Abstract
Objective
To examine the associations between income and education and three markers of inflammation: interleukin-6 (IL-6), C-reactive protein (CRP), and fibrinogen. Socioeconomic status is inversely linked with health outcomes, but the biological processes by which social position “gets under the skin” to affect health are poorly understood.
Method
Cross-sectional analyses involved participants (n = 704) from the second wave of the national population-based Survey of Midlife Development in the United States (MIDUS). Data on pretax household-adjusted income and educational attainment were collected by questionnaire and telephone interview, respectively. Detailed medical history interviews, inventories of medication, and fasting blood samples for assessment of inflammatory proteins were obtained during an overnight clinic stay.
Results
All three inflammatory proteins were inversely associated with both income and education in bivariate analyses. However, multivariate regression models, adjusting for potential confounds, showed that only low income predicted higher levels of inflammatory proteins. Moreover, inclusion of IL-6 in the regression models for CRP and fibrinogen eliminated the associations with income.
Conclusion
These results suggest that income explains the association between education and peripheral inflammation. In short, the reason that higher education is linked to reduced peripheral inflammation is because it reduces the risk for low income status, which is what is directly associated with reduced peripheral inflammation. The findings also suggest that the links between income and both CRP and fibrinogen are mediated by IL-6. These observations help to sharpen our understanding of the relationship between social position and biological markers of illness in the United States.
Keywords: inflammation, income, education
INTRODUCTION
In the United States, lacking a high school degree or living below the poverty line is associated with up to 12 fewer years of life and up to 16 fewer years of life without disease or disability (1,2). But although strong positive associations between socioeconomic status (SES) and health are well documented (3,4), the biological processes that link social position to health outcomes are poorly understood. How do poverty and low educational attainment “get under the skin”? This study employs a nationally representative sample to test the links between income, education, and three key inflammatory markers linked to a range of morbidity and mortality outcomes.
If we want to understand how SES gets under the skin, inflammatory markers are a logical place to start. Inflammatory proteins are strongly associated with cardiovascular disease, which kills nearly one in three Americans, and cardiovascular disease is strongly patterned by both educational attainment and income (5). Serum levels of interleukin (IL)-6, a proinflammatory cytokine, the acute phase C-reactive protein (CRP), and the clotting factor fibrinogen all predict increased risk of later cardiovascular events in healthy individuals as well as increased risk of mortality in patients with a history of cardiovascular disease (6–13). There is a sufficiently strong link between CRP and later cardiovascular disease (14), especially in women (10), that limited clinical screening for CRP levels is now recommended (15). Importantly, flammatory markers independently predict cardiovascular disease above and beyond traditional risk factors.
Both education and income are also strongly associated with cardiovascular disease. SES predicts risk factors for heart disease, such as obesity, limited physical activity, and smoking (5). But it is also more directly associated with heart disease. Even after controlling for risk factors ranging from smoking and obesity to high blood pressure and cholesterol levels, SES remains a strong predictor of cardiovascular mortality (16).
Inflammation has been linked to SES in a number of clinical and population-level studies and in multiple countries. Results from National Health and Nutrition Examination Survey (17), the Multi-Ethnic Study of Atherosclerosis (18), the Heart and Soul Study (19), the Health, Aging, and Body Composition Study (20), and study of Coronary Artery Risk Development in Adolescents (21) have all shown that lower levels of education and/or income are associated greater burden of inflammation. Studies in Finland (22), Greece (23), and Israel (24) have yielded similar conclusions, whereas results from the Whitehall II study in the United Kingdom have linked higher levels of inflammation with lower occupational status (25).
Although important for documenting associations between SES and inflammation, these prior studies share three significant limitations. First, they often treat different SES indicators as interchangeable by only testing education, income, or occupational status. There is growing evidence, however, that different SES indicators, particularly education and income, may have different associations with health (26,27). For example, the relationship between income and health is generally nonlinear in the United States, with the bulk of the relationship concentrated at the bottom of the income distribution (28–36). In contrast, the relationship between schooling and health is generally linear and, in some cases, there are greater differences between college graduates and high school graduates as compared with differences between high school graduates and high school dropouts (37,38). Second, because income and education may be differentially related to health, it is important to determine whether they both predict biological markers of disease, such as inflammation. Whereas some studies have found, for example, that income and education are independently associated with health (27,39), only one study has examined their shared or independent associations with inflammation; lower education predicted higher CRP levels independent of income in a sample of patients with heart disease (19). Determining whether education, income, or both predict inflammation will provide insight into the processes by which social position affects health and, thereby, potential opportunities for intercession. Proximate factors associated with income, for example, may be stronger predictors of inflammation than more distal factors related to education.
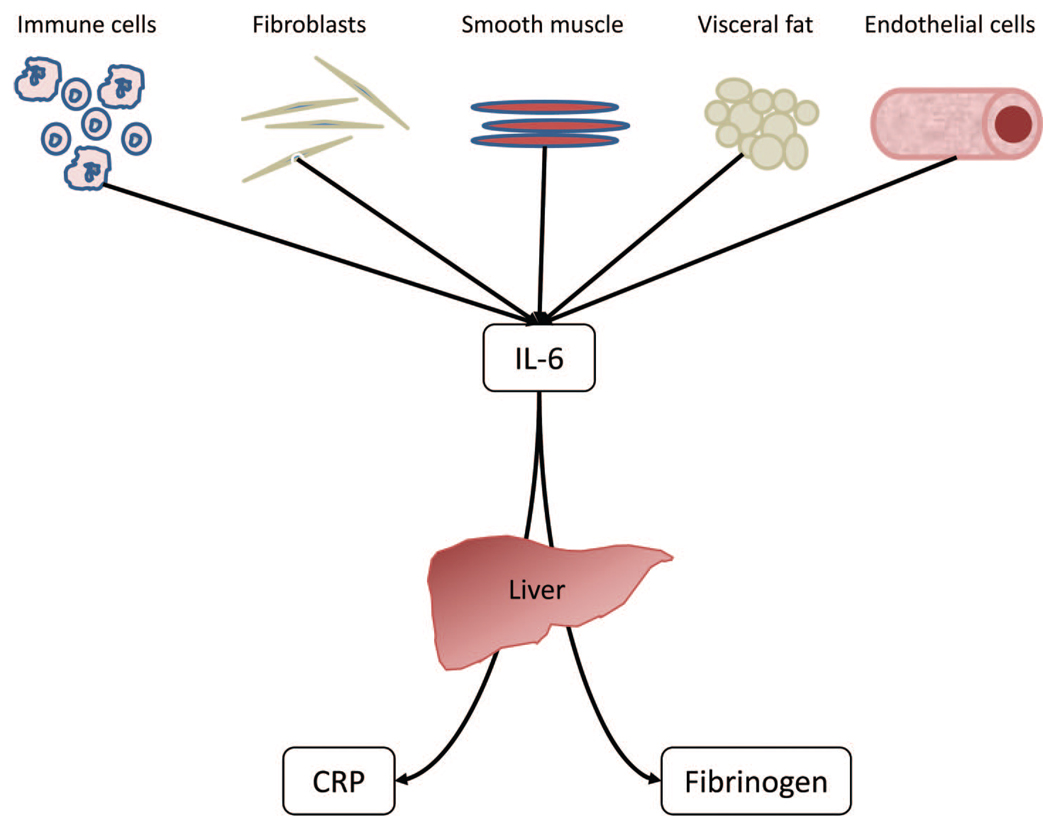
Finally, studies of SES and inflammation rarely account for the close biological relationships among inflammatory proteins (Fig. 1). Because IL-6 is one of the most potent drivers of hepatocyte production of both CRP and fibrinogen (40–43), serum levels of CRP and fibrinogen may increase with declining SES merely because SES is linked to IL-6 and not because of independent associations with SES. There is evidence IL-6 may have a stronger association with some health outcomes than do CRP and fibrinogen. For example, despite the relative clinical popularity of CRP, IL-6 seems to have a more robust association with age-related cardiovascular disease than CRP or fibrinogen (44). If IL-6 does prove to have a unique role in the SES-inflammation relationship, future research on social stratification of health might focus on the factors that modulate IL-6 levels specifically, as opposed to CRP and fibrinogen.
