Abstract
Hippocampal inhibitory interneurons expressing glutamate decarboxylase‐67 (GAD‐67) considerably decline in number during old age. Studies in young adult animals further suggest that hippocampal GAD‐67+ interneuron population is highly vulnerable to excitotoxic injury. However, the relative susceptibility of residual GAD‐67+ interneurons in the aged hippocampus to excitotoxic injury is unknown. To elucidate this, using both adult and aged F344 rats, we performed stereological counting of GAD‐67+ interneurons in different layers of the dentate gyrus and CA1 & CA3 sub‐fields, at 3 months post‐excitotoxic hippocampal injury inflicted through an intracerebroventricular administration of kainic acid (KA). Substantial reductions of GAD‐67+ interneurons were found in all hippocampal layers and sub‐fields after KA‐induced injury in adult animals. Contrastingly, there was no significant change in GAD‐67+ interneuron population in any of the hippocampal layers and sub‐fields following similar injury in aged animals. Furthermore, the stability of GAD‐67+ interneurons in aged rats after KA was not attributable to milder injury, as the overall extent of KA‐induced hippocampal principal neuron loss was comparable between adult and aged rats. Interestingly, because of the age‐related disparity in vulnerability of interneurons to injury, the surviving GAD‐67+ interneuron population in the injured aged hippocampus remained comparable to that observed in the injured adult hippocampus despite enduring significant reductions in interneuron number with aging. Thus, unlike in the adult hippocampus, an excitotoxic injury to the aged hippocampus does not result in significantly decreased numbers of GAD‐67+ interneurons. Persistence of GAD‐67+ interneuron population in the injured aged hippocampus likely reflects an age‐related change in the response of GAD‐67+ interneurons to excitotoxic hippocampal injury. These results have implications towards understanding mechanisms underlying the evolution of initial precipitating injury into temporal lobe epilepsy in the elderly population.
Keywords: brain injury, chronic epilepsy, dentate gyrus, GABA‐ergic interneurons, hippocampus, kainic acid, neurodegeneration, seizures, temporal lobe epilepsy
Introduction
Interneurons in the hippocampus are gamma‐amino butyric acid (GABA) expressing non‐principal neurons distributed in different strata of the dentate gyrus (DG) and hippocampal CA1 and CA3 sub‐fields. Inhibitory input from various populations of GABA‐ergic interneurons to principal neurons in different sub‐fields of the hippocampus serves to maintain the network stability [1]. Any disinhibition of the principal excitatory neurons due to compromised inhibitory input from the GABA‐ergic interneurons (such as that occurs in temporal lobe epilepsy [TLE]) leads to hyperexcitability [2, 3, 4]. The above conclusion is also confirmed by the observations that GABA agonists suppress seizures, GABA antagonists and drugs that inhibit GABA synthesis induce seizures, and drugs that increase synaptic GABA are potent anticonvulsants [5]. Thus, maintenance of critical number of GABA‐synthesizing interneurons in different sub‐fields of the hippocampus appears critical for stabilizing excitatory influences and synchronizing principal excitatory neuron populations in the hippocampus [1, 4].
The GABA‐ergic interneuron population in the hippocampus is vulnerable to changes such as aging and excitotoxic hippocampal injury. Several studies have demonstrated that aging leads to decreased numbers of GABA‐ergic interneurons in all sub‐fields of the hippocampus [6, 7, 8]. Aging is also associated with increased excitability of principal hippocampal neurons [9, 10, 11, 12, 13], and pyramidal neurons in the aging hippocampus exhibit higher frequency bursting comprising sharp‐wave–ripple complexes during large irregular activity [14]. When taken together, the above findings suggest that decreased number of GABA‐ergic interneurons contributes to increased excitability of principal neurons in the aged hippocampus. A reduced functional inhibition observed in the aging hippocampus [15, 16] also supports the above possibility. Thus, the hippocampal circuitry in the aged hippocampus appears to be pro‐excitatory and highly vulnerable to conditions such as epilepsy. Indeed, the aged hippocampus exhibits increased vulnerability to epileptic seizures after injury or exposure to excitotoxins [17, 18, 19].
Furthermore, studies in young and adult animals suggest that excitotoxic hippocampal injury reduces the GABA‐ergic interneuron population considerably [20]. From this perspective, it is plausible that excitotoxic injury to the aged hippocampus might dramatically deplete the residual interneuron population and result in rapid progression of the initial precipitating injury into chronic epilepsy. Currently, it is however unknown whether or not the residual GABA‐ergic interneuron population in the aged hippocampus remains vulnerable to seizures or injury induced by excitotoxins. Nevertheless, such studies are needed, as analyses of the survival of interneurons in the aged hippocampus following administration of excitotoxins have importance for understanding the increased vulnerability of the aged population for developing chronic epilepsy after brain injury resulting from stroke, seizures and head injury [21, 22].
Therefore, in this study, we comprehensively quantified the numbers of GABA‐ergic interneurons in different strata of the DG and CA1 and CA3 sub‐fields of adult and aged F344 rats using stereology, at 3 months after an excitotoxic hippocampal injury inflicted through a unilateral intracerebroventricular kainic acid (ICV KA) administration. To ascertain the relative reductions in GABA‐ergic interneuron numbers following an excitotoxic hippocampal injury in the two age groups, we compared the counts of GABA‐ergic interneurons from KA‐injured hippocampi with age‐matched intact hippocampi. Kainic acid (KA), the prototype agonist for the kainate sub‐type of glutamate receptor, is a widely used excitatory compound in experimental studies of the hippocampus [23, 24, 25, 26]. Intracerebroventricular administration of KA in rat is an animal model used extensively for studying lesion recovery, hippocampal hyperexcitability and TLE, as this model exhibits loss of substantial fractions of CA3 pyramidal neurons and dentate hilar cells [24, 25, 26, 27, 28, 29, 30, 31, 32, 33]. This pattern of neurodegeneration also leads to diffuse and pervasive synaptic re‐organization, hyperexcitability and reduced numbers of GABA‐ergic interneurons in both CA1 sub‐field and DG of the hippocampus in young adult animals [20]. For visualizing GABA‐ergic interneurons in this study, we performed immunohistochemistry using an antibody against glutamate decarboxylase‐67 (GAD‐67), a synthesizing enzyme of GABA found in soma of interneurons [34, 35]. As studies suggest that GAD‐67 provides the basal pool of GABA, antibodies to GAD‐67 are considered as dependable immunocytochemical markers for cell bodies of GABA‐ergic neurons in the CNS [34, 35, 36, 37, 38].
Materials and methods
Animals
Both adult and aged male F344 rats were acquired from the National Institute of Aging colony located at Harlan Sprague–Dawley (Indianapolis, IN). Four groups of rats that received regular diet (i.e. rat chow) for the entire duration of experiments were used in this study. These include adult rats receiving ICV KA (4 months old, n= 6) and aged rats receiving ICV KA (20 months old, n= 6). As ICV KA–treated rats were analyzed at 3 months post‐KA, age‐matched naïve adult (7 months old, n= 5) and naïve aged (23 months old, n= 5) rats were used for comparison. We chose naïve rats instead of rats treated with ICV vehicle solution for comparison in this study because we demonstrated earlier that sham surgery in adult rats involving injection of saline (instead of KA) into the lateral ventricle does not induce hippocampal neurodegeneration [27]. In the above study, an ICV saline administration (comprising the surgical procedure that is identical to that of ICV KA administration performed in this study) did not induce any changes in hippocampal cell layers, hippocampal interneuron population positive for calbindin, the calbindin expression within dentate granule cells and CA1 pyramidal cell layer and the expression of non‐phosphorylated neurofilament protein in the entire hippocampus. Thus, the experimental procedures such as anaesthesia and sham surgery do not alter numbers of interneurons in the adult hippocampus. All experiments were performed as per the animal protocol approved by the animal studies sub‐committee of the Durham Veterans Affairs Medical Center and the institutional animal care and use committee of the Duke University Medical Center. Moreover, all experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23). Furthermore, all efforts were made to minimize the number of animals used and their suffering.
Unilateral kainic acid injections into the lateral ventricle
The KA was purchased from Tocris bioscience (Ellisville, MO), and the detailed methods for unilateral ICV KA administration are described in our previous reports [29, 30, 31]. In brief, each rat was first anaesthetized via an intramuscular injection of an anaesthetic mixture comprising ketamine (50 mg/ml), xylazine (6 mg/ml) and acepromazine (0.5 mg/ml) at a dose of 1.25 ml/kg body weight. Following this, the head was fixed into a stereotaxic apparatus with incisor bars set at 3.7 mm below the interaural line. The dorsal surface of the skull was exposed through a midline incision and a burr hole was drilled in the skull using the following stereotaxic coordinates: antero‐posterior (AP) = 3.7 mm caudal to bregma; lateral (L) = 4.1 mm right lateral to the midline. A 10 μl Hamilton syringe fitted with a 25‐gauge needle and filled with KA solution in saline was placed over the burr hole using stereotaxis, and the needle was lowered through the cerebral cortex and corpus callosum into the lateral ventricle (equivalent to a depth of 4.5 mm from the surface of the brain). One microlitre (containing 0.5 μg of KA) of the solution was injected slowly (0.2 μl/min.) into the lateral ventricle, the needle was left in place for 15 min. and withdrawn slowly afterwards.
NeuN immunostaining and selection of animals for quantification of NeuN+ neurons in different regions of the hippocampus
All rats were deeply anaesthetized with halothane and perfused through the heart with 150 ml of physiological saline containing 0.1% heparin for 5–8 min. followed by 500 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4) for 30 min. All KA‐treated rats were perfused at 3 months post‐KA administration. The brains were removed, post‐fixed in the same 4% paraformaldehyde solution for 18 hrs at 4°C, cryoprotected using sucrose solution, and 30‐μm‐thick cryostat sections were cut coronally through the entire hippocampus and collected in PB. To visualize the extent of hippocampal injury following unilateral ICV KA administration in adult and aged rats, every 15th section through the hippocampus in each rat belonging to KA‐treated groups was stained for Nissl, and every 20th section was processed for neuron‐specific nuclear antigen (NeuN) immunostaining [39], using a mouse monoclonal antibody from Chemicon (anti‐NeuN, clone A60, Temecula, CA). Negative control sections were processed in the same manner except that the primary antibody incubation step was replaced by continued incubation in the normal serum. Neither immunostaining nor any recognizable background staining was observed under these conditions in negative control sections.
