Abstract
Purpose
Molecular biomarkers in blood are promising for assessment of tumor progression and treatment response. We hypothesized that serial monitoring of circulating tumor cells (CTC) using multimarker quantitative real-time reverse transcriptase-PCR (qRT) assays could be a surrogate predictor of outcome for melanoma patients enrolled in a multicenter phase II clinical trial of biochemotherapy (BCT) combined with maintenance biotherapy (mBT).
Experimental Design
Blood specimens were collected from 87 patients before and during induction BCT and mBT for stage IV melanoma. Expression of five melanoma-associated CTC biomarkers (MART-1, GalNAc-T, PAX-3, MAGE-A3, and Mitf) was assessed by qRT and correlated with treatment response and disease outcome.
Results
The number of positive CTC biomarkers decreased overall during induction BCT (P<0.0001). CTC biomarker detection after two cycles of BCT was correlated with treatment response (P=0.005) and overall survival (OS) (P=0.001): an increase in the number of CTC biomarkers was associated with poor response (P=0.006) and OS (P<0.0001). Multivariate analyses using a Cox proportional-hazards model identified the change in CTC biomarkers after two cycles of BCT as an independent prognostic factor for disease progression (risk ratio, 12.6; 95% CI, 4.78 to 33.4; P<0.0001) and OS (risk ratio, 6.11; 95% CI, 2.37 to 15.7; P=0.0005).
Conclusion
Serial monitoring of CTC during induction BCT may be useful for predicting the therapeutic efficacy and disease outcome in patients receiving BCT and mBT for stage IV melanoma.
Keywords: qRT-PCR, Melanoma, Circulating Tumor Cell, Metastasis, Biochemotherapy, Maintenance Biotherapy
INTRODUCTION
Circulating tumor cells (CTC) in blood are a promising surrogate biomarker of treatment response and outcome in metastatic melanoma (1–6). We successfully used a multimarker, quantitative realtime reverse-transcriptase polymerase chain reaction (qRT) assay to correlate CTC detection with treatment outcome in patients receiving neoadjuvant biochemotherapy (BCT) before complete surgical resection of American Joint Committee on Cancer (AJCC) stage III melanomas (7, 8). This assay relied on serial blood sampling to quantify biomarker expression levels at specific time points and to identify biomarker changes during treatment. The same approach might be useful to monitor response to treatment for AJCC stage IV melanoma, because surgical resection of distant metastases is often limited and systemic regimens are prolonged, potentially toxic, and increasingly complex. Moreover, median survival of patients with stage IV melanoma is only 6 to 9 months (9, 10) so early determination of treatment efficacy might enable timely treatment modification.
The heterogeneous expression of melanoma-related genes in blood favors a multimarker qRT assay that uses melanoma-associated biomarkers or therapeutic agents that are functionally distinct and therefore nonoverlapping (1, 11, 12). Our sensitive and specific qRT assay for detection of CTC in blood uses five such biomarkers: MART-1(melanoma antigen recognized by T cells-1), MAGE-A3 (melanoma antigen gene-A3 family), GalNAc-T (β1→4-N-acetylgalactosaminyltransferase), PAX-3 (paired box homeotic gene transcription factor 3), and Mitf (microphthalmia transcription factor) (7, 13, 14). In this study, we used this assay to detect CTC biomarkers in blood specimens from a recently reported cohort of patients undergoing a novel regimen of BCT and maintenance biotherapy (mBT) for stage IV melanoma (15). We hypothesized that the response to treatment would be inversely correlated with the CTC biomarkers detected at various time points during treatment and that this correlation would predict overall survival (OS).
