Abstract
Differences in sensitivity are observed between mouse strains, C57 (sensitive) and SWV (resistant) when exposed to cadmium (Cd) during the neurulation period. In this study, we investigated the toxicokinetics of Cd in relation with toxicodynamic responses to identify factors affecting differential Cd-sensitivity in C57 and SWV. Using a level of exposure which induced developmental toxicity and differential effects between strains, we assessed maternal and embryonic Cd uptake and evaluated biomarkers of response previously linked with Cd exposure, specifically metal ion regulators (Mt1, Mt2, DMT1) and markers of cell cycle arrest/apoptosis induction (p53, Cdkn1a, c-Casp3). Greater Cd uptake was observed in C57 embryos compared to SWV and these observations of differential uptake were associated with increased alterations in expression of biomarkers of metal response (e.g. c-Casp3) and strain sensitivity. Using sensitive and resistant mouse strains, we have identified toxicokinetic and dynamic differences which underlie observed differences in Cd-embryonic sensitivity and response.
Keywords: Metal, Cadmium, Neural Tube Defect, Exencephaly, Toxicokinetics, Metallothionein, Biomarker, Neurulation
1. Introduction
Neurulation represents a critical period in embryonic development where aberrations due to genetic and environmental factors can underlie neural tube defect (NTD) formation and other adverse developmental outcomes [1]. In animal models, exposure to the heavy metal, cadmium (Cd), during neurulation results in a wide array of teratogenic outcomes, including NTD formation [2, 3]. Cd distributes to both the placenta and the developing embryo, dependent on the route of exposure, gestational period, strain, dose, genetic background and nutritional status [4–8]. Limited quantitative information exists regarding the toxicokinetics of Cd during early embryonic development, yet, qualitative studies suggest that Cd reaches the embryo as early as 1h post-injection (p.i.) (2.4mg/kg BW, exposed on GD9.0), concentrating in the neuroepithelial cells of the neural tube as well as the limb buds and embryonic gut [9], resulting in biochemical, cellular and morphological alterations [10, 11]. Investigations regarding the distribution and kinetics of Cd in the developing embryo and its relationship with alterations in neurulation are needed in order to further our understanding of the link between environmental exposure and developmental disease (ex. NTDs).
Comparative studies between inbred mouse strains, C57 and SWV, indicate differences in sensitivity to heavy metals when exposure occurs during early gestational development [6, 12]. For example, C57 mice are more sensitive to Cd than SWV mice during the period of neurulation. Exposed to the same dose of Cd (4mg/kg BW), C57 mice, in comparison with SWV mice, display a ~2 to 7 fold increase in NTD incidence as well as greater increases in resorptions and effects on growth [6, 13]. Our group has used this comparative model of resistant and sensitive mouse strains to identify toxicogenomic responses which correlate with differences in sensitivity during the neurulation period [13]. These studies showed that Cd impacts genes involved in cell cycle arrest, apoptosis (including p53-mediated genes) and CNS development differentially between these two strains in association with differences in strain sensitivity [13]. An objective of this study aims at investigating potential toxicokinetic differences between these two strains (C57 and SWV) that may explain, in part, differences in response and sensitivity.
Our studies as well as related published papers indicate that Cd uptake in maternal and embryonic tissues results in multiple biological and cellular alterations, including zinc or metal ion dysregulation, altered redox status, DNA damage, apoptosis, cell cycle arrest, and/or changes in CNS development signaling [10, 11, 13–15]. Based on these observations, several biomarkers of response have been proposed for use with Cd, including established markers associated with alterations in metal ion regulation, such as metallothioneins (e.g. Mt1 and Mt2) characterized in their involvement in Cd sequesteration [16, 17] and specific divalent ion transporters (ex. DMT1) which regulate Cd cellular uptake [18]. Likewise, markers associated with Cd-induced cell cycle arrest and apoptosis, such as p53, Cdkn1a and Casp3, have been well-documented in their response to Cd [10, 13, 14, 18]. In this study, we evaluate these dynamic biomarkers of Cd response in conjugation with toxicokinetic measurements to understand and assess the relationship between Cd kinetics and dynamic responses in differentially sensitive mouse strains.
In C57 and SWV mouse strains, we investigate Cd uptake in both maternal and embryonic tissues via Inductively Coupled Plasma Mass Spectrometry (ICPMS). In parallel, with 12 and 24h Cd concentration measurements, we assess markers of Cd-response, previously linked with Cd exposure, examining embryonic protein expression of cell cycle arrest and apoptosis markers (c-Casp3, p53, Cdkn1a) and gene expression of metal ion regulators (Mt1, Mt2, DMT1) in both control and Cd-exposed C57 and SWV embryos. In this study, we have identified and characterized Cd-toxicokinetic differences between C57 and SWV embryos, which in relation, with Cd-toxicodynamic factors, associated with increased Cd-sensitivity and -response in C57 versus SWV embryos during this window of development.
2. Methods
2.1 Animals and Cadmium Exposure
As described in Robinson et al., 2009 [13], C57BL/6J (C57) and SWV strains were maintained at the University of Washington, Department of Environmental and Occupational Health Sciences. C57 mice were supplied from Jackson laboratories and SWV mice were originally provided by Dr. Richard Finnell (Texas A&M University). Housed in filter covered transparent plastic cages, animals were held in climate-controlled rooms under an alternating 12h light/dark cycle. Water and food were available ad libitium. All animal care and experiments were completed in agreement with the University of Washington Institute of Animal Care Committee. Timed matings were generated by introducing individual male mice into cages with two females. Copulatory plugs were identified in the early morning (8:00am ± 1h) the following day and designated as GD0. Pregnant mice were administered single doses via intraperitoneal injection (ip) on GD 8.0, 8:00am (±1h) with either cadmium chloride (4mg/kg/BW, Alfa Aesar, Ward Hill, MA) dissolved in deionized water (working concentration of 2mM), or water (vehicle control) (10ul/g). Our exposure time and injection method (ip) used was chosen due to previous observations indicating exposure to Cd on GD8 results in significant developmental toxicity (NTDs, growth effects) and differential effects between C57BL/6 and SWV strains [6, 13].