Only those animals that exhibited classic CA3 lesion in hippocampus ipsilateral to the KA administration (as described in references [29, 30, 31], and as illustrated in Fig. 1 B1 and D1) were chosen for further analyses. Kainic acid administration site was consistent in both young and aged rats chosen for further analyses, as needle track could be traced into the lateral ventricle in Nissl‐stained sections. Furthermore, maximal loss of CA3 pyramidal neurons and moderate loss of CA1 pyramidal neurons observed in hippocampus ipsilateral to KA administration (but not in hippocampus contralateral to KA administration) in both age groups supported the above conclusion. This is because, the above pattern of lesion represents the classic hippocampal lesion after unilateral ICV KA administration [29, 30, 31] and an accidental delivery of KA directly into the hippocampus leads to a massive hippocampal lesion, characterized by almost complete loss of both CA1 and CA3 pyramidal neurons bilaterally. Four animals in each of the two age groups exhibited classic CA3 lesion in hippocampus ipsilateral to ICV KA administration. To ascertain the extent of hippocampal injury after a unilateral ICV KA administration, we measured neuron numbers in different regions of the hippocampus ipsilateral to ICV KA administration in both age groups using the StereoInvestigator system (Microbrightfield Inc., Williston, VT). These numbers were then compared with neuronal counts from similar regions of the hippocampus of age‐matched naïve rats. Specifically, neuron numbers were measured for the dentate hilus (a region comprising both excitatory mossy cells and inhibitory interneurons), the granule cell layer (the major cell layer of the dentate gyrus comprising mostly granule cells) and CA1 and CA3 pyramidal cell layers. Every 20th section through the entire antero‐posterior extent of the hippocampus was utilized for these counts and the methodology used for cell counting is described along with the description for GAD‐67+ cell counts below. In this study, we chose to focus on the hippocampus ipsilateral to ICV KA administration as this side consistently exhibits a clear neurodegeneration. On the other hand, the hippocampus contralateral to ICV KA, though undergoes significant deafferentation (due to loss of commissural axons with the death of CA3 pyramidal neurons in hippocampus ipsilateral to KA administration), does not exhibit an apparent neurodegeneration.
Figure 1.
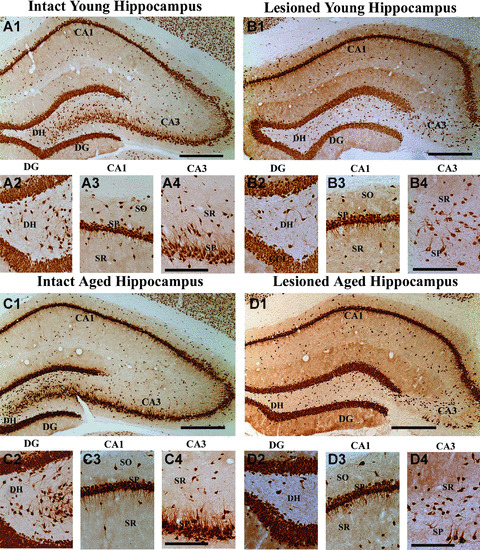
Neuronal loss in the hippocampus of adult and aged rats after an intracerebroventricular (ICV) administration of kainic acid (KA), visualized by NeuN immunostaining. Examples show hippocampal cytoarchitecture in a naïve adult rat (A1), an adult rat that received ICV KA (B1), a naïve aged rat (C1) and an aged rat that received ICV KA (D1). Substantial loss of neurons occurs in the CA3 pyramidal cell layer (B1 and D1) of both adult and aged rats following ICV KA administration. Figures A2–A4, B2–B4, C2–C4 and D2–D4 illustrate magnified views of the dentate hilus (A2, B2, C2, D2), the CA1 sub‐field (A3, B3, C3, D3) and CA3 sub‐field (A4, B4, C4, D4) from figures A1, B1, C1, D1. Note that the extent of neuron loss is comparable in different regions of the hippocampus between adult (B1–B4) and aged (D1–D4) rats following ICV KV administration. DG, dentate gyrus; DH, dentate hilus; SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum. Scale bar, A1, B1, C1, D1 = 400 μm; A2–A4, B2–B4, C2–C4, D2–D4 = 200 μm.
GAD‐67 immunocytochemistry, and quantification of GAD‐67+ interneurons in different layers of the dentate gyrus, and CA1 and CA3 sub‐fields
For GAD‐67 immunostaining, serial sections (every 10th section) through the entire hippocampus were selected in each of the KA‐treated animals (exhibiting classic hippocampal injury described earlier) belonging to the two age groups (n= 4/group). These sections were processed with serial sections from age‐matched naïve adult (n= 4) and naïve aged (n= 4) rats for comparison. The GAD‐67 immunostaining was performed using an indirect immunoperoxidase method, as described in our earlier studies [6, 8]. In brief, sections were rinsed in 0.1 M phosphate‐buffered saline (PBS) and incubated in 1% sodium borohydride solution for 15 min. Sodium borohydride reduces double bonds and free aldehyde groups, which results in enhanced immunoreactivity of protein antigens, including GAD‐67 [6, 20]. Sections were washed in PBS, treated with 10% normal goat serum in PBS for 30 min., incubated overnight in a polyclonal GAD‐67 antibody (1:2000 dilution, Chemicon), which is raised in the rabbit and has been shown to specifically recognize GAD‐67 with Western blot. Following this, sections were rinsed in PBS, treated with peroxidase‐conjugated goat anti‐rabbit IgG (1:500 dilution) for 2 hrs, washed in PBS and the peroxidase reaction was developed using 3,3‐diaminobenzidine (DAB) and nickel chloride as chromogen (Vector, Burlingame, CA). The chromogen reaction was initially standardized under a microscope to find the length of incubation that would result in optimal cell staining with minimal background. This same incubation length was subsequently used for staining across the age groups. The sections were mounted on gelatinized slides, dehydrated, cleared and cover‐slipped with vectamount (Vector). To exclude any possible effects of staining protocol on the number of GAD‐67 positive neurons, sections from different groups of rats were processed in parallel with identical concentrations of primary and secondary antibody solutions. Furthermore, the concentration of DAB and hydrogen peroxide for the chromogen reaction was kept constant. Negative control sections were processed in the same manner except that the primary antibody incubation step was replaced by continued incubation in the normal serum. Neither immunostaining nor any recognizable background staining was observed under these conditions in negative control sections.
The numbers of GAD‐67+ interneurons in each animal belonging to the four groups (n= 4/age group) were measured separately in two regions of the DG (dentate hilus plus GCL [DH+GCL] and the dentate molecular layer [ML]) and three distinct layers (strata oriens [SO], pyramidale [SP] and radiatum [SR]) of CA1 and CA3 sub‐fields. In CA1 and CA3 sub‐fields, the stratum lacunosum moleculare (SLM) was included with the SR. This is because SLM is a very narrow zone of tissue, and the demarcation between SR and SLM was not clear in sections immunostained for GAD‐67 in some of the groups. Thus, we measured GAD‐67+ interneurons from all layers of the DG, and CA1 and CA3 sub‐fields. The layers of smaller CA2 sub‐field were included with the layers of CA1 sub‐field. Cells were counted in every 10th section through the entire antero‐posterior extent of the hippocampus using the StereoInvestigator system.
Stereological quantification of NeuN+ neurons and GAD‐67+ interneurons
The StereoInvestigator system consisted of a colour digital video camera (Optronics Inc., Muskogee, OK) interfaced with a Nikon E600 microscope (Nikon Instrument Inc., Melville, NY, USA). In each hippocampus, for every layer listed earlier, NeuN+ neurons/GAD‐67+ interneurons were counted from 50–400 randomly and systematically selected frames (measuring 25 × 25 μm for NeuN+ cell counts, and 50 × 50 μm for GAD‐67+ cell counts) in each of the selected sections (every 20th for NeuN+ cell counts and every 10th for GAD‐67+ cell counts) using the 100× oil immersion objective lens. The systematic random sampling procedure provides an unbiased and efficient sampling technique. In this scheme, once the region of interest is marked with a contour, sampling sites are evenly distributed throughout the region of interest. The distribution pattern of the sampling sites in each tissue section is systematic, as the distance from each sampling site to the next is the same. The placement of this pattern of sampling sites is the random component. The number and density of frames were selected using the optical fractionator component of the StereoInvestigator system. For each section, the number of frames per section counted varied because the overall area of different strata and regions changed from section to section. For instance, different strata and regions in anterior‐most sections through the hippocampus were smaller than areas in sections through the intermediate and posterior regions of the hippocampus; as a result, cells were counted from a larger number of frames in sections through the intermediate and posterior regions of the hippocampus. The above sampling scheme is consistent with the principle of the optical dissector counting method that the density of sampling units must remain constant for each section. Thus, counting of cells from 50 to 400 randomly and systematically selected frames in every chosen section through different layers of the hippocampus guaranteed that effectively every NeuN+/GAD‐67+ cell in these layers had equal odds of being counted. This is especially imperative for measurement of GAD‐67+ interneurons, as the distribution of interneurons in all layers of the hippocampus was characteristically heterogeneous.
In every section, the contour of every hippocampal layer selected was first delineated for counting using tracing function of the StereoInvestigator. Following this, the optical fractionator component was activated, and the number and location of counting frames and the counting depth for that section was determined by entering parameters such as the grid size, the thickness of guard zone (4 μm) and the optical dissector height (i.e. 8 μm). A computer‐driven motorized stage then allowed the section to be analyzed at each of the counting frame locations. In every counting frame location, the top of the section was set, after which the plane of the focus was moved 4 μm deeper through the section (guard zone) to eliminate the problem of uneven section surface. This plane served as the first point of the counting process. Continuing to focus down, any NeuN+/GAD‐67+ positive cell bodies that came into focus in the next 8‐μm section thickness were counted if they were entirely within the counting frame or touching the upper or right side of the counting frame. Thus, all NeuN+/GAD‐67+ positive cells that were present in the middle 8‐μm section depths were counted in every chosen section.
