Abstract
Noscapine, a benzylisoquinoline alkaloid derived from opium, was recently reported to exhibit activity against a variety of cancers through a poorly understood mechanism. Because the transcription factor nuclear factor-kappaB (NF-κB) has been linked with inflammation, survival, proliferation, invasion, and angiogenesis in tumors, we hypothesized that noscapine mediates its effects by modulating the NF-κB activation pathway. We found that noscapine potentiates apoptosis induced by cytokines and chemotherapeutic agents in tumor cells. Noscapine alone suppressed proliferation of human leukemia and myeloma cells and downregulated the constitutive expression of cell survival proteins. Noscapine also abrogated the inducible expression of proteins involved in survival, proliferation, invasion, and angiogenesis, all of which are regulated by NF-κB. Noscapine suppressed both inducible and constitutive NF-κB activation in tumor cells through inhibition of IκB kinase (IKK), leading to inhibition of phosphorylation and degradation of IκBα. Noscapine also suppressed phosphorylation and nuclear translocation of p65, leading to inhibition of NF-κB reporter activity induced by various components of the NF-κB activation pathway. Activity of the NF-κB-containing COX-2 promoter was also inhibited by noscapine. Thus, noscapine inhibits the proliferation of leukemia cells and sensitizes them to TNF and chemotherapeutic agents by suppressing the NF-κB signaling pathway.
Introduction
Noscapine (also called narcotine, nectodon, nospen, and anarcotine) is a benzylisoquinoline alkaloid derived from the opium poppy Papaver somniferum. This alkaloid lacks sedative activity and has been used as a cough suppressant for decades, with no evidence of toxicity or side effects(1). In 1998, noscapine was shown to have potent antitumor activity against murine lymphoid tumors, through its binding to tubulin and inhibition of microtubule assembly(2). Since then, noscapine has been shown to exhibit activity against a wide variety of tumors in vitro and in vivo, including leukemia and lymphoma(3, 4), melanoma(5), ovarian cancer (6), gliomas(7), breast cancer(8), lung cancer(9), and colon cancer(10). This alkaloid also sensitizes human gliomas to taxol and radiation(11). Noscapine inhibits the growth of tumor cells with little toxicity to normal cells(12).
How noscapine exhibits its antitumor effects is not fully understood, but various mechanisms including its ability to inhibit microtubule assembly(2), suppress expression of hypoxia-inducible factor-1α(13), induce p21 and p53(10), induce apoptosis-inducing factor (AIF)(14), activate c-Jun-N-terminal kinase (JNK)(6), repress Bcl-2(9), and activate p34(cdc2); have been implicated. Like paclitaxel, noscapine is a microtubule-interacting agent, but it can efficiently inhibit the growth of both paclitaxel-sensitive and paclitaxel-resistant ovarian cancer cells(6), indicating that noscapine may mediate anticancer effects through other mechanisms. Because of the critical role of nuclear factor (NF)-κB in inflammation and tumor cell survival, proliferation, invasion, angiogenesis, radioresistance and chemoresistance(15, 16), we hypothesized that noscapine mediates its effects through modulation of the NF-κB signaling pathway.
NF-κB is a transcription factor that consists of 5 proteins: c-Rel, RelA (p65), RelB, NF-κB1 (p50 and p105), and NF-κB2 (p52)(17). Under resting conditions, NF-κB consists of a heterotrimer of p50, p65, and IκB subunit α (IκBα) residing in the cytoplasm(18). NF-κB is activated by a sequence involving the phosphorylation, ubiquitination, and degradation of IκBα, and the phosphorylation of p65, which in turn lead to the translocation of NF-κB to the nucleus, where it binds to specific response elements in the DNA(19). NF-κB regulates the expression of several genes whose products are involved in tumorigenesis, including antiapoptotic proteins (e.g., Bcl-2, Bcl-xl, cIAP, survivin, and TRAF1); COX-2; MMP-9; genes encoding adhesion molecules, chemokines, and inflammatory cytokines; and cell cycle regulatory genes (e.g., cyclin D1 and c-Myc)(15).
Because of the critical role of NF-κB in most cancers and leukemia in particular, we investigated the effects of noscapine on proliferation of leukemia cells, on apoptosis induced by tumor necrosis factor α (TNF) and chemotherapeutic agents, on NF-κB-regulated gene products, and on the NF-κB activation pathway. We found that noscapine is a potent inhibitor of activation of IκBα kinase (IKK), which is required for NF-κB activation. Noscapine suppressed the NF-κB pathway, inhibited various gene products, inhibited proliferation, and potentiated apoptosis in leukemia cells.
Methods
Reagents
A 50 mM solution of (S,R)-noscapine (Sigma Aldrich) was prepared in 100% dimethylsulfoxide, stored at -20°C, and then diluted as needed in cell culture medium. Bacteria-derived recombinant human TNF-α was kindly provided by Genentech (South San Francisco). Penicillin, streptomycin, IMDM, DMEM, RPMI 1640 medium, and FBS were purchased from Invitrogen (Carlsbad). Antibodies against p65, IκBα, cyclin D1, MMP-9, PARP, cIAP1, TRAF1, Bcl-2, c-Myc, caspase-3, caspase-8, ICAM-1, COX-2, Bax and the annexin V staining kit were purchased from Santa Cruz Biotechnology. Antibody to VEGF was purchased from Thermo Scientific. Antibody to survivin was purchased from R&D System. Antibody to XIAP was purchased from BD Biosciences. Antibodies to Ser32/36-phosphorylated IκBα, Ser536-phosphorylated p65, cleaved caspase-3, and cleaved caspase-8 were purchased from Cell Signaling Technology. Antibodies to IKKα, IKKβ, and cellular caspase-8 (FLICE)-like inhibitory protein (c-FLIP) were kindly provided by Imgenex.
Cell lines
KBM-5, HL-60, Jurkat, HuT-78, U266 and RPMI-8226, H1299, A293, DU145, and SCC4 cells were purchased from the American Type Culture Collection. KBM-5 cells were cultured in IMDM supplemented with 15% FBS; H1299, DU145, HuT-78, U266, RPMI-8226 and Jurkat cells were cultured in RPMI 1640 medium; and A293 cells were cultured in DMEM supplemented with 10% FBS. SCC4 cells were cultured in DMEM containing 10% FBS, nonessential amino acids, pyruvate, glutamine, and vitamins.
Electrophoretic mobility shift assay
To assess NF-κB activation, we performed electrophoretic mobility shift assay (EMSA) essentially as described previously(20).
Western blot analysis
To determine the levels of protein expression, we prepared extracts and fractionated them by SDS-PAGE. After electrophoresis, the proteins were electrotransferred to nitrocellulose membranes, blotted with the relevant antibody, and detected with an electrogenerated chemiluminescence reagent (GE Healthcare).
IKK assay
To determine the effect of noscapine on TNF-induced IKK activation, we analyzed IKK essentially as described previously(21).
NF-κB-dependent reporter gene expression assay
The effect of noscapine on TNF-induced NF-κB-dependent reporter gene transcription in A293 cells was measured as described previously(21).
