Abstract
Continuous etching of aggressive all-in-one adhesives occurs in wet dentin tubules after polymerization of the adhesives. This study challenged the hypothesis that unpolymerized acidic monomers from an aggressive all-in-one self-etching adhesive continue to etch beyond dentin hybrid layers. Dentin surfaces bonded with Adper Prompt L-Pop were sectioned into 0.3-mm-thick slabs. Some of the slabs were stored in water (pH 6.8) or glycine buffer (pH 11.1) for six weeks and then examined by CLSM, SEM, and TEM. The rest were immersed in a biomimetic remineralizing medium for up to 4 months. Morphologic analysis indicated no difference in demineralization thickness between the two 6-week storage groups. However, increased permeability and loss of integrity occurred along the base of the hybrid layers in the glycine buffer group, but not in the water storage group. These findings were also confirmed by the results of biomimetic remineralization along the bases of those hybrid layers.
Keywords: continuous etching, glycine buffer, hybrid layer thickness, remineralization, self-etching adhesive
Introduction
All-in-one self-etching adhesives are the most user-friendly among the commercially available dentin adhesives. However, they exhibit compromised bonding effectiveness when compared with the three- step and two-step adhesives (Van Meerbeek et al., 2003; Peumans et al., 2005). They have been associated with several shortcomings, including severe nanoleakage and water entrapment within the adhesive (Tay et al., 2002, 2005), incompatibility with chemically cured composites (Tay et al., 2003), and phase separation of resin components (Van Landuyt et al., 2005).
Another potential disadvantage associated with the use of aggressive self-etching adhesives is that poorly polymerized acidic resin monomers may continue to etch the mineralized dentin beneath hybrid layers (Wang and Spencer, 2005). These resin-sparse zones of inadvertently etched dentin are susceptible to degradation during aging. It is doubtful, however, whether acidic monomers that are already partially neutralized (Spencer et al., 2000) as they diffuse through dentin with high buffering capacity (Camps and Pashley, 2000) remain acidic enough to continue demineralizing dentin beneath hybrid layers. Moreover, this potential problem has not been detected in clinical (Bradna et al., 2008; van Dijken and Pallesen, 2008) and long-term nanoleakage studies (Reis et al., 2007; do Amaral et al., 2009). Thus, the continuous etching potential of aggressive self-etching adhesives requires further clarification.
A nanotechnology-inspired dentin remineralization strategy based on the incorporation of dual biomimetic analogs in a Portland-cement-based medium has been reported (Tay and Pashley, 2008). This strategy was adopted for remineralization of completely demineralized hybrid layers that are devoid of seed crystallites for promoting epitaxial apatite growth within the collagen matrix (Tay and Pashley, 2009). Although the technique is not yet clinically applicable, it requires a high pH that would effectively buffer the acidic groups in aggressive self-etching adhesives, thereby preventing prolonged etching.
Thus, the first objective of the present study was to determine the interfacial ultrastructure and changes in hybrid layer thickness of an aggressive all-in-one self-etching adhesive after 6 wks of storage in either de-ionized water or a glycine buffer. The first hypothesis tested was that there are inherent deficiencies in hybrid layers created by the all-in-one adhesive that are not associated with the continuous etching of residual acidic resin monomers. The second objective was to correlate the events observed in those artificial aging schemes with the results of the biomimetic remineralization strategy. The second hypothesis tested was that the biomimetic remineralization strategy is effective in confirming the presence of resin-infiltration-related deficiencies in hybrid layers created by the adhesive.
Materials & Methods
Specimen Preparation
Twenty recently extracted human third molars were collected after patients’ informed consents were obtained under a protocol reviewed and approved by the Human Assurance Committee of the Medical College of Georgia. A flat dentin surface was prepared perpendicular to the longitudinal axis of each tooth by means of an Isomet saw (Buehler Ltd., Lake Bluff, IL, USA) and an Ecomet III polisher (Buehler Ltd.) to 320-grit roughness under water cooling. Adper Prompt L-Pop (3M ESPE, St. Paul, MN, USA) was applied to the dentin surface and agitated for 15 sec or 60 sec (fresh adhesive applied after 30 sec). The rationale for the extended application time was that any continuous etching beyond hybrid layers in the 15-second group would have been exaggerated and more clearly identified in the 60-second group. After gentle air-drying, light-curing of the adhesive was performed for 40 sec in a light-curing unit with an output intensity of 600 mW/cm2. A microfilled resin composite was placed in three 1-mm-thick increments over the light-cured adhesive and light-cured incrementally. The bonded teeth were stored at 100% relative humidity for 24 hrs at 37°C.