An option in the StereoInvestigator program allowed the experimenter to remain unaware of the running cell count totals until all sections for each animal were completed. The actual numbers of NeuN+ neurons counted varied from 152 to 286 (coefficient of error [CE]= 0.06–0.09) for GCL, 60–167 for the dentate hilus and the CA1 pyramidal cell layer (CE = 0.08–0.1) and 12–134 for the CA3 pyramidal cell layer (CE = 0.09–0.29). Because of substantial loss of CA3 pyramidal neurons after ICV KA–induced injury in both age groups, a greater range of CE value was apparent for the CA3 pyramidal cell layer. The actual numbers of GAD‐67+ interneurons counted for individual layers of the DG, and CA1 and CA3 sub‐fields per animal varied considerably between different groups because of both age‐related and KA‐injury–related reductions in numbers. In the DG, it varied from 61 to 196 for DH+GCL (CE = 0.07–0.15) and 56 to 172 for ML (CE = 0.09–0.14). In the CA1 sub‐field, it varied from 32 to 131 for SO (CE = 0.09–0.18), 36 to 180 for SP (CE = 0.08–0.17) and 49 to 240 for SR (CE = 0.07–0.15). In the CA3 sub‐field, numbers ranged from 12 to 79 for SO (CE = 0.11–0.29), 18 to 86 for SP (CE = 0.11–0.24] and 21 to 140 for SR (CE = 0.08–0.22). A wider range of CE values for layers of the CA3 sub‐field reflect greater reductions in the numbers of GAD‐67+ interneurons after KA‐induced injury in the adult group.
Based on the above parameters and cell counts, the StereoInvestigator program calculated the total number of NeuN+/GAD‐67+ cells per selected hippocampal layer by utilizing the optical fractionator formula, N = 1/ssf.1/ asf.1/hsf.EQ−. The abbreviation ssf represents the section sampling fraction, which is 20 for NeuN+ cell counting and 10 for GAD‐67+ cell counting; asf symbolizes the area sampling fraction, which is calculated by dividing the area sampled with the total area of the layer; hsf stands for the height sampling fraction, which is calculated by dividing the height sampled (i.e. 8 μm in this study) with the section thickness at the time of analysis (i.e. 19–20 μm in sections from adult rats, 15–16 μm in sections from aged animals); EQ− denotes the total count of particles sampled for the entire layer. The value for different layers was calculated separately for every animal before calculating the mean and standard errors for different groups. In each sub‐field, cumulative interneuron counts from different layers were expressed as the number per sub‐field, and cumulative interneuron counts from the three sub‐fields (DG and CA1 and CA3 sub‐fields) were expressed as the number for the entire hippocampus. The data between intact adult (n= 4), ICV KA–treated adult (n= 4), intact aged (n= 4) and ICV KA–treated aged (n= 4) animals were compared using one‐way analysis of variance (ANOVA) with a Student–Newman–Keuls multiple comparisons post hoc test. All data are presented as means ± standard errors (S.E.M.). In addition, to determine the possible interaction between aging and injury, values from the above four groups were also analyzed using two‐way ANOVA with Bonferroni post‐tests. Specifically, we examined whether the effects of excitotoxic injury on NeuN+ neurons and GAD‐67+ interneurons differ between adult and aged groups.
Measurement of the ratio of GAD‐67+ interneurons to NeuN+ neurons in the hippocampus
Using the total numbers of GAD‐67+ interneurons measured for the entire hippocampus (i.e. the sum of GAD‐67+ neurons in the DG, CA1 and CA3 sub‐fields) and total numbers of NeuN+ neurons measured in the hippocampal principal cell layers (i.e. the sum of NeuN+ neurons in the GCL, and CA1 and CA3 pyramidal cell layers), we calculated the ratio of GAD‐67+ interneurons to NeuN+ neurons in all principal cell layers of the entire hippocampus. The values are then presented as numbers of GAD‐67+ interneurons per 100 NeuN+ neurons in principal cell layers of the hippocampus. The possible change in ratio during the normal course of aging (naïve adult versus naïve aged hippocampi), and the extent of change in ratio after ICV KA induced injury in each age group (naïve adult versus injured adult hippocampi, and naïve aged versus injured aged hippocampi) were first determined using one‐way ANOVA with a Student–Newman–Keuls multiple comparisons post hoc test. Furthermore, to ascertain the role of interaction between aging and injury in altering the ratio, values from the four groups were also analyzed using two‐way ANOVA with Bonferroni post‐tests.
Results
Behaviour of animals and structural changes in the hippocampus after unilateral ICV KA administration
Both adult and aged rats were observed for 4 hrs after a unilateral ICV KA administration for identifying any age‐related differences in behavioural activity following ICV KA injections. The behaviour suggestive of motor seizures, typically seen following intraperitoneal administration of KA in conscious rats [39], were absent in all rats receiving ICV KA injections under anaesthesia. This observation is consistent with multiple previous studies using the ICV KA model of hippocampal injury [29, 30, 32, 33, 40]. In both age groups, unilateral ICV KA caused neuronal loss in hippocampus ipsilateral to the KA administration (Fig. 1). Visualization of the extent of hippocampal lesion size through examination of serial sections immunostained for NeuN revealed extensive loss of CA3 pyramidal neurons in hippocampus ipsilateral to the KA administration in both age groups of rats (Fig. 1B1, B4, D1 and D4]), in comparison to hippocampi from age‐matched intact rats (Fig. 1A1 and C1]). This comprised most of the CA3 cell layer except for a smaller zone adjacent to the CA2 cell layer, where larger pyramidal neurons of CA3a sub‐region were preserved. There was no apparent loss of neurons in the dentate granule cell layer but dentate hilus appeared to exhibit a considerable loss of neurons in both age groups of rats receiving KA (Fig. 1B1, B2, D1 and D2). The CA1 pyramidal cell layer was mostly spared in the anterior half of the hippocampus (Fig. 1B1, B3, D1 and D3) but fractions of CA1 pyramidal cells were lost in the posterior half of the hippocampus (data not illustrated) in both age groups. These observations are consistent with our previous studies that documented neurodegeneration after ICV KA administration in young and aged rats at earlier time‐points after KA administration [29, 30, 41]. In hippocampus contralateral to the KA administration, all cell layers remained intact in both age groups (data not illustrated). Because of this, analyses of injury‐induced changes in GAD‐67+ interneurons in this study were performed for hippocampi ipsilateral to the KA administration. The hippocampus ipsilateral to the KA administration is referred hereafter as ‘the injured hippocampus’ in both age groups.
Extent of NeuN+ neuron loss in different regions of the hippocampus after ICV KA–induced injury
Measurement of NeuN+ neurons in the dentate hilus revealed that ICV KA administration significantly reduced dentate hilar neuron population in the hippocampus ipsilateral to KA in adult rats (35% loss; P < 0.01) but not in aged rats (21% loss, P > 0.05; Fig. 2A1). However, as aging itself reduces the numbers of dentate hilar neurons (30% loss, P < 0.01), the residual population of dentate hilar neurons after ICV KA becomes highly comparable between adult and aged groups (P > 0.05; Fig. 2A1). Quantification of NeuN+ neurons in the GCL demonstrated that ICV KA administration did not change the numbers of dentate granule cells in both age groups (P > 0.05; Fig. 2B1). The granule cell population was also stable during the process of aging as the population of dentate granule cells was comparable between naïve adult rats and naïve aged rats (P > 0.05; Fig. 2B1). The CA1 pyramidal cell layer exhibited a moderate loss of neurons in both adult (31% loss, P > 0.05) and aged (46% loss, P < 0.05) groups (Fig. 2C1). On the other hand, the CA3 pyramidal cell layer displayed an extensive loss of neurons in both adult and aged groups (80–87% loss, P < 0.001; Fig. 2D1). However, both CA1 and CA3 pyramidal neuron populations did not change significantly during the process of normal aging (p > 0.05; Fig. 2C1, and D1). Thus, the overall principal neuron loss (i.e. in the GCL and CA1 & CA3 pyramidal cell layers) in the hippocampus ipsilateral to ICV KA administration was mostly comparable between adult and aged rats with massive loss of neurons in the CA3 pyramidal cell layer and moderate loss of neurons in the CA1 pyramidal cell layer. When taken as a whole (i.e. when all principal cell layers [GCL and CA1 and CA3 pyramidal cell layers] were combined), the principal neuron loss after ICV KA was 22% in the adult hippocampus (P > 0.05) and 26% in the aged hippocampus (P < 0.05). Further analyses with two‐way ANOVA demonstrated that there was no interaction between age and ICV KA–induced injury for any of the principal cell layers (GCL, F= 0.03, P= 0.85; CA1 pyramidal cell layer, F= 1.31, P= 0.27; CA3 pyramidal cell layer, F= 0.41, P= 0.53). These results suggest that the excitotoxic injury inflicted via ICV administration of 0.5 μg of KA causes mostly similar loss of principal neurons in different regions of the adult and aged hippocampus. However, this does not rule out the possible increased vulnerability of CA1 and CA3 pyramidal neurons in the aged hippocampus to lower doses of KA, as only the long‐term effects of one particular dose of KA is examined in this study. Additional neurodegeneration analyses (in combination with EEG recordings for detection of seizures) at early time‐points following administration of lower doses of KA are needed in future studies to address this issue.
Figure 2.