Luciferase assay
The effect of noscapine on COX-2 promoter activity induced by TNF was analyzed using a luciferase assay. A293 cells were transfected with COX-2 promoter-luciferase reporter DNA. After 24 h of transfection, the cells were incubated with noscapine for 12 h, exposed to 1 nM TNF for 20 h, and harvested. Luciferase activity was measured using a luciferase assay system (Promega).
Cytotoxicity assay
Cytotoxicity was assayed by the modified tetrazolium salt 3-(4-5-dimethylthiozol-2-yl)2-5-diphenyl-tetrazolium bromide (MTT) assay as described previously(21).
Live/dead assay
To assess cytotoxicity, we used a live/dead assay (Invitrogen), which determines intracellular esterase activity and plasma membrane integrity. In brief, 1 × 106 cells were incubated with 25 μM noscapine for 12 h and then treated with 1 nM TNF for 16 h at 37°C. Cells were stained with the live/dead reagent (5 μM ethidium homodimer and 5 μM calcein-AM) and incubated at 37°C for 30 min. Cells were analyzed under a fluorescence microscope (Labophot-2, Nikon).
Annexin V assay
To detect apoptosis, we used annexin V antibody conjugated to fluorescein isothiocyanate (FITC). In brief, 106 cells were pretreated with 25 μM noscapine for 12 h, treated with 1 nM TNF for 24 h, and subjected to annexin V staining. Cells were washed, stained with FITC-conjugated antibody to annexin V, and analyzed with a flow cytometer (FACSCalibur; BD Biosciences).
Terminal deoxynucleotidyl transferase dUTP nick-end labeling assay
We assayed cytotoxicity by the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) method using an in situ cell death detection reagent (Roche Pharmaceuticals). In brief, 1× 106 cells were pretreated with 25 μM noscapine for 12 h and with 1 nM TNF for 24 h at 37°C. Thereafter, cells were incubated with reaction mixture for 60 min at 37°C. Stained cells were quantified by flow cytometry (FACSCalibur; BD Biosciences).
Results
The aim of this study was to investigate the effect of noscapine (Figure 1A) on the NF-κB signaling pathway, NF-κB-regulated gene products, and NF-κB-mediated cellular responses such as survival, proliferation, chemosensitization, invasion, and angiogenesis in leukemia cells. The concentration of noscapine we used, and the duration of exposure, had minimal effect on cell viability as determined by the Trypan blue dye exclusion test (data not shown), suggesting that the effects of noscapine on the NF-κB signaling pathway are not due to its cytotoxic effects. To examine the effect of noscapine on the NF-κB activation pathway, we used TNF because the pathway activated by this agent is relatively well investigated. For most studies, we used human leukemia and myeloma cells.
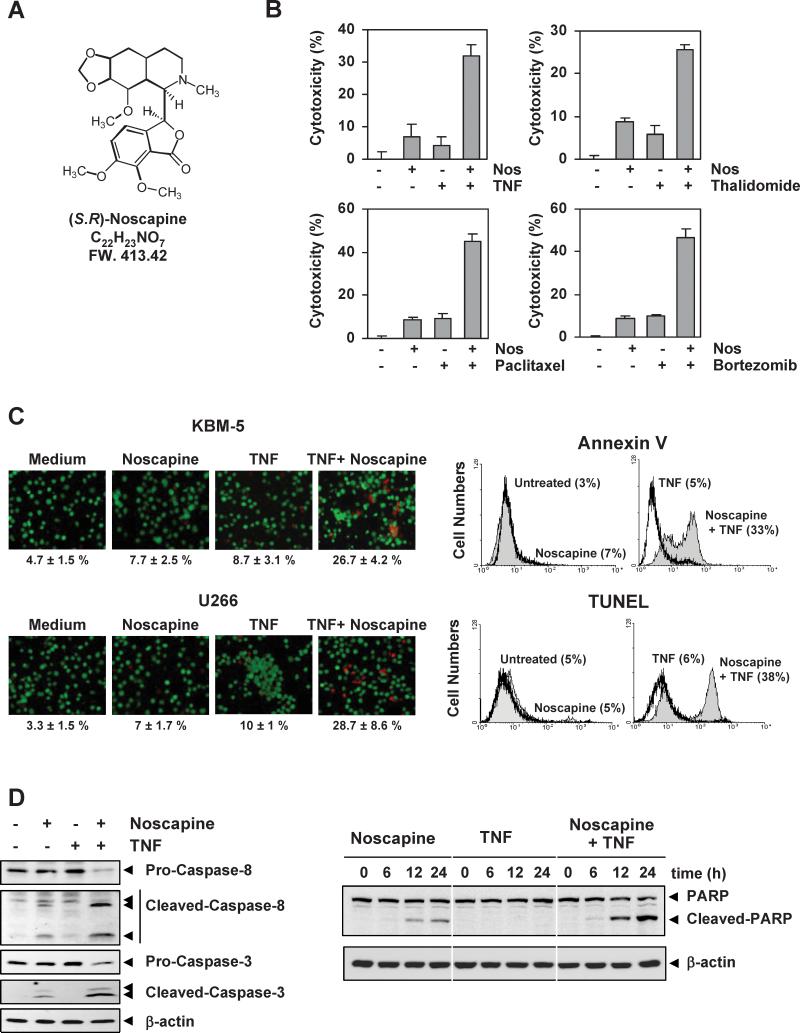
Figure 1. Noscapine potentiates apoptosis induced by TNF and chemotherapeutic agents.
(A) Chemical structure of noscapine. (B) Noscapine potentiates cytotoxicity induced by TNF and chemotherapeutic agents. KBM-5 cells were pretreated with 25 μM noscapine for 12 h and then incubated with 1 nM TNF, 10 μg/mL thalidomide, 5 nM paclitaxel, and 20 nM bortezomib for 24 h. Cell viability was then analyzed by MTT assay. (C, left) Noscapine potentiates TNF-induced apoptosis. KBM-5 or U266 cells (1 × 106) were pretreated with 25 μM noscapine for 12 h and then incubated with 1 nM TNF for 24 h. Cells were stained with live/dead assay reagent for 30 min and then analyzed under a fluorescence microscope. (right) Cells were pretreated with 25 μM noscapine for 12 h and then incubated with 1 nM TNF for 16 h. Cells were incubated with FITC-conjugated antibody to annexin V, or with TUNEL assay reagent, and then analyzed by flow cytometry for early apoptotic effects. (D, left) Noscapine enhances TNF-induced caspase activation. Cells were pretreated with 25 μM noscapine for 12 h and then incubated with 1 nM TNF for 16 h. Whole-cell extract were prepared and analyzed by western blotting using the indicated antibodies. (right) Cells were incubated with 1 nM TNF, alone or with 25 μM noscapine, for the indicated times. PARP cleavage was determined by western blot analysis.
