). While these methods are useful in identifying brain networks, however, they do not provide information concerning the directionality of the functional links. This factor tends to limit the usefulness of such techniques when large areas of cortex are highly synchronous, as in generalized epileptic seizures. Recently, directed connectivity measures based upon the concept of Granger causality (Granger, 1969) have emerged as a means to discern neuronal sources and their targeted cortical regions (Goebel et al., 2003, Astolfi et al., 2004, Baccala et al., 2004, Brovelli et al., 2004, Chen et al., 2004). These measures could prove useful in the identification of the generators of the ictal activity.
Traditional pairwise causality measures have an inherent disadvantage when applied to multivariate systems. In these situations, spurious causal links may arise between pairs of electrode channels or regions of interest (ROIs) when no such coupling physically exists (Kus et al., 2004). To overcome this problem, Kaminski and Blinowska introduced the concept of the directed transfer function (DTF), which is a type of multivariate application of Granger causality (Kaminski and Blinowska, 1991). They demonstrated that the DTF was able to identify the direction of propagated information between simulated ROIs. Additionally, they illustrated the ability of the DTF to accurately reproduce the connectivity profile in a modeled system composed of simulated ictal waveforms (Kaminski and Blinowska, 1991).
The DTF was first applied to epileptic recordings by Franaszczuk et al. (Franaszczuk et al., 1994). Here, it was demonstrated in three patients with mesial temporal lobe epilepsy that the DTF was able to correctly identify locations within the mesial temporal structures as the generators giving rise to the ictal activity. In a follow-up study, Franaszczuk et al. examined several additional patients with temporal lobe epilepsy originating in either the mesial or lateral cortical structures (Franaszczuk and Bergey, 1998). Even though the seizures which arose from the lateral temporal cortical structures propagated more rapidly, the DTF method identified the sources of the ictal activity which were highly correlated with the epileptologist-determined SOZ. Additional studies have further shown the DTF method to be capable of differentiating primary from secondary sources of interictal spike activity in EEG recordings from patients with epilepsy (Ding et al., 2007a).
While the results of these studies are encouraging and suggest that the DTF method may provide a useful tool in the localization of ictal activity, there has not been an examination of the efficacy of the DTF method in patients with neocortical onset extra-temporal lobe epilepsy. Unlike mesial temporal lobe epilepsy, the ictal activity in this group of patients typically propagates throughout the cortex much more quickly. Investigation of the applicability of the DTF method to neocortical-onset seizures is therefore warranted to ascertain its usefulness in these situations.
In this study, we examined the ability of the DTF to detect the neuronal generators responsible for the initiation of the neocortical seizures. Here, we identify the seizure foci by calculating the ictal activity which originates at each ECoG electrode. This method has been utilized successfully in previous studies applying the DTF to temporal lobe epilepsy (Franaszczuk et al., 1994, Franaszczuk and Bergey, 1998).
II. Methods
Patients
Eleven pediatric patients (5M/6F, ages 7-13; Table 1) with medically intractable epilepsy were studied under a protocol approved by the Institutional Review Boards of the University of Minnesota and the University of Chicago. All of the selected patients presented with complex partial seizures with secondary generalizations and underwent surgical evaluation at the University of Chicago Children’s Hospital. Each patient had resection of the epileptogenic foci and follow-up was performed at 6-12 months.
The location of the SOZ in each patient was identified from intracranial ictal recordings by trained epileptologists. Each of the selected patients was determined to have neocortical foci responsible for the generation of the ictal activity. Furthermore, of the eleven patients studied, six were identified as possessing two or more independent foci.
A single focus was identified by the epileptologists in Patients 3, 6, 8, 9 and 11. The focus was located within the frontal lobes of Patients 9 and 11; the parietal lobes of Patients 6 and 9; and the parietal/occipital lobes of Patient 3. A pair of foci was identified in Patients 1, 2, 5, 7 and 10, while three separate foci were identified in the left frontal and parietal lobes of Patient 4.