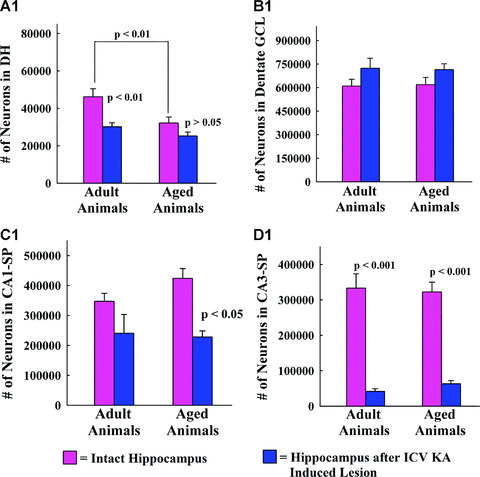
The bar charts (A1, B1, C1 and D1) compare numbers of NeuN+ neurons in the dentate hilus (A1), the granule cell layer (B1), the CA1 pyramidal cell layer (C1) and the CA3 pyramidal cell layer (D1) between different groups. Note that, ICV KA administration induces a significant (35%) loss of neurons in the dentate hilus of the adult group (P < 0.01; A1), no changes in the granule cell layer of both age groups (B1), significant (46%) loss of neurons in the CA1 pyramidal cell layer of the aged group (P < 0.05; C1), and substantial (80%–87%) loss of neurons in the CA3 pyramidal cell layer of both age groups (P < 0.001, D1).
Distribution of GAD‐67+ interneurons in the hippocampus of different groups
Immunostaining of hippocampal sections with GAD‐67 antibody revealed interneurons within all layers and sub‐fields of the hippocampus in both age groups (Figs. 3, 4 and 5A1–D2). Closer examination of the distribution of GAD‐67+ interneurons suggested a considerable decrease in numbers of GAD‐67+ interneurons in different regions of the injured hippocampus in adult rats (B1 in 3, 4, 5), in comparison with respective regions of the intact hippocampus in age‐matched naïve control rats (A1 in 3, 4, 5). This was apparent in virtually all strata of the DG, and CA1 and CA3 sub‐fields. Interestingly, the distribution of GAD‐67+ interneurons in different regions of the injured hippocampus of aged rats (D1 in 3, 4, 5) did not appear different from the distribution of GAD‐67+ interneurons in respective regions of the intact hippocampus in age‐matched naïve control aged rats (C1 in 3, 4, 5).
Figure 3.

Distribution of GAD‐67+ interneurons in the dentate hilus and granule cell layer (DH and GCL) of a naïve adult hippocampus (A1), a hippocampus of an adult rat with kainic acid (KA)‐induced injury (B1), a naïve aged hippocampus (C1) and a hippocampus of an aged rat with KA‐induced injury (D1). A2, B2, C2 and D2 are magnified views of regions from A1, B1, C1 and D1 showing the morphology of GAD‐67+ interneurons. Scale bar, A1, B1, C1, D1 = 100 μm; A2, B2, C2, D2, = 50 μm. The bar charts (E1 and F1) compare numbers of GAD‐67+ interneurons in the DH + GCL (E1) and the dentate molecular layer (F1) between different groups. Note that, in the hippocampus of adult rats with KA‐induced injury, numbers of GAD‐67+ interneurons exhibit considerable (56%–62%) decline in different regions of the dentate gyrus, in comparison with numbers in similar regions of the age‐matched intact rats (P < 0.01 to P < 0.001). In contrast, numbers of GAD‐67+ interneurons in the dentate gyrus are mostly comparable between hippocampus of naïve aged rats and the hippocampus of aged rats with KA‐induced injury (P > 0.05).
Figure 4.
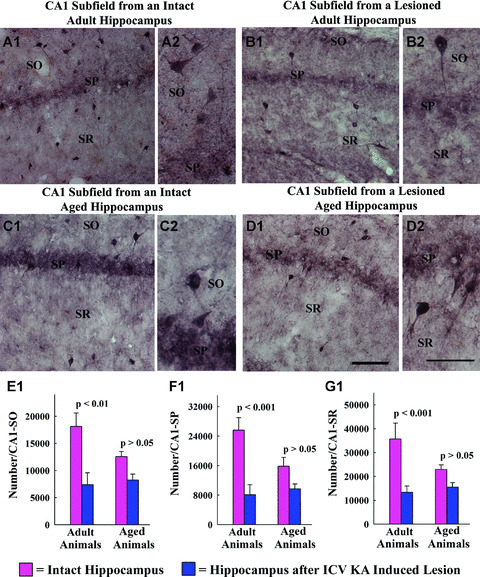
Distribution of GAD‐67+ interneurons in different layers of the CA1 sub‐field of a naïve adult hippocampus (A1), a hippocampus of an adult rat with kainic acid (KA)‐induced injury (B1), a naïve aged hippocampus (C1) and a hippocampus of an aged rat with KA‐induced injury (D1). A2, B2, C2 and D2 are magnified views of regions from A1, B1, C1 and D1 showing the morphology of GAD‐67+ interneurons. Scale bar, A1, B1, C1, D1 = 100 μm; A2, B2, C2, D2, = 50 μm. The bar charts (E1, F1 and G1) compare numbers of GAD‐67+ interneurons in the stratum oriens (SO), stratum pyramidale (SP) and stratum radiatum (SR) of the CA1 sub‐field between different groups. Note that, in the hippocampus of adult rats with KA‐induced injury, numbers of GAD‐67+ interneurons exhibit significant (59%–68%) decrease in different regions of the CA1 sub‐field, in comparison with numbers in similar regions of age‐matched intact rats (P < 0.01 to P < 0.001). On the other hand, numbers of GAD‐67+ interneurons in the CA1 sub‐field are mostly similar between the hippocampus of naïve aged rats and the hippocampus of aged rats with KA‐induced injury (P > 0.05).
Figure 5.
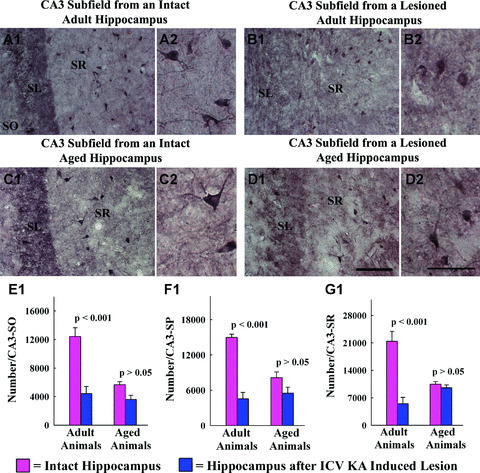
Distribution of GAD‐67+ interneurons in the stratum radiatum of CA3 sub‐field of a naïve adult hippocampus (A1), a hippocampus of an adult rat with kainic acid (KA)‐induced injury (B1), a naïve aged hippocampus (C1) and a hippocampus of an aged rat with KA‐induced injury (D1). A2, B2, C2 and D2 are magnified views of regions from A1, B1, C1 and D1 showing the morphology of GAD‐67+ interneurons. Scale bar, A1, B1, C1, D1 = 100 μm; A2, B2, C2, D2, = 50 μm. The bar charts (E1, F1 and G1) compare numbers of GAD‐67+ interneurons in the stratum oriens (SO), stratum pyramidale (SP) and stratum radiatum (SR) of the CA3 sub‐field between different groups. Note that in the hippocampus of adult rats with KA‐induced injury, numbers of GAD‐67+ interneurons are reduced considerably (64%–74% reduction) in different regions of the CA3 sub‐field, in comparison with numbers in similar regions of the age‐matched intact rats (P < 0.001). In the aged group, numbers of GAD‐67+ interneurons in all strata of CA3 sub‐field are comparable between the hippocampus of naïve rats and the hippocampus of rats with ICV KA–induced injury (P > 0.05). SL, stratum lucidum.
Changes in GAD‐67+ interneuron numbers in layers of the dentate gyrus after ICV KA–induced injury
In the dentate gyrus of the adult hippocampus, ICV KA–induced injury resulted in considerably reduced numbers of GAD‐67+ interneurons in all layers, in comparison with their counterparts in age‐matched intact adult hippocampus. The overall reductions were 62% in the DH‐GCL (P < 0.001; Fig. 3E1) and 56% in the dentate molecular layer (P < 0.01; Fig. 3F1). However, a similar ICV
KA–induced injury to the aged hippocampus did not significantly alter the numbers of GAD‐67+ interneurons in either the DH‐GCL (11% reduction, P > 0.05; Fig. 3E1) or the dentate molecular layer (18% reduction, P > 0.05; Fig. 3F1). Further analyses using two‐way ANOVA demonstrated a significant interaction between age‐ and excitotoxic injury‐related interneuron loss for both DH‐GCL (F= 31.06; P= 0.0001) and the dentate molecular layer (F= 6.82; P= 0.023). Thus, advanced age at the time of excitotoxic injury results in a greatly reduced interneuron loss in the dentate gyrus. However, this reduced loss of interneurons in different layers of the dentate gyrus of the aged hippocampus after ICV KA is not linked to diminished loss of principal neurons, as ICV KA administration did not induce any cell loss in the GCL of both adult and aged groups (Fig. 1B1).
Changes in GAD‐67+ interneuron numbers in various strata of CA1 sub‐field after ICV KA–induced injury
Different strata of the CA1 sub‐field in the adult hippocampus also exhibited greatly reduced numbers of GAD‐67+ interneurons after ICV KA–induced injury, in comparison with GAD‐67+ interneuron numbers in respective layers of the age‐matched intact adult hippocampus. The extent of reductions varied from 59% in the stratum oriens (P < 0.01; Fig. 4E1), 68% in the stratum pyramidale (P < 0.001; Fig. 4F1) and 63% in the stratum radiatum (P < 0.001; Fig. 4G1). On the contrary, a comparable ICV KA–induced injury to the aged hippocampus did not induce statistically significant reductions in numbers of GAD‐67+ interneurons in any of three layers of the CA1 sub‐field (stratum oriens, 35% reduction, P > 0.05, Fig. 4E1; stratum pyramidale, 40% reduction, P > 0.05, Fig. 4F1 and stratum radiatum, 32% reduction, P > 0.05, Fig. 4G1). Analyses using two‐way ANOVA revealed the presence of significant interaction between age‐ and excitotoxic injury‐related interneuron loss for stratum pyramidale (F= 4.78; P= 0.049) and stratum radiatum (F= 8.75; P= 0.012). Thus, increased age at the time of excitotoxic injury is associated with reduced interneuron loss in the CA1 region. Moreover, this diminished loss of interneurons in different layers of the CA1 sub‐field of the aged hippocampus after ICV KA is not due to a reduced loss of principal neurons, as ICV KA administration caused a relatively greater loss of CA1 pyramidal neurons (46% loss, Fig. 1C1) in the aged hippocampus than in the adult hippocampus (31% loss, Fig. 1C1).