Figure 1.
Biological relationships among interleukin (IL)-6, C-reactive protein (CRP), and fibrinogen. Serum IL-6 has a number of biological sources, most notably adipocytes, which account for as much as 30% of circulating IL-6 levels (71) and immunocompetent cells. CRP and fibrinogen are principally synthesized in and released from the liver (72,73), and IL-6 is one of the most potent stimulators of their production.
To sharpen our understanding of how inflammatory conditions arise in the socioeconomically disadvantaged as well as the potential biological pathways involved, the current study focuses on two issues. First, we examine the extent to which income and education are uniquely associated with inflammation. Second, because IL-6 is a potent stimulator of CRP and fibrinogen synthesis and release (Fig. 1), we tested the hypothesis that IL-6 would mediate associations between SES and both CRP and fibrinogen.
We examined these issues, using data from the Survey of Midlife Development in the United States (MIDUS) (45). Existing population-based studies, such as National Health and Nutrition Examination Survey, typically involve a single inflammatory marker (17,19), although studies that have included more than one marker often have nonrepresentative samples (19,20). MIDUS comprises a national sample of 35- to 84-year-old men and women and assessments of multiple indices of inflammation, making it suitable for a population-based examination of shared and unique associations between different SES measures and multiple inflammatory proteins.
METHODS
Participants
The MIDUS study consists, in part, of telephone and mail surveys that were conducted in 1995 to 1996 and 2004 to 2006 on a national random digit dial sample of noninstitutionalized adults living in the 48 contiguous states. A subsample of MIDUS respondents, who had completed the second wave of surveys (MIDUS 2), also completed detailed clinic-based assessments of health, disease-related biomarkers, and physiologic function (“biomarker sample”). With the exception of two MIDUS subsamples—siblings of respondents and inner city oversamples—all MIDUS 2 respondents who had completed the telephone and mail surveys were eligible for the biomarker sample, and they were recruited by letter and with a follow-up telephone call. An additional sample of African Americans from Milwaukee, Wisconsin was recruited during the second wave of MIDUS data collection, and individuals who completed in-home interviews and self-administered questionnaires were eligible for participation in the biomarker study.
Three regional General Clinical Research Centers (GCRCs) participated in the MIDUS biomarker study—one on the West Coast, one in the Midwest, and one on the East Coast—and participants were invited to stay overnight at whichever GCRC imposed the least travel burden. On arrival at the GCRC, each respondent provided a detailed medical history interview with a GCRC clinician and completed a set of self-administered questionnaires. Participants were also asked to bring all current medications to the GCRC, and these were inventoried by project staff. Fasting blood samples were obtained the next morning. Serum was isolated from all samples, aliquoted, and frozen at −80°C, shipped on dry ice to the appropriate laboratory, and stored at −80°C for assay.
The MIDUS biomarker sample includes a subsample of twin pairs, but concern that genetic relatedness might artificially inflate the associations of interest led to the exclusion of these participants. Current analyses are based on data from nontwin respondents who had completed biomarker assessments as of July 2008 (n = 721); biomarker data collection is ongoing. Finally, values of CRP >10 pg/mL are often indicative of acute infection, and excluding such data are recommended (46). Thirty-six (4.8% of the sample) participants had CRP values >10 mg/L and their data were excluded from analyses, leaving a final sample of 704 participants. Compared with MIDUS 2 respondents who did not complete the biomarker study (n = 3237), the study sample had greater educational attainment (5.8% had less than a high school education compared with 6.4% for MIDUS 2 respondents, p < .05). On other measures relevant to these analyses that were assessed in both samples—age, pretax household income, gender, marital status, and number of chronic health problems—the two samples were statistically comparable.
Collection of data for MIDUS and analysis of those data for the current study were both approved by the Health Sciences Institutional Review Board at the University of Wisconsin-Madison.
Education
As part of the telephone interview, participants were asked their highest level of educational attainment. Responses were grouped into 12 categories ranging from “no school/some grade school” (category 1) to “PhD, MD, JD, or other professional degree” (category 12). This 12-category variable was used to examine linear associations between education and inflammatory proteins. To examine nonlinear associations, we created a set of dummy-coded variables: less than a high school education, graduated high school or obtained GED, and graduated from a 4-year college. Those with some college education, but no degree, or with a degree from a 2-year or vocational institution were grouped with high school graduates. College graduate status served as the reference category in multivariate analyses.
Income
Information on pretax household income from wages, pensions, social security, and government assistance was obtained from mail surveys. Income was adjusted for household size by dividing by the square root of the number of individuals in the household. Income was treated as a continuous variable in analyses examining linear associations between income and inflammatory proteins. However, results from earlier studies have shown that low income groups, but not moderate income groups, had significantly higher levels of inflammatory markers compared with high income groups (17,19). To allow for such nonlinear associations, we also stratified the sample into income quintiles. The income ranges that corresponded to each quintile were: Q1: ≤$17,838; Q2: $17,839–$35,037; Q3: $35,038–$50,161; Q4: $50,162–$76,809; Q5: ≥$76,810. Each quintile was dummy-coded and included in statistical analyses with the top quintile serving as the referent.
Inflammatory Markers
Serum IL-6 was measured, using high-sensitivity enzyme-linked immunosor-bent assay according to manufacturer guidelines (R&D Systems, Minneapolis, MN). CRP was measured, using a particle enhanced immunonepholometric assay (BNII nephelometer, Dade Behring Inc., Deerfield, IL). Fibrinogen was measured, using a semiautomated modification of the Clauss method (Clauss, 1957) on a BNII nephelometer (Dade Behring). Assays for IL-6 were completed in the laboratory of Dr. Christopher Coe at the University of Wisconsin-Madison, and those for CRP and fibrinogen were completed in the laboratory of Dr. Russell Tracy at the University of Vermont. The laboratory intra- and inter-assay coefficients of variance for all protein assays were in acceptable ranges (<10%). Distributions for IL-6 and CRP values were positively skewed and were log-transformed for statistical analyses.
Covariates
In addition to age, gender, marital status, and race, analyses controlled for the potential confounding influences of health status and health behavior.
Health Status
Health status was assessed, using both self-reported and objective measures. Participants completed questionnaires, assessing whether they had received a physician’s diagnosis for any of 20 chronic conditions. From these responses, an index of chronic disease burden was constructed that consisted of the number of diseases specifically linked to inflammation (autoimmune disorders, cardiovascular and cerebrovascular disease, hypertension, arthritis, asthma, diabetes, gastrointestinal diseases, liver disease, and cancer). Height and weight were measured by GCRC staff and used to calculate body mass index (BMI) (weight in kilograms divided by the square of height in meters). A continuous measure of BMI was included in all analyses; values were log-transformed to normalize the distribution. Finally, antihypertensive (47,48), cholesterol-lowering (49), and antidepressant (50) medications have all been shown to have anti-inflammatory properties, and steroid medications, particularly as part of a hormone replacement regimen, have been shown to increase CRP levels (51,52). Dummy-coded variables indicating use of these medications were included in all analyses.
Health Behavior
Health behavior indicators are based on self-reported information from questionnaires completed at the GCRC. Variables for cigarette smoking (never smoked, former smoker, and current smoker), the number of drinks consumed during a typical week, number of minutes engaging in vigorous or moderate exercise per week, and number of caffeinated beverages consumed per day were included in all models.