2.2 Maternal Liver and Embryo Preparation for Kinetic Assessments
C57 and SWV dams were euthanized on GD8.25 (6h p.i.), GD 8.5 (12h p.i.) and GD 9.0 (24h p.i.). The uterine horns were extracted from the abdomen region and placed in cold CMF-EBSS. Embryos were isolated, in cold CMF-EBSS, placed in liquid nitrogen, and stored at −80°C. Pooled litters were kept separate in a volume of <100ul CMF-EBSS. To conduct parallel maternal Cd uptake assessments, maternal livers were isolated and placed in liquid nitrogen and stored at −80°C. Cd extraction was completed using the EPA 200.3 method with modifications [19]. Ultra pure grade concentrated nitric acid (HNO3) (200ul) was added directly to each sample (maternal liver or litter of embryos) in 2ml polypropylene tubes and heated at 90°C (water bath) for 30 min. Samples were cooled at room temperature (~5 min), then an additional 50ul of HNO3 was added. Samples were heated at 90°C (water bath) for at least 15 min. Visibly assured for complete digestion, samples were cooled at 20°C (~5min) then transferred to 25ml Erlenmeyer flasks. Each tube was rinsed 3× with de-ionized water (500ul). An additional 50ul HNO3 was added and flasks were heated on a hot plate for 30 min and left overnight to cool. In each flask, volumes were reduced to ~500ul via evaporation by hot plate. ACS reagent grade, 30% hydrogen peroxide (H2O2) (40ul) was added to each flask. Volumes were again reduced to ~500ul, then 30% H2O2 was added again, repeated (5×). Samples were cooled and transferred to 15ml tubes. Each sample was brought up to a final equal volume of 6ml with de-ionized water, twice the minimum volume needed for ICPMS analysis (3ml). Before ICPMS analysis, each sample was centrifuged and displaced in a new tube to eliminate any remaining undigested sample. Cd standards were prepared in 6% HNO3/ 4.0% H2O2.
2.3 Inductively Coupled Plasma Mass Spectrometry (ICPMS)
ICPMS analysis was conducted using the EPA 200.8 method [20] to measure Cd content in collected maternal liver and embryo samples by the Environmental Health Laboratory at the University of Washington (Seattle, WA). Cd concentrations (ppb) were calculated for liver and embryonic samples using the Cd concentration standard curve. Concentrations below the detection level of 0.1 ppb were determined to be equal to 0.1 ppb. We detected Cd concentrations above our minimum detection level of 0.1 ppb for all maternal liver (Cd exposed and control), 47% of control embryo and 87% of Cd-exposed embryo samples (SWV and C57). For maternal liver samples, we divided the total ng Cd calculated in each sample by the original liver mass (mg). For embryo (litter) samples, we calculated the ratio of total ng of Cd in each sample by the number of embryos assessed to determine ng Cd on a per embryo basis. A selected Cd stock was assessed using ICPMS and found to be 99% accurate using our current methodology. Acid digestion led to an estimated reduction in 8–11% of total Cd.
2.4 Embryo RNA and Protein isolation
To conduct embryo RNA and protein assessments, Cd exposed and control pregnant C57 and SWV females were euthanized GD8 – GD10. Cd exposed embryos were collected specifically at 12 and 24h p.i. (GD8.5, 9.0), while control embryos were collected at 0, 12, 24, and 48h p.i. (GD8, 8.5, 9.0, 10.0). Embryos were collected exactly as above in preparation for our kinetic assessments. Embryos were placed in 500ul of RTL Cell Lysis buffer (Qiagen, Valencia, CA) and lysed with a 30G needle to homogenize the tissue. RNA was purified using the RNAeasy kit (Qiagen, Valencia, CA). The Nanodrop ND-100 spectrophotometer (Nanodrop Technologies, Wilmington, DE) was used to assess initial RNA quantity (~5 – 30ug/litter of embryos) and quality (Abs260/280 = 1.9 – 2.1).
2.5 Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Using Superscript II, cDNA was prepared from purified total RNA. To assess gene expression of Mt1, Mt2, DMT1, and BActin, 4uL of cDNA was amplified using the Taqman Universal PCR Master Mix and 6-FAM dye-labeled Taqman assays (Applied Biosystems, Foster City, CA). BActin was utilized as an internal control to normalize our data.
2.6 Western blot
Western blot procedures for selected proteins were conducted according to previously described methods with modifications [21]. Equal amounts of proteins (15ug) were loaded onto criterion gels (12.5%) and separated according to size (Biorad, Hercules, CA). Proteins were then transferred to a nitrocellulose membrane and incubated with specific primary and secondary antibodies. Primary probes and respective dilutions used, included Trp53 (p53) (sc-100) mouse monoclonal 1:500, Cdkn1a (sc-6246) mouse monoclonal, c-Casp3 (rabbit polyclonal) 1:500, Actin (sc-1616) goat polyclonal from Santa Cruz Biotechnologies (Santa Cruz, CA) and GAPDH (MAB374) mouse monoclonal from Chemicon (Billerica, MA). Raw intensities (volume) were generated by calculating the intensity of each band minus its representative background within each film (Jimage). Intensities were adjusted either by GADPH or Actin. No significant differences were observed between using either GADPH or Actin to adjust intensities.
2.7 Statistical Analysis
To determine the significance of comparisons between treatment, time and strain as well as their respective interactions, we conducted ANOVA for both measures of toxicokinetic and toxicodynamic changes. For example, we assessed the effect of treatment (Cd, Control), strain (C57, SWV) and time (6, 12, 24h p.i.) on Cd uptake in mouse livers or embryos using the ANOVA Model: [ng Cd/mg liver tissue or ng Cd/embryo = (BCdx1) + (BStrainx2) + (BTimex3) + (BCd_Strainx1x2) + (BCd_Timex1x3) + (BStrain_Timex2x3) + (BCd_Strain_Timex1x2x3)] Significant effects were identified using an F-test (p<0.05) for each effect variable. Additional post-hoc analyses (Student one-sided t-tests) were completed to make direct comparisons between groups at respective timepoints (p<0.05). For gene expression assessments (Mt1, Mt2, DMT1), data was presented as a bar graph with the mean + SEM. For protein expression assessments, we normalized adjusted intensity values by the average of the 12 + 24h controls within each gel to make comparisons across blots. We adjusted by other means and showed similar distributions and significance in our relationships. The geometric mean for each group was determined between separate litters for each treatment/timepoint. Data was presented as a bar graph with the geometric mean + SEM. Three to six samples (n=3–6) for each strain were assessed within each exposure group for all kinetic and dynamic (RNA or protein) assessments, with the exception of GD10 control embryo (C57 and SWV) RNA assessments (n=2).
