Abstract
Golgi Phosphoprotein 3 (GOLPH3, also known as GPP34/GMx33/MIDAS) represents an exciting new class of oncoproteins involved in vesicular trafficking. Encoded by a gene residing on human chromosome 5p13, which is frequently amplified in multiple solid tumor types, GOLPH3 was initially discovered as a phosphorylated protein localized to the Golgi apparatus. Recent functional, cell biological and biochemical analyses demonstrate that GOLPH3 can function as an oncoprotein to promote cell transformation and tumor growth by enhancing activity of the mammalian target of rapamycin (mTOR), a serine/threonine protein kinase known to regulate cell growth, proliferation, and survival. While its precise mode-of-action in cancer remains to be elucidated, the fact that GOLPH3 has been implicated in protein trafficking, receptor recycling and glycosylation points to potential links of these cellular processes to tumorigenesis. Understanding how these processes may be deregulated and contribute to cancer pathogenesis and drug response will uncover new avenues for therapeutic intervention.
Background
Emerging evidence supports an important role for vesicular trafficking pathways in cancer. While still in its infancy, this area of cancer research has largely focused on cancer cells' ability to manipulate rudimentary endocytotic pathways to alter cell adhesion, migration and growth signaling (1). In the case of the later, endocytotic pathways serve to regulate signal transduction cascades downstream of growth factor receptors internalized from the cell surface (2). Receptor internalization is critical for attenuating signaling from the cell surface by decreasing the number of available receptors for a given extracellular ligand. The ensuing sorting pathways destine cargo proteins for lysosome-mediated degradation or recycling back to the plasma membrane for reactivation, thereby serving as an important means for regulating homeostasis in receptor signaling. Given their importance in maintaining a balance in growth factor signaling, it is reasonable to expect that deregulated receptor trafficking might provide a mechanism to promote oncogenesis, either through delaying receptor internalization, favoring recycling over degradation or enhancing compartmentalized signaling at vesicles. Indeed, the identification of several bona fide oncoproteins functioning in endocytotic pathways supports a role for aberrant trafficking in the development of cancer. One of the earliest known examples of an endocytotic protein with transforming activity is Huntingtin interacting protein-1 (HIP1), a cofactor involved with clathrin-mediated endocytosis, over-expression of which alters clathrin trafficking leading to delayed endosome-mediated degradation of receptor tyrosine kinases (RTK) and prolonged downstream signaling through the MAPK and PI3K pathways (3, 4). The significance of HIP1 up-regulation is further evidenced through its correlation with poor clinical outcome in a variety of epithelial and lymphoid malignancies (5, 6, 7). Other genes involved in vesicular trafficking have been shown to be directly targeted for amplification in the cancer genome. Among those is RAB25, a small GTPase whose protein family are primary regulators of intracellular protein trafficking pathways (8). RAB25's role in cancer was originally discovered using an integrated genomics approach to identify recurrent amplifications in breast and ovarian carcinoma (9), and subsequent work has documented RAB25 over-expression in numerous epithelial cancers in a manner consistent with increased tumor stage and aggressiveness (10). Using a different genomics approach, Zhang and colleagues identified RAB-coupling protein (RCP/RAB11FIP1) as a target of 8p11–12 amplification in breast cancer (11). RCP is an effector of RAB GTPases that include RAB11 (12, 13), interaction with which facilitates endocytic protein sorting and enhances receptor recycling (14). Similar to RAB25, modulation of RCP levels elicits an array of pro-proliferative and oncogenic phenotypes that include cell proliferation, anchorage-independence, cell migration and tumor growth. Despite these and other advances in identifying endocytotic pathway components that are deregulated or genomically targeted in cancer, altered protein trafficking remains a relatively understudied area of tumorigenesis.