Noscapine potentiates apoptosis induced by TNF and chemotherapeutic agents
Inflammatory cytokines, such as TNF, and chemotherapeutic agents have been shown to activate NF-κB. Because NF-κB activation has been shown to suppress the apoptosis induced by various chemotherapeutic agents(22-24), we examined the effect of noscapine on apoptosis induced by TNF and chemotherapeutic drugs. As determined by the MTT assay, noscapine significantly potentiated the cytotoxic effects of TNF, thalidomide, paclitaxel and bortezomib, in human leukemia KBM-5 cell lines (Figure 1B). We also determined the dose of noscapine required to inhibit cell growth by 50% (IC50) either alone or in combination with chemotherapeutic agents. We found that the IC50 of noscapine for KBM-5 cells decreased from 84.4 μM when used alone to 53.6 μM, 18.9 μM, 15.2 μM and 16.5 μM when combined with TNF (1 nM), thalidomide (10 μg/mL), paclitaxel (5 nM) and bortezomib (16.5 μM), respectively. Similarly, for U266 cells, the IC50 was 155 μM, 72 μM, 47.5 μM, 64.5 μM and 62.8 μM when used alone or in combination with TNF, thalidomide, paclitaxel or Bortezomib, respectively.
To determine whether noscapine potentiates apoptosis, we used the live/dead assay, which examines intracellular esterase activity and plasma membrane integrity. Noscapine enhanced TNF-induced apoptosis in KBM-5 human chronic myeloid leukemia cells (Figure 1C, left-upper panel) and U266 human multiple myeloma cells (Figure 1C, left-lower panel). We also used annexin V staining to examine apoptosis by membrane phosphatidylesterase exposure and found that noscapine potentiated TNF-induced early apoptosis from 5% to 33% (Figure 1C, right-upper panel). Similarly, when we examined apoptosis by DNA strand breaks using the TUNEL method, we found that noscapine enhanced apoptosis from 6% to 38% (Figure 1C, right-lower panel).
Noscapine potentiates TNF-induced caspase activation and PARP cleavage
TNF binds to TNFR1, which then sequentially recruits TNFR-associated death domain (TRADD) and TNFR-associated factor 2 (TRAF2), leading to activation of NF-κB and recruitment of Fas-associated death domain, which ultimately leads to activation of caspases(23). We investigated whether noscapine affects TNF-induced activation of caspase-8 (also called FLICE) and caspase-3. TNF alone had a minimal effect on activation of caspase-8 or caspase-3, whereas treatment with noscapine potentiated the activation, as indicated by the presence of cleaved caspases (Figure 1D, left). We also used the PARP cleavage assay to detect TNF-induced apoptosis. Again, noscapine potentiated the effect of TNF-induced PARP cleavage, even though noscapine alone resulted in minimal PARP cleavage (Figure 1D, right). These assays demonstrate that noscapine enhances the apoptotic effects of TNF and chemotherapeutic drugs.
Noscapine suppresses cell proliferation and enhances apoptosis in cancer cells
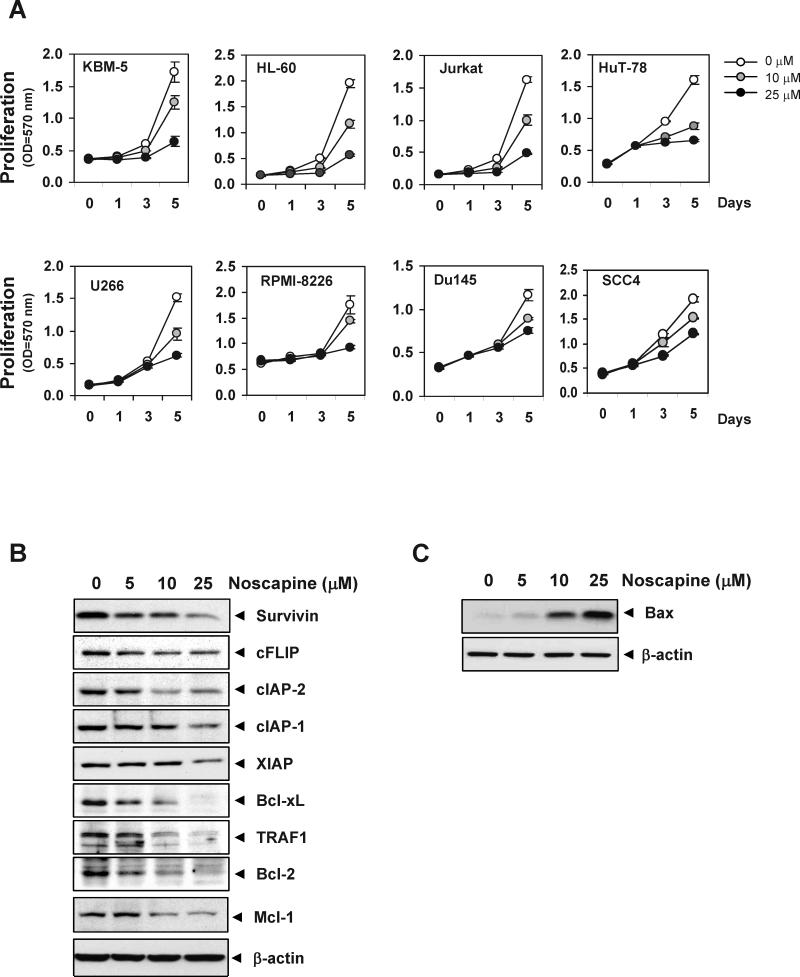
We examined whether noscapine alone can modulate the proliferation of various tumor cell types. Noscapine by itself suppressed the proliferation of human leukemic cells (such as KBM-5 and HL-60), T-cell lymphoma cells (Jurkat and HuT-78), and multiple myeloma cells (U266 and RPMI-8226; Figure 2A). Besides the hematologic tumor cells, noscapine also suppressed the proliferation of solid tumor cells, such as human prostate cancer DU145 and head and neck cancer SCC4 cells.
Figure 2. Noscapine suppresses tumor cell proliferation, downregulates antiapoptotic and upregulates proapoptotic protein expression.
(A) Cells (5 × 103/100 μL) were plated in triplicate, treated with 10 or 25 μM noscapine, and subjected to MTT assay on days 1, 3, or 5 to analyze cell proliferation. Optical density (OD) was measured at 570 nm. (B and C) U266 cells were treated with various concentrations of noscapine for 24 h. Whole-cell extracts were prepared, separated on SDS-PAGE, and subjected to western blotting using antibodies against the indicated proteins.
We then assessed whether the suppression of cell proliferation by noscapine results from modulation of constitutive expression of tumor-cell survival proteins, such as cFLIP, cIAP-1, cIAP-2, XIAP, survivin, Bcl-2, Bcl-xL, Mcl-1, and TRAF1. In U266 multiple myeloma cells, noscapine downregulated the expression of all of the aforementioned antiapoptotic proteins (Figure 2B). Next, we also examined whether noscapine affects the expression of proapoptotic proteins. Noscapine induced the expression of proapoptotic bax in human multiple myeloma, U266 cells (Figure 2C). These results suggest that noscapine causes inhibition of proliferation of tumor cell and further enhances apoptosis.