3. Results
3.1 Maternal Liver - Cd Kinetics
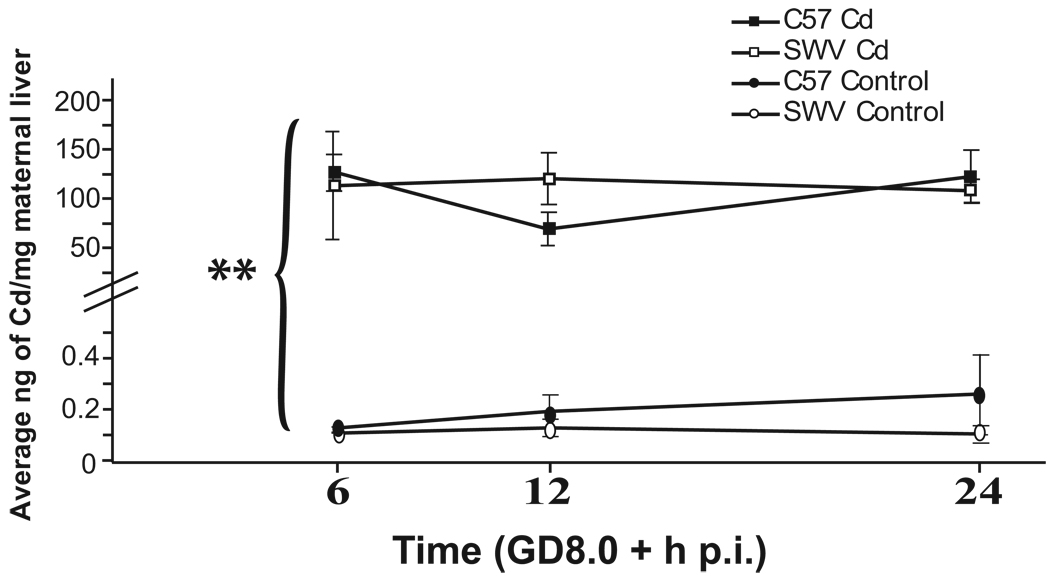
In Figure 1, in both C57 and SWV dams, we observed a significant increase in Cd uptake in Cd-exposed compared to control livers across all three timepoints (6, 12, 24h p.i.) (ANOVA, BCd p<0.005). No significant differences were observed in Cd concentrations between strains or timepoints measured (ANOVA, p> 0.05).
Figure 1. Cd uptake in maternal liver of C57 and SWV mice.
Cd-exposed and control maternal liver of pregnant dams of C57 and SWV were assessed for total Cd uptake via ICPMS 6, 12, 24 h p.i. (GD8.0). Data are shown as mean ± SEM. ANOVA was conducted to determine significant primary and interactive effects across time, exposure, and strain. Asterisks indicates significant Cd uptake in maternal liver (p<0.001 (**)). Livers of Cd-exposed C57 and SWV showed similar Cd concentrations across time (ANOVA, p>0.05)
3.2 Embryos - Cd Kinetics
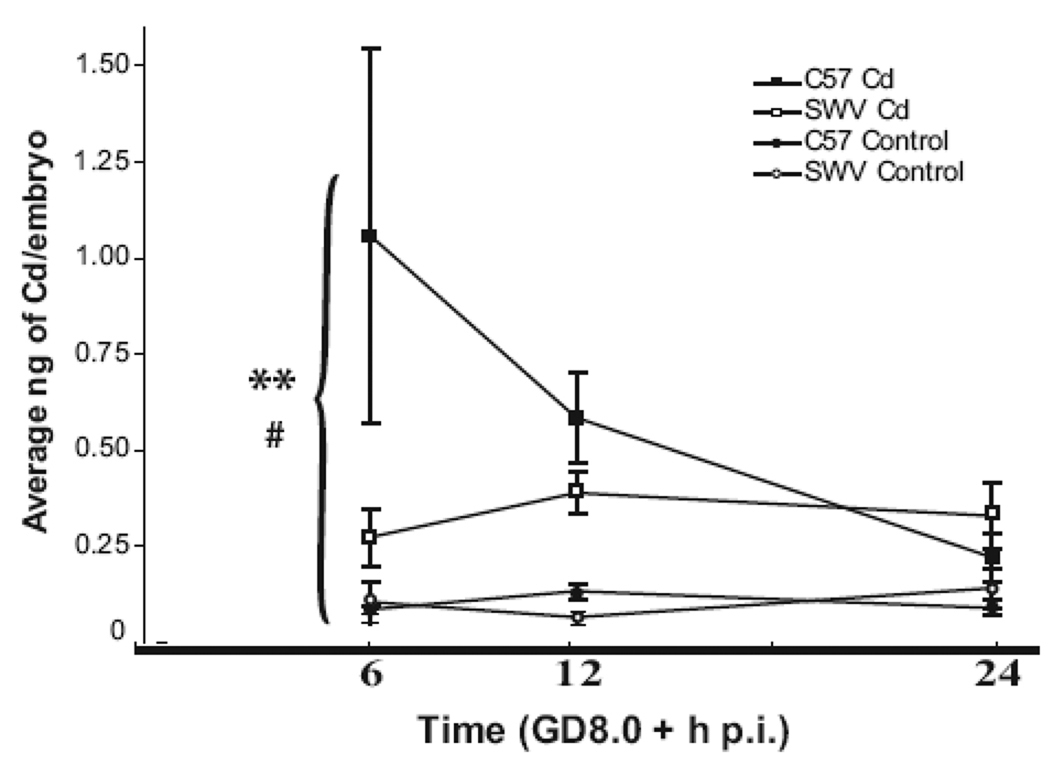
Likewise in Figure 2, in both C57 and SWV embryos, we observed a significant increase in Cd uptake in exposed compared to control embryos across time (ANOVA, BCd p<0.005). However, in contrast to observations in Cd-exposed C57 and SWV maternal livers, we observed differential embryonic Cd uptake at specific timepoints between strains as indicated by the significant interaction between time, treatment, and strain effects (ANOVA, BCd_Strain_Time p<0.05). At 6h p.i., we observed a ~4 fold greater increase in mean Cd uptake in embryos of C57 (1.06± 0.49 ng/embryo) compared to SWV (0.27 ± 0.07 ng/embryo). All Cd measurements at 6h p.i. were identified to be higher in the C57 compared to the SWV in Cd exposed embryos (n=3) (data not shown). At 12h and 24h p.i., Cd levels were lower compared to 6h p.i. in the C57 and differed between strains to a lesser degree (~1.5 fold difference between strains). At 12h p.i., Cd concentrations were higher in the C57 (0.59 ± 0.12 ng/embryo) compared to the SWV (0.39 ± 0.06 ng/embryo), whereas at 24h p.i., Cd levels were higher in the SWV (0.33 ± 0.09 ng/embyo, C57, 0.22 ± 0.06 ng/embryo).