Recently we described a gene discovery approach based on comparing array comparative genomic hybridization profiles of multiple tumor types to prioritize common copy number alterations that are hence more likely to be pathogenetically relevant (15). This analysis identified frequent regional amplification at 5p13, and fluorescence in situ hybridization on tumor tissue microarrays confirmed amplification in multiple tumor types that include breast, colorectal and non-small cell lung cancer (NSCLC) among others. Detailed mapping of one focally-amplified melanoma specimen delimited an informative minimal common region (MCR) of gain comprised of four genes (GOLPH3, MTMR12, ZFR, and SUB1). We showed that 5p13 copy number status was significantly correlated with gene expression of two genes (GOLPH3 and SUB1) in human lung cancer specimens, and subsequent functional studies (RNAi knockdown and cDNA over-expression) of both genes pointed to GOLPH3 as one gene likely targeted for activation in cancers with 5p gain. Specifically, depletion of GOLPH3 abrogates transformation and tumor cell proliferation in GOLPH3-amplified cell lines, and conversely, GOLPH3 over-expression drives transformation of primary cell lines and enhances mouse xenograft tumor growth in vivo. Biochemical and cell biological studies revealed that GOLPH3 physically interacts with the VPS35 subunit of the retromer protein recycling complex and enhances signaling through mTOR, which integrates input from multiple signaling pathways to control cell growth, proliferation and survival (16). Importantly, 5p13 copy number correlates with increased phosphorylation of the mTOR substrate p70 S6 Kinase in NSCLC tumor specimens, which further links GOLPH3 function to mTOR activity. When transplanted into immunodeficient hosts, human tumor cell lines over-expressing GOLPH3 not only develop tumors faster than control, but also are markedly more sensitive to rapamycin, a potent inhibitor of mTOR (17), providing pharmacological proof that GOLPH3's pro-tumorigenic activity is mediated through mTOR signaling. This also raises the possibility that GOLPH3 expression level or copy number status may serve as a predictive biomarker for sensitivity to mTOR inhibitors. While the precise mechanistic basis for GOLPH3's activity on mTOR signaling remains to be fully elucidated, several lines of evidence suggest that GOLPH3 plays a role in vesicular trafficking and protein glycosylation pathways.
GOLPH3 is a highly conserved 34KDa protein initially identified through proteomic characterizations of the Golgi apparatus (18, 19). These efforts found GOLPH3 to be a peripheral membrane protein primarily enriched at the trans face of Golgi cisternae. Moreover, GOLPH3 is highly post-translationally modified, having numerous predicted phosphorylation and myristylation modification sites that possibly influence the protein's activity or localization (18). Detergent partitioning assays suggested that GOLPH3 is dynamically associated with the Golgi, localizing at the Golgi membrane as well as in a cytosolic pool. Cell-based assays confirmed GOLPH3's ability to rapidly exchange between cytosolic and Golgi-associated pools, and the protein was additionally found to associate with tubules and vesicles leaving the Golgi (20). Complementary vesicle budding assays indicated that GOLPH3 associates with vesicles in a manner promoted by ATP and, similar to other Golgi-bound proteins whose localization is regulated by GTPases, GOLPH3 can be sequestered to vesicles through use of non-hydrolizable GTP analogs (20). The same authors additionally found GOLPH3 to localize to peripheral structures that include endosomal compartments and the plasma membrane, which suggests that GOLPH3 may traffic to these structures hence facilitating cargo sorting from the Golgi. This hypothesis is supported by work in Saccharomyces cerevisiae, where deletion of the yeast homolog of GOLPH3, VPS74, resulted in defective trafficking and secretion of vacuolar proteases (21). Furthermore, mutations in VPS74 result in synthetic growth defects when combined with mutations in GET1/GET2 and RIC1/YPT6, all components of protein transport complexes (22, 23).