PATIENTS AND METHODS
Patients and treatment
Subjects for this qRT study were enrolled in a recently reported prospective multicenter phase II trial of concurrent decrescendo BCT (4 to 6 cycles) followed by mBT (up to 12 cycles) (15). The BCT regimen was repeated every 21 days and comprised cisplatin (20 mg/m2, intravenously [i.v.], on day 1–4), dacarbazine (800 mg/m2, i.v., on day 1), vinblastine (1.5 mg/m2, i.v., on day 1–4), IL-2 (Chiron Corporation, CA; 18 MU/m2, continuous infusion, over 24 hrs, on day 1, 9 MU/m2, over 24 hrs, on day 2, and 4.5 MU/m2, over 24 hrs, on day 3 and 4), IFN-α2b(Schering-Plough, NJ; 5 MU/m2, subcutaneously[s.c.], on day 1–5) and granulocyte-macrophage colony stimulating factor (GM-CSF) (Amgen Inc., CA; 500 mcg, s.c., on day 6–15). The mBT regimen was a 28-day cycle of low-dose IL-2 (2 MU/m2, s.c., daily) and GM-CSF (250 mcg, s.c., on day 1–14), which included intermittent pulses of high-dose decrescendo IL-2 (18 MU/m2, continuous infusion, over the first 6 hrs, the next 12 hrs, and the final 24 hrs) on day 1–2 of mBT cycles 1–6, 8, 10, and 12 (15).
Response was assessed by Response Evaluation Criteria in Solid Tumors (RECIST) criteria every 6 weeks (2 cycles) during BCT and every 3 months during mBT. Patients with stable disease(SD), partial response(PR), or complete response(CR) continued on the study treatment; those with progressive disease(PD) during BCT or mBT did not receive further study treatment but were followed for survival. RECIST for progression on mBT was modified from a 20–30% increase to allow non-clinically significant progression without a deterioration of performance status (less than 5% of the study population). Patients who developed new central nervous system (CNS) lesions were allowed to remain on study if they had stable or responding systemic disease (non-CNS).
All blood specimens were coded for double-blind study and processed within 24 hours. Peripheral blood was drawn regularly during treatment, processed and cryostored until used as previously described (8). Patients gave written informed consent for the use of their blood specimens before treatment, and the qRT study was approved and carried out in accordance with guidelines set forth by the individual institutional review board committees and reporting recommendations for tumor marker prognostic studies (REMARK criteria) (16).
Blood processing for qRT assay
We selected four sampling times for multimarker qRT study: immediately before the first cycle of BCT (BCT1, n=87), before the third cycle of BCT (BCT3, n=87), before the first cycle of mBT (mBT1, n=64), and before the third cycle of mBT (mBT3, n=54). The interval between each of the four sampling times was approximately 6–8 weeks. The interval between BCT3 and mBT1 was approximately 9–12 weeks in patients who received 5 or 6 cycles of BCT.
Peripheral blood specimens (10 ml) were collected in sodium citrate-containing tubes and the first several ml was discarded to eliminate skin-plug contamination, as previously described (11, 17). Nucleated cell fractions were isolated from blood specimens using the Purescript RBC Lysis Solution (Gentra, MN) and cryopreserved in liquid nitrogen until thawed for the study, as previously described (18).
Tri-Reagent (Molecular Research Center, OH) was used to isolate total cellular RNA from blood specimens, as previously described (11). RNA was quantified and assessed for purity by ultraviolet spectrophotometry. RT reactions were performed using Moloney murine leukemia virus reverse transcriptase (Promega, WI) with oligo-dT primer (19).
Multimarker qRT assay measured mRNA levels of MART-1, MAGE-A3, GalNAc-T, PAX-3, and Mitf. In previous studies, we validated the sensitivity and specificity of this qRT assay for detection of CTC in blood specimen (7, 8, 13, 14). In those studies, all five CTC biomarkers were frequently detected in melanoma cell lines but not detected in blood specimens from healthy donors. In the present study, the qRT assay was performed using ABI Prism 7900HT thermocycler (Applied Biosystems, CA) (18). We transferred 4 μL cDNA from 200 ng total RNA to individual wells of a 384-well PCR plate; 0.5 μmol/L of each primer, 0.3 μmol/L probe, and 5 μL iTaq custom supermix with ROX (Bio-Rad Laboratories, CA) were added to a final volume of 10 μL. Samples were amplified with a precycling hold at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 sec, and then 1 min of annealing/extension at 55 °C for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), at 59 °C for MART-1, at 58 °C for MAGE-A3 and Mitf, and at 62 °C for GalNAc-T and PAX-3. The standard curve was generated by using threshold cycle (Ct) of seven serial dilutions of plasmid templates (100–106 copies). The Ct of each sample was interpolated from the standard curve, and the number of mRNA copies was calculated. PCR efficiency, assessed from the slopes of the curves, was 90 – 100%. The correlation coefficient for all standard curves (Ct versus log copy number) was ≥0.99.