Figure 2. Differential Cd uptake in embryos of C57 and SWV mice.
Cd-exposed and control litters of embryos of C57 and SWV were assessed for total Cd uptake via ICPMS 6, 12, 24h p.i. (GD8.0). Data are shown as mean ± SEM. Asterisks indicate significant (ANOVA) Cd concentrations in Cd exposed embryos compared to controls (p<0.001 (**)). A significant interaction between Cd uptake, time and strain was identified (p<0.05 (#)), indicating differential Cd uptake between strains at specific timepoints. The largest difference between strains was observed at 6h p.i.
3.3 Biomarkers of Cd Response - Metal Ion Regulators
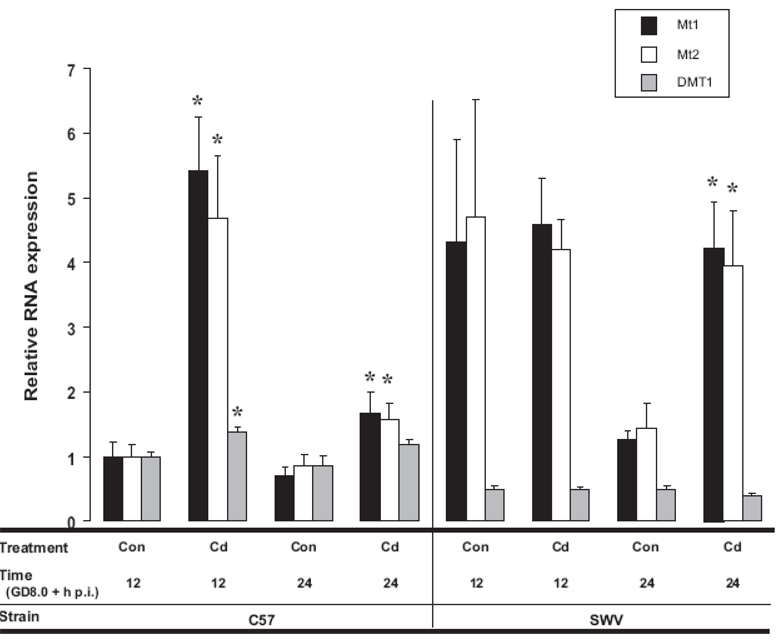
In Figure 3, we show relative RNA expression of Mt1, Mt2 and DMT1 in Cd-exposed and control C57 versus SWV embryos 12 and 24h p.i. corresponding with GD8.5 and 9.0. Moving left to right in this figure, in the C57 (12h p.i.), Cd-induced upregulation of Mt1, Mt2 and DMT1 (p<0.05) compared to their concurrent control (12h). At 24h, increased Mt1 and Mt2 expression was reduced, but still significantly elevated (p<0.05) and a statistical tendency for increased DMT1 (p=0.05) was observed in comparison with 24h controls. In the SWV, we did not observe any significant changes with Cd at 12h p.i., however at 24h p.i., we observed significant Mt1 and Mt2 induction compared to their respective 24h control (p<0.05). For all three genes (MT1, MT2, and DMT1), significant differences in background expression between control C57 and SWV embryos were observed on GD8.5 (p<0.05), an important timepoint in neural tube closure.
Figure 3. RNA expression of biomarkers of Cd response associated with metal ion regulation (Mt1, Mt2, DMT1)in Cd-treated and control C57 and SWV embryos.
Homogenized litters of Cd- exposed and control C57 and SWV embryos were assessed for RNA expression at 12 and 24h p.i. via RT-PCR. Raw intensities were adjusted by Bactin and then normalized to the average C57 12h control value to be able to display comparisons between the three probes. Data are shown as mean ± SEM. An asterisk indicates significant differences between Cd exposed versus control at each respective timepoint (Ttest, p<0.05).