Changes in GAD‐67+ interneuron numbers in various layers of CA3 sub‐field after ICV KA–induced injury
The ICV KA–induced reductions in GAD‐67+ interneuron numbers were also conspicuous in different layers of the CA3 sub‐field in the adult hippocampus. The overall reductions were 64% in the stratum oriens (P < 0.001; Fig. 5E1), 69% in the CA3 stratum pyramidale (P < 0.001; Fig. 5F1) and 74% in the CA3 stratum radiatum (P < 0.001; Fig. 5G1). In contrast, a similar ICV KA–induced injury to the aged hippocampus did not cause statistically significant changes in numbers of GAD‐67+ interneurons in any of three layers of the CA3 sub‐field (stratum oriens, 36% reduction, P > 0.05; Fig. 5E1; stratum pyramidale, 32% reduction, P > 0.05; Fig. 5F1 and stratum radiatum, 9% reduction, P > 0.05; Fig. 5G1). Additional analyses using two‐way ANOVA demonstrated a significant interaction between age‐ and excitotoxic injury‐related interneuron loss for all three layers (stratum oriens, F= 11.59, P= 0.005; stratum pyramidale, F= 15.61, P= 0.002; and stratum radiatum, F= 22.82, P= 0.001). Thus, increased age considerably reduces the excitotoxic injury–induced interneuron loss in the CA3 sub‐field. Furthermore, decreased interneuron loss in different layers of the CA3 sub‐field of the aged hippocampus in response to ICV KA is not due to reduced principal neuron loss, as ICV KA administration caused highly comparable (80–87%) loss of CA3 pyramidal neurons in both adult and aged groups (Fig. 2D1).
Changes in GAD‐67+ interneuron numbers in different sub‐fields and the entire hippocampus after ICV KA–induced injury
The ICV KA administration caused considerable overall reductions in numbers of GAD‐67+ interneurons in the DG of the adult hippocampus (59% reduction, P < 0.001; Fig. 6A1) but not in the DG of the aged hippocampus (15% reduction, P > 0.05; Fig. 6A1). Similar trend was observed in both CA1 and CA3 sub‐fields after ICV KA–induced injury. The overall reduction was 64% in the CA1 sub‐field of the adult hippocampus (P < 0.01; Fig. 6B1) and 35% in the CA1 sub‐field of the aged hippocampus (P > 0.05; Fig. 6B1). In the CA3 sub‐field, the overall reduction was 73% for the adult hippocampus (P < 0.001; Fig. 6C1) and 23% for the aged hippocampus (P > 0.05; Fig. 6C1). Thus, in comparison with GAD‐67+ interneuron populations in the adult hippocampus, GAD‐67+ interneurons in the aged hippocampus exhibit considerably reduced vulnerability to ICV KA–induced injury in all three sub‐fields of the hippocampus. Comparison of the total numbers of GAD‐67+ interneurons in the entire hippocampus between different groups further substantiated this finding. The overall reduction in GAD‐67+ interneuron number after ICV KA–induced injury was 65% for the entire adult hippocampus (P < 0.001; Fig. 6D1) but only 26% for the entire aged hippocampus (P > 0.05; Fig. 6D1). Additional analyses using two‐way ANOVA revealed a significant interaction between age‐ and excitotoxic injury‐related interneuron loss for all three regions and the entire hippocampus (dentate gyrus, F= 16.62, P= 0.002; CA1 sub‐field, F= 7.5, P= 0.018; CA3 sub‐field, F= 33.7, P= 0.0001; entire hippocampus, F= 18.21, P= 0.001). The above analyses further confirm that increased age at the time of excitotoxic injury is associated with significantly reduced loss of GAD‐67+ interneurons in the hippocampus. Interestingly, despite the above differences in the vulnerability of GAD‐67+ interneurons to ICV KA–induced injury between adult and aged groups, the numbers of remaining GAD‐67+ interneurons are highly comparable between the injured adult hippocampus and the injured aged hippocampus (Fig. 6 A1–D1). This outcome is due to a substantial decrease in the GAD‐67+ interneuron population between adult and old age [8].
Figure 6.
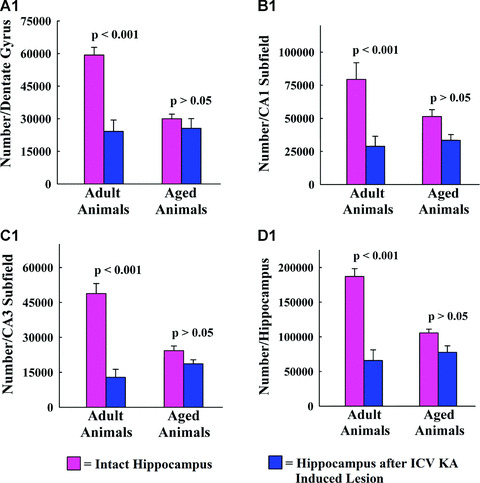
Comparison of the total number of GAD‐67+ interneurons in the entire dentate gyrus (A1), the entire CA1 sub‐field (B1), the entire CA3 sub‐field (C1) and the whole hippocampus between different groups. Note that in the hippocampus of adult rats with KA‐induced injury, numbers of GAD‐67+ interneurons exhibit considerable decline in all sub‐fields (59–73% decline, P < 0.01 to P < 0.001) and the entire hippocampus (65% decrease, P < 0.001), in comparison with numbers in the age‐matched intact rats. In the aged group, numbers of GAD‐67+ interneurons in the dentate gyrus, and CA1 and CA3 sub‐fields (and in the entire hippocampus) are comparable between the hippocampus of naïve rats and the hippocampus of rats with KA‐induced injury.
Changes in the ratio of GAD‐67+ interneurons to principal neurons in the hippocampus with aging and after ICV KA–induced injury
Aging alone decreases the ratio of GAD‐67+ interneurons to all principal neurons in the hippocampus. This is a consequence of substantial reductions in GAD‐67+ interneuron numbers during the course of aging [8] with no changes in numbers of neurons in hippocampal principal cell layers (GCL, and CA1 and CA3 pyramidal cell layers). The intact adult hippocampus contains an average of 1,289,800 (mean ± S.E.M. = 1,289,800 ± 82,683) NeuN+ principal cells (i.e. the sum of NeuN+ neurons in GCL, and pyramidal cell layers of CA1 and CA3) and an average of 187,050 (187,050 ± 11,227) GAD‐67+ interneurons (i.e. the sum of GAD‐67+ cells in DG, CA1 and CA3 sub‐fields). Thus, there is an average of 14.5 GAD‐67+ cells to 100 NeuN+ principal cells in the intact adult hippocampus. In contrast, the intact aged hippocampus contains an average of 1,364,800 ± 95,907 NeuN+ principal cells and an average of 105,676 ±5,413 GAD‐67+ interneurons, which is equivalent to having an average of 7.7 GAD‐67+ cells to 100 NeuN+ principal cells. Thus, when the hippocampus is taken as a whole, the ratio of GAD‐67+ interneurons to 100 NeuN+ principal neurons declines from 14.5 to 7.7 (47% reduction, P < 0.001; Fig. 7) with aging.
Figure 7.
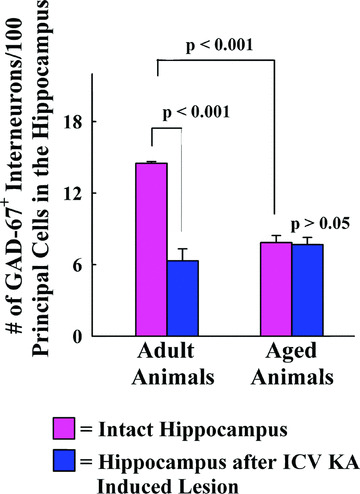
Comparison of the overall ratio of GAD‐67+ interneurons to NeuN+ principal neurons in the hippocampus between different groups. The values are presented as number of GAD‐67+ interneurons per 100 NeuN+ principal neurons. Aging alone decreases the ratio of GAD‐67+ interneurons to NeuN+ principal neurons in the hippocampus (47% reduction; P < 0.001). Intracerebroventricular kainic acid (ICV KA)–induced injury in adult rats considerably decreases the ratio of GAD‐67+ interneurons to NeuN+ principal neurons (55% reduction; P < 0.001) in the hippocampus. In contrast, similar ICV KA–induced injury to the aged hippocampus does not induce any change in the ratio of GAD‐67+ interneurons to NeuN+ principal neurons (P > 0.05).