Statistical Analyses
Bivariate analyses examined associations among continuous SES indicators, inflammatory markers, and covariates. A continuous measure of income was then used in multivariate regression analyses to estimate linear associations between income and inflammation in both adjusted and unadjusted models. As women tend to have higher circulating levels of some inflammatory proteins than men (53), and socioeconomic gradients in some aspects of health, such as BMI, are more robust in women than in men (54), we included interaction terms in each model to determine whether income-inflammation association differed by gender. Nonlinear associations of SES and inflammation were then estimated in multivariate models, using dummy-coded education categories and pretax household income quintiles. Income and education were modeled independently and then in combination to determine the extent to which their associations with inflammation were independent. Demographic data, health status variables, and health behavior variables were entered in successive steps. Finally, to test for IL-6 mediation, serum IL-6 level was entered at the final step of CRP and fibrinogen multivariate analyses. The threshold for identifying statistically significant associations was set at α = 0.05.
RESULTS
Descriptive statistics for the biomarker sample are shown in Table 1. It is worth noting that the largest proportion of the sample (41.4%) was classified as obese (BMI above 30). Descriptive statistics for the inflammatory proteins stratified by educational attainment and pre-tax household income are shown in Table 2.
TABLE 1.
Descriptive Statistics for Biomarker Sample (n = 704)
| Variable | Mean (SD) | Range | % |
|---|---|---|---|
| Socioeconomic measures |
|||
| Education | |||
| <HS | 5.8 | ||
| HS graduate or GED | 72.2 | ||
| College graduate | 21.8 | ||
| Pretax household income ($; median) |
43,501 (39,535) | 0–212, 132 | |
| Demographics | |||
| Age | 57.9 (11.6) | 35–86 | |
| Gender (% female) | 53.6 | ||
| Race/ethnicity (% non-white) |
25.1 | ||
| Health status | |||
| BMI | |||
| Normal weight | 22.6 | ||
| (<24.99) | |||
| Overweight | 36.0 | ||
| (25–29.99) | |||
| Obese (>30) | 41.4 | ||
| Number of chronic conditions |
2.8 (2.1) | 0–13 | |
| Medications (% yes) | |||
| Antihypertensive | 37.2 | ||
| Cholesterol lowering | 27.5 | ||
| Steroids (including estrogen therapy) |
12.2 | ||
| Antidepressants | 13.1 | ||
| Health behavior | |||
| Smoking status | |||
| Never smoked | 48.2 | ||
| Former smoker | 36.2 | ||
| Current smoker | 15.6 | ||
| Caffeine consumption (drinks per day) |
3.0 (3.2) | 0 to 30 | |
| Alcohol (drinks/wk) | 3.3 (5.7) | 0 to 46 | |
| Vigorous/moderate exercise (mins/wk) |
322.6 (609.8) | 0 to 5,040 |
SD = standard deviation; HS = high school; GED = General Educational Development test; BMI = body mass index.
TABLE 2.
Geometric Means (and IQRs) for IL-6, CRP, and Fibrinogen at Different Levels of Educational Attainment and Income Quintiles
| IL-6 (pg/mL) | CRP (µg/mL) | Fibrinogen (mg/dL) | ||||
|---|---|---|---|---|---|---|
| Geometric Mean |
(IQR) | Geometric Mean |
(IQR) | Geometric Mean |
(IQR) | |
| Educational attainment | ||||||
| <HS | 2.7 | (1.3, 5.0) | 1.8 | (0.9, 3.9) | 350.1 | (285.0, 428.0) |
| HS graduate or GED | 2.2 | (1.4, 3.7) | 1.4 | (0.7, 3.5) | 325.5 | (285.3, 396.0) |
| College graduate | 2.0 | (1.3, 2.9) | 1.3 | (0.6, 2.6) | 321.2 | (277.0, 370.5) |
| Pretax household-adjusted income | ||||||
| Lowest quintile | 3.1 | (1.9, 5.1) | 1.9 | (0.9, 4.7) | 353.9 | (304.5, 426.5) |
| Q2 | 2.3 | (1.3, 3.7) | 1.3 | (0.6, 3.2) | 326.6 | (276.0, 393.0) |
| Q3 | 2.1 | (1.3, 3.3) | 1.4 | (0.7, 3.3) | 327.4 | (278.0, 387.5) |
| Q4 | 2.0 | (1.2, 3.2) | 1.4 | (0.7, 3.1) | 314.0 | (271.3, 376.8) |
| Highest quintile | 1.7 | (1.1, 2.5) | 1.1 | (0.6, 2.0) | 313.7 | (282.3, 368.3) |
IQRs = interquartile ranges; IL = interleukin; CRP = C-reactive protein; HS = high school; GED = General Educational Development test.
As expected, income and education were significantly correlated in bivariate analyses (r = 0.37, P < .001). Also as expected, IL-6 was significantly correlated with both CRP (r = 0.50, P < .001) and fibrinogen (r = 0.46, P < .001), and CRP and fibrinogen were also correlated (r = 0.57, P < .001). Table 3 shows additional correlational relationships between the SES indicators and demographic, health status, and health behavior covariates.
TABLE 3.
Zero-Order Correlations Between SES Indicators and Covariates
| Educational Attainment |
Pretax Household- Adjusted Income |
|
|---|---|---|
| Demographics | ||
| Age | 0.01 | 0.36** |
| Gender (1 = female) | −0.09* | −0.09* |
| Race (1 = African American) | −0.21** | −0.32** |
| Marital status (1 = married) | 0.12** | 0.32** |
| Health Status | ||
| BMI | −0.11** | −0.04 |
| Chronic conditions | −0.05 | −0.17** |
| Medications (1 = yes) | ||
| Antihypertensive | 0.11** | 0.13** |
| Cholesterol-lowering | −0.03 | −0.06 |
| Steroids | 0.07 | 0.03 |
| Antidepressants | −0.09* | 0.02 |
| Health behavior | ||
| Smoking | −0.24** | −0.13** |
| Alcohol (drinks/wk) | 0.04 | 0.04 |
| Caffeine (drinks/day) | −0.06 | 0.05 |
| Exercise (mins/wk) | −0.10* | −0.01 |
p < .05;
p < .01.
Pearson r values shown.
SES = socioeconomic status; BMI = body mass index.
Multivariate regression analyses using a continuous measures of pre-tax household income showed that income was significantly negatively associated with IL-6 in the unadjusted model (β = −0.21, P < .001), and while inclusion of demographic, health status, and health behavior variables in the model reduced the magnitude of this association, it remained statistically significant (β = −0.08, P < .05). In contrast, while CRP and fibrinogen were significantly negatively associated with income in unadjusted models (CRP: β = −0.11, P < .01; fibrinogen: β = −0.12, P < .01), inclusion of covariates reduced these associations to marginal (CRP: β = −0.07, P = .09) or non-significance (fibrinogen: β = −0.03, P = .58). Finally, interaction terms were included in all models to test for differential associations by gender, but none of these was statistically significant (data not shown).
To examine non-linear associations between SES indicators and inflammation, we estimated regression models using dummy-coded variables for educational attainment and income. Table 4 shows the multivariate analysis regressing IL-6 on education and income. As shown in Model 1, those with less than a high school degree (β = 0.10, P < .05) had significantly higher levels of IL-6 than college graduates, although when education and income were included in the same model, the associations between education and IL-6 were eliminated (Model 3). Dummy-coded income categories were included in Model 2, and compared to those in the highest income quintile (the reference category) those in each of the lower income strata had significantly higher levels of IL-6 (Q1: β = 0.30, P < .001; Q2: β = 0.16, P < .001; Q3: β = .11, P < .05). These associations were essentially unchanged after inclusion of education in the model (Model 3). However, after adjusting for age, gender, and race (Model 4), and after the inclusion of health status and health behavior variables (Models 5 and 6), IL-6 remained significantly elevated only in the lowest income quintile.