GOLPH3's potential role in trafficking is further supported by its interaction with phosphatidylinositol-4-phosphate (PtdIns(4)P) (24, 25), which is a phosphatidylinositol lipid molecule whose synthesis is catalyzed by PtdIns 4-kinases (PI4K) (26). PtdIns(4)P is found in most subcellular locations (27) and is known to play important roles in Golgi function (28). GOLPH3 co-localizes with PtdIns(4)P at the Golgi, tubules and vesicles, and direct binding of GOLPH3 to PtdIns(4)P is required for GOLPH3's localization and normal vesicular sorting. This is reinforced by the recent finding that Pik1, the sole Golgi-localized PI4K in S. cerevisiae, is required for targeting Vps74 to the Golgi in yeast (25). Wood and colleagues additionally solved the x-ray crystal structure of human GOLPH3, which allowed for prediction of a putative PtdIns(4)P-binding sites that when disrupted leads to grossly mislocalized GOLPH3 protein and disrupted trafficking. This later finding is consistent with other studies showing that over-expression and depletion of PI4-kinases, which catalyzes formation of PtdIns(4)P, can increase and decrease Golgi to plasma membrane sorting, respectively (29, 30). In addition to the trafficking defects, Dippold and colleagues also noted an altered Golgi architecture upon GOLPH3 depletion (24). The change in Golgi morphology likely results from GOLPH3's interaction with the unconventional myosin, MYO18A, which links GOLPH3 to the actin cytoskeleton. Moreover, the authors suggest a model whereby GOLPH3 binds to PtdIns(4)P, MYO18A and F-actin to form a complex required for maintaining a tensile force necessary for Golgi architecture and vesicular trafficking. Collectively, these data point to a role for GOLPH3 in maintaining Golgi function and protein sorting pathways.
Similar to RAB25 and RCP (9, 11, 31), GOLPH3 enhances downstream growth signaling in response to RTK activation (15). Possible explanations may derive from the finding that GOLPH3 interacts with VPS35, a component of the retromer complex that participates in endosomes-to-Golgi trafficking of transmembrane cargo (15, 32, 33). Proper retromer function has been shown to be required for Wnt signaling during development in five recent studies (34, 35, 36, 37, 38). Briefly, it was shown that inhibition of retromer function through depletion of VPS35 destabilizes Wntless by impeding its recycling following internalization, and retromer-mediated recycling of the Wntless receptor is essential for proper secretion of the Wnt morphogens. Given the well-recognized role of Wnt signaling in cancer (39), it is reasonable to speculate that the deregulation of retromer function via GOLPH3 (and its interaction with VPS35) may impact on function of Wnt and other cell-surface receptors, particularly the RTKs, leading to its cancer-relevant activities. This is supported by the finding that sorting nexin-1 (SNX1), a component of the mammalian retromer complex, is important for transport of epidermal growth factor receptor (EGFR) (40). Moreover, depletion of Drosophila VPS35 was found to inhibit endocytosis of several transmembrane proteins that include the Toll receptor, EGFR and the PDGF and VEGF-receptor-related receptor (PVR) with concomitant increase in plasma membrane localization and correspondingly increase in signaling via downstream components promoting cell proliferation (41). These observations together raise the possibility that GOPLH3 may function with VPS35 to regulate receptor recycling of key surface molecules thus influencing downstream signaling.
A primary function of the Golgi apparatus involves the processing and trafficking of glycosylated proteins and lipid molecules. Another route through which GOLPH3 might regulate signaling stems from two recent reports identifying a function for the yeast homolog (Vps74) in regulating Golgi-localization of protein glycosyltransferases (42, 43). Both studies found that Vps74 interacts with cytosolically-exposed N-terminal tails of protein glycosyltransferases thereby anchoring the enzymes at the Golgi and ultimately regulating glycoprotein processing. These findings are supported by the work of Wood and colleagues, who showed that PtdIns(4)P-binding site mutations (discussed above) in VPS74 phenocopied the glycosyltransferase anchoring defect observed in VPS74-deleted cells and led to secretion of under-glycosylated proteins (25). These observations are of interest given that altered glycosylation is a hallmark feature of tumorigenesis (44). Glycan structures are well-known markers for tumor progression and are associated with numerous pathological events in cancer that include cell growth, adhesion, migration and invasion, immune recognition and signal transduction. In the case of signal transduction, it is noteworthy that glycosylation has been proven important for growth factor-activation of transmembrane receptors (45). For example, the RTK epidermal growth factor receptor (EGFR) contains multiple glycan moieties (46), and glycosylation at these residues is necessary for both EGFR sorting and subsequent ligand binding (47, 48). Moreover, Golgi glycosyltransferase activity can alter endocytosis of transmembrane receptors, which can lead to altered sensitivity to receptor ligand (44). In the case of EGFR, modified N-glycosylation sequesters EGFR at the plasma membrane by resisting internalization thereby resulting in prolonged responsiveness to growth factor (49). Future work will be required to determine whether GOLPH3 modulates glycosyltransferase retention and/or receptor internalization in mammalian cells, and more importantly whether these processes provide a mechanism to regulate the mTOR signaling response mediated by GOLPH3.