With ICV KA–induced injury in the adult hippocampus, the population of NeuN+ principal neurons reduces from 1,289,800 ± 82,683 to 1,005,600 ± 110,916 (22% loss) and the population of GAD‐67+ interneurons reduces from 187,050 ± 11,227 to 65,756 ± 15,444 (65% loss). This asymmetrical loss between principal neurons and interneurons after ICV KA in the adult hippocampus alters the ratio of GAD‐67+ interneurons to 100 NeuN+ principal neurons from 14.5 to 6.5 (55% reduction, P < 0.001). The scenario is quite different with ICV KA–induced injury in the aged hippocampus. The population of NeuN+ principal neurons reduces from 1,364,800 ± 95,907 to 1,005,750 ± 55,227 (26% loss) and the population of GAD‐67+ interneurons reduces from 105,676 ± 5413 to 77,715 ± 9279 (26% loss). This symmetrical loss between principal neurons and interneurons after ICV KA in the aged hippocampus does not change the ratio of GAD‐67+ interneurons to 100 NeuN+ principal neurons, which remains at 7.7 even after ICV KA–induced injury (P > 0.05; Fig. 7). Thus, the overall ratio of GAD‐67+ interneurons to NeuN+ principal neurons in the adult hippocampus declines considerably with ICV KA–induced injury due to widespread (65%) loss of interneurons in comparison with moderate (22%) loss of principal neurons. On the other hand, the overall ratio of GAD‐67+ interneurons to NeuN+ principal neurons in the aged hippocampus exhibits no change with ICV KA–induced injury, as a consequence of both proportioned and moderate loss (26% each) of principal neurons and interneurons. Two‐way ANOVA analysis further revealed that there is significant interaction between age and injury pertaining to reductions in ratio between GAD‐67+ interneurons and NeuN+ principal neurons (i.e. GAD‐67+ interneuron to NeuN+ principal neuron ratio decreases considerably with excitotoxic injury in the adult hippocampus but not in the aged hippocampus; F= 36.94; P= 0.0001). Despite the above, the overall ratio of GAD‐67+ interneurons to NeuN+ neurons in the injured aged hippocampus is highly similar to ratio in the injured adult hippocampus. This is due to substantial declines in the ratios of GAD‐67+ interneurons to NeuN+ principal neurons occurring with aging alone.
Discussion
This study provides novel evidence that an excitotoxic injury to the aged hippocampus is associated with considerably decreased loss of GAD‐67+ inhibitory interneurons, in comparison with the widespread loss of GAD‐67+ interneurons observed after such an injury in the adult hippocampus. Specifically, an ICV KA administration caused substantial reductions in GAD‐67+ interneuron numbers in all hippocampal layers and sub‐fields of adult animals with an overall 65% reduction for the entire injured adult hippocampus. However, similar ICV KA administration in aged animals did not significantly reduce the GAD‐67+ interneuron population in any of the hippocampal layers and sub‐fields. Furthermore, the overall 26% reduction in GAD‐67+ interneuron population observed for the entire injured aged hippocampus was statistically insignificant. This differential vulnerability of GAD‐67+ interneuron population between the two age groups is not related to variability in the lesion size, as stereological quantification of surviving NeuN+ principal neurons revealed that the overall neuron loss in the hippocampus ipsilateral to ICV KA administration is mostly comparable between adult and aged rats. This comprised massive loss of neurons in the CA3 pyramidal cell layer (87% in the adult and 80% in the aged), moderate loss of neurons in the CA1 pyramidal cell layer (31% in the adult and 46% in the aged) and no significant loss of neurons in the GCL. The overall principal neuron loss for the entire hippocampus was 22% in the adult hippocampus and 26% in the aged hippocampus. Thus, an asymmetrical loss between principal neurons and interneurons occurs after an ICV KA administration in the adult hippocampus, which decreases the ratio of GAD‐67+ interneurons to NeuN+ principal neurons by 55%. On the other hand, as a result of both proportioned and moderate loss (26% each) of principal neurons and interneurons, the overall ratio of GAD‐67+ interneurons to NeuN+ principal neurons in the aged hippocampus exhibits no change with ICV KA–induced injury. A reduced loss of GAD‐67+ interneurons in the aged hippocampus after ICV KA administration likely reflects an age‐related change in the response of GAD‐67+ interneurons to excitotoxic hippocampal injury. This altered response may be linked to their prolonged exposure to increased excitability of principal neurons prevailing in the aged hippocampus and/or changes in intrinsic properties of hippocampal interneurons with aging.
Changes in GAD‐67+ interneuron population after ICV KA–induced injury in the two age groups
Multiple previous studies have suggested that hippocampal GABA‐ergic interneuron population is vulnerable to excitotoxic injury in young and adult animals [20, 27, 31, 42, 43, 44, 45]. However, this study represents the first stereological investigation to comprehensively document the extent of long‐term reductions in GABA‐ergic interneuron numbers in different strata of all hippocampal sub‐fields of adult animals following excitotoxic injury. The results clearly demonstrate that excitotoxic injury substantially reduces the population of GABA‐ergic interneurons in all layers and regions of the adult hippocampus. The overall decreases ranged from 59% in the DG, 64% in the CA1 sub‐field, 73% in the CA3 sub‐field and 65% in the entire hippocampus. Because of these widespread reductions (which are greater than the overall 22% reduction observed for NeuN+ principal neurons), the ratio of GAD‐67+ interneuron numbers to NeuN+ principal neurons declined by 55% in the injured adult hippocampus. In sharp contrast, a similar injury to the aged hippocampus induced much reduced and insignificant reductions in the GAD‐67+ interneuron population in all layers and sub‐fields, which varied from 15% in the DG, 35% in the CA1 sub‐field, 23% in the CA3 sub‐field and 26% in the entire hippocampus. These moderate reductions are similar to the overall 26% reduction observed for NeuN+ principal neurons. Overall, the loss of principal neurons and interneurons after ICV KA in the aged hippocampus was symmetrical, and hence the ratio of GAD‐67+ interneurons to NeuN+ principal neurons did not change in the aged hippocampus after injury. Thus, unlike in the adult hippocampus, the vulnerability of GAD‐67+ interneurons to excitotoxic injury declines considerably in the aged hippocampus. However, to determine whether distinct populations of GAD‐67+ interneurons are resistant to excitotoxic injury in different layers of the aged hippocampus, further experiments that comprise dual labelling for GAD‐67 and markers of sub‐classes of interneurons (such as parvalbumin, calbindin, calretinin, neuropeptide Y, somatostatin etc.) are needed in future.
Interestingly, despite the decreased susceptibility to excitotoxic injury, both GAD‐67+ interneuron population and the ratio of GAD‐67+ interneurons to NeuN+ principal neurons in the injured aged hippocampus remain comparable to that observed in the injured adult hippocampus. This is because both GAD‐67+ interneuron population and the ratio of GAD‐67+ interneurons to NeuN+ principal neurons decline substantially during the normal process of aging in the hippocampus. Thus, GAD‐67+ interneurons in the DG, and CA1 and CA3 sub‐fields of the adult hippocampus, although manifest in greater numbers in the intact condition, exhibit increased vulnerability to ICV KA–induced injury. In contrast, GAD‐67+ interneurons in these regions of the aged hippocampus, although manifest in reduced numbers in the intact condition, display considerably diminished susceptibility to ICV KA–induced injury. As both population size of surviving GAD‐67+ interneurons and ratio of GAD‐67+ interneurons to NeuN+ principal neurons are mostly similar between the injured adult hippocampus and the injured aged hippocampus, it is possible that the overall effects of excitotoxic injury on inhibitory GABA‐ergic neurotransmission will be similar between the adult and aged hippocampi. However, there may be differences between adult and aged groups for other parameters of post‐injury plasticity such as changes in post‐synaptic GABA receptors, sprouting of GABA‐ergic axons, GABA release and re‐uptake. Rigorous neurochemical and electrophysiological studies of the hippocampi after excitotoxic injury are, however, needed from both age groups to understand any post‐excitotoxic injury‐related differences in GABA‐ergic neurotransmission between adult and aged groups.
Potential reasons of decreased vulnerability of GAD‐67+ interneurons in the aged hippocampus to excitotoxic injury
We examined whether the stability of GAD‐67+ interneurons in aged rats is linked to the presence of potentially milder hippocampal injury than adult rats following ICV KA administration through stereological quantification of surviving NeuN+ neurons in the GCL and CA1 and CA3 pyramidal cell layers. This analysis, however, ruled out the above possibility, as the extent of neuron loss after an ICV KA administration was found to be mostly comparable between adult and aged rats in principal cell layers of both CA3 sub‐field (80%–87%) and CA1 sub‐field (31%–46%). The GCL in both age groups did not exhibit any reductions in neuron number. This finding is consistent with our earlier observation that both volume of damage to CA3 and CA1 cell layers and hippocampal inflammation after ICV KA administration are comparable between adult and aged rats [41]. Considering the above, it is tempting to speculate that the reduced vulnerability of the residual GAD‐67+ interneuron population in the aged hippocampus to excitotoxic injury reflects adaptability of the residual GAD‐67+ interneurons to increased excitability of principal cells prevailing in the aged hippocampus. However, the association between aging and increased excitability of principal cells in the hippocampus is somewhat controversial [46, 47, 48, 49, 50]. Some studies suggest that aging is associated with reduced intrinsic excitability of individual hippocampal pyramidal neurons, reflected by an enlarged post‐burst afterhyperpolarization and an increased spike‐frequency adaptation [51]. In contrast, another study suggests that all three sub‐fields of the hippocampus exhibit increased dye coupling with age, reflecting increased electrical connections through gap junctions in the aged hippocampus [11]. This was also supported by the observation of an apparent increase in post‐synaptic excitability as assessed by the ratio of the population spike to EPSP components of the extracellularly recorded field potential [11]. Furthermore, an enhanced field potential coupling and higher frequency bursting during large irregular activity have been observed in aged animals [13, 14]. Overall, it is likely that multiple changes in principal hippocampal neurons during aging contribute to an overall lowering of the threshold for action potential discharge, that is, a mechanism demonstrating the potential for increased excitability in aged neurons [52]. From this perspective, it is plausible that the residual GABA‐ergic interneurons in the aged hippocampus are exposed frequently to increased excitability of principal neurons that has resulted in increased adaptability of these interneurons to excitatory stimulation by principal neurons. Indeed, GABA‐ergic interneurons in the hippocampus receive afferents from principal hippocampal neurons, which facilitates feedback inhibition. According to one estimate, a single pyramidal neuron innervates approximately 100 interneurons and 100 interneurons in turn innervate approximately 1000–3000 pyramidal neurons [4].