After storage, each tooth was sectioned into 0.3-mm-thick serial composite-dentin slabs. Three central slabs were selected from each tooth and randomly divided into 3 groups: de-ionized water, glycine buffer, and biomimetic remineralization (N = 10). The rationale for using a glycine buffer (pH 11.1) was to buffer all acidic monomers to prevent potential continuous etching beyond hybrid layers, as seen in specimens that were aged in de-ionized water (Wang and Spencer, 2005). We prepared the latter by mixing 0.15 M glycine/NaCl and 1.1 M NaOH in de-ionized water. The specimens were aged for 6 wks in either de-ionized water or the glycine buffer and examined with confocal laser scanning microscopy (CLSM), scanning electron microscopy (SEM), and transmission electron microscopy (TEM).
Biomimetic Remineralization
We have found that defects in resin-bonded dentin remineralized in a biomimetic medium (Tay and Pashley, 2009). Briefly, each slab was placed over a set Portland cement block inside a glass scintillation vial. The vial was filled with 15 mL of a phosphate-containing fluid containing 500 µg/mL polyacrylic acid and 200 µg/mL polyvinylphosphonic acid as dual biomimetic analogs. Each slab was turned every 2 wks to ensure that both sides of the slab were in contact with the cement block. We retrieved the experimental slabs after 3 and 6 mos to examine the extent of remineralization with TEM.
Confocal Laser Scanning Microscopy
The CLSM was used for non-destructive identification of the presence of porous dentin zones beneath the hybrid layers that were permeable to an aqueous fluorescent dye. Each slab was polished with a wet 1200-grit silicon carbide paper, ultrasonicated for 5 min, and immersed in a 0.1 wt% Lucifer yellow solution (Sigma-Aldrich, St. Louis, MO, USA; pH = 7.4). After 24 hrs, the dye-infiltrated slabs were rinsed with de-ionized water and examined with a two-photon CLSM (LSM 510 META, Carl Zeiss MicroImaging, Thornwood, NY, USA) that was coupled to a 10 W mode-locked Ti:sapphire laser (MIRA 900 System, Coherent Laser Group, Santa Clara, CA, USA). The excitation wavelength was 800 nm, with a pulse repetition rate of 90 MHz. A water immersion objective (C-Apochromat 63x/1.2 Zeiss, Toronto, ON, Canada) was used for capturing images commencing at 5 µm beneath the polished surface, to avoid superficial specimen preparation artifacts.
Scanning Electron Microscopy
For each of the 4 aged specimen groups (de-ionized-water-aged vs. Glycine-buffer-aged; 15-second-etched vs. 60-second-etched), 5 slabs were randomly selected after CLSM imaging for examination of the resin-dentin interfacial morphology. The surface of each slab was treated with 37% H3PO4 gel for 5 sec, followed by immersion in 5.25% NaOCl for 10 min to bring the resin-dentin interface into relief. The specimens were dehydrated in an ascending ethanol series (50-100%), immersed in hexamethyldisilazane, sputter-coated with gold/palladium, and examined with a field-emission SEM (XL-30 FEG; Philips, Eindhoven, The Netherlands) at 5 kV.
Transmission Electron Microscopy
The rest of the specimens from the 4 aged groups were processed for TEM together with the specimen slabs that had undergone biomimetic remineralization, according to a TEM protocol reported previously (Tay et al., 1999). Briefly, specimen slabs were dehydrated in an ascending ethanol series (50-100%), immersed in propylene oxide, and embedded in epoxy resin. Non-demineralized, 90- to 100-nm-thick sections were examined unstained by means of a JEM-1230 TEM (JEOL, Tokyo, Japan) at 110 kV.
Determination of Hybrid Layer Thickness
Ten CLSM and 10 TEM images from each of the 4 aged groups were analyzed with ImageJ (NIH, Bethesda, MD, USA). For each image, 10 measurements of the hybrid layer thickness were performed, resulting in 100 images per group. Since the intention of the study was not to compare hybrid layer thicknesses created by 15 sec vs. 60 sec of etching, CLSM and TEM data derived from the water-aged and glycine-buffer-aged groups of each etching time-period were analyzed separately by Student t tests with α = 0.05.