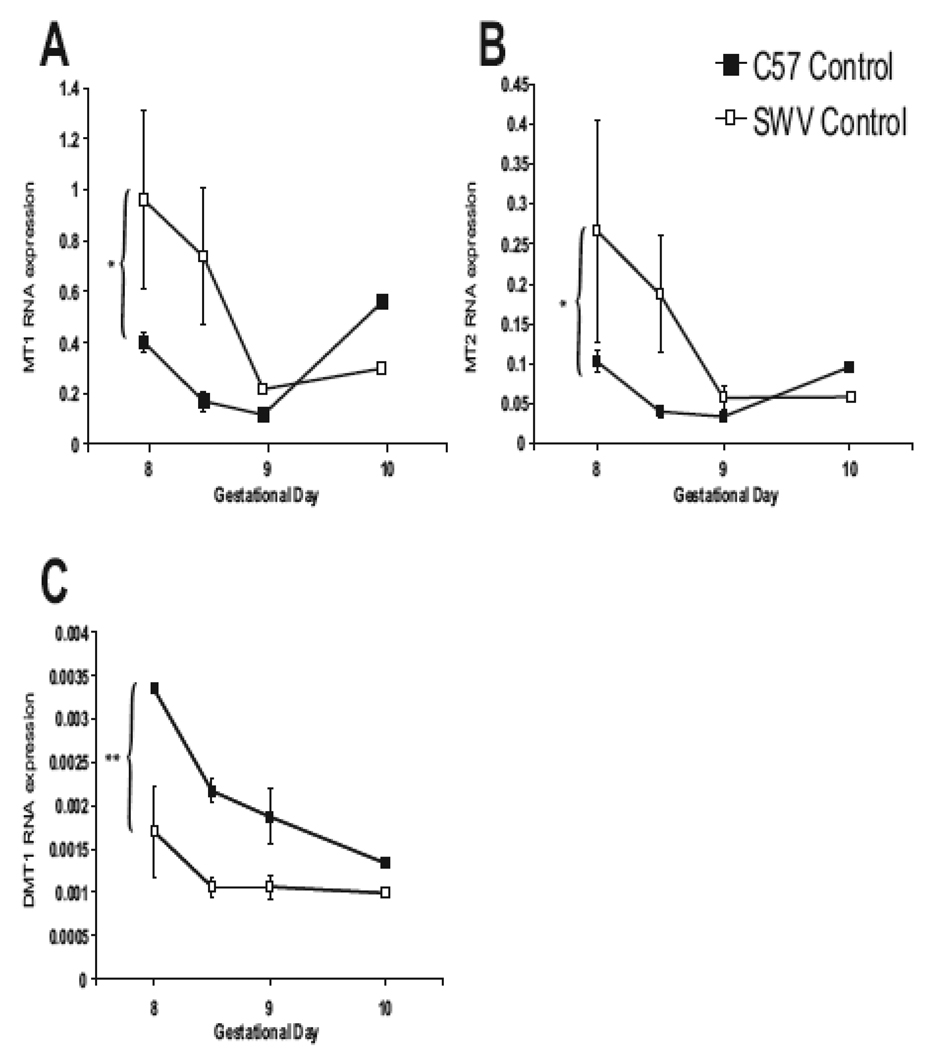
In Figure 4, we present RNA expression of Mt1 (A), Mt2 (B) and DMT1 (C) across early development (GD8–10) in control C57 and SWV embryos. For Mt1 and Mt2, we observed higher expression in SWV compared to the C57 across time (GD8 – 10) between strains (ANOVA, p>0.05) (Figure 4A and B). The largest differences in MT1 and MT2 expression were observed on GD8.5 (~4 fold difference in expression in the SWV). In contrast, we observed significant differential expression of the metal transporter, DMT1 between control C57 and SWV embryos (GD8-GD10, ANOVA, p<0.001) (Figure 4C). C57 embryos contained ~2× the amount of DMT1 RNA compared to the SWV during GD8.0 – 9.0.
Figure 4. Differential RNA expression of Mt1, Mt2 and DMT1 between C57 and SWV embryos during neurulation (GD8.0 – 10).
We conducted RT-PCR for C57 and SWV embryos over the neurulation period (GD8–10) for Mt1 (A), Mt2 (B), and DMT1 (C). Data are shown as mean ± SEM. For Mt1 and Mt2, we observed higher expression in the SWV across time (ANOVA, p<0.05). In contrast, we observed significantly higher DMT1 expression in C57 versus SWV embryos (ANOVA, p<0.001).
3.4 Biomarkers of Cd Response - Cell Cycle Arrest and Apoptosis
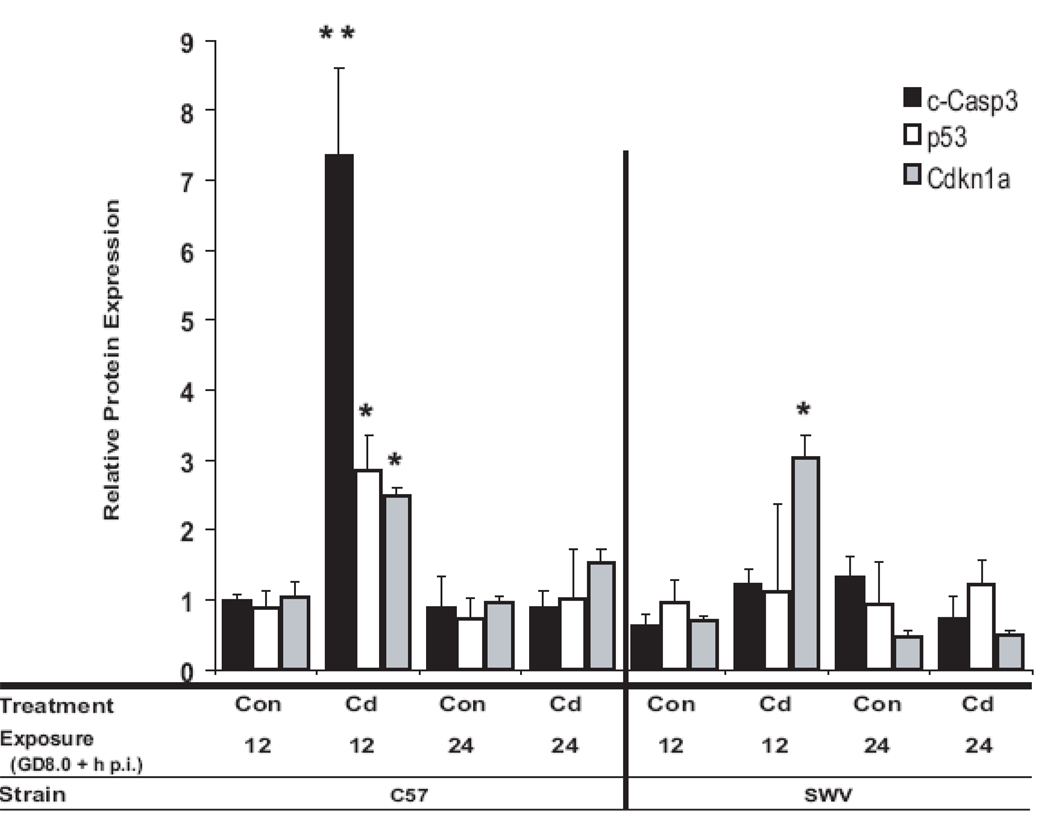
Protein expression of markers of apoptosis and/or cell cycle arrest (c-Casp3, p53 and Cdkn1a) in Cd-exposed and control C57 and SWV embryos are presented in Figure 5. At 12h p.i., in Cd exposed C57 embryos, we observed a significant 7.4 fold increase in the cleaved Caspase 3 protein product, c-Casp3 (p<0.001), while in the SWV, we observed a modest 1.9 fold increase in c-Casp 3 (p>0.05). Cd significantly induced more c-Casp3 in the C57 compared to the SWV (ANOVA, BCd_Strain, p<0.005), correlated with greater Cd uptake in C57 embryos. At 12h p.i., Cd induced a significant increase in total p53 protein in the C57 (3.2-fold increase) (p<0.05), while in the SWV, no significant changes were observed. Cdkn1a protein was significantly upregulated in both C57 (~2.4-fold increase) and SWV (~4.3-fold increase) embryos at 12h p.i (p<0.05). At 24p.i., we did not observe a significant increase in c-Casp3, p53 or Cdkn1a protein in either strain comparing Cd-exposed to control. For both p53 and Cdkn1a protein, no significant interactions were observed between strain and treatment or strain, treatment and time (BCd_Strain or BCd_Strain_Time, p>0.05).