Noscapine suppresses TNF-induced, NF-κB-dependent antiapoptotic proteins
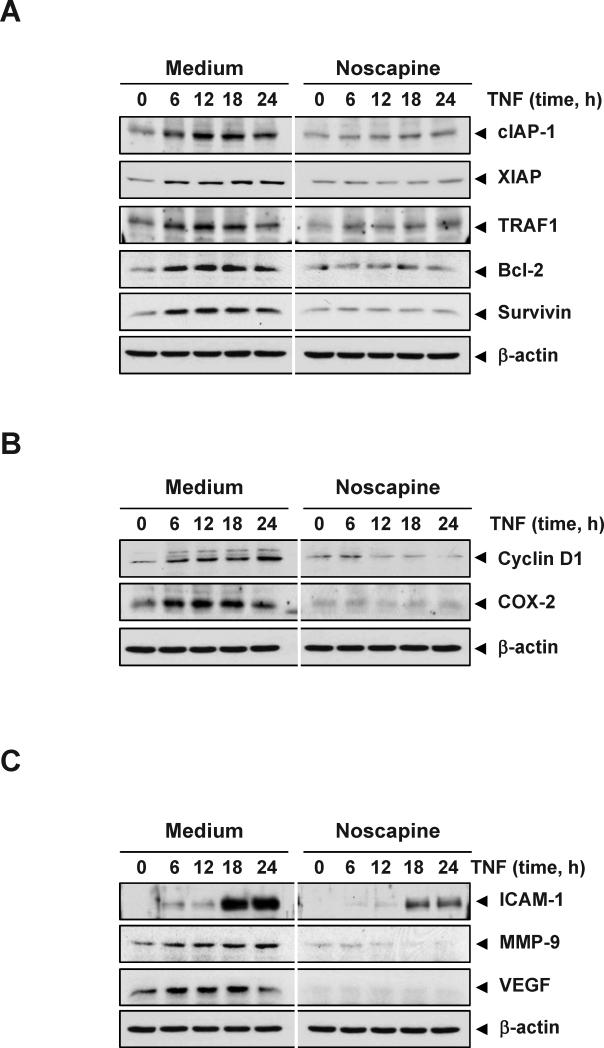
Our results above show that noscapine potentiates the apoptotic effects of TNF and chemotherapeutic agents, both known to activate NF-κB. The latter is known to regulate expression of antiapoptotic proteins such as cIAP-1, XIAP, TRAF1, Bcl-2 and survivin, all of which play a major role in evading apoptosis and prolonging survival of cancer cells(15). We examined whether noscapine modulates TNF-induced expression of these antiapoptotic proteins. TNF induced the expression of antiapoptotic proteins in a time-dependent manner, whereas noscapine markedly suppressed TNF-induced expression of these proteins (Figure 3A).
Figure 3. Noscapine represses TNF-induced, NF-κB-dependent expression of proteins involved in apoptosis resistance, proliferation, and metastasis.
KBM-5 cells were incubated with 25 μM noscapine for 12 h and then treated with 1 nM TNF for the indicated times. Whole-cell extracts were prepared and subjected to western blot analysis using the relevant antibodies. Antiapoptotic (A), proliferative (B), and metastatic (C) gene products are shown.
Noscapine represses inducible NF-κB-dependent cell proliferation proteins
Various gene products, including cyclin D1 and COX-2, are induced by TNF and have been linked with proliferation of tumor cells(19). Cyclin D1 regulates the cell cycle transition from G1 to S phase and is overexpressed in a variety of tumors(25). COX-2 is an enzyme that catalyzes the production of prostaglandin E2 and has been linked to proliferation and metastasis of tumor cells(26). We examined whether noscapine modulates the expression of proliferative gene products induced by TNF. TNF induced the expression of cyclin D1 and COX-2 in a time-dependent manner, whereas noscapine significantly inhibited their expression (Figure 3B). These results suggest a molecular mechanism that how noscapine suppresses tumor cell proliferation.
Noscapine suppresses TNF-induced proteins involved in invasion and angiogenesis
Invasion and angiogenesis are critical for tumor metastasis and are induced by TNF. ICAM-1, MMP-9, and VEGF have been implicated in invasion and angiogenesis(27-29). We therefore examined whether noscapine can suppress the expression of these proteins. TNF treatment induced the expression of VEGF, MMP-9, and ICAM-1 in a time-dependent manner, whereas noscapine inhibited their expression (Figure 3C). These results suggest a potential mechanism how this alkaloid suppresses invasion and angiogenesis.
Noscapine inhibits TNF-induced NF-κB activation
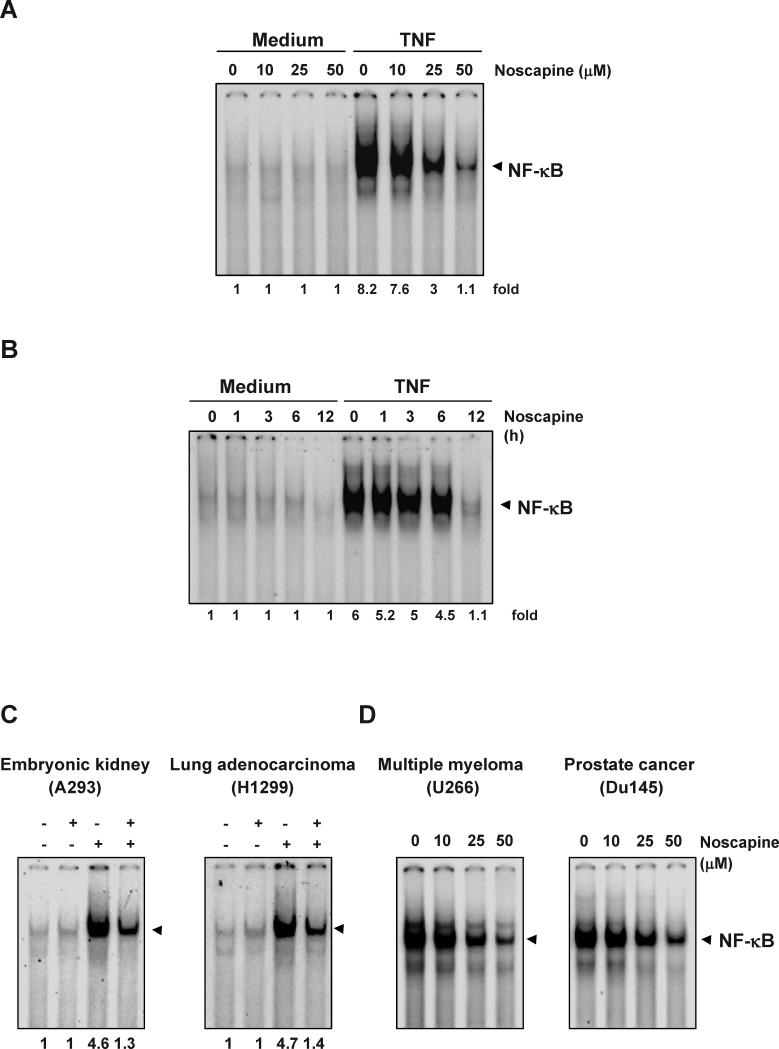
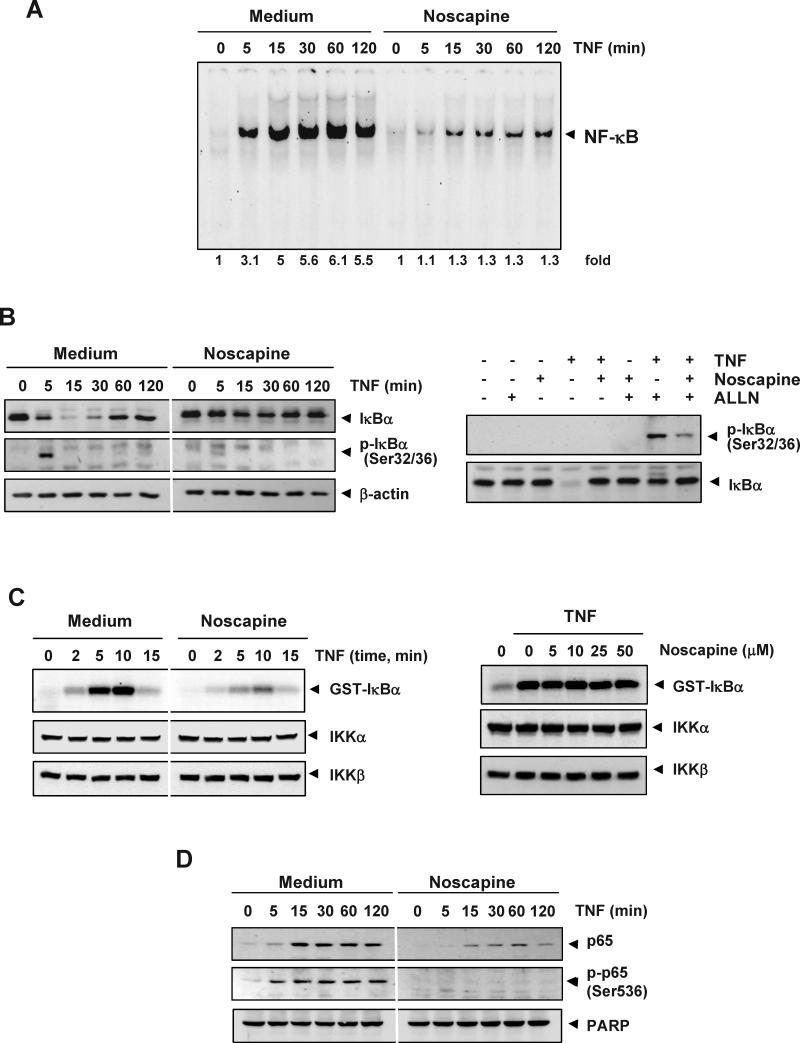
Because all of the gene products mentioned above are regulated by NF-κB, we examined whether noscapine modulates the activation of NF-κB. EMSA revealed that noscapine alone had no effect on NF-κB activation; however, noscapine inhibited TNF-mediated NF-κB activation in a dose-dependent manner (Figure 4A). The suppression of NF-κB activation by noscapine was also time dependent (Figure 4B).
Figure 4. Noscapine inhibits TNF-dependent NF-κB activation.
(A) Effect of noscapine dose. KBM-5 cells were preincubated with indicated concentrations of noscapine for 12 h, treated with 0.1 nM TNF for 30 min, and subjected to EMSA to test for NF-κB activation. (B) Effect of noscapine exposure time. Cells were preincubated with 50 μM noscapine for the indicated times, treated with 0.1 nM TNF for 30 min, and then subjected to EMSA to test for NF-κB activation. Inhibition of NF-κB activation by noscapine is not cell-type specific. To determine the effect of noscapine on inducible NF-κB activation (C), A293 and H1299 cells were incubated with 50 μM noscapine for 12 h and then incubated with 0.1 nM TNF for 30 min. To determine the effect of noscapine on constitutive NF-κB activation (D), U266 and Du145 cells were incubated with indicated concentrations of noscapine for 12 h. Nuclear extracts were then prepared and assayed for NF-κB activation by EMSA.