Clinical-Translational Advances
While the studies reviewed here offer initial insight into GOLPH3's function, much work is still needed to determine how this protein influences malignant transformation and cell growth (Figure 1). It will be important to determine whether association with the retromer complex is required for GOLPH3-mediated signaling through mTOR, a finding that would obligate much more comprehensive examination of retromer function in cancer. Compelling work in S. cerevisiae (50) already support an mTOR-retromer link. Using a proteomics approach, the yeast mTOR orthologs (TOR1 and TOR2) were found to associate with multiple regulators of endocytosis in unique membrane fraction isolates. Subsequent genetic analyses using cells mutated for components of the TOR complexes 1 and 2 (TORC1 and TORC2) revealed many synthetic interactions between TORC1, which is the rapamycin-sensitive TOR complex, and regulators of receptor-mediated endocytosis. Based on these findings, the authors defined a genetic network for TORC1 and endocytosis genes, leading to identification of key vesicular transport genes. Remarkably, two of the top four scoring hits were VPS29 and VPS35, both of which are retromer subunits additionally shown to exhibit synthetic growth defects when mutated in TOR1-deleted cells. Moreover, the authors discovered that TOR1/2 associates with endosome-like compartments, and inhibiting TORC1 by rapamycin treatment significantly delayed endocytosis thus further supporting a novel connection between TOR and vesicular trafficking.
Figure 1. Illustration depicting possible routes for GOLPH3-mediated oncogenic signaling.
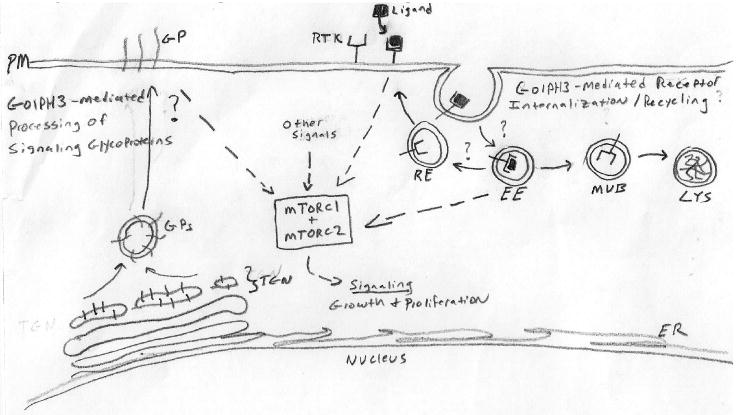
Following ligand-binding, cell surface receptors such as receptor tyrosine kinases (RTK) are destined for internalization for receptor recycling or lysosome-mediated destruction. GOLPH3 localization to the Golgi apparatus and likely other non-Golgi structures is dependent on its interaction with PtdIns(4)P, synthesis of which is requires one of four mammalian PI4-kinases (PI4KIIα/β) spatially distributed at the Golgi, ER, plasma membrane, and endosomes (27). Over-expression of GOLPH3 exerts its gain-of-function effect on signaling through mTOR complex 1 and 2 (mTORC1 and mTORC2), possibly by way of delaying receptor internalization or enhancing receptor recycling. Alternatively, GOLPH3's role in glycosyltransferase anchoring may influence signaling through enhanced glycosylation/altered secretion of cancer-relevant glycoproteins (GP) participating in signaling events upstream of mTORC1/2. Abbreviations: EE, early endosomes; RE, recycling endosome; MVB, multivesicular body; Lys, lysosome; TGN, trans-Golgi network; ER, endoplasmic reticulum; PM, plasma membrane.