Each qRT assay was performed at least twice and included marker-positive and marker-negative controls and reagent controls (reagent without RNA or cDNA). If only one of the duplicates was positive, qRT assay was repeated. Any specimen with inadequate mRNA copies (<1×104) of the GAPDH housekeeping gene was excluded. The mean mRNA copy number was used for analysis. Blood processing, RNA extraction, qRT assay set-up, and post qRT product analysis were carried out in separate designated rooms to prevent cross-contamination.
Biostatistical analysis
Biomarker change and OS were primary endpoints; marker expression, treatment response and progression-free survival (PFS) were secondary endpoints.
Wilcoxon signed rank test was used to compare the number of biomarkers detected before and during treatment. Chi-square test and exact T-test were used to examine the association between biomarker detection and treatment response. Survival was measured from the start of BCT (BCT1). The correlation between biomarker detection, biomarker change, and survival was examined by log-rank test. Survival curves were generated by using a Kaplan-Meier method. A Cox proportional-hazards model was developed to examine the association of biomarker detection with PFS and OS and used for multivariate analysis.
McNemar’s test compared the detection of individual CTC biomarkers between any two time points. Mann-Whitney U test assessed CTC biomarker differences according to site of metastasis. The analysis was performed using SAS statistical software and all tests were two-sided with significance level ≤ 0.05.
RESULTS
Patients Assessed for qRT
Our qRT study included 87 of 133 patients participating in the clinical trial biomarker assay. The 133 patients comprised 94 males and 39 females, with a median age of 50 years (range, 18–76); our cohort comprised 60 males and 27 females, with a median age of 48 years (range, 18–73 years). The patient cohort had histopathologically confirmed M1a (n=8), M1b (n=19), or M1c (n=60) melanoma. The cycles of BCT received were 2, 3, 4, and 6 in 10, 2, 65, and 10 patients, respectively. The cycles of mBT were 1–3, 4–6, and 7–12 in 18, 23, and 23 patients, respectively. Twenty-three patients did not receive mBT. These distributions paralleled those in the parent population. All 87 patients had BCT1 and BCT3 blood specimens; the remaining 46 patients were excluded because of lack of blood procurement and inadequate samples to process at BCT1 or BCT3. Of the 87 patients, 64 had mBT1 blood specimens and 23 patients had no blood specimens at mBT1 (11 patients developed PD after BCT, 6 patients did not receive mBT for other reasons, 3 had inadequate specimens for qRT analysis, and 3 had no blood sampling at mBT1). Of 64 patients with blood samples at mBT1, 54 patients had blood samples at mBT3, 5 discontinued mBT because of PD before mBT3, and 5 had no sampling at mBT3. Overall 292 blood samples were studied from 87 patients that participated or qualified for entry into the companion biomarker assay.
Detection of CTC biomarkers in blood
Before treatment, blood specimens from 66 (76%) of 87 patients expressed at least one CTC biomarker and specimens from 45 (52%) patients expressed more than one CTC biomarker. Overall, the number of CTC biomarkers gradually decreased during treatment: at mBT3, specimens from 24(44%) patients had no CTC biomarkers and only 11 (21%) specimens expressed more than one CTC biomarker (P<0.0001). However, this decrease was significant only during the first two cycles of BCT among the specified sampling intervals (Table 1).
Table 1.