Seizure Data
The ictal electrocorticogram (ECoG) recordings were obtained from silastic electrode grids (interelectrode distance: 1 cm) implanted on the cortical surface. The recordings were referenced to the contralateral mastoid, passed through a 200 Hz anti-aliasing filter and sampled at 400 Hz (BMSI 6000; Nicolet Biomedical Inc, Madison, WI, USA). Following acquisition of the data, off-line pre-processing was performed and included additional band-pass filtering and automated artifact rejection. Visual inspection was also performed on the data and any channels exhibiting the presence of artifact were discarded from the analysis. For each recording, the seizure onset was identified from the epileptologists’ notes. A 3-7 second time window following the onset of each seizure was selected for analysis. A total of 51 seizures (range 1-8 per patient) were analyzed in this manner.
The DTF method is based upon the framework of an autoregressive model, and is best applied to linear systems. As such, it is necessary that this method be applied to quasistationary datasets (Kaminski and Blinowska, 1991). The ictal epochs in the analyzed datasets were carefully selected to ensure that they satisfied this criterion. Each of the ictal epochs was selected several seconds following the onset of the seizure during the regular, highly synchronous activity. The time-varying power spectrum was analyzed for each selected epoch to ensure that the frequency content remained relatively stable over the chosen time period. In addition to visual analysis of the epochs, the Bayesian Information Criterion (BIC) (Schwarz, 1978) was applied to each selected time series. The appropriate model order was selected by identification of a minimum in the BIC. If a clear minimum was not identified in the BIC plot, the epoch was not utilized in the current analysis. This method has been utilized in previous studies to verify that the selected intervals satisfied the quasistationary assumption (Kaminski and Blinowska, 1991, Franaszczuk et al., 1994, Franaszczuk and Bergey, 1998).
Localization of Ictal Generators
In each of the selected seizure recordings, a multivariate autoregressive (MVAR) model was fit to the time series. Morlet wavelet-based time-frequency analysis was performed on each ictal segment and the frequency containing the maximum power corresponding to the ictal activity was identified. The upper and lower bounds of the frequency band were selected such that they encompassed frequencies with ≥50% of the maximum power. The frequency bands displaying the increased energy were visually compared to the recorded time series to verify that the identified frequencies were correlated with the seizure waveforms. These selected frequency bands were then studied in the subsequent DTF analysis. An example of the time-frequency representation for a selected seizure and the subsequent selection of the ictal frequency band is shown in supplementary figure 1.
The DTF was calculated from the autoregressive coefficients obtained from the MVAR model as described by Kaminski and Blinowska (Kaminski and Blinowska, 1991). From the DTF calculation, the causal relationships among the ECoG channels in the selected ictal frequency bands were identified (see Supplementary Material). Once the causal interactions from the DTF calculation for the analyzed epoch were obtained, statistical significance testing was performed in order to remove the links which formed spurious interactions between ECoG channels. A surrogate data method was applied to each analyzed epoch in which the temporal correlation between the ECoG channels was destroyed (Theiler et al., 1992, Palus and Hoyer, 1998). The DTF method was applied to the surrogate datasets and a distribution of DTF values was obtained which corresponded to the null hypothesis of no causal interactions. From this distribution, a threshold was set (p=0.01) and links in which the strength of the causal interaction did not exceed this threshold were discarded from further analysis.
Following calculation of the causal links, the sum was obtained of the DTF-calculated activity which arose from each channel. The resulting value can be interpreted as the degree to which each electrode acts as a generator of the observed ictal activity (Franaszczuk et al., 1994). This value, which we termed the causal source activity, was calculated for each ECoG channel in each seizure. The causal source activity was normalized such that the electrode(s) with the maximum activity in each analyzed seizure had unit strength. In each patient, this procedure was repeated for each seizure and the causal source activity was summed across all of the analyzed epochs. Thresholds were set at 50% and 80% of the maximum summed source value and the resulting spatial map of the DTF-calculated causal source activity was compared to the cortical generators identified by the epileptologists. A diagram outlining the methods utilized in this study is shown in Figure 1.
Figure 1.