In addition to follow-up studies examining GOLPH3 and retromer-mediated mTOR signaling, it will also be important to assess the relevance of GOLPH3's PtdIns(4)P-binding activity in the context of cancer signaling, which may offer clues to whether GOLPH3's oncogenic activity requires recruitment to the Golgi or stems from Golgi-independent gain-of-function activities outside of the Golgi. Along these lines of reasoning, it is worth noting the emerging importance of “non-Golgi pools” of PtdIns(4)P at the plasma membrane and other non-Golgi membranous structures (28). Brought about by spatially-distributed PI4K isoforms (27), these non-Golgi PtdIns(4)P pools have been tied to vesicular trafficking, degradation and recycling. When coupled with GOLPH3's interaction with PtdIns(4)P and association with endosomes and the plasma membrane, these findings together suggest that GOLPH3 may provide a functional role away from the Golgi. GOLPH3's tight association with PtdIns(4)P also warrants a more careful examination of PI4K activity in cancer, including the screening of tumor specimens for cancer-relevant mutations in PI4K family members. Given the finding that disruption of VPS74 impairs glycosyltransferase docking and results in under-glycosylation of secreted proteins, it will also be important to determine whether GOLPH3's oncogenic activity occurs by way of mediating glycosylation changes on cancer-relevant molecules. In this scenario GOLPH3 may exert its influence on mTOR signaling by an indirect means, perhaps by hyper-activating an upstream regulator of mTOR through increased glycosylation, rather than direct association with the mTOR itself. Last but not the least, it will be of great interest to assess human GOLPH3's broader impact on total glycan structures given Vps74's important role in regulating Golgi glycosyltransferases, potentially revealing new insights on how glycans influence host-tumor interactions and tumor progression.
In addition to examining GOLPH3's potential roles in regulating protein trafficking and glycosylation pathways, insight into the role of its phosphorylation may provide clues into its regulation and function. Future work should also include a careful examination of the protein's expression in cancer. It will be especially important to measure GOLPH3's expression in the context of mTOR-inhibition to test GOLPH3's potential utility in predicting response as suggested by pre-clinical studies in mice (15). Additional positive findings in this regard would warrant assessing GOLPH3 copy number or expression status in cancer clinical trials using rapamycin-related analogs, response to which have been for the most part disappointing (17).
In summary, GOLPH3's role in vesicular trafficking and glycosylation, against the backdrop of its proven oncogenicity, has charted new paths to elucidating how these cellular pathways can influence malignancy. Components making up these pathways might represent novel therapeutic targets against cancer; therefore, additional insight into GOLPH3's biological activity may ultimately serve as an entry point to translate modifiers of these pathways into clinical endpoints
Acknowledgments
Grant support: This work was primarily supported by grants from the NIH to L.C. (RO1 CA93947 and P50 CA93683) and grants from the NIH and American Cancer Society to K.L.S. (5-T32-AR07098-31 and PF-07-039-01-CSM, respectively).
