Abstract
Chorda tympani (CT) and glossopharyngeal (IXth) nerves relay taste information from anterior and posterior tongue to brainstem where they synapse with second order neurons in the rostral nucleus of solitary tract (rNST). rNST neurons monosynaptically connected to afferent gustatory input were identified both by anatomical labeling and synaptic latency measures. Anterograde tracing was used to label the CT and IXth terminal fields, and neurons surrounded by fluorescent neural profiles visualized with differential interference contrast (DIC) optics in horizontal brainstem slices. Anatomically identified neurons were patch-clamped and excitatory postsynaptic currents (EPSCs) evoked by electrically stimulating the solitary tract (ST) under GABAA receptor blockade. Monosynaptic connections were confirmed by measures of the standard deviation of synaptic latency (jitter). rNST neurons responded to ST stimulation with either all-or-none or graded amplitude EPSCs. Most (70%) of the rNST neurons with CT input and 30% with IX input responded with all-or-none EPSCs. The remainder of the neurons with CT and IX input responded with increasing EPSC amplitudes to greater intensity stimulus shocks. EPSCs evoked in rNST neurons by increasing shock frequency to both CT and IXth nerves resulted in reduced amplitude EPSCs characteristic of frequency dependent synaptic depression. Our results suggest that the second order rNST neurons respond to afferent input with different patterns of EPSCs that potentially influence transmission of gustatory information. Frequency dependent synaptic depression would act as a low pass filter important in the initial processing of gustatory derived sensory messages.
Keywords: Taste, Nucleus of the solitary tract, synaptic responses, frequency dependent depression, chorda tympani nerve, glossopharyngeal nerve
1. Introduction
Taste information from the anterior and posterior tongue is relayed to the CNS by the chorda tympani (CT) branch of the VIIth and lingual-tonsillar branch of the glossopharyngeal (IXth) nerves (Hamilton and Norgren, 1984). The brainstem connections and morphology of the rNST has been studied in a number of mammals including rats (Mangold and Hill, 2007; May and Hill, 2006), and hamsters (Whitehead, 1986; Whitehead and Frank, 1983). The central processes of the CT and IX nerves enter the brainstem to form the solitary tract (ST) which descends in a rostral-caudal direction giving off collateral branches that synapse with second order neurons of the rostral nucleus of the solitary tract (rNST) (Whitehead and Frank, 1983). The terminal fields of the CT and IX nerves form a dense overlapping rostral caudal neuropil surrounding second order rNTS neurons (Mangold and Hill, 2007; May and Hill, 2006). At the ultastructural level the afferent endings are observed to have multiple synaptic specializations with the dendrites of rNST neurons. Two types of synaptic ending have been defined associated with the afferent terminals: the first type contains large round vesicles and the second are smaller endings densely filled with small vesicles (Whitehead, 1986).
The properties of synapses between primary afferent gustatory input and second order rNST neurons have been largely ignored by the many investigators using extracellular recordings in rNST. Some details have emerged based on correlations between the average discharge frequency of CT fibers and the average frequency response of rNST neurons to the same concentration of orally applied chemical stimuli. Results of these studies revealed that there was an apparent gain between the afferent input and the second order NST neurons (Doetsch and Erickson, 1970; Ganchrow and Erickson, 1970; Vogt and Mistretta, 1990). On average second order neuron responses are larger than the frequencies recorded in the CT by a factor of 4.3 explained by convergence of primary afferent fibers onto second order rNST neurons. However, these early experiments were based on recordings from unidentified rNST neurons with an unknown role in rNST sensory processing circuits. Moreover, the techniques used did not permit examination of the transfer function of individual synapses and are based on data reported in two separate investigations (Doetsch and Erickson, 1970; Erickson et al., 1965).
Electrophysiological properties of synapses between the gustatory afferent input and the second order rNST neurons have been studied in rat brain slices using whole cell recording (Grabauskas and Bradley, 1996; Wang and Bradley, 1995). Synaptic potentials and currents were initiated in the second order neurons by electrical stimulation of the ST which evokes several types of post synaptic potentials (PSP) in the rNST neurons after a short latency. The majority of the PSPs were depolarizing and the remainder were either hyperpolarizing or mixed excitatory and inhibitory sequences (Grabauskas and Bradley, 1996; Wang and Bradley, 1995). Application of GABAA and glutamate receptor blockers indicated that the evoked PSPs are a composite of summed excitatory and inhibitory potentials. The short latency PSP component was excitatory and the longer latency component inhibitory. Use of glutamate receptor blockers effectively eliminated all the PSP leading to the conclusion that AMPA receptors are the predominant glutamate receptor at the primary afferent synapse in the central taste pathway (Grabauskas and Bradley, 1996; Wang and Bradley, 1995). In all of these studies the recordings were made “blind' from neurons located in the rNST using fixed amplitude ST stimulation currents.