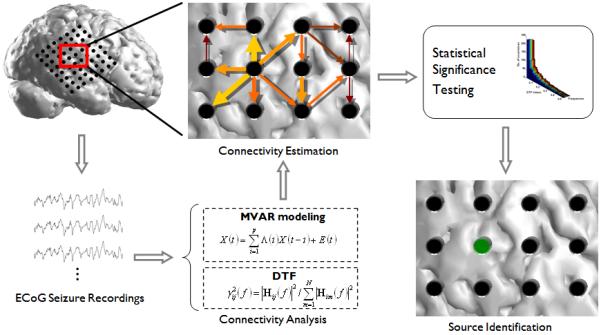
A diagram outlining the DTF source localization method utilized in the present study. First, a time segment following the ictal onset was selected from the ECoG recordings. The DTF method was applied to the time series and the connectivity pattern between the ECoG electrodes was obtained. Significance testing by means of a surrogate data method was performed to obtain the causal interactions which were statistically significant. From here, the amount of information leaving each electrode (strength of the outgoing arrows) was summed and the electrode with the maximum amount of source activity for each seizure was noted. This process was repeated for each seizure and the statistically significant source activity was summed to obtain the total DTF-calculated source activity for each patient.
III. Results
The spatial maps corresponding to the causal source activity from each seizure analyzed in the first patient are shown in Figure 2(a). From this figure, it can be observed that there are several similarities in the spatial pattern of each analyzed seizure. The sum of the causal activity for the six seizures is displayed in Figure 2(b). The dashed lines indicating the 50% and 80% thresholds are also shown.
Figure 2.
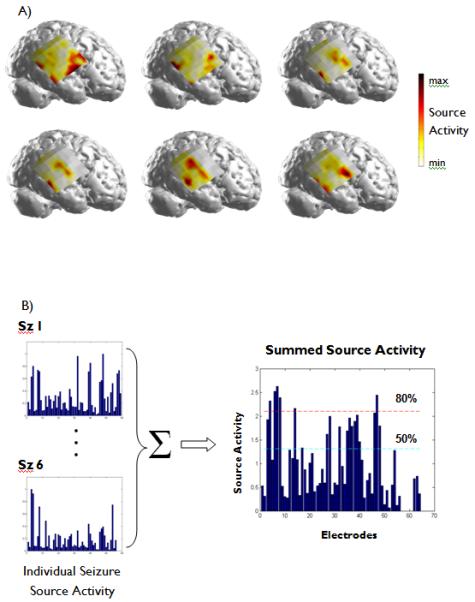
(A)The significant source activity obtained from each of the six seizures analyzed in Patient 1. The red color indicates a high degree of source activity in each seizure. (B) The source activity from each electrode was normalized such that the maximum source activity for each seizure had unit strength. This source activity was summed over all of the analyzed seizures in each patient and thresholding was performed at 50% and 80% of the maximum summed activity. Here, the source activity obtained from the six seizures analyzed in Patient 1 are shown.
From the thresholded DTF results, two regions of source activity can be observed in Patient 1 (Fig. 3). While the spatial map for the 50% thresholded values is fairly broadly distributed, the causal activity is more focally confined when thresholded at 80% of the maximum source activity. Additionally, the spatial map corresponding to the maximum causal source activity obtained from each individual seizure is shown. From this figure, a good correlation can be observed between the spatial locations of the causal source activity and the SOZ identified by the epileptologist. A right temporal lobectomy and resection of the frontal focus were performed. Following surgery, the patient experienced a 70% reduction in seizure frequency.
Figure 3.
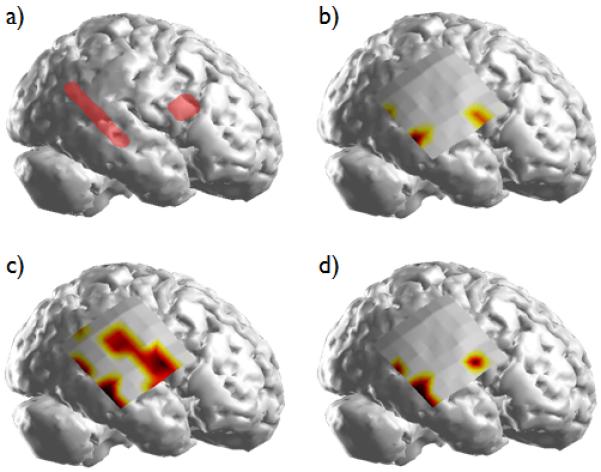
(a) The SOZs in Patient 1 identified clinically by the epileptologists; (b) the DTF-calculated source activity obtained by selecting the cortical regions having the maximum source activity in each of the analyzed seizures; (c) the summed source activity exceeding the 50% threshold; (d) the summed source activity exceeding the 80% threshold.