TABLE 4.
Serum IL-6 Regressed on Educational Attainment and Pretax household Income
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|---|---|---|---|---|---|---|
| Educational attainment | ||||||
| <High school | 0.10* | 0.03 | 0.01 | 0.01 | −0.01 | |
| HS graduate or GED | 0.07 | 0.02 | 0.03 | 0.03 | 0.01 | |
| College graduate | Ref. | Ref. | Ref. | Ref. | Ref. | |
| Pretax household-adjusted income (quintiles) | ||||||
| Q1 (lowest) | 0.30*** | 0.30*** | 0.18*** | 0.16** | 0.17** | |
| Q2 | 0.16*** | 0.16** | 0.08 | 0.06 | 0.05 | |
| Q3 | 0.11* | 0.10* | 0.06 | 0.03 | 0.03 | |
| Q4 | 0.08 | 0.08 | 0.06 | 0.04 | 0.02 | |
| Q5 | Ref. | Ref. | Ref. | Ref. | Ref. | |
| Demographic characteristics | ||||||
| Gender (1 = female) | 0.05 | 0.04 | 0.04 | |||
| Age | 0.23*** | 0.17*** | 0.17*** | |||
| Race/ethnicity (1 = non-white) | 0.15*** | 0.09* | 0.07 | |||
| Marital status (1 = married) | −0.06 | −0.06 | −0.04 | |||
| Health status and health behaviors | ||||||
| BMI | 0.26** | 0.25*** | ||||
| Chronic conditions | 0.13** | 0.11* | ||||
| Prescription medications (1 = yes) | ||||||
| Antihypertensive | −0.03 | −0.03 | ||||
| Cholesterol lowering | 0.03 | 0.02 | ||||
| Steroids | 0.02 | 0.01 | ||||
| Antidepressants | −0.08* | −0.06 | ||||
| Smoking status | 0.06 | |||||
| Caffeine consumption | 0.03 | |||||
| Exercise (mins/wk) | −0.10*** | |||||
| Alcohol consumption | 0.02 | |||||
| Adjusted R2 for model | .02*** | .06*** | .06*** | .12*** | .21*** | .23*** |
p < .05;
p < .01;
p < .001.
Standardized regression coefficients are shown.
HS = high school; GED = General Educational Development test; BMI = body mass index.
Regression models for CRP (Table 5) showed very similar patterns of association with SES indicators. CRP was significantly higher in those who didn’t complete high school (β = 0.09, P < .05) and those with high school (or equivalent) degrees (β = 0.10, P < .01) compared to college graduates (Model 1), but these relationships were reduced to statistical non-significance after the inclusion of income in Model 3. In contrast, CRP was significantly elevated in the lowest income quintile compared to the highest quintile (Model 2; β = 0.19, P < .001), and this association remained statistically significant after adjustments for education (Model 3; β = 0.16, P < .01), demographic characteristics (Model 4; β = 0.14, P < .05), health status (Model 5; β = 0.14, P < .01), and health behaviors (Model 6; β = 0.14, P < .01).
TABLE 5.
Serum CRP Regressed on Educational Attainment and Pretax Household Income
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | |
|---|---|---|---|---|---|---|---|
| Educational attainment | |||||||
| <High school | 0.09* | 0.05 | 0.04 | 0.02 | 0.01 | 0.01 | |
| HS graduate or GED | 0.10** | 0.06 | 0.04 | 0.01 | −0.02 | −0.02 | |
| College graduate | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |
| Pretax household income (quintiles) | |||||||
| Q1 (lowest) | 0.19*** | 0.16** | 0.14* | 0.14** | 0.14** | 0.09 | |
| Q2 | 0.08 | 0.05 | 0.04 | 0.04 | 0.03 | 0.02 | |
| Q3 | 0.10* | 0.07 | 0.08 | 0.06 | 0.06 | 0.05 | |
| Q4 | 0.10* | 0.07 | 0.06 | 0.05 | 0.04 | 0.03 | |
| Q5 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |
| Demographic characteristics | |||||||
| Gender (1 = female) | 0.15*** | 0.11** | 0.12** | 0.11** | |||
| Age | 0.04 | −0.04 | −0.04 | −0.09* | |||
| Race/ethnicity (1 = non-white) | 0.09* | −0.01 | −0.02 | −0.04 | |||
| Marital status (1 = married) | 0.07 | 0.07 | 0.08* | 0.10* | |||
| Health status and health behaviors | |||||||
| BMI | 0.37*** | 0.35*** | 0.27*** | ||||
| Chronic conditions | 0.14** | 0.12** | 0.09* | ||||
| Prescription medications (1 = yes) | |||||||
| Antihypertensive | −0.05 | −0.06 | −0.05 | ||||
| Cholesterol lowering | 0.10* | 0.09* | 0.08* | ||||
| Steroids | −0.10** | −0.11** | −0.11*** | ||||
| Antidepressants | 0.01 | 0.02 | 0.04 | ||||
| Smoking status | 0.04 | 0.02 | |||||
| Caffeine consumption | 0.06 | 0.05 | |||||
| Exercise (mins/wk) | −0.10** | −0.05 | |||||
| Alcohol consumption | −0.01 | −0.02 | |||||
| Serum IL-6 | 0.34*** | ||||||
| Adjusted R2 for model | .01** | .02** | .02** | .04*** | .21*** | .22*** | .31*** |
p < .05;
p < .01;
p < .001.
Standardized regression coefficients are shown.
CRP = C-reactive protein; HS = high school; GED = General Educational Development test; BMI = body mass index; IL = interleukin.
Models for fibrinogen are shown in Table 6. Like the results for IL-6 and CRP, fibrinogen levels were higher in those with less than a high school education (β = 0.09, P < .05) and in high school graduates (β = 0.09, P < .05) compared to those with college degrees (Model 1), but these associations were statistically non-significant after adjustment for income (Model 3). However, fibrinogen levels were higher in those who fell in the lowest income quintile in the unadjusted model (β = 0.19, P < .001; Model 2) as well as the model that adjusted for education (β = 0.18, P < .001; Model 3). Adjustments for demographic , health status , and health behavior covariates reduced this association to statistical non-significance.
TABLE 6.
Serum Fibrinogen Regressed on Educational Attainment and Pretax Household Income
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | |
|---|---|---|---|---|---|---|---|
| Educational attainment | |||||||
| <High school | 0.09* | 0.05 | 0.02 | 0.02 | 0.02 | 0.02 | |
| HS graduate or GED | 0.08* | 0.04 | 0.02 | 0.01 | 0.01 | −0.01 | |
| College graduate | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |
| Pretax household income (quintiles) | |||||||
| Q1 (lowest) | 0.19*** | 0.18*** | 0.06 | 0.06 | 0.07 | 0.03 | |
| Q2 | 0.07 | 0.06 | −0.01 | −0.01 | −0.01 | −0.03 | |
| Q3 | 0.07 | 0.07 | 0.03 | 0.03 | 0.03 | 0.02 | |
| Q4 | 0.02 | 0.02 | −0.02 | −0.03 | −0.04 | −0.04 | |
| Q5 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |
| Demographic characteristics | |||||||
| Gender (1 = female) | 0.15*** | 0.17*** | 0.17*** | 0.16*** | |||
| Age | 0.15*** | 0.11* | 0.10* | 0.06 | |||
| Race/ethnicity (1 = African American) | 0.20*** | 0.14** | 0.14** | 0.12** | |||
| Marital status | 0.02 | 0.02 | 0.02 | 0.03 | |||
| Health status and health behaviors | |||||||
| BMI | 0.24*** | 0.22*** | 0.15*** | ||||
| Chronic conditions | 0.11* | 0.10* | 0.07 | ||||
| Prescription medications (1 = yes) | |||||||
| Antihypertensive | 0.05 | 0.04 | 0.05 | ||||
| Cholesterol lowering | −0.01 | −0.01 | −0.02 | ||||
| Steroids | 0.09* | 0.08* | 0.08* | ||||
| Antidepressants | −0.02 | −0.01 | 0.01 | ||||
| Smoking status | 0.01 | −0.01 | |||||
| Caffeine consumption | 0.06 | 0.05 | |||||
| Exercise (min/wk) | −0.06 | −0.02 | |||||
| Alcohol consumption | −0.06 | −0.07 | |||||
| Serum IL-6 | 0.29*** | ||||||
| Adjusted R2 for model | .01* | .02** | .02** | .08*** | .15*** | .16*** | .22*** |
p < .05;
p < .01;
p < .001.