Figure 5. Protein expression of biomarkers of Cd response associated with cell cycle arrest and apoptosis in Cd-treated and control C57 and SWV embryos.
Homogenized litters (pooled embryos within one litter) of Cd- exposed and control C57 and SWV embryos (n=3–6) were probed for c-Casp3, total p53 and Cdkn1a, 12 and 24h p.i. via Western Blot. Data are shown as geometric mean ± SEM. Asterisks indicate significant differences between Cd exposed versus control at each respective timepoint (Ttest, p<0.05 (*), p<0.005 (**)). Significant differences in c-Casp3 activation were observed between strains (ANOVA, p<0.05).
4. Discussion
The C57 and SWV display differential sensitivity to Cd when exposed during the neurulation period, resulting in increased developmental toxicity in the sensitive strain [6, 13]. In the current study, we investigated potential toxicokinetic and toxicodynamic factors which contribute to previously confirmed differences in Cd-response and –sensitivity. We explored Cd concentrations in C57 and SWV (maternal and embryonic) in relation with biomarkers of Cd response, including genes associated with Cd ion regulation (Mt1, Mt2, DMT1) and proteins involved in cell cycle arrest and apoptosis (p53, Cdkn1a, c-casp3) [10, 13, 14, 17, 18, 22].
Previous studies investigating the distribution of Cd during pregnancy indicate Cd passes through the placenta and accumulates in the embryo [9, 23]. At doses able to induce developmental toxicity during early gestation, Cd distributes to the neural tube as early as 1h following injection (Cd 2.4mg/kg BW, GD9.0, ip), localizing in the neural tube, limb buds and gut [9]. In our study, we observed Cd concentrations in C57 and SWV embryos ~2 – 13× above control levels, dependent on strain and time (Figure 2). We observed Cd peak concentrations at 6h p.i. in the C57 and 12h p.i in the SWV. Peak concentrations (≤12h) support subsequent morphological cellular changes (pyknotic nuclei) which have been reported to occur 10–12h within the neural tube, with minimal changes occurring at 4, 24, and 48h [11]. Our study supports these reports that Cd can cross the placenta into embryonic tissues and also provides significant quantitative knowledge of Cd uptake in two differentially sensitive strains of mouse embryos during a critical period in early development
Despite evidence of equivalent maternal Cd uptake in the liver (6–24h p.i.) (Figure 1), at 6h p.i and to a lesser degree at 12h p.i., we observed higher Cd concentrations in C57 (sensitive) compared to SWV embryos. Observations of increased Cd embryonic uptake and/or higher peak concentrations in C57 versus SWV embryos occurred at times of peak neurulation sensitivity, implicating that both toxicokinetic and toxicodynamic factors underlie differences in sensitivity between C57 and SWV embryos exposed identically to Cd in utero.
In addition to toxicokinetic assessments, we measured biomarkers of response associated with Cd ion regulation, Mt1, Mt2 and DMT1. Due to their involvement in metal ion regulation and oxidant damage, metallothioneins (Mts) are proposed as biomarkers of metal response and human sensitivity [17, 22]. Recent reports suggest Cd induces increases in expression of Mt1 and Mt2 in C57 mouse embryos (4mg/kg, GD8) [14, 16]. In this study, we support these findings that Mt1 and Mt2 serve as a biomarker of response in early developing mouse embryos and provide evidence of differential Mt response between C57 and SWV embryos (temporal aspects and the degree of response) in relation with toxicokinetic assessments (Figure 3). Mt1 and Mt2 (12 and 24h p.i.) were significantly upregulated in the C57, while only Mt1 and Mt2 were upregulated in the SWV at 24h p.i. Higher embryonic Cd concentrations at 6 and 12h p.i. in the C57 compared to the SWV associate with greater Mt1 and Mt2 induction in the C57 compared to the SWV (12h p.i.). Our results suggest the degree of Mt1 and Mt2 induction at 12h p.i., is associated with greater Cd embryonic uptake and furthermore, associates with sensitivity to Cd-induced NTDs in embryos undergoing neurulation.
Increased expression of the divalent metal transporter, DMT1, is associated with increased Cd uptake in adult rat tissues (small intestine, liver, kidney) [18]. In our study, we found Cd to induce embryonic DMT1 RNA expression in only the C57, potentially indicating upregulation due to increased Cd uptake or induced alterations in divalent metal status in the sensitive strain. The ability of Cd to alter DMT1 expression in mouse embryos during neurulation has not been previously repeated. We provide evidence for Cd to alter expression of DMT1 in mouse embryos and are able to identify this response to be associated with differential Cd uptake between resistant and sensitive mouse strains.
For all three of these biomarkers involved in Cd ion regulation (Mt1, Mt2, DMT1), we identified significant differences in expression between C57 and SWV controls across neurulation (GD8, 8.5, 9, 10) (ANOVA, p<0.05 or p<0.001) (Figure 4). These differences were clearly evident at GD8.5, where both Mt1 and Mt2 were found to be ~4× higher in the SWV compared to the C57, while DMT1 was identified to be ~2× higher in the C57 compared to the SWV. Differential levels of these transcripts may be of importance in the predisposition to metal sensitivity due to alterations in Cd ion uptake or distribution. For example, in Mt1 and 2 (−/−) pregnant dams exposed via Cd (oral, daily, beginning on GD0) across gestation, ~100× more Cd accumulates in knockout fetuses compared to wildtype (isolated on GD14 or GD17) [4], indicating a clear necessity for Mt function and Cd embryonic accumulation. The ability of DMT1’s influence on Cd influx has also been observed in genetically modified cell lines, where overexpression of DMT1 results in increased transport of Cd [24] and elimination of DMT leads to decreased Cd uptake [25], suggesting differential expression of DMT1 influences Cd transport. Our results show significant differences in baseline expression of these genes between strains during this window in development, suggesting a need to further investigate the normal background status between these two differentially sensitive strains in pathways important for metal exposure as well as toxicity mediation and normal development.