In the five patients with a single ictal focus, the DTF-calculated causal source locations were in close agreement with the SOZs calculated by the epileptologists. All of the spatial maps of the thresholded DTF calculations (50% and 80%) as well as the maximum DTF-calculated causal source activity in each of the individual seizures analyzed in Patients 6 and 9 (Fig. 4(e) and (h)) were located within the SOZ identified clinically. Both of these patients were seizure-free following surgical resection.
Figure 4.
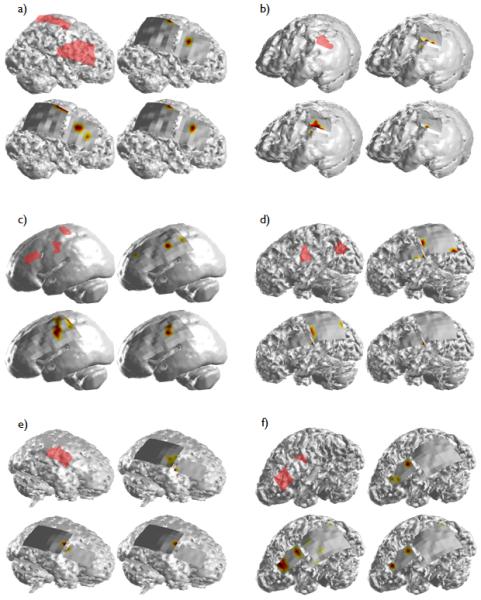
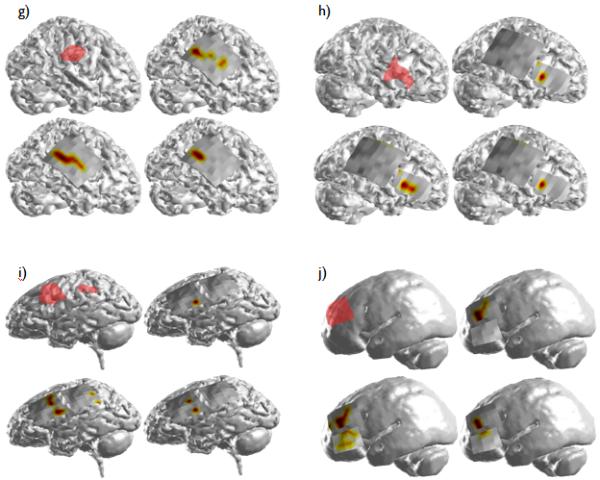
The DTF-calculated source activity for Patient 2 (a) through Patient 11 (j). The layout of the results for each patient is the same as shown in Figure 3. In Patients 4 (c) and 11 (j), whole-brain MRI scans were not available. In these two patients, the causal source activity and SOZ were projected onto an averaged smoothed cortical model.
In the three remaining patients with a single identified focus, portions of the DTF-calculated casual source activity extended beyond the borders of the clinical SOZs. The spatial map corresponding to the maximum causal source activity obtained in each of the six seizures analyzed in Patient 8 (Fig. 4(g)) displayed activity which extended anteriorly from the border of the SOZ. This activity was also observed in the spatial map corresponding to the 50% thresholded activity, but vanished, however when an increased (80%) threshold was applied. Likewise, the causal source activity calculated from the six analyzed seizures in Patient 11 (Fig. 4(j)) revealed activity located inferior to the SOZ in the 50% thresholded spatial map which was also observed to a lesser degree in the spatial map corresponding to the 80% threshold. However, the causal source activity calculated from each of the seizures individually was completely within the clinical SOZ. Finally, a significant amount of the DTF-calculated activity observed in Patient 3 (Fig. 4(b)) was located adjacent to and within the inferior border of the parietal/occipital SOZ identified clinically. Patients 3, 8 and 11 all experienced significant reductions in seizure frequency (97%, 99% and 85% respectively) following surgery.
In addition to Patient 1, Patients 2, 5, 7 and 10 had two ictal foci apiece identified by the epileptologists. As with Patients 6 and 9, the DTF-calculated source activity in Patient 2 (Fig. 4(a)) was exclusively within the borders of the epileptologist-defined SOZ. The patient was seizure-free for a period of time following surgical resection but required a second surgery several years later following recurrence of the seizures. A right functional hemisperectomy was performed at that time following which the patient remained seizure-free.
Two areas of causal activity were observed in Patient 7 (Fig. 4(f)) and were closely correlated with the pair of clinical foci. The causal source activity corresponding to the frontal SOZ was within the borders of the SOZ, while the second focus of causal activity was located immediately anterior to the posterior focus. This patient was seizure-free following surgery.