In the present study, we now characterize the properties of the first central synapse in the central taste pathway with identified monosynaptic connections to rNST neurons. By using anterograde fluorescent labeling of either the CT or IXth nerves we were able to record evoked synaptic responses of rNST neurons with known input. In addition, using graded stimulus intensities and different stimulating frequencies we could further characterize the properties and frequency dependence of the first central synapse in the taste pathway.
2. Results
2.1. Identification of second order neurons in rNTS
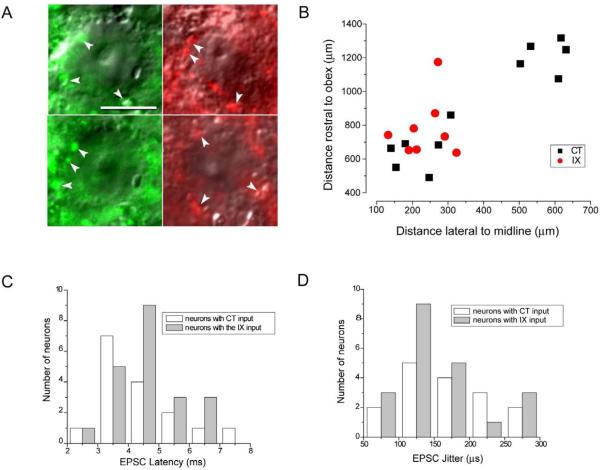
Both anatomical and electrophysiological techniques have been used to identify second order neurons in the caudal non-gustatory NST (Doyle et al., 2004; Doyle and Andresen, 2001). We have used a similar approach to categorize second order neurons in rNST. Neurons in the rNST surrounded by labeled fibers were visualized using fluorescent and IR-DIC optics and patched for current and voltage clamp recordings (Fig. 1A). The neurons were filled with Lucifer yellow during recording and the location of a subset of these neurons could be mapped based on distance rostral to the obex and lateral to the midline of the brainstem (Fig. 1B). The neurons were distributed along the rostral-caudal extent of the rNST: the most rostral were located in the CT terminal field while the more caudally situated neurons were in an area of overlap between the CT and IX terminal fields.
Fig. 1.
Characterization of monosynaptic contacts between gustatory afferent input and second order rNST neurons. A. Examples of DIC images of rNST neurons surrounded by labeled afferent profiles. The profiles surrounding the neurons on the left resulted from Alexa Fluor dextran 488 (green) applied to the CT and the profiles surrounding the neurons on the right resulted from Alexa Fluor dextran 568 (red) applied to the IX nerve. Examples of the labeled profiles are indicated by white arrow heads. Bar = 10 μm. B. Location of a subset of the recorded neurons. Lucifer yellow labeled neurons with definite co-ordinates were mapped relative their position rostral to the obex and lateral to the midline of the brainstem. C Distribution of synaptic latencies evoked by ST stimulation for neurons with input from the CT and IX nerves. D. Distribution of the standard deviation of synaptic latency (jitter) for neurons with input from the CT and IX nerves.
rNST neurons surrounded by labeled fluorescent profiles (arrowheads in Fig. 1A) were selected for patch-clamping and EPSCs evoked by current stimulation of the ST. The mean synaptic latency of rNST neurons in the CT terminal field was 4.3 ± 1.4 ms with a jitter values of 170 ± 63 μs (n = 16) and for neurons in the IXth terminal field mean latency was 4.6 ± 1.1 ms and jitter 147 ± 62 μs (n = 21) (Fig. 1C, D). There was no significant difference in EPSC latency and jitter value between neurons in the CT and IXth terminal field (p > 0.05, n = 16 and 21 respectively). Based on the close proximity of fluorescent labeling surrounding the rNST neurons and measurements of latency and jitter, these EPSC recordings are considered to be from second order rNST neurons with monosynaptic input from the primary afferent gustatory neurons.