Standardized regression coefficients are shown.
HS = high school; GED = General Educational Development test; BMI = body mass index; IL = interleukin.
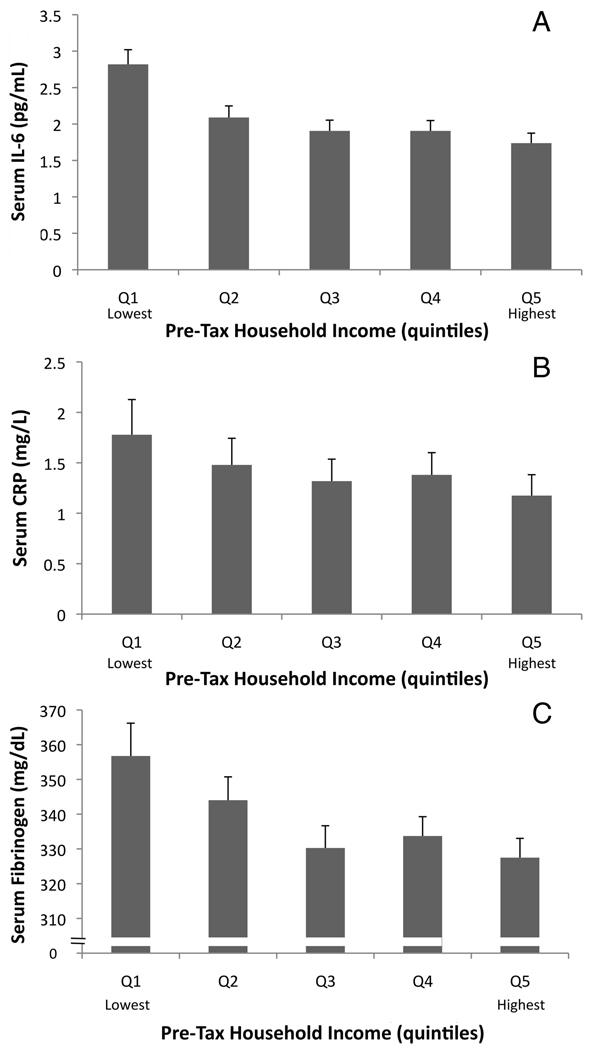
The adjusted associations between income and all three inflammatory proteins are shown in Figure 2.
Figure 2.
Mean (±SE) serum interleukin (IL)-6 (A), serum C-reactive protein (CRP) (B), and serum fibrinogen (C) at each quintile of income. Adjusted means from multivariate analyses are shown. Logged mean values for IL-6 and CRP were back-transformed to facilitate interpretation of results. Results from multivariate regression models showed that the lowest income quintile had levels of IL-6 and CRP that were significantly higher than other quintiles; no other quintile differed significantly from the rest. Levels of fibrinogen in the lowest income quintile were significantly higher than other quintiles in unadjusted analyses, but not in the full regression model.
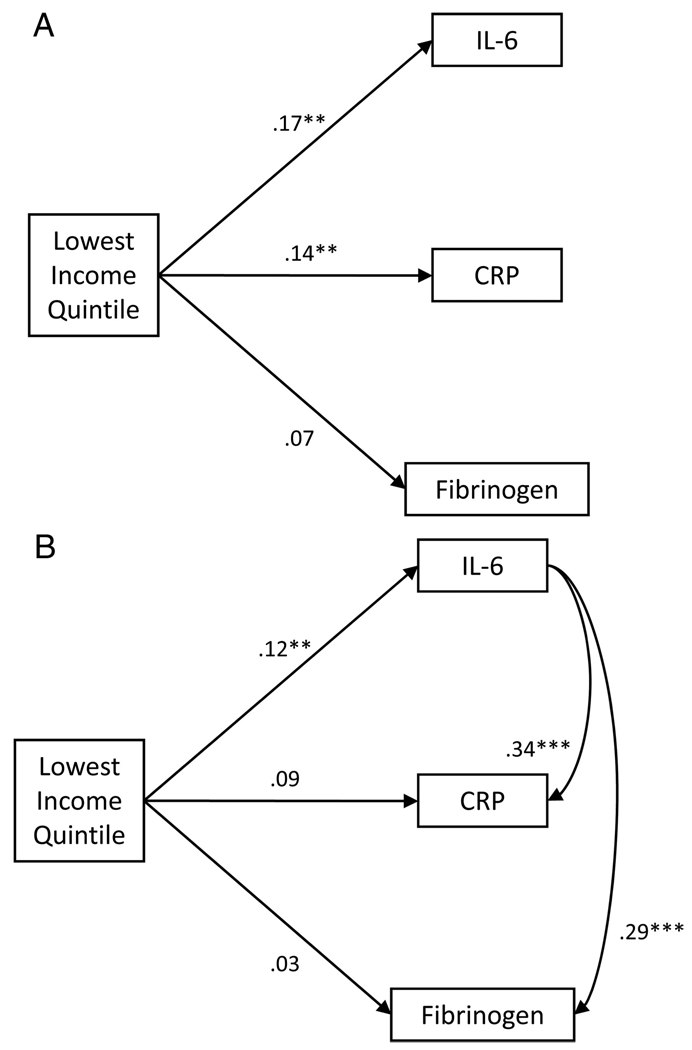
Finally, we tested the hypothesis that IL-6 would mediate associations between income and both CRP and fibrinogen. For mediation to exist, the predictor (income) must be associated with both the outcome variable (CRP or fibrinogen) and the intervening variable (IL-6). The intervening variable must also be associated with the outcome variable, and inclusion of the intervening variable in outcome variable model should attenuate the association between predictor and outcome (55). All of these requirements are met in the current analyses. In adjusted regression models low income significantly predicted all 3 proteins as noted above. Moreover, in bivariate analyses and as seen in Table 3 and Table 4, IL-6 was significantly associated with both CRP and fibrinogen . Finally, after inclusion of IL-6 in the full model, the association of low income and CRP was only marginally significant (β = 0.09; P = .06; Table 5, Model 7). For fibrinogen, inclusion of IL-6 in the fully adjusted model reduced the coefficient for low income to non-significance (β = 0.03, P = .60; Table 6, Model 7). This mediation by IL-6 is shown graphically in Figure 3.
Figure 3.
Graphic representation of meditational analyses. A) Partial correlations of low income status and inflammatory proteins after adjusting for demographic, health status, and health behavior characteristics. B) Partial correlations of low income status and inflammatory proteins after adjusting for demographic, health status, and health behavior characteristics and for associations among inflammatory proteins. The link between low income and interleukin (IL)-6 remained statistically significant in multivariate models that adjusted for C-reactive protein (CRP) and fibrinogen (data not shown), but the association between low income and CRP was reduced to statistical nonsignificance (and the association with fibrinogen reduced by >50%) after inclusion of IL-6 in those respective models (Table 5 and Table 6).