In both Patients 5 and 10 (Fig. 4(d) and (i)), causal source activity was not observed in the posterior focus in the thresholded spatial maps. While analysis of the maximum activity in each of the individual seizures revealed a focal area within the posterior SOZ in Patient 5, none was observed in Patient 10. This was not unexpected, however, as only a single seizure was available for analysis in this patient. The reduction in seizure frequency following surgery was approximately 70 and >90% for Patient 5 and 10 respectively.
Additionally, one of the analyzed patients had three separate clinical foci. From the thresholded causal maps in Patient 4 (Fig. 4(c)), a single causal source was identified which was located within the middle SOZ. This causal activity was also observed in the spatial map corresponding to the maximum source activity from the individual seizures. Also observed from this map, causal activity was seen superior and inferior to the anterior and posterior SOZs respectively. This patient had a 95% reduction in seizure frequency following surgical removal of the three clinical SOZs.
For each patient, the ratio of the DTF-calculated source activity arising from the clinical SOZs to the total causal activity was obtained. In Figure 5, the post-surgical outcome is compared to the ratio of the SOZ-causal activity calculated from the 50% thresholded maps.
Figure 5.
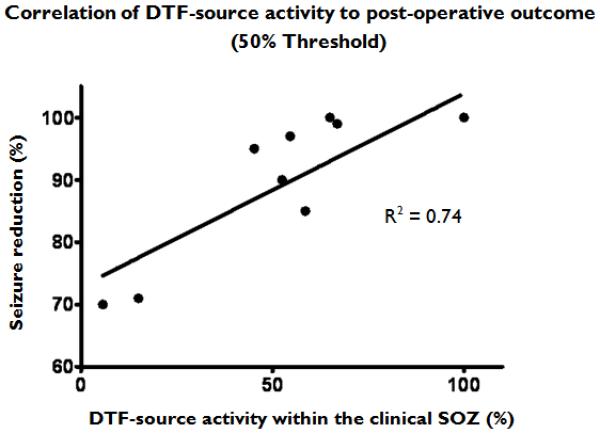
The correlation of the post-surgical seizure outcome in the analyzed patients to the percentage of the causal source activity within the clinical SOZ calculated from the 50% thresholded spatial maps.
IV. Discussion
In this study, we have examined the ability of the DTF-based connectivity method to identify seizure foci in neocortical extra-temporal lobe epilepsy. Specifically, we studied ECoG recordings in eleven pediatric patients undergoing surgical evaluation for treatment of intractable epilepsy. Of the eleven patients, six had two or more distinct foci identified by the epileptologists. All of the patients experienced a ≥70% decrease in the frequency of seizures following surgical resection and four patients were seizure-free following surgery.
For each patient included in the analysis, the DTF-calculated sources of the ictal activity were well-correlated with the epileptogenic foci identified by the epileptologists. Furthermore, in four of the five patients with multiple ictal foci, distinct regions of causal-source activity were observed which closely corresponded to the individual SOZs. From the thresholded results obtained from an analysis of the combined ictal activity in each patient, all of these separate foci were not always observed (ie. Patients 4 and 5). This is likely due to the fact that the seizures may not arise from each SOZ in equal proportions. Thus, the focal areas which are more active may slightly bias this estimator. However, the results from the thresholded DTF source values were still consistent in location with one or more of the clinical foci. From these findings, it appears as though the sources for the functional networks identified by the DTF are closely related to the sources of the ictal activity as determined by the epileptologists. Such a finding could potentially lead to more focused surgical resections through the limited resection of specific seizure-generating nodes given knowledge of the specific seizure propagating networks.
From the results, it was observed that the thresholded DTF sources as well as the maximum DTF-calculated source values for the individual seizures were fairly focal in extent. In the analyzed patients, these DTF-identified sources were often much more focal in nature that the SOZs identified by the epileptologists. It is possible that the seizures in the analyzed patients arise from discrete ictal generators or focal networks which are not readily apparent upon visual inspection of the intracranial recordings. This is supported by the observation that the three patients in which the DTF activity was completely within the clinical SOZ, all remained seizure-free following surgical resection. Furthermore, the patients in which the majority of the DTF-activity calculated at the 50% threshold level was within the SOZ, experienced significant (>85%) reductions in seizure frequency as well. The two patients who experienced the least decline in seizure frequency post-operatively (~70% reduction) likewise had a significant amount of DTF-calculated causal source activity beyond the borders of the clinical SOZs. However, as the current study is a post hoc analysis of patients undergoing presurgical monitoring, it is not known if a more limited resection of the DTF-identified sources alone would produce as favorable of post-surgical outcomes. Based upon the results of this study, additional studies, perhaps involving animal models of epilepsy, are needed to quantitatively address this issue.