Noscapine-induced inhibition of NF-κB activation is not cell-type specific
Because distinct signal transduction pathways can mediate NF-κB induction in different cell types(30), we examined the effect of noscapine on TNF-induced NF-κB activation in human embryonic kidney A293 cells and human lung adenocarcinoma H1299 cells. Noscapine inhibited TNF-induced NF-κB activation in both cell types (Figure 4C).
Noscapine inhibits constitutive NF-κB activation in tumor cells
We next tested the effect of noscapine on NF-κB activation in U266 human multiple myeloma and Du145 human prostate cancer cells, which express constitutively active NF-κB(31, 32). Treatment with various concentrations of noscapine suppressed constitutive NF-κB activation in both cell types (Figure 4D).
Noscapine inhibits TNF-dependent IκBα phosphorylation and degradation
The translocation of NF-κB to the nucleus is preceded by the phosphorylation, ubiquitination, and proteolytic degradation of IκBα(33). To determine whether inhibition of TNF-induced NF-κB activation by noscapine is caused by inhibition of IκBα degradation, we pretreated cells with noscapine and then exposed them to TNF for various time periods. EMSA showed that NF-κB was activated with increasing TNF incubation times, and noscapine pretreatment markedly decreased this activation (Figure 5A). Western blot analysis showed that TNF induced IκBα degradation in control cells within 5 min, reaching maximum degradation at 15-30 min, but TNF could not induce IκBα degradation in noscapine-pretreated cells (Figure 5B, left-top panel). These results indicate that noscapine inhibits both TNF-induced NF-κB activation and IκBα degradation.
Figure 5. Noscapine inhibits TNF-dependent IκBα phosphorylation, IκBα degradation, p65 phosphorylation, and p65 nuclear translocation.
(A) Noscapine inhibits TNF-induced activation of NF-κB. KBM-5 cells were incubated with 50 μM noscapine for 12 h, treated with 0.1 nM TNF for the indicated times, and analyzed for NF-κB activation by EMSA. (B, left) Effect of noscapine on TNF-induced IκBα degradation and phosphorylation. Cells were incubated with 50 μM noscapine for 12 h and treated with 0.1 nM TNF for the indicated times. Cytoplasmic extracts were prepared, fractionated on SDS-PAGE, and electrotransferred to nitrocellulose membrane. Western blot analysis was performed using the indicated antibody. Anti-β-actin antibody was used as loading controls. (right) Effect of noscapine on phosphorylation of IκBα by TNF. Cells were preincubated with 50 μM noscapine for 12 h, incubated with 50 μg/mL ALLN for 30 min, and treated with 0.1 nM TNF for 10 min. Cytoplasmic extracts were fractionated and then subjected to western blot analysis using antibody to phosphorylated IκBα. The same membrane was reblotted with antibody to IκBα. (C, left) Noscapine inhibits TNF-induced IκBα kinase activity. Whole-cell extracts were immunoprecipitated with antibody against IKKα and analyzed by an immune complex kinase assay. To examine the effect of noscapine on expression of IKK protein, whole-cell extracts were fractionated on SDS-PAGE and examined by western blot analysis using antibodies to IKKα and IKKβ. (right) Direct effect of noscapine on IKK activation induced by TNF. Whole-cell extracts were prepared from KBM-5 cells treated with 1 nM TNF and immunoprecipitated with antibody to IKKα. The immune complex kinase assay was performed with the indicated concentrations of noscapine. (D) Effect of noscapine on TNF-induced p65 nuclear translocation and phosphorylation. Cells were incubated with 50 μM noscapine for 12 h and treated with 0.1 nM TNF for the indicated times. Nuclear extracts were prepared and performed for western blot analysis using the indicated antibody. Anti-PARP was used as loading control for nuclear extract, respectively.