Results
No significant difference was found between hybrid layer thickness in the de-ionized-water-aged and glycine-buffer-aged groups for both the 15- and 60-second etching time-periods (p > 0.05), in both CLSM and TEM images (p > 0.05) (Table).
Table.
Thickness of Hybrid Layers Created by Adper Prompt L-Pop after the Resin-Dentin Interfaces were Aged for 6 wks in Either De-ionized Water (pH 6.8) or a Glycine Buffer (pH 11.1)
| CLSM* |
TEM* |
|||
|---|---|---|---|---|
| Aging Medium |
||||
| Application Time | De-ionized Water | Glycine Buffer | De-ionized Water | Glycine Buffer |
| 15 sec | 4.92 ± 0.59a | 5.09 ± 0.81a | 4.52 ± 0.43A | 4.46 ± 0.49A |
| 60 sec | 9.06 ± 0.921 | 8.85 ± 1.221 | 8.12 ± 1.00§ | 8.28 ± 1.30§ |
Data derived by confocal laser scanning microscopy (CLSM) and transmission electron microscopy (TEM) were analyzed separately. Likewise, data acquired from the two etching time-periods (i.e., 15 or 60 sec) were analyzed separately. For each data pair (e.g., de-ionized water vs. glycine buffer, CLSM images, 15-second etching time-period), the use of the same superscript indicates no significant difference (p > 0.05). Values are mean ± SD, in µm. N = 100.
Diffusion of the fluorescent dye into the permeable resin-bonded dentin subsurfaces resulted in baseline CLSM images that displayed pale-yellow hybrid layers in the baseline (i.e., before aging) specimens (Figs. 1A,1D) and de-ionized-water-aged groups (Figs. 1B,1E). Conversely, fluorescence was much brighter at the base of the hybrid layers in the glycine-buffer-aged specimens, indicating the highly permeable nature of those regions (Figs. 1C,1F).
Figure 1.
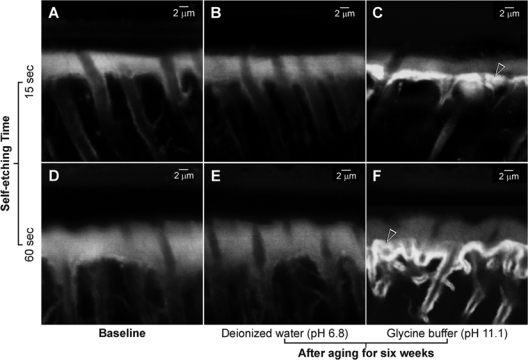
Confocal laser scanning microscopy images of Adper Prompt L-Pop-bonded dentin slabs. (A) A representative 15-second-etched baseline specimen before aging. Diffusion of the fluorescent dye into the permeable resin-bonded dentin subsurface resulted in the identification of a 5- to 6-µm-thick, palely fluorescent hybrid layer. (B) A representative specimen from the 15-second-etched, water-aged subgroup. The thickness of the hybrid layer was similar to that of the baseline specimen. (C) A 15-second-etched, glycine-buffer-aged specimen. Hybrid layer thickness was similar to that of the water-aged subgroup. Intense fluorescence could be identified from the base of the hybrid layer (open arrowhead). (D) A representative 60-second-etched baseline specimen before aging. A 10- to 12-µm-thick, palely fluorescent hybrid layer could be seen. (E) A 60-second-etched, water-aged specimen. Hybrid layer thickness was similar to that of the baseline specimen. (F) A 60-second-etched, glycine-buffer-aged specimen. Although the thickness of the hybrid layer was similar to that of the 60-second-etched, water-aged specimens, intense fluorescence could be identified from the basal area of the hybrid layer (open arrowhead). Collectively, there was no evidence to support that the self-etch adhesive etched continuously beyond hybrid layers during the period of prolonged water aging. However, increased fluorescence in the glycine-buffer-aged subgroups indicated that the base of those hybrid layers became more permeable after prolonged immersion in the alkaline glycine buffer.
Scanning electron microscopy of specimens from the de-ionized-water-aged groups (Figs. 2A, 2D) revealed acid-/base-resistant hybrid layers that closely approximated the underlying mineralized dentin base. There was no evidence of continuous etching beneath those hybrid layers. Conversely, specimens that were aged in glycine buffer depicted thin (0.5-2 µm thick), remnant acid-/base-resistant hybrid layers (Figs. 2B, 2E) that were accompanied by wide spaces between the bottoms of those hybrid layers and the mineralized dentin (Figs. 2C, 2F).