Combined with kinetic measurements and assessments of biomarkers of response associated with Cd ion regulation, we examined biomarkers of Cd response linked with Cd-induced apoptosis and cell cycle arrest. Apoptosis and cell cycle arrest-related molecular events are associated with Cd-induced NTDs and have been proposed as biomarkers of metal response [10, 13, 14]. In this study, we examined the impact of Cd on protein expression of three markers of interest, c-Casp3, p53 and Cdkn1a, known to play critical roles in cell cycle arrest and apoptosis (Figure 5). Here, we reconfirm earlier observations of Cd to induce activation of the important mediator of apoptosis, Casp3, in C57 mouse embryos undergoing neurulation [10]. Furthermore, we show that c-Casp3 is associated with increased toxicity and sensitivity in comparisons between the C57 and SWV as c-Casp3 was induced by a significantly greater amount with Cd exposure in the C57 compared to the SWV.
Previous studies from our lab suggest Cd induces RNA expression of p53 and the p53-downstream mediator, Cdkn1a, in both C57 and SWV embryos undergoing neurulation [13]. In the current study, we observed Cd to significantly induce upregulation of p53 protein in only the C57 (12h p.i.) and not the SWV (Figure 4). Differential Cd effects between strains on p53 expression were only modest as indicated by ANOVA (BCd_Strain, p=0.15), however, increased p53 at 12h, much like c-Casp3, associated with increased toxicity as well as greater Cd uptake in the C57 in comparison with the SWV. Differential increases in protein expression of total p53 in the C57 compared to the SWV may implicate differential induction of p53 downstream mediated genes. Interestingly, p53-mediators, Pmaip1 and Ccng1 at 12h p.i. were observed to be upregulated in the C57 and not in the SWV exposed similarly to the current study [13]. Also, in our study, Cd induced Cdkn1a protein, however, unlike p53 and c-Casp3, we observed significant increases in both C57 and SWV mouse embryos (12h p.i.). The magnitude of Cdkn1a protein expression at 12h p.i. did not reflect the level of toxicity between these two strains, or for that matter, p53 protein expression, implying that Cdkn1a may be upregulated through p53-independent mechanisms.
Limited quantitative information exists about Cd accumulation to make proper assessments between animal models and human during early development. With the degree of maternal exposure (4mg/kg BW) and route (ip) in this study differing from typical environmental human exposures, extrapolating our results from a risk assessment standpoint proves to be complex as illustrated with other teratogenic agents (e.g. arsenic) with similar experimental design (high dose, acute exposures) [26]. Extrapolations using an approximation for embryonic weight suggest embryonic Cd levels observed in this study to be within a 10–100× range of early assessments of Cd accumulation (using atomic absorption) in aborted 1st trimester human embryos [27]. However, these comparisons should be made with caution due to differing experimental techniques. Few quantitative studies exist which compare Cd accumulation during neurulation in rodents. Measured by atomic absorption, SWV embryos, 24h following Cd repeat exposures (4mg/kg BW, GD7–9, ip injection) contain ~2× Cd as controls, approximately 1000× higher than concentrations (0.6 ug/embryo) observed in our study [15]. Issues surrounding sensitivity of measurements due to technique (ICPMS versus atomic absorption) and design (whole embryo, animal model, gestational day) need to be resolved to make valid comparisons between human and rodent. Using a combination of toxicokinetic and dynamic factors will improve comparisons between rodent models and human.
In summary, we suggest that differential kinetic profiles of Cd contribute to sensitivity to Cd during neurulation between sensitive (C57) and resistant (SWV) mouse strains and that these differences are supported using the combination of both toxicokinetic and dynamic-based assessements. Differential expression of biomarkers of Cd response may play a role in observed differential effects and their interpretation requires detailed dose and temporal assessments within such a framework.
Acknowledgements
Funding
This work was supported in part by the National Institute of Environmental Health Sciences (NIEHS) (Toxicogenomics, U10 ES 11387 and R01-ES10613, grant number 5-P01-ES009601 from the National Institute of Environmental Health Sciences (NIEHS), NIH and RD-83170901-0 from the Environmental Protection Agency (EPA), the National Science Foundation (NSF: OCE-0434087), and the UW NIEHS Center for Ecogenetics and Environmental Health (5 P30 ES07033). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH, NSF or EPA.
The authors wish to thank Dr. Phillip Mirkes and Dr. Richard Finnell for supplying the original SWV mice to establish our colony at the University of Washington, Russell Dills, Jianbo Yu, and Maureen Cornell Endres for their assistance in protocol development of Cd kinetic measurements and ICPMS analysis, and finally to Alison Laing for her editorial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, Speer MC. Human neural tube defects: developmental biology, epidemiology, and genetics. Neurotoxicology and teratology. 2005;27:515–524. doi: 10.1016/j.ntt.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barr M., Jr The teratogenicity of cadmium chloride in two stocks of Wistar rats. Teratology. 1973;7:237–242. doi: 10.1002/tera.1420070304. [DOI] [PubMed] [Google Scholar]
- 3.Ferm VH. Developmental malformations induced by cadmium. A study of timed injections during embryogenesis. Biology of the neonate. 1971;19:101–107. doi: 10.1159/000240405. [DOI] [PubMed] [Google Scholar]
- 4.Brako EE, Wilson AK, Jonah MM, Blum CA, Cerny EA, Williams KL, Bhattacharyya MH. Cadmium pathways during gestation and lactation in control versus metallothoinein 1,2-knockout mice. Toxicological sciences. 2003;71:154–163. doi: 10.1093/toxsci/71.2.154. [DOI] [PubMed] [Google Scholar]
- 5.Goyer RA. Toxic and essential metal interactions. Annual review of nutrition. 1997;17:37–50. doi: 10.1146/annurev.nutr.17.1.37. [DOI] [PubMed] [Google Scholar]