2.2. Synaptic characteristics of rNST neurons with input from CT and the IXth nerve
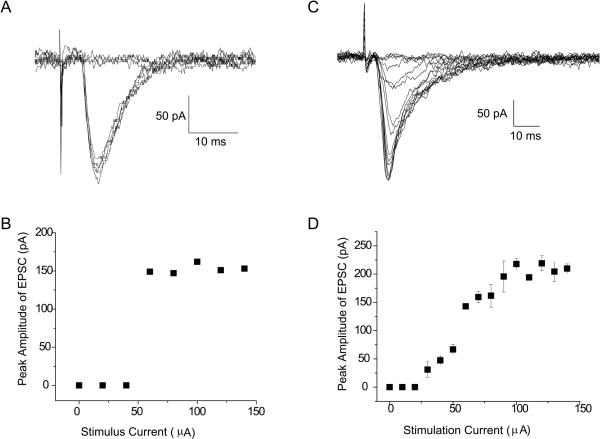
After second order neurons were identified, EPSCs were evoked with an increasing ST stimulus intensities from 0–20, 10–100, and 100–1000 μA. Two types of EPSCs were recorded. The first type responded to increased ST stimulus intensities with an all-or-none EPSC in which once threshold stimulus intensity was achieved the EPSC abruptly reached its maximum amplitude (Fig. 2A, 2B). The second EPSC type responded with a graded increase in amplitude until the EPSC reached a saturated level (Fig. 2, C,D). 70% of the neurons in the CT terminal field had all-or-none EPSCs and the remainder were graded EPSCs (n = 20). In contrast, 70% of neurons with IXth input had graded EPSCs and the remaining neurons had all-or-none EPSCs (n = 27). Investigators of the caudal NST do not report any incidence of graded responses and most if not all synapses respond with an all-or-none response (Andresen et al., 2004; Doyle and Andresen, 2001) to increasing current stimulation of the ST.
Fig. 2.
A. EPSC evoked by increasing intensity stimulation of the ST. Once threshold is exceeded a constant amplitude all-or-none EPSC is evoked. B. Relationship between stimulus current intensity and EPSC amplitude that is characteristic of all-or-none EPSCs. C. EPSC evoked by increasing intensity stimulation of the ST characterized by a graded amplitude response. D. Relationship between stimulus current intensity and EPSC amplitude that is characteristic of graded EPSCs
2.3. Frequency-dependent synaptic characteristics of rNST neurons with input from CT and IXth nerves
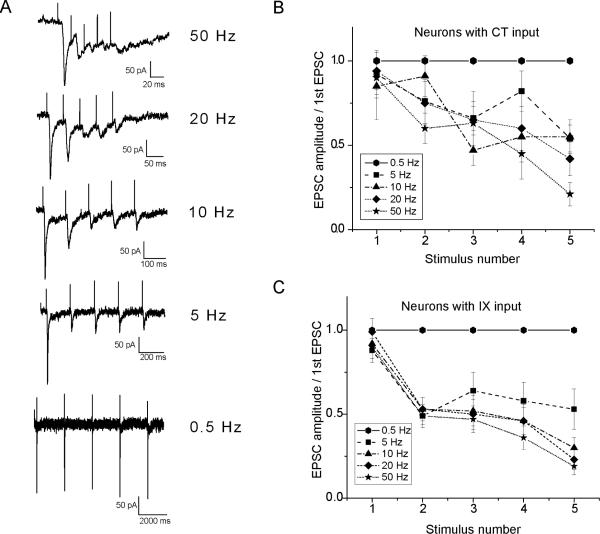
Frequency-dependent depression of synaptic transmission is a characteristic of EPSCs recorded from neurons located in the caudal non-gustatory NTS (Chen et al., 1999; Miles, 1986). We examined the EPSC responses of rNST neurons to trains of 5 stimuli recorded at 0.5, 5, 10, 20 and 50Hz. Stimulus intensity was set just above threshold to evoke an EPSC. While ST stimulation trains at 0.5Hz evoked EPSCs of similar amplitudes, when ST stimulus frequencies were increased to 5, 10, 20 and 50 Hz, neuron responses could be divided into two groups. In the first group neurons responded with a depression of the EPSC amplitude as stimulus frequency increased (Fig. 3A). At 50Hz stimulation, the amplitude of the EPSC was reduced by more than 80% when compared to the amplitude of the first EPSC in the train of stimuli (Fig. 3A). Neurons with CT and IXth input have EPSCs with similar frequency dependent amplitude depression characteristics (Fig. 3 B, C). A second small group of neurons responded to increasing ST stimulus frequency with EPSC amplitudes that were of similar magnitudes.
Fig. 3.
Frequency dependence of EPSCs evoked by ST stimulation. A. EPSCs evoked in an rNST neuron at frequencies of 0.5, 5, 10, 20 and 50 Hz. At higher frequencies the amplitude of the EPSCs declined when compared to the first evoked EPSC. Note the different timescale for the 0.5 Hz trace. B. and C. Relationship of EPSC amplitude at each stimulus frequency shock to the ST as a percentage of the amplitude of the EPSC evoked by the first (control) stimulus.