DISCUSSION
This study had two principal goals. The first was to examine the unique associations of income and education with inflammatory markers. In analyses using dummy-coded education and income categories, we found that educational attainment was negatively associated with all three inflammatory proteins in unadjusted regression models, but these associations were consistently eliminated after adjustment for income. In contrast, those in the lowest quintile of pretax household-adjusted income had significantly higher levels of IL-6, CRP, and fibrinogen in unadjusted models and in models adjusting for educational attainment, and with the exception of fibrinogen, these associations remained statistically significant after adjustments for demographic characteristics, health status, and health behaviors. It is worth noting, however, that the sizes of the coefficients for low income were substantially reduced in the full models compared with the unadjusted models, suggesting that characteristics, such as age, gender, race, BMI, chronic illness, and exercise, explain some of the association between income and inflammation. These results did conflict with a recent study of heart disease patients where income and education were both negatively related to CRP levels, even after adjustments for the other SES indicator (19). The most likely explanation of this discrepancy is the nature of the two samples. Although the current study focused on a community-dwelling national probability sample with heterogeneous clinical status, the earlier study involved a clinical sample. The clinical sample likely had different results because low education is associated with poorer adherence to treatment (56); thus, clinical disease status may amplify the relationship between education and inflammation. Nonetheless, this is one of the first studies to focus specifically on the extent to which links between SES indicators and inflammation are unique or shared among indicators, and our results suggest that the relationship between education and inflammation is completely explained by income.
Our analyses included both continuous and dummy-coded measures of SES, and these measures yielded different results. Inflammation was negatively related to a continuous measure of income in unadjusted models, but with the exception of IL-6, adjustment for covariates eliminated these associations. In contrast, those in the lowest income quintile had higher levels of IL-6 and CRP than those in the remaining four quintiles, even after adjustment for covariates. These results suggest that the relationship between inflammation and income is best characterized as nonlinear, with the bulk of the linear association being captured by higher levels of IL-6 in the lowest income quintile; this association was most likely masked in the analyses with the continuous income measure. This result is consistent with prior research, showing that higher inflammation (17) and adverse health in general (28–38,57) tends to cluster at the low end of the income distribution rather than being distributed linearly.
What might explain the link between income and inflammatory markers? One possibility is that there are higher rates of illness and health-damaging behaviors among the poor that are responsible for higher levels of inflammation. There is ample evidence that low SES is associated with greater illness (3,4), more obesity (in developed countries like the United States) (58), and higher rates of smoking and alcohol consumption (3,59)—all of which are linked to greater inflammation. Rates of infectious illness are also higher at lower levels of the SES distribution (60,61). We attempted to account for many of these potential confounds both by excluding participants with levels of CRP >10 pg/mL (a potential indicator of acute infection) (46) and through inclusion of control variables in our regression models, however, and although the coefficient for low income predicting IL-6, in particular, was reduced in adjusted models, the associations between low income and inflammation remained statistically significant. This was true in spite of robust associations between some covariates, such as BMI, and each inflammatory factor. These results echo those of prior studies (17,19,20) and support the conclusion that income-inflammation relationships are not completely explained by differences in health or health behavior across income strata.
A second possibility is that income may be linked to the kinds of instrumental and psychosocial resources that drive (and can be driven by) biological processes. We examined the extent to which financial strain—difficulty paying monthly bills—mediated the link between income and inflammation, and although it did not (data not shown), there are a number of other psychosocial factors that may explain this association. A variety of psychosocial resources—sense of control over events, social support—can buffer the impact of stressors and help to maintain health, and these resources are less abundant with decreasing income and education (62). Studies (63) focused on daily stress processes found that, although participants with less educational attainment reported fewer stressors on a day-to-day basis, the stressors they experienced were rated as being more severe, using both objective and subjective rating methods. In addition, results from the first wave of MIDUS data collection showed that perceived severity of stressors mediated the relationship between SES and both psychological distress and physical symptoms (64). It is important to note that this earlier research focused on education. Additional work will be needed to extend these observations to include income. Similar to studies on health, it will be critical to determine the extent to which income is moderating the relationship between education and psychosocial resources. Nevertheless, these studies converge on the hypothesis that poorer psychosocial resources at low SES levels may exacerbate the physiological impact of stressors, an effect that may be partially independent of physical health or health behaviors. When coupled with a large literature linking chronic psychological stress to higher levels of inflammatory proteins (25,65–68), these studies suggest that those with the lowest incomes may be at greater risk of stress-related inflammation. We intend to examine this possibility in future work.
A second goal of the current study was to test the hypothesis that IL-6 would mediate the links between SES and both CRP and fibrinogen. IL-6 is a potent stimulator of the synthesis and release of both fibrinogen and CRP from the liver (40,41,69), and IL-6 is hypothesized to a central component of disease-related inflammation (70). Given these biological relationships, we examined the possibility that the associations of income and education with both CRP and fibrinogen in the present study would be eliminated by the inclusion of IL-6 in the statistical models, and the results showed clearly that they were. These observations suggest that IL-6 is in the pathway by which income, in particular, is linked to CRP and fibrinogen, although this possibility requires rigorous testing before it is established. Should this result stand up to further testing, it would suggest that IL-6 should be a specific target for research on how social disadvantage “gets under the skin” to affect health. Such a focus would be more precise than one involving multiple inflammatory markers and more likely to reveal important mediators of the link between income and inflammation. On a more general note, these results also highlight the importance of considering the physiological relationships among biological measures that are becoming increasingly popular in studies of social stratification of health.
Although these results help to illuminate the relationship between income and specific inflammatory markers in a national sample of middle-aged and older adults in the United States, their interpretation is tempered by the fact that although the sample was racially heterogeneous, the African American sample was drawn from the population of Milwaukee, and the links between SES and inflammation may be different in African Americans from other areas of the United States. Moreover, the current analyses focused on only two of a number of possible metrics of socioeconomic position, such as occupational status. Our understanding of how social factors are differentially or interactively associated with biological processes related to health will be advanced by the inclusion of more of these metrics in future studies.
This study joins prior research showing modest but consistent associations between SES and inflammation that are not explained by demographic characteristics, health status, or health behavior, and in so doing they support a potential role for inflammatory proteins as mediators of the links between social position and health outcomes. As research demonstrating biological imprints of SES accumulates, it becomes increasingly important to view social position as a critical determinant of health.
Acknowledgments
We thank the three anonymous reviewers for their insightful comments on earlier versions of this manuscript.
The present analyses were supported, in part, by Grant K01-AG029381 (E.M.F.) and the longitudinal follow-up of the MIDUS investigation was supported, in part, by Grant P01-AG020166 from the National Institute on Aging. The original study was supported, in part, by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development.