- 6.Hovland DN, Jr, Machado AF, Scott WJ, Jr, Collins MD. Differential sensitivity of the SWV and C57BL/6 mouse strains to the teratogenic action of single administrations of cadmium given throughout the period of anterior neuropore closure. Teratology. 1999;60:13–21. doi: 10.1002/(SICI)1096-9926(199907)60:1<13::AID-TERA6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima K, Kawamata A, Matsuoka M, Wakisaka T, Fujiki Y. [Dose-response relationship of cadmium or radiation-induced embryotoxicity in mouse whole embryo culture] Gifu Shika Gakkai zasshi = The Journal of Gifu Dental Society. 1988;15:412–419. [PubMed] [Google Scholar]
- 8.Sonawane BR, Nordberg M, Nordberg GF, Lucier GW. Placental transfer of cadmium in rats: influence of dose and gestational age. Environmental health perspectives. 1975;12:97–102. doi: 10.1289/ehp.751297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christley J, Webster WS. Cadmium uptake and distribution in mouse embryos following maternal exposure during the organogenic period: a scintillation and autoradiographic study. Teratology. 1983;27:305–312. doi: 10.1002/tera.1420270304. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez EL, Gustafson AL, Andersson M, Hellman B, Dencker L. Cadmium-induced changes in apoptotic gene expression levels and DNA damage in mouse embryos are blocked by zinc. Toxicological sciences. 2003;76:162–170. doi: 10.1093/toxsci/kfg208. [DOI] [PubMed] [Google Scholar]
- 11.Webster WS, Messerle K. Changes in the mouse neuroepithelium associated with cadmium-induced neural tube defects. Teratology. 1980;21:79–88. doi: 10.1002/tera.1420210110. [DOI] [PubMed] [Google Scholar]
- 12.Machado AF, Hovland DN, Jr, Pilafas S, Collins MD. Teratogenic response to arsenite during neurulation: relative sensitivities of C57BL/6J and SWV/Fnn mice and impact of the splotch allele. Toxicol Sci. 1999;51:98–107. doi: 10.1093/toxsci/51.1.98. [DOI] [PubMed] [Google Scholar]
- 13.Robinson JF, Yu X, Hong S, Griffith WC, Beyer R, Kim E, Faustman EM. Cadmium-induced differential toxicogenomic response in resistant and sensitive mouse strains undergoing neurulation. Toxicol Sci. 2009;107:206–219. doi: 10.1093/toxsci/kfn221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kultima K, Fernandez EL, Scholz B, Gustafson AL, Dencker L, Stigson M. Cadmium-induced gene expression changes in the mouse embryo, and the influence of pretreatment with zinc. Reproductive toxicology (Elmsford, N.Y. 2006;22:636–646. doi: 10.1016/j.reprotox.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Paniagua-Castro N, Escalona-Cardoso G, Chamorro-Cevallos G. Glycine reduces cadmium-induced teratogenic damage in mice. Reproductive toxicology (Elmsford, N.Y. 2007;23:92–97. doi: 10.1016/j.reprotox.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez EL, Dencker L, Tallkvist J. Expression of ZnT-1 (Slc30a1) and MT-1 (Mt1) in the conceptus of cadmium treated mice. Reproductive toxicology (Elmsford, N.Y. 2007;24:353–358. doi: 10.1016/j.reprotox.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Cheng ML, Yang Q, Shan KR, Shen J, Zhou Y, Zhang X, Dill AL, Waalkes MP. Blood metallothionein transcript as a biomarker for metal sensitivity: low blood metallothionein transcripts in arsenicosis patients from Guizhou, China. Environ Health Perspect. 2007;115:1101–1106. doi: 10.1289/ehp.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leazer TM, Liu Y, Klaassen CD. Cadmium absorption and its relationship to divalent metal transporter-1 in the pregnant rat. Toxicol Appl Pharmacol. 2002;185:18–24. doi: 10.1006/taap.2002.9505. [DOI] [PubMed] [Google Scholar]
- 19.United States Environmental Protection Agency (USEPA) Methods for the determination of organic compounds in drinking water, Method 200.3. 1988 EPA-600/4-88/039. Cincinnati, OH: Environmental Monitoring systems laboratory Office of Research and Development; (Revised July 1991). [Google Scholar]
- 20.United States. Environmental Protection Agency. Office of Research and Development., Environmental Monitoring and Support Laboratory (Cincinnati Ohio) Methods for the determination of metals in environmental samples. Cincinnati, Ohio Springield, Va.: U.S. Environmental Protection Agency, Office of Research and Development available from the National Technical Information Service; 1991. [Google Scholar]
- 21.Yu X, Sidhu JS, Hong S, Faustman EM. Essential role of extracellular matrix (ECM) overlay in establishing the functional integrity of primary neonatal rat Sertoli cell/gonocyte co-cultures: an improved in vitro model for assessment of male reproductive toxicity. Toxicol Sci. 2005;84:378–393. doi: 10.1093/toxsci/kfi085. [DOI] [PubMed] [Google Scholar]
- 22.Nordberg GF. Biomarkers of exposure, effects and susceptibility in humans and their application in studies of interactions among metals in China. Toxicol Lett. 2009 doi: 10.1016/j.toxlet.2009.06.859. [DOI] [PubMed] [Google Scholar]
- 23.Dencker L. Possible mechanisms of cadmium fetotoxicity in golden hamsters and mice: uptake by the embryo, placenta and ovary. Journal of reproduction and fertility. 1975;44:461–471. doi: 10.1530/jrf.0.0440461. [DOI] [PubMed] [Google Scholar]
- 24.Picard V, Govoni G, Jabado N, Gros P. Nramp 2 (DCT1/DMT1) expressed at the plasma membrane transports iron and other divalent cations into a calcein-accessible cytoplasmic pool. The Journal of biological chemistry. 2000;275:35738–35745. doi: 10.1074/jbc.M005387200. [DOI] [PubMed] [Google Scholar]
- 25.Bannon DI, Abounader R, Lees PS, Bressler JP. Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells. Am J Physiol Cell Physiol. 2003;284:C44–C50. doi: 10.1152/ajpcell.00184.2002. [DOI] [PubMed] [Google Scholar]
- 26.Holson JF, Desesso JM, Jacobson CF, Farr CH. Appropriate use of animal models in the assessment of risk during prenatal development: an illustration using inorganic arsenic. Teratology. 2000;62:51–71. doi: 10.1002/1096-9926(200007)62:1<51::AID-TERA10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 27.Chaube S, Nishimura H, Swinyard CA. Zinc and cadmium in normal human embryos and fetuses: analyses by atomic absorption spectrophotometry. Arch Environ Health. 1973;26:237–240. doi: 10.1080/00039896.1973.10666265. [DOI] [PubMed] [Google Scholar]