Detection of Biomarkers in Blood Sampled at Specific Intervals During Treatment
| BCT1 | BCT3 | |||
|---|---|---|---|---|
| N=87 | (%) | N=87 | (%) | |
| Biomarkers | ||||
| MART-1 | 35 | (40) | 18 | (21) |
| MAGE-A3 | 24 | (28) | 13 | (15) |
| GalNAc-T | 38 | (44) | 30 | (34) |
| PAX-3 | 20 | (23) | 20 | (23) |
| Mitf | 22 | (25) | 25 | (29) |
| Number of biomarkers tested | ||||
| 0 | 21 | (24) | 30 | (35) |
| 1 | 21 | (24) | 24 | (28) |
| 2 | 25 | (29) | 20 | (23) |
| 3 | 13 | (15) | 10 | (11) |
| ≥ 4 | 7 | (8) | 3 | (3) |
Abbreviations: BCT, biochemotherapy; mBT, maintenance biotherapy.
MART-1 and Mitf mRNA detection rates dropped significantly during overall treatment (BCT1 vs. mBT3) (MART-1, P=0.03; Mitf, P=0.005), reflecting a gradual and significant decrease during each sampling interval except for a non-significant decrease in Mitf detection during the first two cycles of BCT. However, there were no significant changes in CTC biomarker detection except for a significant decrease in MAGE-A3 detection between BCT1 and BCT3.
Association between CTC biomarkers and treatment response
The number of patients who achieved CR, PR, SD, and PD as a best response to BCT was 8, 36, 32, and 11, respectively. Also, these distributions almost paralleled those in the parent study population cohort (data not shown). The number of CTC biomarkers before treatment (BCT1) did not predict treatment response (Table 2). However, the number of CTC biomarkers during BCT was significantly lower in the CR/PR group (P=0.005). Twenty-two patients had an increase in the number of CTC biomarkers; seven had an increase of at least two biomarkers (Table 3). The number of biomarkers increased in 10 (31%) and 4 (36%) patients with SD and PD, respectively, as compared with only 8 (18%) patients with CR/PR (P=0.006).
Table 2.
Correlation of CTC Biomarkers with BCT Response
| Number of CTC Biomarkers (%) |
||||||||
|---|---|---|---|---|---|---|---|---|
| BCT Response | BCT1 | BCT3 | ||||||
| 0 | 1 | ≥ 2 | P* | 0 | 1 | ≥ 2 | P* | |
| CR/PR (n =44) | 10 (23) | 12 (27) | 22 (50) | 0.24 | 18 (41) | 16 (36) | 10 (23) | 0.005 |
| SD (n =32) | 6 (19) | 9 (28) | 17 (53) | 10 (31) | 8 (25) | 14 (44) | ||
| PD (n =11) | 5 (45) | 0 (0) | 6 (55) | 2 (18) | 0 (0) | 9 (82) | ||
Chi-square test, exact p-value.
Table 3.
Correlation of Changes in CTC Biomarkers with BCT Response
| BCT Response | Increase in CTC Biomarkers between BCT1 and BCT3 (%) |
|||
|---|---|---|---|---|
| ≤ 0 | 1 | ≥2 | P* | |
| CR/PR (n =44) | 36 (82) | 6 (14) | 2 (4) | 0.006 |
| SD (n =32) | 22 (69) | 9 (28) | 1 (3) | |
| PD (n =11) | 7 (64) | 0 (0) | 4 (36) | |
Chi-square test.
At a median follow-up of 15.4 months (range 2.1 to 43.1 months), the best response to BCT and mBT was CR, PR, SD, and PD in 9, 25, 18, and 30 patients, respectively. Five patients received elective surgery after induction BCT and were excluded from the analysis. As with the best response to BCT, best response to BCT + mBT was significantly correlated with the number of CTC biomarkers detected at BCT3 (P=0.01; Table 4) but not with the number of biomarkers detected at BCT1 (data not shown). The number of CTC biomarkers detected in the last available blood specimen significantly correlated with best response to BCT + mBT (P=0.006; Table 4).
Table 4.
Correlation of CTC Biomarkers with Overall Response
| Number of CTC Biomarkers (%) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Overall Response | BCT3 |
Last blood sampling |
||||||
| 0 | 1 | ≥2 | P* | 0 | 1 | ≥2 | P* | |
| CR/PR (n = 34) | 16 (47) | 13 (38) | 5 (15) | 0.01 | 20 (59) | 9 (26) | 5 (15) | 0.006 |
| SD (n = 18) | 6 (33) | 5 (28) | 7 (39) | 6 (33) | 4 (22) | 8 (45) | ||
| PD (n = 30) | 8 (27) | 5 (17) | 17 (56) | 6 (20) | 7 (23) | 17 (57) | ||
Chi-square test.