In the current study, we analyzed the causal source activity arising from the frequency band associated with the oscillatory ictal activity. For the selected epochs, this typically occurred within the theta to beta frequency range. In addition to this analysis, we had previously explored the application of the DTF to whole band analysis within the theta, alpha, beta and gamma frequencies in a small number of subjects. In these patients, the site of the seizure onset as calculated by the DTF was similar, although in some situations there was slightly more variance in the spatial map of the causal source activity. It is possible that this increased variance is attributed to normal (physiologic) brain activity or possibly attributed to noise arising from integrating the DTF-calculated source activity over an extended frequency range.
Several studies have previously shown that high frequency oscillations (HFOs) (100-500+ Hz) are associated with epileptogenic cortex (Allen et al., 1992, Bragin et al., 1999, Bragin et al., 2002, Worrell et al., 2004, Jirsch et al., 2006, Worrell et al., 2008). However, due to the limitations imposed by the sampling frequency of the data analyzed in the current study, we were unable to extensively study the causal interactions within this frequency band. Acquisition of data with significantly higher sampling rates could allow for the analysis of these HFOs with DTF-based causality measures. This would allow for potentially important insight into the comparison of the cortical networks underlying these HFOs and the lower frequency ictal oscillations analyzed in the current study.
An inherent assumption in the DTF calculation is that the connectivity pattern among the ECoG electrodes does not change over the analyzed time window. In several of the analyzed seizure recordings, many different time windows were analyzed to determine if the calculated DTF source was significantly affected by the choice of the time window. In each case, the DTF calculated source was unaffected by the choice of the time period (data not shown). All of the time series were selected after the onset of the ictal activity when a large increase could be observed in the power spectra related to the seizure. Thus, following the onset of the seizure, it could be assumed that the majority of the analyzed frequency content was due to the ictal activity. DTF analysis was not performed prior to the ictal onset as this assumption was not valid for this time period.
In summary, we have examined the ability of the DTF method to localize neocortical onset seizures in eleven pediatric patients. In these patients, the precise location of the seizure onset zone is typically challenging to obtain due to the rapid spread of the ictal activity throughout the cortex. We have found in this study that, despite the speed in which the epileptiform activity spreads throughout the neocortex, the DTF method is able to accurately localize the source of the ictal activity. In the eleven selected patients, the sources estimated from the DTF method were found to be in agreement with the seizure foci identified by the epileptologists. The use of such causal analysis tools could provide greater insight into the sources of the epileptogenic networks which give rise to the ictal activity and pave the way for better and more focused treatment of patients with intractable epilepsy.
Supplementary Material
Acknowledgments
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The authors would like to thank Dr. V.L. Towle for useful discussions. This work was supported in part by NIH RO1EB007920, and a grant from the Minnesota Partnership for Biotechnology and Medical Genomics. C.W. was supported in part by NIH T32 EB008389.
Footnotes
Disclosure: None of the authors has any conflict of interest to disclose.