We next determined whether noscapine affects the TNF-induced IκBα phosphorylation needed for IκBα degradation. TNF induced IκBα phosphorylation in control cells within 5 min, but noscapine-pretreated cells inhibited the phosphorylation of IκBα. (Figure 5B, left, second panel from top).
Phosphorylated IκBα rapidly undergoes degradation. To stabilize phosphorylated IκBα, we used N-acetyl-leucyl-leucyl-norleucinal (ALLN), which suppresses degradation of IκBα by the 26S proteasome. Western blot analysis using an antibody to the serine-phosphorylated form of IκBα showed that TNF induced IκBα phosphorylation, whereas noscapine suppressed it (Figure 5B, right).
Noscapine inhibits TNF-induced activation of IKK
Because IKK is needed for the phosphorylation of IκBα, we tested the effect of noscapine on TNF-induced IKK activation. Noscapine suppressed TNF-induced activation of IKK (Figure 5C, left upper panel). Neither TNF nor noscapine had any effect on the expression of IKKα or IKKβ proteins (Figure 5C, left bottom 2 panels).
We next examined whether noscapine suppresses IKK activity directly by binding to the IKK protein. An immune-complex kinase assay of whole-cell extracts from untreated and TNF-treated cells showed that noscapine did not directly affect the activity of IKK, suggesting that noscapine does not directly inhibit IKK, but rather modulates the activation of IKK induced by TNF (Figure 5D, right).
Noscapine inhibits nuclear translocation of p65
Suppression of IκBα degradation is known to inhibit the translocation of p65 to the nucleus. We examined whether noscapine inhibits nuclear translocation of p65 induced by TNF. TNF induced the nuclear translocation of p65 in as little as 5 min of incubation, whereas noscapine suppressed p65 translocation (Figure 5D, upper panel).
The phosphorylation of p65 is required for the transcriptional activity of NF-κB. We assessed whether noscapine affects TNF-induced phosphorylation of p65. In the nuclear fraction of TNF-treated cells, there was a time-dependent increase in the phosphorylation of p65 at Ser536; this phosphorylation was suppressed by noscapine (Figure 5D, middle panel).
Noscapine represses TNF-induced, NF-κB-dependent reporter gene expression
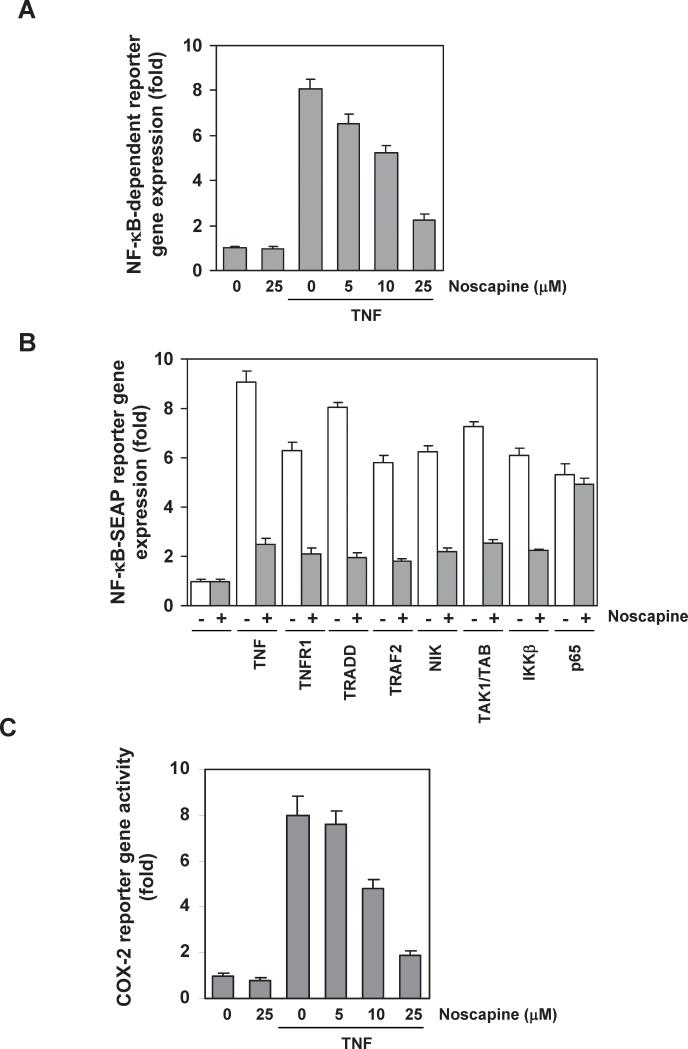
We investigated whether noscapine modulates NF-κB-dependent reporter gene transcription. TNF produced an 8-fold increase in NF-κB-dependent expression of secretory alkaline phosphatase (SEAP) activity over vector control (Figure 6A). When the cells were pretreated with various concentration of noscapine, TNF-induced NF-κB-dependent expression of SEAP was inhibited in a dose-dependent manner (Figure 6A). These results show that noscapine inhibits NF-κB-dependent reporter gene expression induced by TNF.
Figure 6. Noscapine represses NF-κB-dependent reporter gene expression induced by TNF and various plasmids.
(A) Noscapine inhibits the NF-κB-dependent reporter gene expression induced by TNF. A293 cells were transiently transfected with a NF-κB-expressing plasmid for 24 h. After transfection, cells were incubated with noscapine for 12 h and then treated with 1 nM TNF for an additional 24 h. The supernatants of the culture media were assayed for SEAP activity. (B) Noscapine inhibits the NF-κB-dependent reporter gene expression induced by TNF, TNFR1, TRADD, TRAF2, NIK, TAK1/TAB1, IKKβ, and p65. Cells were transiently transfected with a NF-κB-expressing plasmid alone or with plasmids expressing the indicated proteins. After transfection, cells were incubated with 25 μM noscapine for 12 h and then incubated with the relevant plasmid for additional 24 h. TNF-treated cells were incubated with 25 μM noscapine for 12 h and then treated with 1 nM TNF for an additional 24 h. The supernatants of the culture media were assayed for SEAP activity. (C) Noscapine inhibits the COX-2 promoter activity induced by TNF. Cells were transiently transfected with a plasmid expressing a luciferase reporter gene under control of the COX-2 promoter for 24 h and then treated with noscapine for 12 h. Cells were then treated with 1 nM TNF for an additional 24 h, lysed, and subjected to a luciferase assay. Data in panels A-C are shown as fold activity over the activity of the vector control with the mean plus or minus the SD of the results of at least 3 independent experiments.