3. Discussion
Much recent research has demonstrated that the rNST is a complex sensory relay nucleus consisting of several subnuclei with different functional roles and neurochemical characteristics (Bradley, 2006). There is accumulating evidence of both ascending and local brainstem targets of rNST second order neurons (Lundy and Norgren, 2004) and rNST neurons have been divided into groups based on repetitive discharge characteristics, morphology and ion channel expression (Bradley and Sweazey, 1992; Suwabe and Bradley, 2009). Our data based on postsynaptic current recordings and EPSCs evoked by trains of stimuli at different frequencies now provide additional information on heterogeneity of rNST synapses. Neurons responded to increasing stimulus currents applied to the ST by either EPSCs of graded amplitudes or an all-or-none response. Neurons with a graded and all-or-none synapses responded to stimulus trains of increasing frequency with EPSCs of decreasing amplitude referred to as frequency dependent synaptic depression.
3.1. rNST primary afferent synapses differ in response characteristics
Our studies show that 70% of the rNST neurons with CT input and 30% with IX input respond to ST stimulation with all-or-none EPSCs while the remainder responded with graded amplitude EPSCs. All-or-none synapses have been extensively investigated in other brain areas including the caudal NST (Araki and De Groat, 1996; Doyle and Andresen, 2001; Stern et al., 1992). These synapses are characterized by having single or few contacts with the afferent fiber input, low failure rates and large amplitude EPSCs. They have been described as “strong” synapses characterized by high fidelity of transmission of afferent sensory information (Andresen et al., 2004; Franks and Isaacson, 2006). In contrast graded synapses have multiple convergent inputs and therefore integrate sensory input from several receptive fields resulting in amplification and modulation of the afferent information.
3.2. Role of rNST primary afferent synapses in gustatory processing
By recording from defined populations of rNST neurons with known inputs and outputs, insight can be gained on the role of the second order neurons in taste processing. In a recent study we have reported that rNST second order neurons that project rostrally to the pontine parabrachial nucleus with known afferent input from either the CT or IX nerves have all-or-none synapses (Suwabe and Bradley, 2009). These projection neurons with all-or-none synapses will pass sensory information relatively unchanged to rostral taste relays. In contrast rNST neurons with graded synapses apparently do not relay rostrally and therefore are likely to send information to oromotor or secretomotor brainstem sites. In addition, rNST neurons with graded synaptic responses have multiple inputs and therefore integrate and modulate the afferent input. Thus, the two types of synapses that characterize the rNST afferent input represent processing pathways that are either involved in the sensory role of the nucleus or as part of the connections responsible for sensory guided reflex activity.
While the CT and IXth synapses with rNST neurons were both all-or-none and graded types, a higher proportion of neurons with CT input were all-or-none. These differences may relate to the fact that anterior tongue input via the CT functions in stimulus identification while posterior tongue input via IXth plays a role in ingestive motivation and orofacial reflex activity (Spector and Glendinning, 2009). For example, investigators have reported that transection of the IXth nerve severely diminishes bitter taste-initiated reflex oral gaping movements that are characteristic of aversive tasting stimuli, whereas transection of the CT nerve has a much less significant effect on oral-motor behavior (Hanamori et al., 1988; King et al., 2008; Travers et al., 1987). Thus, the different synaptic proportions between CT and IXth nerve afferent input may relate to oromotor control circuit connections important in taste initiated acceptance and rejection behavior. Alternatively, the CT contains a high proportion of gustatory fibers while the IXth consists of mixed gustatory and somatosensory fibers (Frank, 1991). It is therefore possible that the chemosensory fibers of the CT and IXth have all-or-none synapses, and the graded synapses are transmitting non-gustatory somatosensory information.
3.3. Role of frequency-dependent-synaptic depression in gustatory processing
The majority of rNST neurons with input from either the CT or IX nerves also responded to stimulation of the ST with increasing frequency of shocks with a time-dependent depression of synaptic amplitude. Based on the results reported here the response frequency recorded from second order rNST neurons to gustatory stimulation of the oral cavity would be likely to decrease as the response frequencies of primary afferent fibers increased. Investigator recording from single CT fibers report response frequencies to taste stimuli as high as 60Hz with higher frequencies in the initial part of the response (Frank et al., 1988; Ganchrow and Erickson, 1970; Sato et al., 1994). However, in extracellular recordings from rNST neurons, investigators have reported higher response frequencies to the same chemical stimuli applied to the tongue (Ganchrow and Erickson, 1970). Unfortunately, most of the extracellular recordings of tongue stimulation are at single concentrations and responses to different concentrations are rarely reported (see for example Smith et al., 1983). The single publication describing responses of rNST neurons to increasing stimulus intensity reports only mean data (Ganchrow and Erickson, 1970).