References
- Allen PJ, Fish DR, Smith SJ. Very high-frequency rhythmic activity during SEEG suppression in frontal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1992;82:155–159. doi: 10.1016/0013-4694(92)90160-j. [DOI] [PubMed] [Google Scholar]
- Astolfi L, Cincotti F, Mattia D, Salinari S, Babiloni C, Basilisco A, Rossini PM, Ding L, Ni Y, He B, Marciani MG, Babiloni F. Estimation of the effective and functional human cortical connectivity with structural equation modeling and directed transfer function applied to high-resolution EEG. Magn Reson Imaging. 2004;22:1457–1470. doi: 10.1016/j.mri.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Babiloni F, Cincotti F, Babiloni C, Carducci F, Mattia D, Astolfi L, Basilisco A, Rossini PM, Ding L, Ni Y, Cheng J, Christine K, Sweeney J, He B. Estimation of the cortical functional connectivity with the multimodal integration of high-resolution EEG and fMRI data by directed transfer function. NeuroImage. 2005;24:118–131. doi: 10.1016/j.neuroimage.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Baccala LA, Alvarenga MY, Sameshima K, Jorge CL, Castro LH. Graph theoretical characterization and tracking of the effective neural connectivity during episodes of mesial temporal epileptic seizure. Journal of integrative neuroscience. 2004;3:379–395. doi: 10.1142/s0219635204000610. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Westerveld M, Ostroff RB, Vanderhill SD, Freeman J, Necochea A, Uranga P, Tanhehco T, Smith A, Seibyl JP, Stokking R, Studholme C, Spencer SS, Zubal IG. Selective frontal, parietal, and temporal networks in generalized seizures. NeuroImage. 2003;19:1556–1566. doi: 10.1016/s1053-8119(03)00204-0. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr. Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL. Beta oscillations in a large-scale sensorimotor cortical network: Directional influences revealed by granger causality. Proc Natl Acad Sci USA. 2004;101:9849–9854. doi: 10.1073/pnas.0308538101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Rangarajan G, Feng J, Ding M. Analyzing multiple nonlinear time series with extended granger causality. Phys Lett A. 2004;324:26–35. [Google Scholar]
- Ding L, Wilke C, Xu B, Xu X, van Drongelen W, Kohrman M, He B. EEG source imaging: Correlating source locations and extents with electrocorticography and surgical resections in epilepsy patients. J Clin Neurophysiol. 2007a;24:130–136. doi: 10.1097/WNP.0b013e318038fd52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Worrell GA, Lagerlund TD, He B. Ictal source analysis: Localization and imaging of causal interactions in humans. NeuroImage. 2007b;34:575–586. doi: 10.1016/j.neuroimage.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Jr, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel J Jr, editor. Surgical treatment of the epilepsies. Raven Press; New York: 1993. pp. 609–622. [Google Scholar]
- Franaszczuk PJ, Bergey GK. Application of the directed transfer function method to mesial and lateral onset temporal lobe seizures. Brain Topogr. 1998;11:13–21. doi: 10.1023/a:1022262318579. [DOI] [PubMed] [Google Scholar]
- Franaszczuk PJ, Bergey GK, Kaminski MJ. Analysis of mesial temporal seizure onset and propagation using the directed transfer function method. Electroencephalogr Clin Neurophysiol. 1994;91:413–427. doi: 10.1016/0013-4694(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS. Time-dependent changes in effective connectivity measured with PET. Hum Brain Mapp. 1993a;1:69–80. [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: The principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993b;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Goebel R, Roebroeck A, Kim DS, Formisano E. Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and granger causality mapping. Magn Reson Imaging. 2003;21:1251–1261. doi: 10.1016/j.mri.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Gotman J. Epileptic networks studied with EEG-fMRI. Epilepsia. 2008;49(Suppl 3):42–51. doi: 10.1111/j.1528-1167.2008.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J. Noninvasive methods for evaluating the localization and propagation of epileptic activity. Epilepsia. 2003;44(Suppl 12):21–29. doi: 10.1111/j.0013-9580.2003.12003.x. [DOI] [PubMed] [Google Scholar]
- Granger CWJ. Investigating causal relations by econometric models and cross-spectra methods. Econometrica. 1969;37:424–428. [Google Scholar]
- Hong KS, Lee SK, Kim JY, Lee DS, Chung CK. Pre-surgical evaluation and surgical outcome of 41 patients with non-lesional neocortical epilepsy. Seizure. 2002;11:184–192. doi: 10.1053/seiz.2001.0616. [DOI] [PubMed] [Google Scholar]