We next determined where noscapine acts in the TNF-induced NF-κB activation pathway that is sequentially linked through TNFR1, TRADD, TRAF2, NF-κB-inducing kinase (NIK)(34, 35), transforming growth factor-β-activated kinase 1 (TAK1)/TAK1-binding protein (TAB1)(36), and IKK recruitment. Transfection of cells with plasmids expressing TNFR1, TRADD, TRAF2, NIK, TAK1/TAB1, IKKβ, or p65 induced NF-κB-dependent reporter gene expression; noscapine suppressed reporter gene expression induced by all plasmids except p65 (Figure 6B), indicating that noscapine acts at a site upstream to p65.
Noscapine inhibits TNF-induced COX-2 promoter activity
TNF induces COX-2, which has NF-κB binding sites in its promoter(37). Because downregulation of NF-κB by noscapine suppressed the expression of NF-κB-regulated gene products, including COX-2, we examined the effect of noscapine on TNF-induced COX-2 promoter activity using a COX-2 promoter-luciferase reporter plasmid. TNF induced COX-2 promoter activity, whereas noscapine suppressed this activity in a dose-dependent manner (Figure 6C). This result suggests that noscapine inhibits NF-κB-regulated gene expression by suppressing NF-κB binding to the COX-2 promoter.
Discussion
The aim of this study was to investigate the mechanism by which noscapine mediates antitumor effects. Because NF-κB has been linked to survival, proliferation, chemoresistance, invasion, and angiogenesis, we investigated the effects of noscapine on the NF-κB signaling cascade. We found that noscapine inhibits the proliferation of various tumor cell lines; potentiated the apoptosis induced by chemotherapeutic agents; suppressed the expression of antiapoptotic gene products that are regulated by NF-κB; and inhibited NF-κB activation through inhibition of IKK activation, IκBα phosphorylation, IκBα degradation, and p65 phosphorylation. Thus, we have identified NF-κB as a novel target for the action of noscapine.
We found that noscapine inhibits the proliferation of various tumor cell lines including T-cell leukemia, chronic myeloid leukemia, lymphoma, multiple myeloma, prostate cancer, and squamous-cell carcinoma. These results are in agreement with previous reports that this alkaloid inhibits the growth of leukemia and lymphoma(3, 4), melanoma(5), ovarian cancer(6), gliomas(7), breast cancer(38), lung cancer(9), and colon cancer(10). We found that the inhibition of cell proliferation by noscapine is associated with downregulation of constitutive NF-κB activation and inhibition of expression of survivin, cIAP-1, cFLIP, XIAP, Bcl-xL, TRAF1, Bcl-2 and Mcl-1. Our results are in agreement with a report that noscapine suppresses Bcl-2 expression(9).
Besides antiapoptotic proteins, noscapine also downregulates the expression of proteins linked to cell proliferation (cyclin D1), inflammation (COX2), invasion (MMP-9), adhesion (ICAM-1), and angiogenesis (VEGF). These observations imply that noscapine has anti-inflammatory, antiangiogenic, and antimetastatic activities. In hypoxic human glioma cells, noscapine has been shown to inhibit the secretion of VEGF(13).
We also found that noscapine suppresses TNF-induced NF-κB activation. The suppression of NF-κB activation was accompanied by inhibition of IκBα phosphorylation and degradation, as result of inhibition of IKK activation. Numerous kinases have been implicated in the activation of IKK, including TAK1, which plays a major role in TNF-induced IKK activation. We found that noscapine inhibits TAK1-induced NF-κB reporter activity. Thus, TAK1 may be a direct target of noscapine in the NF-κB pathway. We found that noscapine also abrogated nuclear translocation and phosphorylation of p65, which has been linked to IKK(39). The inhibition of p65 phosphorylation by noscapine may therefore result from its inhibition of IKK.
Noscapine has been reported to suppress the transcription factor hypoxia-inducible factor 1 in human gliomas(13). We now find that noscapine also inhibits the NF-κB pathway. Although noscapine binds tubulin and suppresses microtubule assembly, like paclitaxel, noscapine also suppresses growth of paclitaxel-resistant cells, suggesting a difference in the mechanisms of action of the two compounds. Whereas noscapine inhibits NF-κB activation, paclitaxel has been shown to activate NF-κB. Our results indicate for the first time that unlike most other inhibitors of microtubule assembly(40), noscapine exhibits anti-inflammatory activities. Whether all these activities are inter-connected, however, is not certain. It is also not clear metabolites of noscapine such as meconine, cortarnine, and hydrocotamine(41), are involved in additional activities. Vinblastine-resistant human T-lymphoblastoid cells have been shown to undergo apoptosis when exposed to noscapine analogues(4). Because NF-κB activation has been closely linked to drug resistance, this noscapine-induced sensitization of tumor cells to chemotherapeutic agents may be mediated through downregulation of NF-κB, as described above. This may also explain how noscapine overcomes resistance to paclitaxel. Unlike paclitaxel, noscapine is highly water-soluble, well tolerated, and highly bioavailable(10). Noscapine alone has been shown to suppress the growth of a variety of tumors in rodent models(5, 8, 9, 42). Preclinical pharmacokinetics and bioavailability of noscapine, has been examined(43). The pharmacokinetics of oral noscapine in 20 healthy volunteers given 100 mg, 200 mg and 300 mg tablets of noscapine or 200 mg as a solution indicated the terminal half-life of noscapine as 4.5 h, which was independent of formulation or dose(44). A Phase I/II clinical trials with noscapine for non-Hodgkin's lymphoma and for multuple myeloma are in progress (www.clinicaltrials.gov). Thus, this agent should be explored further for its anticancer activities, alone or in combination with currently use chemotherapeutic agents. Thus noscapine and analogues are likely to be cost-effective as potential chemotherapeutic agents(45).
Overall, our results suggest that noscapine, a component of opioids, mediates antiproliferative, proapoptotic, anti-invasive, antiangiogenic, and chemosensitive effects through the suppression of NF-κB and NF-κB-regulated gene products. Our study suggests a novel function and a potential use in cancer treatment for this ancient drug.
Acknowledgments
We thank Pierrette Lo for proofreading the manuscript and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a grant from the Clayton Foundation for Research (B.B.A.), a core grant from the National Institutes of Health (CA16672), a program project grant from the National Institutes of Health (CA124787-01A2), and a grant from the Center for Targeted Therapy at M. D. Anderson Cancer Center.
Footnotes
Conflict-of-interest disclosure
The authors declare no competing financial interests.