Glossary
- IL-6
interleukin-6
- CRP
C-reactive protein
- BMI
body mass index
- SES
socioeconomic status
- MIDUS
Survey of Midlife in the United States
- GCRC
General Clinical Research Center
REFERENCES
- 1.Crimmins EM, Saito Y. Trends in healthy life expectancy in the United States, 1970–1990: gender, racial, and educational differences. Soc Sci Med. 2001;52:1629–1641. doi: 10.1016/s0277-9536(00)00273-2. [DOI] [PubMed] [Google Scholar]
- 2.Rogot E, Sorlie PD, Johnson NJ. Life expectancy by employment status, income, and education in the National Longitudinal Mortality Study. Public Health Rep. 1992;107:457–461. [PMC free article] [PubMed] [Google Scholar]
- 3.Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL. Socioeconomic status and health. The challenge of the gradient. Am Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Marmot M, Wilkinson RG. Social Determinants of Health. New York: Oxford University Press; 1999. [Google Scholar]
- 5.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88:1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- 6.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Tracy RP, Rubin SM, Harris TB, Pahor M, Yaffe K, Lindquist K, Simonsick EM, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cardiovascular disease. (The Health, Aging and Body Composition [Health ABC] Study) Am J Cardiol. 2003;92:522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 7.Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, Benderly M, Goldbourt U, Willeit J, Kiechl S, Yarnell JW, Sweetnam PM, Elwood PC, Cushman M, Psaty BM, Tracy RP, Tybjaerg-Hansen A, Haverkate F, de Maat MP, Fowkes FG, Lee AJ, Smith FB, Salomaa V, Harald K, Rasi R, Vahtera E, Jousilahti P, Pekkanen J, D’Agostino R, Kannel WB, Wilson PW, Tofler G, Arocha-Pinango CL, Rodriguez-Larralde A, Nagy E, Mijares M, Espinosa R, Rodriquez-Roa E, Ryder E, Diez-Ewald MP, Campos G, Fernandez V, Torres E, Marchioli R, Valagussa F, Rosengren A, Wilhelmsen L, Lappas G, Eriksson H, Cremer P, Nagel D, Curb JD, Rodriguez B, Yano K, Salonen JT, Nyyssonen K, Tuomainen TP, Hedblad B, Lind P, Loewel H, Koenig W, Meade TW, Cooper JA, De Stavola B, Knottenbelt C, Miller GJ, Cooper JA, Bauer KA, Rosenberg RD, Sato S, Kitamura A, Naito Y, Palosuo T, Ducimetiere P, Amouyel P, Arveiler D, Evans AE, Ferrieres J, Juhan-Vague I, Bingham A, Schulte H, Assmann G, Cantin B, Lamarche B, Despres JP, Dagenais GR, Tunstall-Pedoe H, Woodward M, Ben-Shlomo Y, Davey Smith G, Palmieri V, Yeh JL, Rudnicka A, Ridker P, Rodeghiero F, Tosetto A, Shepherd J. Plasma fibrinogen level and the risk of major cardiovascular diseases and non-vascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 8.Empana JP, Sykes DH, Luc G, Juhan-Vague I, Arveiler D, Ferrieres J, Amouyel P, Bingham A, Montaye M, Ruidavets JB, Haas B, Evans A, Jouven X, Ducimetiere P The Prospective Epidemiological Study of Myocardial Infarction (PRIME) Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men. Circulation. 2005;111:2299–2305. doi: 10.1161/01.CIR.0000164203.54111.AE. [DOI] [PubMed] [Google Scholar]
- 9.Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, Wallace RB, Jackson RD, Pettinger MB, Ridker PM. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women’s Health Initiative observational study. JAMA. 2002;288:980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 12.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D’Agostino RB. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 13.Volpato S, Guralnik JM, Ferrucci L, Balfour J, Chaves P, Fried LP, Harris TB. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women’s health and aging study. Circulation. 2001;103:947–953. doi: 10.1161/01.cir.103.7.947. [DOI] [PubMed] [Google Scholar]
- 14.Abraham J, Campbell CY, Cheema A, Gluckman TJ, Blumenthal RS, Danyi P. C-reactive protein in cardiovascular risk assessment: a review of the evidence. J Cardiometab Syndr. 2007;2:119–123. doi: 10.1111/j.1559-4564.2007.05950.x. [DOI] [PubMed] [Google Scholar]
- 15.Mitka M. Panel endorses limited role for CRP tests. JAMA. 2003;289:973–974. doi: 10.1001/jama.289.8.973. [DOI] [PubMed] [Google Scholar]
- 16.Smith GD, Hart C, Watt G, Hole D, Hawthorne V. Individual social class, area-based deprivation, cardiovascular disease risk factors, and mortality: the Renfrew and Paisley Study. J Epidemiol Community Health. 1998;52:399–405. doi: 10.1136/jech.52.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alley DE, Seeman TE, Ki Kim J, Karlamangla A, Hu P, Crimmins EM. Socioeconomic status and C-reactive protein levels in the US population: NHANES IV. Brain Behav Immun. 2006;20:498–504. doi: 10.1016/j.bbi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Ranjit N, Diez-Roux AV, Shea S, Cushman M, Ni H, Seeman T. Socioeconomic position, race/ethnicity, and inflammation in the multiethnic study of atherosclerosis. Circulation. 2007;116:2383–2390. doi: 10.1161/CIRCULATIONAHA.107.706226. [DOI] [PubMed] [Google Scholar]
- 19.Lubbock LA, Goh A, Ali S, Ritchie J, Whooley MA. Relation of low socioeconomic status to C-reactive protein in patients with coronary heart disease (from the Heart and Soul Study) Am J Cardiol. 2005;96:1506–1511. doi: 10.1016/j.amjcard.2005.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koster A, Bosma H, Penninx BW, Newman AB, Harris TB, van Eijk JT, Kempen GI, Simonsick EM, Johnson KC, Rooks RN, Ayonayon HN, Rubin SM, Kritchevsky SB. Association of inflammatory markers with socioeconomic status. J Gerontol A Biol Sci Med Sci. 2006;61:284–290. doi: 10.1093/gerona/61.3.284. [DOI] [PubMed] [Google Scholar]
- 21.Gruenewald TL, Cohen S, Matthews KA, Tracy R, Seeman TE. Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Soc Sci Med. 2009;69:451–459. doi: 10.1016/j.socscimed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jousilahti P, Salomaa V, Rasi V, Vahtera E, Palosuo T. Association of markers of systemic inflammation, C reactive protein, serum amyloid A, and fibrinogen, with socioeconomic status. J Epidemiol Community Health. 2003;57:730–733. doi: 10.1136/jech.57.9.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panagiotakos DB, Pitsavos CE, Chrysohoou CA, Skoumas J, Toutouza M, Belegrinos D, Toutouzas PK, Stefanadis C. The association between educational status and risk factors related to cardiovascular disease in healthy individuals: The ATTICA study. Ann Epidemiol. 2004;14:188–194. doi: 10.1016/S1047-2797(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 24.Steinvil A, Shirom A, Melamed S, Toker S, Justo D, Saar N, Shapira I, Berliner S, Rogowski O. Relation of educational level to inflammation-sensitive biomarker level. Am J Cardiol. 2008;102:1034–1039. doi: 10.1016/j.amjcard.2008.05.055. [DOI] [PubMed] [Google Scholar]
- 25.Owen N, Poulton T, Hay FC, Mohamed-Ali V, Steptoe A. Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental stress. Brain Behav Immun. 2003;17:286–295. doi: 10.1016/s0889-1591(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 26.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294:2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 27.Herd P, Goesling B, House JS. Socioeconomic position and health: the differential effects of education versus income on the onset versus progression of health problems. J Health Soc Behav. 2007;48:223–238. doi: 10.1177/002214650704800302. [DOI] [PubMed] [Google Scholar]
- 28.Backlund E, Sorlie P, Johnson N. The shape of the relationship between income and mortality in the United States: evidence from the National Longitudinal Mortality Study. Ann Epidemiol. 1996;6:12–23. doi: 10.1016/1047-2797(95)00090-9. [DOI] [PubMed] [Google Scholar]
- 29.Bassuk S, Berkman L, Amick B. Socioeconomic status and mortality among the elderly: findings from four communities. Am J Epidemiol. 2002;155:520–533. doi: 10.1093/aje/155.6.520. [DOI] [PubMed] [Google Scholar]