CTC biomarkers as a predictor of disease progression
Before treatment, there was no correlation between CTC biomarker detection and patient sex, age, Eastern Cooperative Oncology Group (ECOG) performance status, or previous treatment status. Of the 87 patients, 8 had M1a disease, 19 had M1b disease, and 60 had M1c disease before treatment, and 41 patients developed CNS metastasis during treatment. There was no correlation between CTC biomarker detection/increase and M category or CNS metastasis.
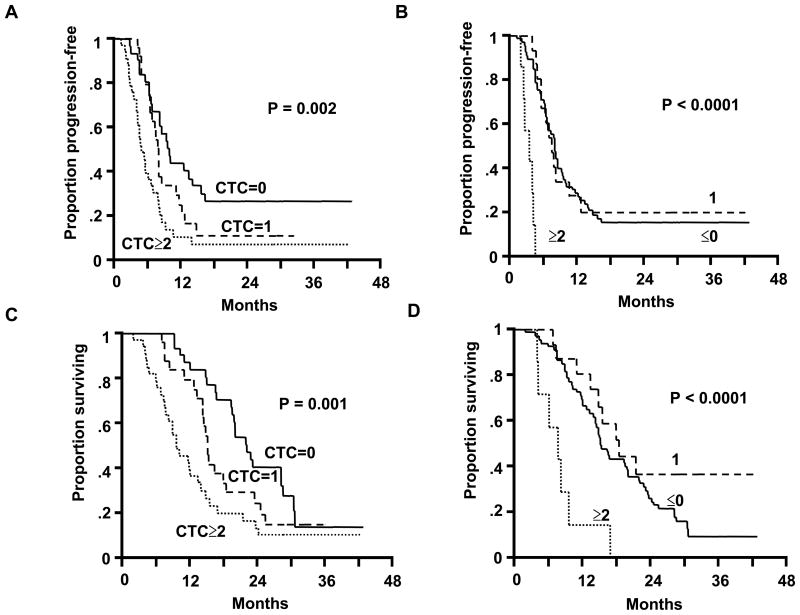
During follow-up, 78 patients showed disease progression and other nine patients were free from disease progression. At BCT1, patients with >1 CTC biomarker tended to have a shorter survival than patients with no or one CTC biomarker (data not shown). At BCT3, PFS significantly decreased when a CTC biomarker was positive (Figure 1A). The size of the decrease was directly correlated with the number of positive CTC biomarkers. Median PFS was 10.02 months (95% CI, 7.10–15.90), 8.10 months (95% CI, 6.67–11.10), and 5.68 months (95% CI, 4.50–7.20) for patients with no positive CTC biomarkers (n=30), one positive CTC biomarker (n=24), and ≥2 positive CTC biomarkers (n=33), respectively (P=0.002).
Figure 1.
(A) Kaplan-Meier curves of PFS based on CTC biomarker detection after two cycles of induction BCT. (B) Kaplan-Meier curves of PFS based on changes in CTC biomarker detection between BCT1 and BCT3. (C) Kaplan-Meier curves of OS based on CTC biomarker detection after two cycles of induction BCT. (D) Kaplan-Meier curves of OS based on changes in CTC biomarker detection between BCT1 and BCT3. In each panel, the solid line corresponds to no CTC biomarkers, the broken line is 1 CTC biomarker, and the dotted line is ≥2 CTC biomarkers.