References
- 1.Mahmoudian M, Mehrpour M, Benaissa F, Siadatpour Z. A preliminary report on the application of noscapine in the treatment of stroke. Eur J Clin Pharmacol. 2003;59:579–81. doi: 10.1007/s00228-003-0676-1. [DOI] [PubMed] [Google Scholar]
- 2.Ye K, Ke Y, Keshava N, et al. Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proc Natl Acad Sci U S A. 1998;95:1601–6. doi: 10.1073/pnas.95.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aneja R, Vangapandu SN, Lopus M, Chandra R, Panda D, Joshi HC. Development of a novel nitro-derivative of noscapine for the potential treatment of drug-resistant ovarian cancer and T-cell lymphoma. Mol Pharmacol. 2006;69:1801–9. doi: 10.1124/mol.105.021899. [DOI] [PubMed] [Google Scholar]
- 4.Aneja R, Zhou J, Vangapandu SN, Zhou B, Chandra R, Joshi HC. Drug-resistant T-lymphoid tumors undergo apoptosis selectively in response to an antimicrotubule agent, EM011. Blood. 2006;107:2486–92. doi: 10.1182/blood-2005-08-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landen JW, Lang R, McMahon SJ, et al. Noscapine alters microtubule dynamics in living cells and inhibits the progression of melanoma. Cancer Res. 2002;62:4109–14. [PubMed] [Google Scholar]
- 6.Zhou J, Gupta K, Yao J, et al. Paclitaxel-resistant human ovarian cancer cells undergo c-Jun NH2-terminal kinase-mediated apoptosis in response to noscapine. J Biol Chem. 2002;277:39777–85. doi: 10.1074/jbc.M203927200. [DOI] [PubMed] [Google Scholar]
- 7.Landen JW, Hau V, Wang M, et al. Noscapine crosses the blood-brain barrier and inhibits glioblastoma growth. Clin Cancer Res. 2004;10:5187–201. doi: 10.1158/1078-0432.CCR-04-0360. [DOI] [PubMed] [Google Scholar]
- 8.Aneja R, Lopus M, Zhou J, et al. Rational design of the microtubule-targeting anti-breast cancer drug EM015. Cancer Res. 2006;66:3782–91. doi: 10.1158/0008-5472.CAN-05-2962. [DOI] [PubMed] [Google Scholar]
- 9.Jackson T, Chougule MB, Ichite N, Patlolla RR, Singh M. Antitumor activity of noscapine in human non-small cell lung cancer xenograft model. Cancer Chemother Pharmacol. 2008;63:117–26. doi: 10.1007/s00280-008-0720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aneja R, Ghaleb AM, Zhou J, Yang VW, Joshi HC. p53 and p21 determine the sensitivity of noscapine-induced apoptosis in colon cancer cells. Cancer Res. 2007;67:3862–70. doi: 10.1158/0008-5472.CAN-06-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altinoz MA, Bilir A, Del Maestro RF, Tuna S, Ozcan E, Gedikoglu G. Noscapine and diltiazem augment taxol and radiation-induced S-phase arrest and clonogenic death of C6 glioma in vitro. Surg Neurol. 2006;65:478–84. doi: 10.1016/j.surneu.2005.06.024. discussion 85. [DOI] [PubMed] [Google Scholar]
- 12.Ke Y, Ye K, Grossniklaus HE, Archer DR, Joshi HC, Kapp JA. Noscapine inhibits tumor growth with little toxicity to normal tissues or inhibition of immune responses. Cancer Immunol Immunother. 2000;49:217–25. doi: 10.1007/s002620000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newcomb EW, Lukyanov Y, Schnee T, Ali MA, Lan L, Zagzag D. Noscapine inhibits hypoxia-mediated HIF-1alpha expression andangiogenesis in vitro: a novel function for an old drug. Int J Oncol. 2006;28:1121–30. [PubMed] [Google Scholar]
- 14.Newcomb EW, Lukyanov Y, Smirnova I, Schnee T, Zagzag D. Noscapine induces apoptosis in human glioma cells by an apoptosis-inducing factor-dependent pathway. Anticancer Drugs. 2008;19:553–63. doi: 10.1097/CAD.0b013e3282ffd68d. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–30. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–56. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med. 2004;82:434–48. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 20.Chaturvedi MM, Mukhopadhyay A, Aggarwal BB. Assay for redox-sensitive transcription factor. Methods Enzymol. 2000;319:585–602. doi: 10.1016/s0076-6879(00)19055-x. [DOI] [PubMed] [Google Scholar]
- 21.Sung B, Pandey MK, Aggarwal BB. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol Pharmacol. 2007;71:1703–14. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 22.Giri DK, Aggarwal BB. Constitutive activation of NF-kappaB causes resistance to apoptosis in human cutaneous T cell lymphoma HuT-78 cells. Autocrine role of tumor necrosis factor and reactive oxygen intermediates. J Biol Chem. 1998;273:14008–14. doi: 10.1074/jbc.273.22.14008. [DOI] [PubMed] [Google Scholar]
- 23.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–3. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 24.Mayo MW, Wang CY, Cogswell PC, et al. Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–5. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 25.Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–21. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 26.Romano M, Claria J. Cyclooxygenase-2 and 5-lipoxygenase converging functions on cell proliferation and tumor angiogenesis: implications for cancer therapy. Faseb J. 2003;17:1986–95. doi: 10.1096/fj.03-0053rev. [DOI] [PubMed] [Google Scholar]
- 27.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 28.John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- 29.Johnson JP. The role of ICAM-1 in tumor development. Chem Immunol. 1991;50:143–63. [PubMed] [Google Scholar]
- 30.Bonizzi G, Piette J, Merville MP, Bours V. Distinct signal transduction pathways mediate nuclear factor-kappaB induction by IL-1beta in epithelial and lymphoid cells. J Immunol. 1997;159:5264–72. [PubMed] [Google Scholar]
- 31.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–62. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 32.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;20:7597–609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 34.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 35.Simeonidis S, Stauber D, Chen G, Hendrickson WA, Thanos D. Mechanisms by which IkappaB proteins control NF-kappaB activity. Proc Natl Acad Sci U S A. 1999;96:49–54. doi: 10.1073/pnas.96.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–51. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem. 1995;270:31315–20. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 38.Aneja R, Vangapandu SN, Joshi HC. Synthesis and biological evaluation of a cyclic ether fluorinated noscapine analog. Bioorg Med Chem. 2006;14:8352–8. doi: 10.1016/j.bmc.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–6. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 40.Kingston DG. Tubulin-interactive natural products as anticancer agents (1). J Nat Prod. 2009;72:507–15. doi: 10.1021/np800568j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsunoda N, Yoshimura H. Metabolic fate of noscapine. II. Isolation and identification of novel metabolites produced by C-C bond cleavage. Xenobiotica. 1979;9:181–7. doi: 10.3109/00498257909038719. [DOI] [PubMed] [Google Scholar]
- 42.Barken I, Geller J, Rogosnitzky M. Noscapine inhibits human prostate cancer progression and metastasis in a mouse model. Anticancer Res. 2008;28:3701–4. [PubMed] [Google Scholar]
- 43.Aneja R, Dhiman N, Idnani J, et al. Preclinical pharmacokinetics and bioavailability of noscapine, a tubulin-binding anticancer agent. Cancer Chemother Pharmacol. 2007;60:831–9. doi: 10.1007/s00280-007-0430-y. [DOI] [PubMed] [Google Scholar]
- 44.Karlsson MO, Dahlstrom B, Eckernas SA, Johansson M, Alm AT. Pharmacokinetics of oral noscapine. Eur J Clin Pharmacol. 1990;39:275–9. doi: 10.1007/BF00315110. [DOI] [PubMed] [Google Scholar]
- 45.Joshi HC, Zhou J. Noscapine and analogues as potential chemotherapeutic agents. Drug News Perspect. 2000;13:543–6. doi: 10.1358/dnp.2000.13.9.858482. [DOI] [PubMed] [Google Scholar]