In the caudal, visceral NST, the transfer of information across the first synapse has been measured directly by recording responses in the second order neuron to electrical stimulation of the carotid sinus nerve at different frequencies (Seller and Illert, 1969). At this caudal synapse transmission was characterized by a marked frequency dependent decrease in synaptic response amplitude. Depression of synaptic potential amplitude begins at low afferent stimulus frequencies and is depressed by 35% at 5 Hz and 60% at 10Hz (Miles, 1986). Subsequent to this early investigation, frequency dependent synaptic depression has been studied in some detail and found to occur in caudal NST neural responses evoked by stimulation of the solitary tract or selective stimulation of the visceral afferents fibers in the carotid sinus, vagus as well as other afferent input relaying multiple sensory modalities (Andresen and Yang, 1995; Doyle and Andresen, 2001; Liu et al., 2000; Miles, 1986). In caudal NST the sensory afferent synapses have a limited transmission of high-frequency input that has been suggested to confine the arterial baroreceptors within their physiological discharge frequency.
An early in vivo study in rats of frequency-following capacity of rNST neurons revealed that failure to respond to each shock applied to the CT typically began to occur at repetition rates of 40 Hz and little or no following occurred at rates of 100 Hz and higher (Doetsch and Erickson, 1970) indicating that frequency dependent depression occurs in the rNST. A more recent similar study of second order rNST neurons to paired pulse stimulation of the CT reported frequency dependent synaptic depression (Rosen and DiLorenzo, 2009). In this in vivo study rNST neurons were isolated and responses to tongue stimulation with chemicals determined and then responses of the same neurons were measured to paired pulse stimulation of the CT at pulse intervals ranging from 10 to 2000 ms (i.e. high to low frequencies). Most of the rNST neurons responded with a decreased number of action potentials to the second pulse when compared to the number of spikes evoked by the first pulse. The percentage decrease in action potential number was inversely related to the length of the interpulse interval. With long interpulse intervals (> 500 ms, low frequency) the reduction in impulse frequency following the second stimulus was minimal and maximum at short (20 – 30 ms, high frequency) intervals. Paired pulse stimulation of the CT in a smaller group of rNST neurons neither decreased nor increased the number of action potentials following the second pulse. The results of this in vivo study are comparative to the intracellular recordings in the current study and demonstrate frequency dependent changes of transmission across the first central synapse in the taste pathway. The authors of the in vivo study while noting that although frequency dependent depression might account for their result, concluded that inhibitory processes are the most likely contributing factor (Rosen and DiLorenzo, 2009). Since we used GABAA synaptic blockers to eliminate inhibitory activity it is more likely that the paired pulse depression was the result of the frequency dependent depression characteristics of the CT-rNST neuron synapses.
The role of frequency dependent depression in rNST gustatory processing is not clear since it implies that higher frequency afferent input would not pass the first synapse. The fact that time dependent depression occurs in vivo indicates that it is of significance in transmission of information between the peripheral and central taste pathways. The time course of the paired pulse depression was correlated with the breadth of tuning of the chemosensitive rNST neurons and the low pass filtering speculated to stabilize the temporal pattern of the afferent input (Rosen and DiLorenzo, 2009). Alternatively, the low pass filtering may isolate the input to frequencies that are in the linear part of the response concentration function thereby enhancing the ability to distinguish differences in stimulus concentration.
The small group of rNST neurons that do not exhibit frequency dependent depression would transmit afferent input in a relatively unaltered state. Similar subsets of neurons that have higher cut-off frequencies that pass the afferent input relatively unchanged have been described in the caudal NST (Mifflin, 2001) and also in the paired pulse study of CT-rNST synapses (Rosen and DiLorenzo, 2009).
4. Experimental procedures
4.1. Anterograde labeling of the chorda and glossopharyngeal nerves
We used the anterograde labeling method of May and Hill (2006) with minor modification to label the CT and IX nerves peripheral to their ganglia. All surgical procedures were carried out under National Institutes of Health and University of Michigan Animal Care and Use Committee approved protocols. Briefly, 14–30-day-old Sprague-Dawley rats (Charles River) were anesthetized with an intraperitoneal injection of a mixture of ketamine and xylazine (10 mg/kg each). The rat's head was fixed by an atraumatic holder (Erickson, 1966) and body temperature maintained by a heating pad. To label the CT nerve, a longitude incision was made in the midline of the neck and the tympanic bulla exposed. A small hole was made in the tympani bulla to exposure the CT in the middle ear. The CT nerve was cut and, Alexa Fluor dextran (molecular weight, 10,000) 488 (green) or 568 (red) (Molecular Probe, Eugene, OR) was applied to the cut central end. A small drop of Kwik-Cast silicone (WPI, FL) was used to prevent dye diffusion. The skin incision was closed and the rat allowed to recover on a heating pad. To label the IXth nerve the procedure was similar to labeling the CT except the lingual-tonsilar branch of IX was exposed and a small piece of parafilm was inserted under the nerve. The fluorescent tracer was applied to the cut end of the nerve and then covered with silicone. The anterograde tracers were allowed to transport for 24–48 hours. Other investigators have failed to show any significant ultrastructural evidence of degenerative changes in the synapses between the CT and IXth nerves and second order rNST neurons with these survival times (May et al., 2007). Moreover, investigators using similar methods to record EPSCs from monosynaptic synapses in the caudal non-gustatory NTS also do not report significant changes in electrophysiological properties of these synapses (Doyle and Andresen, 2001).