Another possibility is that the residual GABA‐ergic interneurons in the aged hippocampus receive fewer synaptic connections from principal hippocampal neurons (such as dentate granule cells, and CA1 and CA3 pyramidal neurons) because of age‐related loss of synapses in the hippocampus. Although GABA‐ergic interneurons in the hippocampus are also innervated by extrahippocampal afferents (promoting feedforward inhibition [4]), the possible reduced afferent connectivity from principal hippocampal neurons during aging might lead to an overall decrease in the excitability of interneurons even when hippocampal principal neurons are exhibiting KA‐induced hyperexcitability. Furthermore, aging may be associated with changes in intrinsic properties of hippocampal interneurons such as reduced excitability following afferent stimulation possibly due to alterations in glutamate and other receptors promoting excitatory neurotransmission. In addition, it is possible that KA‐induced hyperexcitability in principal neurons of the aged hippocampus is not strong enough to induce reductions in GABA‐ergic interneuron numbers. Indeed, a previous study suggests that although both young and aged rats exhibit an increase in the EEG power during the KA treatment, visual inspection and spectral analysis demonstrates a reduction of the faster frequencies in the EEGs of aged animals despite a shorter latency to stage V seizures in comparison to young rats [53]. This altered EEG activity following KA in aged animals, although appears sufficient to induce loss of populations of principal neurons, might not be adequate to induce considerable reductions in GABA‐ergic interneuron numbers. Additional studies are, however, needed in future to ascertain the relative contribution of above possibilities to resistance of GABA‐ergic interneurons in the aged hippocampus to excitotoxic injury.
Implications of reduced vulnerability of GABA‐ergic interneurons to excitotoxic injury in the aged hippocampus
Although there is no consensus regarding the causes of decreased inhibition during chronic epilepsy, it is believed that decreased inhibition is one of the factors contributing to spontaneous seizures during chronic epilepsy [54, 55, 56, 57, 58, 59, 60, 61, 62, 63]. This is evidenced by anticonvulsant actions observed after the administration of drugs that increase synaptic GABA [5] or grafting of cells that release GABA in epilepsy models [64, 65, 66, 67, 68]. From these perspectives, reduced vulnerability of GABA‐ergic interneurons to excitotoxic injury in the aged hippocampus appears beneficial for diminishing the occurrence of chronic epilepsy after the initial brain injury. Furthermore, the aberrant sprouting of mossy fibres into the dentate supragranular layer after hippocampal injury, an injury‐induced synaptic re‐organization believed to contribute towards epileptogenesis and the development of chronic epilepsy [32, 33, 42, 69, 70, 71] is considerably impaired in the aged hippocampus [29]. Similarly, increased genesis and abnormal migration of newly born neurons in the dentate gyrus of young/adult animals after hippocampal injury and/or seizures, an injury‐induced phenomenon in the young/adult hippocampus proposed to contribute towards the development of epileptogenic circuitry and chronic epilepsy [72, 73, 74, 75], are not observed in the aged hippocampus after similar injury/seizures [41, 76]. Thus, considering that some of the major epileptogenic changes that occur after excitotoxic injury in the young and adult hippocampus (i.e. widespread reductions in GAD‐67+ interneuron population, robust aberrant sprouting of aberrant mossy fibres and pervasive but abnormal migration of newly born neurons into the dentate hilus) are greatly diminished after similar injury in the aged hippocampus, one might argue that the possibility for chronic epilepsy development after an initial brain injury in the aged is minimal. However, on the contrary, human studies suggest that the aged population has increased susceptibility for developing chronic epilepsy after brain injury resulting from stroke, seizures and head injury [21, 22]. Therefore, it is possible that factors other than reduced numbers of GABA‐ergic interneurons, aberrant mossy fibre sprouting and abnormal neurogenesis play important roles in the progression of initial precipitating injury into chronic epilepsy in the elderly population.
Acknowledgement
This research was supported by grants from the Department of Veterans Affairs (VA Merit Review Award to A.K.S.) and the National Institute of Neurological Disorders and Stroke (R01 NS054780 to A.K.S.).
References
- 1. Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus . 1996; 6: 347–70. [DOI] [PubMed] [Google Scholar]
- 2. Franck JE, Kunkel DD, Baskin DG, et al . Inhibition in kainate‐lesioned hyperexcitable hippocampi: physiologic, autoradiographic, and immunocytochemical observations. J Neurosci . 1988; 8: 1991–02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cornish SM, Wheal HV. Long‐term loss of paired pulse inhibition in the kainic acid‐lesioned hippocampus of the rat. Neuroscience . 1989; 28: 563–71. [DOI] [PubMed] [Google Scholar]
- 4. Buzsaki G, Geisler C, Henze DA, et al . Interneuron Diversity series: Circuit complexity and axon wiring economy of cortical interneurons. Trends Neurosci . 2004; 27: 186–93. [DOI] [PubMed] [Google Scholar]
- 5. Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia . 2001; 42: 8–12. [DOI] [PubMed] [Google Scholar]
- 6. Shetty AK, Turner DA. Hippocampal interneurons expressing glutamic acid decarboxylase and calcium‐binding proteins decrease with aging in Fischer 344 rats. J Comp Neurol . 1998; 394: 252–69. [PubMed] [Google Scholar]
- 7. Shi L, Argenta AE, Winseck AK, et al . Stereological quantification of GAD‐67‐immunoreactive neurons and boutons in the hippocampus of middle‐aged and old Fischer 344 × Brown Norway rats. J Comp Neurol . 2004; 478: 282–91. [DOI] [PubMed] [Google Scholar]
- 8. Stanley DP, Shetty AK. Aging in the rat hippocampus is associated with widespread reductions in the number of glutamate decarboxylase‐67 positive interneurons but not interneuron degeneration. J Neurochem . 2004; 89: 204–16. [DOI] [PubMed] [Google Scholar]
- 9. Landfield PW, Pitler TA, Applegate MD. The effects of high Mg2+‐to‐Ca2+ ratios on frequency potentiation in hippocampal slices of young and aged rats. J Neurophysiol . 1986; 56: 797–11. [DOI] [PubMed] [Google Scholar]
- 10. Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci . 1994; 17: 13–18. [DOI] [PubMed] [Google Scholar]
- 11. Barnes CA, Rao G, McNaughton BL. Increased electrotonic coupling in aged rat hippocampus: a possible mechanism for cellular excitability changes. J Comp Neurol . 1987; 259: 549–58. [DOI] [PubMed] [Google Scholar]
- 12. Bekenstein JW, Lothman EW. Dormancy of inhibitory interneurons in a model of temporal lobe epilepsy. Science . 1993; 259: 97–100. [DOI] [PubMed] [Google Scholar]
- 13. Papatheodoropoulos C, Kostopoulos G. Age‐related changes in excitability and recurrent inhibition in the rat CA1 hippocampal region. Eur J Neurosci . 1996; 8: 510–20. [DOI] [PubMed] [Google Scholar]
- 14. Smith AC, Gerrard JL, Barnes CA, et al . Effect of age on burst firing characteristics of rat hippocampal pyramidal cells. NeuroReport . 2000; 11: 3865–71. [DOI] [PubMed] [Google Scholar]
- 15. Luebke JI, Rosene DL. Aging alters dendritic morphology, input resistance, and inhibitory signaling in dentate granule cells of the rhesus monkey. J Comp Neurol . 2003; 460: 573–84. [DOI] [PubMed] [Google Scholar]
- 16. Potier B, Jouvenceau A, Epelbaum J, et al . Age‐related alterations of GABAergic input to CA1 pyramidal neurons and its control by nicotinic acetylcholine receptors in rat hippocampus. Neuroscience . 2006; 142: 187–201. [DOI] [PubMed] [Google Scholar]
- 17. Liang LP, Beaudoin ME, Fritz MJ, et al . Kainate‐induced seizures, oxidative stress and neuronal loss in aging rats. Neuroscience . 2007; 147: 1114–8. [DOI] [PubMed] [Google Scholar]
- 18. Patrylo PR, Williamson A. The effects of aging on dentate circuitry and function. Prog Brain Res . 2007; 163: 679–96. [DOI] [PubMed] [Google Scholar]
- 19. McCord MC, Lorenzana A, Bloom CS, et al . Effect of age on kainate‐induced seizure severity and cell death. Neuroscience . 2008; 154: 1143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shetty AK, Turner DA. Glutamic acid decarboxylase‐67‐positive hippocampal interneurons undergo a permanent reduction in number following kainic acid‐induced degeneration of ca3 pyramidal neurons. Exp Neurol . 2001; 169: 276–97. [DOI] [PubMed] [Google Scholar]
- 21. LaRoche SM, Helmers SL. Epilepsy in the elderly. Neurologist . 2003; 9: 241–9. [DOI] [PubMed] [Google Scholar]
- 22. Leppik IE, Kelly KM, deToledo‐Morrell L, et al . Basic research in epilepsy and aging. Epilepsy Res . 2006; 68:S21–37. [DOI] [PubMed] [Google Scholar]
- 23. Ben‐Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience . 1985; 14: 375–403. [DOI] [PubMed] [Google Scholar]
- 24. Nadler JV, Perry BW, Cotman CW. Intraventricular kainic acid preferentially destroys hippocampal pyramidal cells. Nature . 1978; 271: 676–7. [DOI] [PubMed] [Google Scholar]
- 25. Nadler JV, Perry BW, Cotman CW. Selective reinnervation of hippocampal area CA1 and the fascia dentata after destruction of CA3‐CA4 afferents with kainic acid. Brain Res . 1980; 182: 1–9. [DOI] [PubMed] [Google Scholar]
- 26. Nadler JV, Perry BW, Gentry C, et al . Degeneration of hippocampal CA3 pyramidal cells induced by intraventricular kainic acid. J Comp Neurol . 1980; 192: 333–59. [DOI] [PubMed] [Google Scholar]
- 27. Shetty AK, Turner DA. Intracerebroventricular kainic acid administration in adult rat alters hippocampal calbindin and non‐phosphorylated neurofilament expression. J Comp Neurol . 1995; 363: 581–99. [DOI] [PubMed] [Google Scholar]