Between BCT1 and BCT3, 65 patients showed no increase or decrease in number of CTC biomarkers, 15 had an increase of one CTC biomarker, and 7 had an increase of >1 CTC biomarker. Median PFS was 8.18 months (95% CI, 6.93–9.46), 7.82 months (95% CI, 5.88–9.82), and 3.68 months (95% CI, 2.17–4.50) for patients with an increase of 0, 1, and ≥2, respectively (P<0.0001; Figure 1B). Clinical factors did not correlate with disease progression in univariate analyses. Cox proportional-hazards regression model using a stepwise procedure to assess BCT3 versus standard clinical variables showed that only the number of CTC biomarkers at BCT3 was an independent prognostic factor for PFS (risk ratio, 2.58; 95% CI, 1.50 to 4.43; P=0.0006). A similar analysis showed that only the change in the number of positive CTC biomarkers was an independent prognostic factor for PFS (risk ratio, 12.6; 95% CI, 4.78 to 33.4; P<0.0001).
CTC Biomarkers as a predictor of OS
During follow-up, 72 patients died of disease progression. OS showed a significant inverse correlation with the number of positive CTC biomarkers at BCT3 (Figure 1C). Median OS was 22.3 months (95% CI, 19.5–28.6), 15.4 months (95% CI, 13.5–19.1), and 9.8 months (95% CI, 7.7–13.5) for patients with no positive, one, and ≥2 positive CTC biomarkers, respectively (P=0.001). Median OS was 15.4 months (95% CI, 14.2–20.2), 19.1 months (95% CI, 11.1-NA), and 8.0 months (95% CI, 4.5–9.8 months) for patients with an increase of 0, 1, or ≥2 CTC biomarkers, respectively (P<0.0001; Figure 1D). Clinical factors did not correlate with survival in univariate analyses. Cox proportional-hazards regression model using a stepwise procedure to assess BCT3 and clinical factors found that only the number of CTC biomarkers at BCT3 was independent prognostic factor for OS (risk ratio, 2.71; 95% CI, 1.56 to 4.73; P=0.0004). A similar analysis revealed an independent prognostic significance of a change in the number of CTC biomarkers from BCT1 to BCT3 for OS (risk ratio, 4.33; 95% CI, 1.91 to 9.84; P=0.0005).
DISCUSSION
Systemic BCT for stage IV melanoma has promise, but results from phase III trials have been mixed and inconsistent, (20–24) in part because responses to BCT are not durable and do not prevent CNS progression. The novel regimen of mBT and induction BCT/mBT received by patients in our correlative study was developed to amplify and prolong the systemic antitumor immune response elicited by BCT (25). As recently reported, this regimen appears to extend PFS and OS as compared with regimens in recent multicenter trials of BCT or chemotherapy (15).
The present study showed a significant relation between prolonged survival and decreased number of CTC biomarkers during therapy. Although most investigations of CTC to predict disease progression report a single biomarker measured at a single point, a single-biomarker assay is limited by the heterogeneous expression of melanoma-related genes, (11, 19, 26) particularly in advanced disease (27). However, the efficacy of multiple-biomarker assay depends on the careful selection of CTC biomarkers (28), serial rather than single-point assessment, (7, 8, 14) and quantification of CTC to compensate for ectopic and background mRNA (29). The assay system used in the study was the same as that used to assess stage III melanoma patients receiving neo-adjuvant BCT (8). Our assay system demonstrated the clinical utility and robustness of CTC biomarkers in patients with stage IV as well as stage III melanoma who received systemic therapies in phase II multicenter trials.
Our results confirm recent reports that changes in CTC during treatment may indicate therapeutic efficacy for metastatic cancer (30–32). The detection of CTC biomarkers before treatment (BCT1) was not an indicator of treatment response. Subclinical tumor metastasis, particularly to the CNS system, and tumor heterogeneity are likely related to the findings. The metastasis to CNS system is critical and occurred in almost half of our patients. Treatment effects to chemo- or immunotherapeutic agents are often different among the metastatic tumors within an individual patient. Thus, the prediction of tumor response and survival before treatment may be difficult in further advanced metastatic melanoma patients with multiple lesions and metastasis at specific organ sites. If the number of CTC biomarkers increased during treatment, we observed a poor prognosis. Of the five patients who underwent elective surgery after BCT, all five had no increase in biomarker detection after surgery and three survived for 42, 30, 29 months, respectively. Although the number of positive biomarkers decreased across the sampling intervals, however, this decrease was significant only during the first two cycles of BCT. These significant findings may reflect reduced drug efficacy with increasing duration of treatment. These findings are important, as metastatic melanoma patients with CTC at the start of treatment may have a poor outcome.