4.2. Slice preparation
Horizontal brainstem slices were prepared as previously described in detail (Grabauskas and Bradley, 2003; Suwabe and Bradley, 2007). Briefly, 300-μm horizontal brain stem slices were cut using a Vibratome (Technical Products International) in ice-cold physiological saline containing (in mM): 206 sucrose, 2 KCl, 2 MgSO4, 1.25 NaH2PO4, 1 CaCl2, 1 MgCl2, 26 NaHCO3 and 10 D-glucose, oxygenated with 95% O2 and 5% CO2 A sapphire knife (Delaware Diamond Knives) was used to cut the slices. After 1 hour of recovery, a slice was transferred to the recording chamber attached to the stage of a Nikon Eclipse E600FN microscope and continually perfused with physiological saline containing (in mM) 124 NaCl, 5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1.25KH2PO4, 26 NaHCO3 and 10 dextrose at 32°C~34°C.
4.3. Whole cell patch clamp recordings
The terminal fields of the CT and IX nerves were identified by fluorescent illumination. Neurons located in the rNTS surrounded by labeled fibers were identified by infrared-differential interface contrast optics (IR-DIC) via a CCD camera (DAGE-MTI). Patch pipettes pulled in two stages from 1.5mm filament glasses (WPI) were filled with a solution containing (in mM) 130 K-gluconate, 10 n-2-hydroxy-ethylpiperazine- N′-2 ethanesulfonic acid (HEPES), 10 ethylene glycolbis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 1 MgCl2, 1 CaCl2 and 2 ATP, buffered to pH 7.2 with KOH. Lucifer yellow (0.1%, Sigma) was dissolved in the pipette solution to label recorded neurons for later mapping of position. Tip resistance of filled pipettes was 6–8 MΩ. An Axoclamp-2A amplifier (Axon Instruments, CA) was used to perform voltage clamp recording. Membrane potential was held at −60 mV by current injection. Data were passed through a 2 kHz low-pass filter and digitized at 10 kHz (DigiData 1200, Axon Instruments) and recorded on a computer using pCLAMP8 software (Axon Instruments). Synaptic responses were evoked via a concentric bipolar electrode (200 μm outer diameter, Frederick Haer, Bowdoinham, ME), placed on the ST 1~2 mm away from the recording site. Shocks were delivered to the ST using a programmable stimulator (Master-8, AMPI, Israel). When performing EPSC recordings, a single stimulation at 0.1 ms pulse duration was generated by the stimulator. In the frequency dependent EPSC recordings, a train of 0.1 ms pulse currents were generated at 0.5 Hz, 5 Hz, 10 Hz, 20 Hz and 50 Hz with 3~4s intervals between stimulations. EPSCs were recorded after GABAA block by addition of bicuculline (10μM, Biomol Int.) to the physiological saline superfusing the slices. For each neuron we measured basic membrane characteristics as well as synaptic latency. Latency was measured as the time from the beginning of the stimulus artifact to the beginning of the rise of postsynaptic current and jitter was calculated for each neuron as the standard deviation of the latency of repeatedly (> 20) elicited PSCs (Doyle and Andresen, 2001). No leak subtractions, liquid junction potentials or series resistance compensations were performed. Data were analyzed using CLAMPFIT (Axon Instruments), Origin (Microcal), and SPSS. Numerical value are presented as mean ± SD and statistic significant set at p<0.05. All drugs except where indicated were obtained from Sigma (St. Louis, MO).