References
- 1.Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8:835–50. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]
- 2.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–22. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao DS, Bradley SV, Kumar PD, et al. Altered receptor trafficking in Huntingtin Interacting Protein 1-transformed cells. Cancer Cell. 2003;3:471–82. doi: 10.1016/s1535-6108(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 4.Hyun TS, Rao DS, Saint-Dic D, et al. HIP1 and HIP1r stabilize receptor tyrosine kinases and bind 3-phosphoinositides via epsin N-terminal homology domains. J Biol Chem. 2004;279:14294–306. doi: 10.1074/jbc.M312645200. [DOI] [PubMed] [Google Scholar]
- 5.Rao DS, Hyun TS, Kumar PD, et al. Huntingtin-interacting protein 1 is overexpressed in prostate and colon cancer and is critical for cellular survival. J Clin Invest. 2002;110:351–60. doi: 10.1172/JCI15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyun TS, Ross TS. HIP1: trafficking roles and regulation of tumorigenesis. Trends Mol Med. 2004;10:194–9. doi: 10.1016/j.molmed.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Bradley SV, Smith MR, Hyun TS, et al. Aberrant Huntingtin interacting protein 1 in lymphoid malignancies. Cancer Res. 2007;67:8923–31. doi: 10.1158/0008-5472.CAN-07-2153. [DOI] [PubMed] [Google Scholar]
- 8.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–25. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 9.Cheng KW, Lahad JP, Kuo WL, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10:1251–6. doi: 10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R, Jurisica I, Mills GB, Cheng KW. The Emerging Role of the RAB25 Small GTPase in Cancer. Traffic. 2009 doi: 10.1111/j.1600-0854.2009.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Liu X, Datta A, et al. RCP is a human breast cancer-promoting gene with Ras-activating function. J Clin Invest. 2009;119:2171–83. doi: 10.1172/JCI37622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hales CM, Griner R, Hobdy-Henderson KC, et al. Identification and characterization of a family of Rab11-interacting proteins. J Biol Chem. 2001;276:39067–75. doi: 10.1074/jbc.M104831200. [DOI] [PubMed] [Google Scholar]
- 13.Lindsay AJ, Hendrick AG, Cantalupo G, et al. Rab coupling protein (RCP), a novel Rab4 and Rab11 effector protein. J Biol Chem. 2002;277:12190–9. doi: 10.1074/jbc.M108665200. [DOI] [PubMed] [Google Scholar]
- 14.Peden AA, Schonteich E, Chun J, Junutula JR, Scheller RH, Prekeris R. The RCP-Rab11 complex regulates endocytic protein sorting. Mol Biol Cell. 2004;15:3530–41. doi: 10.1091/mbc.E03-12-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott KL, Kabbarah O, Liang MC, et al. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature. 2009;459:1085–90. doi: 10.1038/nature08109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 18.Wu CC, Taylor RS, Lane DR, Ladinsky MS, Weisz JA, Howell KE. GMx33: a novel family of trans-Golgi proteins identified by proteomics. Traffic. 2000;1:963–75. [PubMed] [Google Scholar]
- 19.Bell AW, Ward MA, Blackstock WP, et al. Proteomics characterization of abundant Golgi membrane proteins. J Biol Chem. 2001;276:5152–65. doi: 10.1074/jbc.M006143200. [DOI] [PubMed] [Google Scholar]
- 20.Snyder CM, Mardones GA, Ladinsky MS, Howell KE. GMx33 associates with the trans-Golgi matrix in a dynamic manner and sorts within tubules exiting the Golgi. Mol Biol Cell. 2006;17:511–24. doi: 10.1091/mbc.E05-07-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonangelino CJ, Chavez EM, Bonifacino JS. Genomic screen for vacuolar protein sorting genes in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:2486–501. doi: 10.1091/mbc.02-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong AH, Lesage G, Bader GD, et al. Global mapping of the yeast genetic interaction network. Science. 2004;303:808–13. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- 23.Pan X, Ye P, Yuan DS, Wang X, Bader JS, Boeke JD. A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell. 2006;124:1069–81. doi: 10.1016/j.cell.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 24.Dippold HC, Ng MM, Farber-Katz SE, et al. GOLPH3 bridges phosphatidylinositol-4- phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell. 2009;139:337–51. doi: 10.1016/j.cell.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood CS, Schmitz KR, Bessman NJ, Setty TG, Ferguson KM, Burd CG. PtdIns4P recognition by Vps74/GOLPH3 links PtdIns 4-kinase signaling to retrograde Golgi trafficking. J Cell Biol. 2009 doi: 10.1083/jcb.200909063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong K, Cantley LC. Cloning and characterization of a human phosphatidylinositol 4-kinase. J Biol Chem. 1994;269:28878–84. [PubMed] [Google Scholar]