As treatment regimens become multi-modal and multi-phasic, CTC detection in serial blood specimens might be used to determine which component of treatment is most effective and which needs to be improved.
Current prognostic systems, such as TNM staging criteria and molecular features of primary tumors, are probably inadequate for managing metastatic melanoma patients receiving systemic chemo- and/or immunotherapy. CTC detection may be a better tool to monitor the treatment efficacy, because blood assessment can be serially performed and change in CTC may be observed immediately after administration of systemic therapy (31, 32). Serial qRT assay can assess CTC changes during different phases of treatment, and this makes CTC assessment a promising method to evaluate treatment efficacy in controlling systemic disease. In patients being treated in these clinical trials, the benefit of changing therapy in early course of the treatment using CTC detection as a surrogate of responsiveness needs to be further examined in clinical trials.
This study demonstrates CTC change as an independent surrogate for survival and treatment efficacy in metastatic melanoma patients who received mBT after BCT. CTC blood biomarkers could be used as surrogates in developing multimodal therapeutic trials. The strategy including CTC change for early identification of treatment-resistant patients may be important not only to discontinue non-effective systemic therapy, but also to develop individualized treatment regimens in metastatic melanoma patients.
Footnotes
STATEMENT OF TRANSLATIONAL RELEVANCE
There is a lack of validated blood tests for assessment of malignant melanoma patients during multimodal therapy. In this study, circulating tumor cells (CTCs) were assessed to monitor serial bloods from a phase II multicenter clinical trial of biochemotherapy followed with maintenance biotherapy. The established multimarker melanoma biomarkers assessed by quantitative realtime PCR was sensitive to detect CTCs in blood directly. The assay robustness allows monitoring of patients in a multicenter setting. The monitoring of CTC during early stage therapy was predictive of disease progression. These studies demonstrate that CTCs have clinical utility in monitoring melanoma patients in multimodal therapy.
References
- 1.Hoon DS, Bostick P, Kuo C, et al. Molecular markers in blood as surrogate prognostic indicators of melanoma recurrence. Cancer Res. 2000;60:2253–7. [PubMed] [Google Scholar]
- 2.Mellado B, Gutierrez L, Castel T, et al. Prognostic significance of the detection of circulating malignant cells by reverse transcriptase-polymerase chain reaction in long-term clinically disease-free melanoma patients. Clin Cancer Res. 1999;5:1843–8. [PubMed] [Google Scholar]
- 3.Mocellin S, Hoon D, Ambrosi A, Nitti D, Rossi CR. The prognostic value of circulating tumor cells in patients with melanoma: a systematic review and meta-analysis. Clin Cancer Res. 2006;12:4605–13. doi: 10.1158/1078-0432.CCR-06-0823. [DOI] [PubMed] [Google Scholar]
- 4.Palmieri G, Strazzullo M, Ascierto PA, Satriano SM, Daponte A, Castello G. Polymerase chain reaction-based detection of circulating melanoma cells as an effective marker of tumor progression. Melanoma Cooperative Group. J Clin Oncol. 1999;17:304–11. doi: 10.1200/JCO.1999.17.1.304. [DOI] [PubMed] [Google Scholar]
- 5.Pantel K, Cote RJ, Fodstad O. Detection and clinical importance of micrometastatic disease. J Natl Cancer Inst. 1999;91:1113–24. doi: 10.1093/jnci/91.13.1113. [DOI] [PubMed] [Google Scholar]
- 6.Voit C, Kron M, Rademaker J, et al. Molecular staging in stage II and III melanoma patients and its effect on long-term survival. J Clin Oncol. 2005;23:1218–27. doi: 10.1200/JCO.2005.04.098. [DOI] [PubMed] [Google Scholar]