Acknowledgement
Supported by National Institute on Deafness and Other Communication Disorders Grant number DC000288 to R.M.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andresen MC, Doyle MW, Bailey TW, Jin YH. Differentiation of autonomic reflex control begins with cellular mechanisms at the first synapse within the nucleus tractus solitarius. Braz. J. Med. Biol. Res. 2004;37:549–558. doi: 10.1590/s0100-879x2004000400012. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Yang MY. Dynamics of sensory afferent synaptic transmission in aortic baroreceptor regions of nucleus tractus solitarius. J. Neurophysiol. 1995;74:1518–1528. doi: 10.1152/jn.1995.74.4.1518. [DOI] [PubMed] [Google Scholar]
- Araki I, De Groat WC. Unitary excitatory synaptic currents in preganglionic neurons mediated by two distinct groups of interneurons in neonatal rat sacral parasympathetic nucleus. J. Neurophysiol. 1996;76:215–226. doi: 10.1152/jn.1996.76.1.215. [DOI] [PubMed] [Google Scholar]
- Bradley RM. The Role of the Nucleus of the Solitary Tract in Gustatory Processing. CRC Press; Boca Raton: 2006. pp. 1–158. [PubMed] [Google Scholar]
- Bradley RM, Sweazey RD. Separation of neuron types in the gustatory zone of the nucleus tractus solitarii based on intrinsic firing properties. J. Neurophysiol. 1992;67:1659–1668. doi: 10.1152/jn.1992.67.6.1659. [DOI] [PubMed] [Google Scholar]
- Chen CY, Horowitz JM, Bonham AC. A presynaptic mechanism contributes to depression of autonomic signal transmission in NTS. Am. J. Physiol. Heart Circ. Physiol. 1999;277:H1350–H1360. doi: 10.1152/ajpheart.1999.277.4.H1350. [DOI] [PubMed] [Google Scholar]
- Doetsch GS, Erickson RP. Synaptic processing of taste-quality information in the nucleus tractus solitarius of the rat. J. Neurophysiol. 1970;33:490–507. doi: 10.1152/jn.1970.33.4.490. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vitro. J. Neurophysiol. 2001;85:2213–2223. doi: 10.1152/jn.2001.85.5.2213. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin YH, Appleyard SM, Low MJ, Andresen MC. Strategies for cellular identification in nucleus tractus solitarius slices. J. Neurosci. Methods. 2004;137:37–48. doi: 10.1016/j.jneumeth.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Erickson RP. Nontraumatic headholders for mammals. Physiol. Behav. 1966;1:97–98. [Google Scholar]
- Erickson RP, Doetsch GS, Marshall DA. The gustatory neural response function. J. Gen. Physiol. 1965;49:247–263. doi: 10.1085/jgp.49.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME. Taste-responsive neurons of the glossopharyngeal nerve of the rat. J. Neurophysiol. 1991;65:1452–1463. doi: 10.1152/jn.1991.65.6.1452. [DOI] [PubMed] [Google Scholar]
- Frank ME, Bieber SL, Smith DV. The organization of taste sensibilities in hamster chorda tympani nerve fibers. J. Gen. Physiol. 1988;91:861–896. doi: 10.1085/jgp.91.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks KM, Isaacson JS. Strong single-fiber sensory inputs to olfactory cortex: Implications for olfactory coding. Neuron. 2006;49:357–363. doi: 10.1016/j.neuron.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Ganchrow JR, Erickson RP. Neural correlates of gustatory intensity and quality. J. Neurophysiol. 1970;33:768–784. doi: 10.1152/jn.1970.33.6.768. [DOI] [PubMed] [Google Scholar]
- Grabauskas G, Bradley RM. Frequency-dependent properties of inhibitory synapses in the rostral nucleus of the solitary tract. J. Neurophysiol. 2003;89:199–211. doi: 10.1152/jn.00963.2001. [DOI] [PubMed] [Google Scholar]
- Grabauskas G, Bradley RM. Synaptic interactions due to convergent input from gustatory afferent fibers in the rostral nucleus of the solitary tract. J. Neurophysiol. 1996;76:2919–2927. doi: 10.1152/jn.1996.76.5.2919. [DOI] [PubMed] [Google Scholar]
- Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J. Comp. Neurol. 1984;222:560–577. doi: 10.1002/cne.902220408. [DOI] [PubMed] [Google Scholar]
- Hanamori T, Miller IJ, Jr., Smith DV. Gustatory responsiveness of fibers in the hamster glossopharyngeal nerve. J. Neurophysiol. 1988;60:478–498. doi: 10.1152/jn.1988.60.2.478. [DOI] [PubMed] [Google Scholar]
- King CT, Garcea M, Stolzenberg DS, Spector AC. Experimentally cross-wired lingual taste nerves can restore normal unconditioned gaping behavior in response to quinine stimulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R738–R747. doi: 10.1152/ajpregu.00668.2007. [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen CY, Bonham AC. Frequency limits on aortic baroreceptor input to nucleus tractus solitarii. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H577–H585. doi: 10.1152/ajpheart.2000.278.2.H577. [DOI] [PubMed] [Google Scholar]