Figure 2.
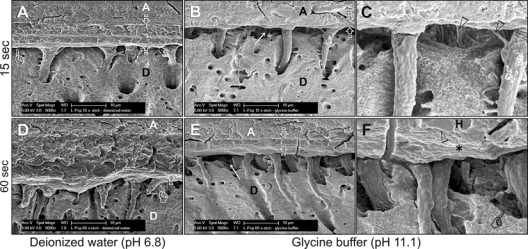
Scanning electron micrographs of Adper Prompt L-Pop-bonded dentin slabs. Generic abbreviations: A, adhesive; D, dentin. (A) A 15-second-etched, water-aged specimen. The 5- to 6-µm-thick hybrid layer (between open arrows) closely approximated the underlying mineralized dentin base (pointer), indicating that no continuous etching occurred beyond the hybrid layer. (B) A 15-second-etched, glycine-buffer-aged specimen. Only the top 1- to 2-µm of the hybrid layer was visible (between open arrowheads). The basal part of the hybrid layer disappeared, creating a gap (arrow) between the remnant hybrid layer and the mineralized dentin base. (C) A high-magnification view of (B). Intact lateral branches of resin tags (open arrowheads) within the hybrid-layer-free zone indicated that the latter was not a dehydration artifact. (D) A 60-second-etched, de-ionized-water-aged specimen with a 10- to 12-µm-thick hybrid layer (between open arrows) that closely approximated the underlying mineralized dentin base (pointer). (E) A 60-second-etched, glycine-buffer-aged specimen. The base of the hybrid layer disappeared (arrow), exposing resin tags that extended into the underlying mineralized dentin. Only the top 2-3 µm of the original hybrid layer remained (between open arrows). (F) A high-magnification view of (E) showing the junction (open arrowhead) between the polished surface (H) and the bottom (asterisk) of the remnant hybrid layer. Pointer: edge of the mineralized dentin base. Collectively, the similar results obtained in the 15- and 60-second-etched specimens indicated that partial disappearance of the hybrid layers could not be attributed to over-aggressive application of the self-etch adhesive. Likewise, the loss of the base of the hybrid layers in the alkaline glycine buffer subgroups indicated that the phenomenon was unlikely to be caused by continuous etching of poorly converted acidic resin monomers.
Transmission electron microscopy of specimens not subjected to biomimetic remineralization revealed hybrid layers that were approximately 5 µm thick for the 15-second (Fig. 3A) and 9 µm thick for the 60-second etching time-periods (Fig. 3D). Specimens that were placed in the biomimetic remineralization medium demonstrated remineralization along the base of the hybrid layer at 3 mos (Figs. 3B, 3E), with almost complete remineralization of the hybrid layers at 6 mos (Figs. 3C, 3F).
Figure 3.
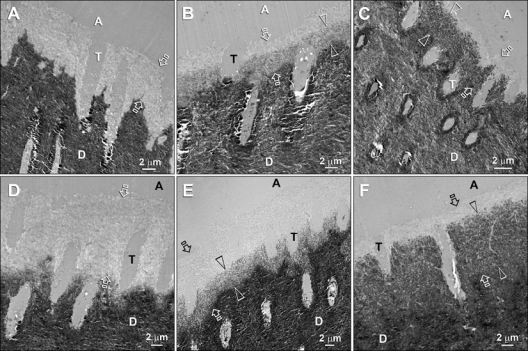
Transmission electron micrographs of unstained, non-demineralized sections of Adper Prompt L-Pop-bonded dentin slabs after biomimetic remineralization. Generic abbreviations: A, adhesive; T, dentinal tubule; D, mineralized intertubular dentin. (A) A 15-second-etched control specimen depicting the resin-dentin interface before remineralization. The aggressive self-etch adhesive created a 4- to 5-µm-thick zone of completely demineralized dentin (between open arrows). (B) A 15-second-etched specimen after 3 mos of remineralization. A 3- to 4-µm-thick remineralized zone (between open arrowheads) could be identified along the base of the original hybrid layer (between open arrows). The top of the original hybrid layer was probably better infiltrated by adhesive resin and did not remineralize because it was free of water-filled voids. (C) A 15-second-etched specimen after 6 mos of remineralization. Only the base (between open arrowheads) of the original hybrid layer (between open arrows) was remineralized. (D) A 60-second-etched control specimen revealing a 9- to 10-µm-thick zone of demineralized dentin. (E) A 60-second-etched specimen after 3 mos of remineralization. Partial remineralization had occurred along the basal 5 µm of the original 10-µm-thick hybrid layer (between open arrows). (F) A 60-second-etched specimen after 6 mos of remineralization. The remineralized base of the hybrid layer was 9 µm thick (between open arrowheads) and was almost as dense as the underlying naturally mineralized dentin. Despite such heavy remineralization in the basal portion, the superficial 2 µm of the original hybrid layer (between open arrows) did not remineralize. Collectively, the basal locations of the remineralized hybrid layers corresponded to the locations where the hybrid layers disappeared after prolonged aging of the specimen slabs in glycine buffer.