- 30.Benzeval M, Judge K, Shouls S. Understanding the relationship between income and health: how much can be gleaned from cross-sectional data? Soc Policy Admin. 2001;35:376–396. [Google Scholar]
- 31.Ellison G. Letting the genie out of the bottle? Challenges facing the relative income hypothesis. Soc Sci Med. 2002;54:561–576. doi: 10.1016/s0277-9536(01)00052-1. [DOI] [PubMed] [Google Scholar]
- 32.House J, Kessler R, Herzog A. Age, socioeconomic status and health. Milbank Q. 1990;68:383–411. [PubMed] [Google Scholar]
- 33.House J, Lepkowski J, Kinney A, Mero R, Kessler R, Herzog A. The social stratification of aging and health. J Health Soc Behav. 1994;35:213–234. [PubMed] [Google Scholar]
- 34.Martikainen P, Makela S, Koskinen S, Valkonen T. Income differences in mortality: a register-based follow-up study of three million men and women. Int J Epidemiol. 2001;30:1397–1405. doi: 10.1093/ije/30.6.1397. [DOI] [PubMed] [Google Scholar]
- 35.McDonough P, Duncan G, Williams D, House J. Income dynamics and adult mortality in the United States, 1972 through 1989. Am J Pub Health. 1997;87:1476–1483. doi: 10.2105/ajph.87.9.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norris J, van der Laan M, Lane S, Anderson J, Block G. Nonlinearity in demographics and behavioral determinants of morbidity. Health Serv Res. 2003;38:1791–1818. doi: 10.1111/j.1475-6773.2003.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cutler D, Lleras-Muney A. Education and health. In: Schoeni R, House J, Kaplan G, Pollack H, editors. The Effects of Social and Economic Policy on Health. New York: Russell Sage; 2008. [Google Scholar]
- 38.Mirowsky J, Ross C. Education, Social Status, and Health. New York: Gruyter; 2003. [Google Scholar]
- 39.Ross CE, Mirowsky J. Refining the association between education and health: the effects of quantity, credential, and selectivity. Demography. 1999;36:445–460. [PubMed] [Google Scholar]
- 40.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moshage H. Cytokines and the hepatic acute phase response. J Pathol. 1997;181:257–266. doi: 10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 42.Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001;38:189–197. doi: 10.1016/s0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 43.Ganapathi MK, Rzewnicki D, Samols D, Jiang SL, Kushner I. Effect of combinations of cytokines and hormones on synthesis of serum amyloid A and C-reactive protein in Hep 3B cells. J Immunol. 1991;147:1261–1265. [PubMed] [Google Scholar]
- 44.Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res. 2005;66:265–275. doi: 10.1016/j.cardiores.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 45.Brim OG, Ryff CD, Kessler RC. How Healthy Are We? A National Study of Well-Being at Mid-Life. Chicago: University of Chicago Press; 2004. [Google Scholar]
- 46.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 47.Fliser D, Buchholz K, Haller H. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004;110:1103–1107. doi: 10.1161/01.CIR.0000140265.21608.8E. [DOI] [PubMed] [Google Scholar]
- 48.Tatli E, Kurum T. A controlled study of the effects of carvedilol on clinical events, left ventricular function and proinflammatory cytokines levels in patients with dilated cardiomyopathy. Can J Cardiol. 2005;21:344–348. [PubMed] [Google Scholar]
- 49.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 50.Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol. 2002;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- 51.Ridker PM, Hennekens CH, Rifai N, Buring JE, Manson JE. Hormone replacement therapy and increased plasma concentration of C-reactive protein. Circulation. 1999;100:713–716. doi: 10.1161/01.cir.100.7.713. [DOI] [PubMed] [Google Scholar]
- 52.Silvestri A, Gebara O, Vitale C, Wajngarten M, Leonardo F, Ramires JA, Fini M, Mercuro G, Rosano GM. Increased levels of C-reactive protein after oral hormone replacement therapy may not be related to an increased inflammatory response. Circulation. 2003;107:3165–3169. doi: 10.1161/01.CIR.0000074208.02226.5E. [DOI] [PubMed] [Google Scholar]
- 53.Lakoski SG, Cushman M, Criqui M, Rundek T, Blumenthal RS, D’Agostino RB, Jr, Herrington DM. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J. 2006;152:593–598. doi: 10.1016/j.ahj.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 54.Ball K, Crawford D. Socioeconomic status and weight change in adults: a review. Soc Sci Med. 2005;60:1987–2010. doi: 10.1016/j.socscimed.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 55.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 56.Goldman DP, Smith JP. Can patient self-management help explain the SES health gradient? Proc Natl Acad Sci U S A. 2002;99:10929–10934. doi: 10.1073/pnas.162086599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.House J. Understanding social factors and inequalities in health: 20th century progress and 21st century prospects. J Health Soc Behav. 2002;43:125–142. [PubMed] [Google Scholar]
- 58.McLaren L. Socioeconomic status and obesity. Epidemiol Rev. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- 59.Marmot MG. Understanding social inequalities in health. Perspect Biol Med. 2003;46:S9–S23. [PubMed] [Google Scholar]
- 60.Dowd JB, Aiello AE, Alley DE. Socioeconomic disparities in the sero-prevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol Infect. 2009;137:58–65. doi: 10.1017/S0950268808000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zajacova A, Dowd JB, Aiello AE. Socioeconomic and race/ethnic patterns in persistent infection burden among U.S. adults. J Gerontol A Biol Sci Med Sci. 2009;64:272–279. doi: 10.1093/gerona/gln012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor SE, Seeman TE. Psychosocial resources and the SES-health relationship. Ann N Y Acad Sci. 1999;896:210–225. doi: 10.1111/j.1749-6632.1999.tb08117.x. [DOI] [PubMed] [Google Scholar]
- 63.Grzywacz JG, Almeida DM, Neupert SD, Ettner SL. Socioeconomic status and health: a micro-level analysis of exposure and vulnerability to daily stressors. J Health Soc Behav. 2004;45:1–16. doi: 10.1177/002214650404500101. [DOI] [PubMed] [Google Scholar]
- 64.Almeida DM, Neupert SD, Banks SR, Serido J. Do daily stress processes account for socioeconomic health disparities? J Gerontol B Psychol Sci Soc Sci. 2005;60:34–39. doi: 10.1093/geronb/60.special_issue_2.s34. [DOI] [PubMed] [Google Scholar]
- 65.Brydon L, Edwards S, Mohamed-Ali V, Steptoe A. Socioeconomic status and stress-induced increases in interleukin-6. Brain Behav Immun. 2004;18:281–290. doi: 10.1016/j.bbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 66.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robles TF, Glaser R, Kiecolt-Glaser JK. Out of balance: a new look at chronic stress, depression, and immunity. Curr Dir Psych Sci. 2005;14:111–115. [Google Scholar]
- 68.Steptoe A, Kunz-Ebrecht S, Owen N, Feldman PJ, Rumley A, Lowe GD, Marmot M. Influence of socioeconomic status and job control on plasma fibrinogen responses to acute mental stress. Psychosom Med. 2003;65:137–144. doi: 10.1097/01.psy.0000039755.23250.a7. [DOI] [PubMed] [Google Scholar]
- 69.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 70.McCarty MF. Interleukin-6 as a central mediator of cardiovascular risk associated with chronic inflammation, smoking, diabetes, and visceral obesity: down-regulation with essential fatty acids, ethanol and pentoxifylline. Med Hypotheses. 1999;52:465–477. doi: 10.1054/mehy.1997.0684. [DOI] [PubMed] [Google Scholar]
- 71.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 72.Mortensen RF. C-reactive protein, inflammation, and innate immunity. Immunol Res. 2001;24:163–176. doi: 10.1385/IR:24:2:163. [DOI] [PubMed] [Google Scholar]
- 73.Tennent GA, Brennan SO, Stangou AJ, O’Grady J, Hawkins PN, Pepys MB. Human plasma fibrinogen is synthesized in the liver. Blood. 2007;109:1971–1974. doi: 10.1182/blood-2006-08-040956. [DOI] [PubMed] [Google Scholar]