- Lundy RF, Norgren R. Gustatory system. In: Paxinos G, editor. The Rat Nervous System. Elsevier Academic Press; Amsterdam: 2004. pp. 891–921. [Google Scholar]
- Mangold JE, Hill DL. Extensive reorganization of primary afferent projections into gustatory brainstem induced by feeding a sodium restricted diet during development: less is more. J. Neurosci. 2007;27:4650–4662. doi: 10.1523/JNEUROSCI.4518-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May OL, Erisir A, Hill DL. Ultrastructure of primary afferent terminals and synapses in the rat nucleus of the solitary tract: Comparison among the greater superficial petrosal, chorda tympani, and glossopharyngeal nerves. J.Comp.Neurol. 2007;502:1066–1078. doi: 10.1002/cne.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May OL, Hill DL. Gustatory terminal field organization and developmental plasticity in the nucleus of the solitary tract revealed through triple-fluorescence labeling. J. Comp. Neurol. 2006;497:658–669. doi: 10.1002/cne.21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin SW. What does the brain know about blood pressure? News Physiol. Sci. 2001;16:266–271. doi: 10.1152/physiologyonline.2001.16.6.266. [DOI] [PubMed] [Google Scholar]
- Miles R. Frequency dependence of synaptic transmission in nucleus of the solitary tract in vitro. J. Neurophysiol. 1986;55:1076–1090. doi: 10.1152/jn.1986.55.5.1076. [DOI] [PubMed] [Google Scholar]
- Rosen AM, DiLorenzo PM. Two types of inhibitory influences target different groups of taste- responsive cells in the nucleus of the solitary tract of the rat. Brain Res. 2009;1275:24–32. doi: 10.1016/j.brainres.2009.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Ogawa H, Yamashita S. Gustatory responsiveness of chorda tympani fibers in the cynomolgus monkey. Chem. Senses. 1994;19:381–400. doi: 10.1093/chemse/19.5.381. [DOI] [PubMed] [Google Scholar]
- Seller H, Illert M. The localization of the first synapse in the carotid sinus baroreceptor reflex pathway and its alteration of the afferent input. Pflugers Arch. 1969;306:1–19. doi: 10.1007/BF00586608. [DOI] [PubMed] [Google Scholar]
- Smith DV, Van Buskirk RL, Travers JB, Bieber SL. Gustatory neuron types in hamster brain stem. J. Neurophysiol. 1983;50:522–540. doi: 10.1152/jn.1983.50.2.522. [DOI] [PubMed] [Google Scholar]
- Spector AC, Glendinning JI. Linking peripheral taste processes to behavior. Curr. Opin. Neurobiol. 2009;19:370–377. doi: 10.1016/j.conb.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P, Edwards FA, Sakmann B. Fast and slow components of unitary EPSCs on stellate cells elicited by focal stimulation in slices of rat visual cortex. J. Physiol. (Lond.) 1992;449:247–278. doi: 10.1113/jphysiol.1992.sp019085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwabe T, Bradley RM. Effects of 5-hydroxytryptamine and substance P on neurons of the inferior salivatory nucleus. J. Neurophysiol. 2007;97:2605–2611. doi: 10.1152/jn.00859.2006. [DOI] [PubMed] [Google Scholar]
- Suwabe T, Bradley RM. Characteristics of rostral solitary tract nucleus neurons with identified afferent connections that project to the parabrachial nucleus in rats. J. Neurophysiol. 2009;102:546–555. doi: 10.1152/jn.91182.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Grill HJ, Norgren R. The effects of glossopharyngeal and chorda tympani nerve cuts on the ingestion and rejection of sapid stimuli: an electromyographic analysis in the rat. Behav. Brain Res. 1987;25:233–246. doi: 10.1016/0166-4328(87)90071-4. [DOI] [PubMed] [Google Scholar]
- Vogt MB, Mistretta CM. Convergence in mammalian nucleus of solitary tract during development and functional differentiation of salt taste circuits. J. Neurosci. 1990;10:3148–3157. doi: 10.1523/JNEUROSCI.10-09-03148.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Bradley RM. In vitro study of afferent synaptic transmission in the rostral gustatory zone of the rat nucleus of the solitary tract. Brain Res. 1995;702:188–198. doi: 10.1016/0006-8993(95)01062-6. [DOI] [PubMed] [Google Scholar]
- Whitehead MC. Anatomy of the gustatory system in the hamster: synaptology of facial afferent terminals in the solitary nucleus. J. Comp. Neurol. 1986;244:72–85. doi: 10.1002/cne.902440106. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Frank ME. Anatomy of the gustatory system in the hamster: central projections of the chorda tympani and the lingual nerve. J. Comp. Neurol. 1983;220:378–395. doi: 10.1002/cne.902200403. [DOI] [PubMed] [Google Scholar]