Discussion
It is clear that the aggressive all-in-one self-etching adhesive did not continue to etch beyond hybrid layers during the six-week period of aging in de-ionized water. However, similar resin-dentin interfaces that were aged in glycine buffer exhibited severe loss of integrity and increased dye permeability along the base of the hybrid layers. Since the hybrid layers derived from both groups were similar in thickness, we have to accept the first hypothesis, that there are inherent deficiencies in hybrid layers created by the all-in-one adhesive that are not associated with the continuous etching of residual acidic resin monomers. Apparently, those inherent deficiencies were identified only after the resin-dentin interfaces were aged in the alkaline glycine buffer. The resemblance between those inherent deficiencies and the remineralized regions of the hybrid layers led us to acknowledge the second hypothesis, that the biomimetic remineralization strategy is effective in confirming the presence of resin-infiltration-related deficiencies in hybrid layers created by the adhesive.
Continuous etching of highly acidic, unpolymerized acidic resin monomers into the underlying dentin should be accompanied by increases in permeability and thickness of the etched regions beneath the original hybrid layers (Wang and Spencer, 2005). These phenomena could not be confirmed in our water-aged specimens, even when etching times were increased from 15 sec to 60 sec. We attributed our results to the excellent buffering capacity of mineralized dentin. It has been observed (Camps and Pashley, 2000) that the amount of hydronium ions diffusing across dentin was the same regardless of application time of 37% H3PO4. They speculated that precipitation of calcium salts in dentinal tubules limited acid penetration. It has been further demonstrated (Maeda et al., 2008) that when self-etching adhesives were mixed with dentin powder for up to 600 sec, their pH values rapidly increased to above the critical pH (6.0-6.8) of dentin, preventing any further apatite dissolution (Hoppenbrouwers et al., 1986).
The acidic monomer in Adper Prompt L-Pop (di-HEMA phosphate) has a pKa value (1.7) that is comparable with that of H3PO4 (pKa1 = 2.1). Although the adhesive could have contained H3PO4 as impurities, di-HEMA phosphate and H3PO4 are comparably aggressive in terms of etching potential (Grégoire and Ahmed, 2007). However, di-HEMA phosphate exhibited a lower apatite-dissolving capacity than H3PO4 (Salz et al., 2006). This is probably due to the higher molecular weight of the acidic resin monomer di-HEMA phosphate (322 g/mol), giving it a lower molar concentration. Although water is required for ionization of acidic monomers, residual water also lowers their concentration (Hiraishi et al., 2005). This probably resulted in a resin-sparse, less highly polymerized region along the base of the hybrid layers.
Sodium hypochlorite has been used to expedite the degradation of resin-dentin bonds in simulated longevity studies (Yamauti et al., 2003; Toledano et al., 2006). For example, Toledano et al. (2006) reported that tensile bond strengths were reduced by 65-77% after resin-dentin beams were immersed in 10% NaOCl for 5 hrs. Since NaOCl is dissolved in NaOH, it is thought that NaOH is responsible for the reduction in hardness of resin composites (Bagheri et al., 2007). It appears that the NaOH-containing glycine buffer was able to exert a similar, albeit milder, degradation effect (i.e., 6 wks compared with 5 hrs for NaOCl) on the resin-dentin interfaces created by Adper Prompt L-Pop. This explains why the highly dye-permeable basal zone could be observed only in the glycine-buffer-aged groups and not in the de-ionized-water-aged groups. More importantly, these results indicate that there are variations in the quality of the hybrid layers created by the all-in-one adhesive, even when it etched and infiltrated the dentin simultaneously. The quality of the basal portion of these hybrid layers is clearly inferior to the quality of the surface portion. The present study further provided convincing evidence that these defects are not associated with the continuous etching effect of residual acidic resin monomers.