- 28. Shetty AK, Turner DA. Development of long‐distance efferent projections from fetal hippocampal grafts depends upon pathway specificity and graft location in kainate‐lesioned adult hippocampus. Neuroscience . 1997; 76: 1205–19. [DOI] [PubMed] [Google Scholar]
- 29. Shetty AK, Turner DA. Aging impairs axonal sprouting response of dentate granule cells following target loss and partial deafferentation. J Comp Neurol . 1999; 414: 238–54. [DOI] [PubMed] [Google Scholar]
- 30. Shetty AK, Turner DA. Vulnerability of the dentate gyrus to aging and intracerebroventricular administration of kainic acid. Exp Neurol . 1999; 158: 491–503. [DOI] [PubMed] [Google Scholar]
- 31. Shetty AK, Turner DA. Fetal hippocampal grafts containing CA3 cells restore host hippocampal glutamate decarboxylase‐positive interneuron numbers in a rat model of temporal lobe epilepsy. J Neurosci . 2000; 20: 8788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shetty AK, Zaman V, Shetty GA. Hippocampal neurotrophin levels in a kainate model of temporal lobe epilepsy: a lack of correlation between brain‐derived neurotrophic factor content and progression of aberrant dentate mossy fiber sprouting. J Neurochem . 2003; 87: 147–59. [DOI] [PubMed] [Google Scholar]
- 33. Shetty AK, Zaman V, Hattiangady B. Repair of the injured adult hippocampus through graft‐mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy. J Neurosci . 2005; 25: 8391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Esclapez M, Tillakaratne NJ, Kaufman DL, et al . Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci . 1994; 14: 1834–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dupuy ST, Houser CR. Prominent expression of two forms of glutamate decarboxylase in the embryonic and early postnatal rat hippocampal formation. J Neurosci . 1996; 16: 6919–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Erlander MG, Tobin AJ. The structural and functional heterogeneity of glutamic acid decarboxylase: a review. Neurochem Res . 1991; 16: 215–26. [DOI] [PubMed] [Google Scholar]
- 37. Kaufman DL, Houser CR, Tobin AJ. Two forms of the gamma‐aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem . 1991; 56: 720–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Esclapez M, Houser CR. Up‐regulation of GAD65 and GAD67 in remaining hippocampal GABA neurons in a model of temporal lobe epilepsy. J Comp Neurol . 1999; 412: 488–505. [PubMed] [Google Scholar]
- 39. Rao MS, Hattiangady B, Reddy DS, et al . Hippocampal neurodegeneration, spontaneous seizures, and mossy fiber sprouting in the F344 rat model of temporal lobe epilepsy. J Neurosci Res . 2006; 83: 1088–105. [DOI] [PubMed] [Google Scholar]
- 40. Shetty AK, Rao MS, Hattiangady B, et al . Hippocampal neurotrophin levels after injury: relationship to the age of the hippocampus at the time of injury. J Neurosci Res . 2004; 78: 520–32. [DOI] [PubMed] [Google Scholar]
- 41. Hattiangady B, Rao MS, Shetty AK. Plasticity of hippocampal stem/progenitor cells to enhance neurogenesis in response to kainate‐induced injury is lost by middle age. Aging Cell . 2008; 7: 207–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate‐treated rats. J Comp Neurol . 1997; 385: 385–404. [PubMed] [Google Scholar]
- 43. Morin F, Beaulieu C, Lacaille JC. Selective loss of GABA neurons in area CA1 of the rat hippocampus after intraventricular kainate. Epilepsy Res . 1998; 32: 363–9. [DOI] [PubMed] [Google Scholar]
- 44. Buckmaster PS, Jongen‐Relo AL. Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate‐induced epileptic rats. J Neurosci . 1999; 19: 9519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ratte S, Lacaille JC. Selective degeneration and synaptic reorganization of hippocampal interneurons in a chronic model of temporal lobe epilepsy. Adv Neurol . 2006; 97: 69–76. [PubMed] [Google Scholar]
- 46. Moyer JR Jr., Thompson LT, Black JP, et al . Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age‐ and concentration‐dependent manner. J Neurophysiol . 1992; 68: 2100–9. [DOI] [PubMed] [Google Scholar]
- 47. Potier B, Rascol O, Jazat F, et al . Alterations in the properties of hippocampal pyramidal neurons in the aged rat. Neuroscience . 1992; 48: 793–806. [DOI] [PubMed] [Google Scholar]
- 48. Potier B, Lamour Y, Dutar P. Age‐related alterations in the properties of hippocampal pyramidal neurons among rat strains. Neurobiol Aging . 1993; 14: 17–25. [DOI] [PubMed] [Google Scholar]
- 49. Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol . 2003; 69: 143–79. [DOI] [PubMed] [Google Scholar]
- 50. Turner DA, Deupree DL. Functional elongation of CA1 hippocampal neurons with aging in Fischer 344 rats. Neurobiol Aging . 1991; 12: 201–10. [DOI] [PubMed] [Google Scholar]
- 51. Disterhoft JF, Oh MM. Alterations in intrinsic neuronal excitability during normal aging. Aging Cell . 2007; 6: 327–36. [DOI] [PubMed] [Google Scholar]
- 52. Kelly KM, Nadon NL, Morrison JH, et al . The neurobiology of aging. Epilepsy Res . 2006; 68: S5–20. [DOI] [PubMed] [Google Scholar]
- 53. Darbin O, Naritoku D, Patrylo PR. Aging alters electroencephalographic and clinical manifestations of kainate‐induced status epilepticus. Epilepsia . 2004; 45: 1219–27. [DOI] [PubMed] [Google Scholar]
- 54. Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science . 1987; 235: 73–6. [DOI] [PubMed] [Google Scholar]
- 55. Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the ‘dormant basket cell’ hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus . 1991; 1: 41–66. [DOI] [PubMed] [Google Scholar]
- 56. Obenaus A, Esclapez M, Houser CR. Loss of glutamate decarboxylase mRNA‐containing neurons in the rat dentate gyrus following pilocarpine‐induced seizures. J Neurosci . 1993; 13: 4470–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mathern GW, Babb TL, Pretorius JK, et al . Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci . 1995; 15: 3990–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Houser CR, Esclapez M. Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res . 1996; 26: 207–18. [DOI] [PubMed] [Google Scholar]
- 59. Marco P, Sola RG, Pulido P, et al . Inhibitory neurons in the human epileptogenic temporal neocortex. An immunocytochemical study. Brain . 1996; 119: 1327–47. [DOI] [PubMed] [Google Scholar]
- 60. Dudek FE, Spitz M. Hypothetical mechanisms for the cellular and neurophysiologic basis of secondary epileptogenesis: proposed role of synaptic reorganization. J Clin Neurophysiol . 1997; 14: 90–101. [DOI] [PubMed] [Google Scholar]
- 61. Esclapez M, Hirsch JC, Khazipov R, et al . Operative GABAergic inhibition in hippocampal CA1 pyramidal neurons in experimental epilepsy. Proc Natl Acad Sci USA . 1997; 94: 12151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rempe DA, Bertram EH, Williamson JM, et al . Interneurons in area CA1 stratum radiatum and stratum oriens remain functionally connected to excitatory synaptic input in chronically epileptic animals. J Neurophysiol . 1997; 78: 1504–15. [DOI] [PubMed] [Google Scholar]
- 63. Bernard C, Esclapez M, Hirsch JC, et al . Interneurons are not so dormant in temporal lobe epilepsy: a critical reappraisal of the dormant basket cell hypothesis. Epilepsy Res . 1998; 32: 93–103. [DOI] [PubMed] [Google Scholar]
- 64. Loscher W, Ebert U, Lehmann H, et al . Seizure suppression in kindling epilepsy by grafts of fetal GABAergic neurons in rat substantia nigra. J Neurosci Res . 1998; 51: 196–209. [DOI] [PubMed] [Google Scholar]
- 65. Gernert M, Thompson KW, Loscher W, et al . Genetically engineered GABA‐producing cells demonstrate anticonvulsant effects and long‐term transgene expression when transplanted into the central piriform cortex of rats. Exp Neurol . 2002; 176: 183–92. [DOI] [PubMed] [Google Scholar]
- 66. Thompson KW. Genetically engineered cells with regulatable GABA production can affect afterdischarges and behavioral seizures after transplantation into the dentate gyrus. Neuroscience . 2005; 133: 1029–37. [DOI] [PubMed] [Google Scholar]
- 67. Thompson KW, Suchomelova LM. Transplants of cells engineered to produce GABA suppress spontaneous seizures. Epilepsia . 2004; 45: 4–12. [DOI] [PubMed] [Google Scholar]
- 68. Castillo CG, Mendoza S, Freed WJ, et al . Intranigral transplants of immortalized GABAergic cells decrease the expression of kainic acid‐induced seizures in the rat. Behav Brain Res . 2006; 171: 109–15. [DOI] [PubMed] [Google Scholar]
- 69. Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid‐treated rats. J Neurosci . 1985; 5: 1016–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wuarin J‐P, Dudek, FE . Excitatory synaptic input to granule cells increases with time after kainate treatment. J Neurophysiol . 2001; 85: 1067–77. [DOI] [PubMed] [Google Scholar]
- 71. Sutula TP, Dudek FE. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system. Prog Brain Res . 2007; 163: 541–63. [DOI] [PubMed] [Google Scholar]
- 72. Parent JM. Adult neurogenesis in the intact and epileptic dentate gyrus. Prog Brain Res . 2007; 163: 529–817. [DOI] [PubMed] [Google Scholar]
- 73. Parent JM, Yu TW, Leibowitz RT, et al . Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci . 1997; 17: 3727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Scharfman HE, Goodman JH, Sollas AL. Granule‐like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure‐induced neurogenesis. J Neurosci . 2000; 20: 6144–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Scharfman HE, Gray WP. Relevance of seizure‐induced neurogenesis in animal models of epilepsy to the etiology of temporal lobe epilepsy. Epilepsia . 2007; 48: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rao MS, Hattiangady B, Shetty AK. Status epilepticus during old age is not associated with enhanced hippocampal neurogenesis. Hippocampus . 2008; 18: 931–44. [DOI] [PMC free article] [PubMed] [Google Scholar]