- Horwitz B. The elusive concept of brain connectivity. NeuroImage. 2003;19:466–470. doi: 10.1016/s1053-8119(03)00112-5. [DOI] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Oliver A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–15608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Kaminski MJ, Blinowska KJ. A new method of the description of the information flow in the brain structures. Biol Cybern. 1991;65:203–210. doi: 10.1007/BF00198091. [DOI] [PubMed] [Google Scholar]
- Kus R, Kaminski M, Blinowska KJ. Determination of EEG activity propagation: Pair-wise versus multichannel estimate. IEEE Trans Biomed Eng. 2004;51:1501–1510. doi: 10.1109/TBME.2004.827929. [DOI] [PubMed] [Google Scholar]
- Lee L, Harrison LM, Mechelli A. A report of the functional connectivity workshop, dusseldorf 2002. NeuroImage. 2003;19:457–465. doi: 10.1016/s1053-8119(03)00062-4. [DOI] [PubMed] [Google Scholar]
- Lee SA, Spencer DD, Spencer SS. Intracranial EEG seizure-onset patterns in neocortical epilepsy. Epilepsia. 2000;41:297–307. doi: 10.1111/j.1528-1157.2000.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Lutkepohl H. Comparison of criteria for estimating the order of a vector autoregressive process. J Time Ser Anal. 1985;6:5–52. [Google Scholar]
- Michel CM, Lantz G, Spinelli L, DePeralta RG, Landis T, Seeck M. 128-channel EEG source imaging in epilepsy: clinical yield and localization precision. J Clin Neurophysiol. 2004;21:71–83. doi: 10.1097/00004691-200403000-00001. [DOI] [PubMed] [Google Scholar]
- Palus M, Hoyer D. Detecting nonlinearity and phase synchronization with surrogate data. IEEE Eng Med Biol Mag. 1998;17:40–45. doi: 10.1109/51.731319. [DOI] [PubMed] [Google Scholar]
- Schiller Y, Cascino GD, Busacker NE, Sharbrough FW. Characterization and comparison of local onset and remote propagated electrographic seizures recorded with intracranial electrodes. Epilepsia. 1998;39:380–388. doi: 10.1111/j.1528-1157.1998.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, Cavalcanti D, Baulac M. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51:1256–1262. doi: 10.1212/wnl.51.5.1256. [DOI] [PubMed] [Google Scholar]
- Spencer SS. Cortical and intercortical seizure spread. In: Ferendelli J, Wieser HG, Meldrum B, editors. Anatomy of epilepsy. John Libbey; London: 1988. pp. 139–154. [Google Scholar]
- Tao JX, Baldwin M, Ray A, Hawes-Ebersole S, Ebersole JS. The impact of cerebral source area and synchrony on recording scalp electroencephalography ictal patterns. Epilepsia. 2007;48:2167–2176. doi: 10.1111/j.1528-1167.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, Krause PJ, Hrybyk M. Cortico-cortical associations and EEG coherence: A two-compartmental model. Electroencephalogr Clin Neurophysiol. 1986;64:123–143. doi: 10.1016/0013-4694(86)90107-0. [DOI] [PubMed] [Google Scholar]
- Theiler J, Eubank S, Longtin A, Galdrikian B, Farmer JD. Testing for nonlinearity in time series: The method of surrogate data. Physica D. 1992;58:77–94. [Google Scholar]
- Timofeev I, Steriade M. Neocortical seizures: Initiation, development and cessation. Neuroscience. 2004;123:299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- Towle VL, Carder RK, Khorasani L, Lindberg D. Electrocorticographic coherence patterns. J Clin Neurophysiol. 1999;16:528–547. doi: 10.1097/00004691-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Towle VL, Syed I, Berger C, Grzesczcuk R, Milton J, Erickson RK, Cogen P, Berkson E, Spire JP. Identification of the sensory/motor area and pathologic regions using ECoG coherence. Electroencephalogr Clin Neurophysiol. 1998;106:30–39. doi: 10.1016/s0013-4694(97)00082-5. [DOI] [PubMed] [Google Scholar]
- Tsau Y, Guan L, Wu JY. Epileptiform activity can be initiated in various neocortical layers: An optical imaging study. J Neurophysiol. 1999;82:1965–1973. doi: 10.1152/jn.1999.82.4.1965. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–1506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Guan L, Tsau Y. Propagating activation during oscillations and evoked responses in neocortical slices. J Neurosci. 1999;19:5005–5015. doi: 10.1523/JNEUROSCI.19-12-05005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner J, Hufnagel A, Ostertun B, Wolf HK, Behrens E, Campos MG, Solymosi L, Elger CE, Wiestler OD, Schramm J. Surgical treatment of extratemporal epilepsy: Clinical, radiologic, and histopathologic findings in 60 patients. Epilepsia. 1996;37:1072–1080. doi: 10.1111/j.1528-1157.1996.tb01027.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