The striking resemblance between the expedited degradation (6 wks) of hybrid layers seen in the glycine-buffered specimens and the subsequent results (3-6 mos) of biomimetic remineralization of the hybrid layers provided further support of the variation in quality of the resin-dentin interfaces created by the aggressive all-in-one adhesive. As implied by our results, it may take a relatively long time (months to years) for these deficiencies to be eventually detected by water-aging, with the presumption that those hybrid layers are of high quality and resistant to acid-base challenge. During this “innocuous period”, the inferior regions of the hybrid layers could become degraded by endogenous collagenolytic enzymes (Pashley et al., 2004; Carrilho et al, 2008) that are retained within the demineralized dentin matrix (Mazzoni et al., 2007) and activated by the buffered acidic resin monomers (Nishitani et al., 2006). It is impossible for a degraded collagen matrix to be remineralized (Xu et al., 2007), even in the presence of dentin non-collagenous proteins or their biomimetic analogs, to the hierarchy of apatite deposition that is found in mineralized hard tissues (Traub et al., 1992). The present biomimetic remineralization scheme represents a proof-of-concept only, since remineralization was achieved via sideways diffusion of amorphous calcium phosphate nanoprecursors, instead of from the top of the resin-bonded dentin. The successful remineralization of those inferior regions within the hybrid layers that are undetectable with a conventional water-aging protocol highlights our urgent need to understand the mechanism of biomimetic remineralization for clinically applicable remineralization delivery systems to be designed. It is important that biomimetic remineralization commence before degradation of the collagen matrix.
In conclusion, the incompletely resin-infiltrated zone in hybrid layers created by an aggressive all-in-one self-etching adhesive was not associated with continuous etching of remnant acidic resin monomers. Degradation of this incompletely resin-infiltrated zone was expedited with the use of an alkaline glycine buffer as the aging medium. This incompletely resin-infiltration zone was amenable to biomimetic remineralization prior to degradation of its collagen matrix.
Acknowledgments
The authors are grateful to Mrs. Michelle Barnes for secretarial support.
Footnotes
This work was supported by grant R21 DE019213 from the National Institute of Dental and Craniofacial Research (F.R. Tay).
References
- Bagheri R, Tyas MJ, Burrow MF. (2007). Comparison of the effect of storage media on hardness and shear punch strength of tooth-colored restorative materials. Am J Dent 20:329-334 [PubMed] [Google Scholar]
- Bradna P, Vrbova R, Dudek M, Roubickova A, Housova D. (2008). Comparison of bonding performance of self-etching and etch-and-rinse adhesives on human dentin using reliability analysis. J Adhes Dent 10:423-429 [PubMed] [Google Scholar]
- Camps J, Pashley DH. (2000). Buffering action of human dentin in vitro. J Adhes Dent 2:39-50 [PubMed] [Google Scholar]
- Carrilho MR, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, et al. (2008). Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. J Biomed Mater Res B Appl Biomater 90:373-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Amaral RC, Stanislawczuk R, Zander-Grande C, Michel MD, Reis A, Loguercio AD. (2009). Active application improves the bonding performance of self-etch adhesives to dentin. J Dent 37:82-90 [DOI] [PubMed] [Google Scholar]
- Grégoire G, Ahmed Y. (2007). Evaluation of the enamel etching capacity of six contemporary self-etching adhesives. J Dent 35:388-397 [DOI] [PubMed] [Google Scholar]
- Hiraishi N, Nishiyama N, Ikemura K, Yau JY, King NM, Tagami J, et al. (2005). Water concentration in self-etching primers affects their aggressiveness and bonding efficacy to dentin. J Dent Res 84:653-658 [DOI] [PubMed] [Google Scholar]
- Hoppenbrouwers PM, Driessens FC, Borggreven JM. (1986). The vulnerability of unexposed human dental roots to demineralization. J Dent Res 65:955-958 [DOI] [PubMed] [Google Scholar]