- 27.Wong K, Meyers dd R, Cantley LC. Subcellular locations of phosphatidylinositol 4-kinase isoforms. J Biol Chem. 1997;272:13236–41. doi: 10.1074/jbc.272.20.13236. [DOI] [PubMed] [Google Scholar]
- 28.D'Angelo G, Vicinanza M, Di Campli A, De Matteis MA. The multiple roles of PtdIns(4)P -- not just the precursor of PtdIns(4,5)P2. J Cell Sci. 2008;121:1955–63. doi: 10.1242/jcs.023630. [DOI] [PubMed] [Google Scholar]
- 29.Wang YJ, Wang J, Sun HQ, et al. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114:299–310. doi: 10.1016/s0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- 30.Hausser A, Storz P, Martens S, Link G, Toker A, Pfizenmaier K. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex. Nat Cell Biol. 2005;7:880–6. doi: 10.1038/ncb1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D, Norman JC. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol. 2008;183:143–55. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seaman MN. Recycle your receptors with retromer. Trends Cell Biol. 2005;15:68–75. doi: 10.1016/j.tcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20:427–36. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belenkaya TY, Wu Y, Tang X, et al. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14:120–31. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Franch-Marro X, Wendler F, Guidato S, et al. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10:170–7. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan CL, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell. 2008;14:132–9. doi: 10.1016/j.devcel.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Port F, Kuster M, Herr P, et al. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol. 2008;10:178–85. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- 38.Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell. 2008;14:140–7. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Kurten RC, Cadena DL, Gill GN. Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science. 1996;272:1008–10. doi: 10.1126/science.272.5264.1008. [DOI] [PubMed] [Google Scholar]
- 41.Korolchuk VI, Schutz MM, Gomez-Llorente C, et al. Drosophila Vps35 function is necessary for normal endocytic trafficking and actin cytoskeleton organisation. J Cell Sci. 2007;120:4367–76. doi: 10.1242/jcs.012336. [DOI] [PubMed] [Google Scholar]
- 42.Schmitz KR, Liu J, Li S, et al. Golgi localization of glycosyltransferases requires a Vps74p oligomer. Dev Cell. 2008;14:523–34. doi: 10.1016/j.devcel.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tu L, Tai WC, Chen L, Banfield DK. Signal-mediated dynamic retention of glycosyltransferases in the Golgi. Science. 2008;321:404–7. doi: 10.1126/science.1159411. [DOI] [PubMed] [Google Scholar]
- 44.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–67. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi M, Tsuda T, Ikeda Y, Honke K, Taniguchi N. Role of N-glycans in growth factor signaling. Glycoconj J. 2004;20:207–12. doi: 10.1023/B:GLYC.0000024252.63695.5c. [DOI] [PubMed] [Google Scholar]
- 46.Cummings RD, Soderquist AM, Carpenter G. The oligosaccharide moieties of the epidermal growth factor receptor in A-431 cells. Presence of complex-type N-linked chains that contain terminal N-acetylgalactosamine residues. J Biol Chem. 1985;260:11944–52. [PubMed] [Google Scholar]
- 47.Soderquist AM, Carpenter G. Glycosylation of the epidermal growth factor receptor in A-431 cells. The contribution of carbohydrate to receptor function. J Biol Chem. 1984;259:12586–94. [PubMed] [Google Scholar]
- 48.Gamou S, Shimizu N. Glycosylation of the epidermal growth factor receptor and its relationship to membrane transport and ligand binding. J Biochem. 1988;104:388–96. doi: 10.1093/oxfordjournals.jbchem.a122478. [DOI] [PubMed] [Google Scholar]
- 49.Partridge EA, Le Roy C, Di Guglielmo GM, et al. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004;306:120–4. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- 50.Aronova S, Wedaman K, Anderson S, Yates J, 3rd, Powers T. Probing the membrane environment of the TOR kinases reveals functional interactions between TORC1, actin, and membrane trafficking in Saccharomyces cerevisiae. Mol Biol Cell. 2007;18:2779–94. doi: 10.1091/mbc.E07-03-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]