- Maeda T, Yamaguchi K, Takamizawa T, Rikuta A, Tsubota K, Ando S, et al. (2008). pH changes of self-etching primers mixed with powdered dentine. J Dent 36:606-610 [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, et al. (2007). Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. J Dent Res 86:436-440; erratum in J Dent Res 86:792, 2007 [DOI] [PubMed] [Google Scholar]
- Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, et al. (2006). Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci 114:160-166 [DOI] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. (2004). Collagen degradation by host-derived enzymes during aging. J Dent Res 83:216-221 [DOI] [PubMed] [Google Scholar]
- Peumans M, Kanumilli P, De Munck J, Van Landuyt K, Lambrechts P, Van Meerbeek B. (2005). Clinical effectiveness of contemporary adhesives: a systematic review of current clinical trials. Dent Mater 21:864-881 [DOI] [PubMed] [Google Scholar]
- Reis AF, Bedran-Russo AK, Giannini M, Pereira PN. (2007). Interfacial ultramorphology of single-step adhesives: nanoleakage as a function of time. J Oral Rehabil 34:213-221 [DOI] [PubMed] [Google Scholar]
- Salz U, Mucke A, Zimmermann J, Tay FR, Pashley DH. (2006). pKa value and buffering capacity of acidic monomers commonly used in self-etching primers. J Adhes Dent 8:143-150 [PubMed] [Google Scholar]
- Spencer P, Wang Y, Walker MP, Wieliczka DM, Swafford JR. (2000). Interfacial chemistry of the dentin/adhesive bond. J Dent Res 79: 1458-1463 [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH. (2008). Guided tissue remineralisation of partially demineralised human dentine. Biomaterials 29:1127-1137 [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH. (2009). Biomimetic remineralization of resin-bonded acid-etched dentin. J Dent Res 88:719-724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay FR, Moulding KM, Pashley DH. (1999). Distribution of nanofillers from a simplified-step adhesive in acid-conditioned dentin. J Adhes Dent 1:103-117 [PubMed] [Google Scholar]
- Tay FR, Pashley DH, Yoshiyama M. (2002). Two modes of nanoleakage expression in single-step adhesives. J Dent Res 81:472-476 [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH, Yiu CK, Sanares AM, Wei SH. (2003). Factors contributing to the incompatibility between simplified-step adhesives and chemically-cured or dual-cured composites. Part I. Single-step self-etching adhesive. J Adhes Dent 5:27-40 [PubMed] [Google Scholar]
- Tay FR, Pashley DH, Suh BI, Hiraishi N, Yiu CK. (2005). Water treeing in simplified dentin adhesives—déjà vu? Oper Dent 30:561-579 [PubMed] [Google Scholar]
- Toledano M, Osorio R, Albaladejo A, Aguilera FS, Osorio E. (2006). Differential effect of in vitro degradation on resin-dentin bonds produced by self-etch versus total-etch adhesives. J Biomed Mater Res A 77:128-135 [DOI] [PubMed] [Google Scholar]
- Traub W, Arad T, Weiner S. (1992). Origin of mineral crystal growth in collagen fibrils. Matrix 12:251-255 [DOI] [PubMed] [Google Scholar]
- Xu L, Anderson AL, Lu Q, Wang J. (2007). Role of fibrillar structure of collagenous carrier in bone sialoprotein-mediated matrix mineralization and osteoblast differentiation. Biomaterials 28:750-761 [DOI] [PubMed] [Google Scholar]
- van Dijken JW, Pallesen U. (2008). Long-term dentin retention of etch-and-rinse and self-etch adhesives and a resin-modified glass ionomer cement in non-carious cervical lesions. Dent Mater 24:915-922 [DOI] [PubMed] [Google Scholar]
- Van Landuyt KL, De Munck J, Snauwaert J, Coutinho E, Poitevin A, Yoshida Y, et al. (2005). Monomer-solvent phase separation in one-step self-etch adhesives. J Dent Res 84:183-188 [DOI] [PubMed] [Google Scholar]
- Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, et al. (2003). Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent 28:215-235 [PubMed] [Google Scholar]
- Wang Y, Spencer P. (2005). Continuing etching of an all-in-one adhesive in wet dentin tubules. J Dent Res 84:350-354 [DOI] [PubMed] [Google Scholar]
- Yamauti M, Hashimoto M, Sano H, Ohno H, Carvalho RM, Kaga M, et al. (2003). Degradation of resin-dentin bonds using NaOCl storage. Dent Mater 19:399-405 [DOI] [PubMed] [Google Scholar]