- 7.Koyanagi K, Kuo C, Nakagawa T, et al. Multimarker quantitative real-time PCR detection of circulating melanoma cells in peripheral blood: relation to disease stage in melanoma patients. Clin Chem. 2005;51:981–8. doi: 10.1373/clinchem.2004.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koyanagi K, O’Day SJ, Gonzalez R, et al. Serial monitoring of circulating melanoma cells during neoadjuvant biochemotherapy for stage III melanoma: outcome prediction in a multicenter trial. J Clin Oncol. 2005;23:8057–64. doi: 10.1200/JCO.2005.02.0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–48. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 10.Greene FL, Sobin LH. The staging of cancer: a retrospective and prospective appraisal. CA Cancer J Clin. 2008;58:180–90. doi: 10.3322/CA.2008.0001. [DOI] [PubMed] [Google Scholar]
- 11.Hoon DS, Wang Y, Dale PS, et al. Detection of occult melanoma cells in blood with a multiple-marker polymerase chain reaction assay. J Clin Oncol. 1995;13:2109–16. doi: 10.1200/JCO.1995.13.8.2109. [DOI] [PubMed] [Google Scholar]
- 12.Siena S, Sartore-Bianchi A, Di Nicolantonio F, Balfour J, Bardelli A. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst. 2009;101:1308–24. doi: 10.1093/jnci/djp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitago M, Koyanagi K, Nakamura T, et al. mRNA expression and BRAF mutation in circulating melanoma cells isolated from peripheral blood with high molecular weight melanoma-associated antigen-specific monoclonal antibody beads. Clin Chem. 2009;55:757–64. doi: 10.1373/clinchem.2008.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyanagi K, O’Day SJ, Gonzalez R, et al. Microphthalmia transcription factor as a molecular marker for circulating tumor cell detection in blood of melanoma patients. Clin Cancer Res. 2006;12:1137–43. doi: 10.1158/1078-0432.CCR-05-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Day SJ, Atkins MB, Boasberg P, et al. Phase II multicenter trial of maintenance biotherapy after induction concurrent Biochemotherapy for patients with metastatic melanoma. J Clin Oncol. 2009;27:6207–12. doi: 10.1200/JCO.2008.20.3075. [DOI] [PubMed] [Google Scholar]
- 16.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–4. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 17.Miyashiro I, Kuo C, Huynh K, et al. Molecular strategy for detecting metastatic cancers with use of multiple tumor-specific MAGE-A genes. Clin Chem. 2001;47:505–12. [PubMed] [Google Scholar]
- 18.Koyanagi K, Mori T, O’Day SJ, Martinez SR, Wang HJ, Hoon DS. Association of circulating tumor cells with serum tumor-related methylated DNA in peripheral blood of melanoma patients. Cancer Res. 2006;66:6111–7. doi: 10.1158/0008-5472.CAN-05-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeuchi H, Kuo C, Morton DL, Wang HJ, Hoon DS. Expression of differentiation melanoma-associated antigen genes is associated with favorable disease outcome in advanced-stage melanomas. Cancer Res. 2003;63:441–8. [PubMed] [Google Scholar]
- 20.Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008;26:5748–54. doi: 10.1200/JCO.2008.17.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eton O, Legha SS, Bedikian AY, et al. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: results from a phase III randomized trial. J Clin Oncol. 2002;20:2045–52. doi: 10.1200/JCO.2002.07.044. [DOI] [PubMed] [Google Scholar]
- 22.Ives NJ, Stowe RL, Lorigan P, Wheatley K. Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: a meta-analysis of 18 trials involving 2,621 patients. J Clin Oncol. 2007;25:5426–34. doi: 10.1200/JCO.2007.12.0253. [DOI] [PubMed] [Google Scholar]
- 23.Keilholz U, Goey SH, Punt CJ, et al. Interferon alfa-2a and interleukin-2 with or without cisplatin in metastatic melanoma: a randomized trial of the European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Clin Oncol. 1997;15:2579–88. doi: 10.1200/JCO.1997.15.7.2579. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Prospective randomized trial of the treatment of patients with metastatic melanoma using chemotherapy with cisplatin, dacarbazine, and tamoxifen alone or in combination with interleukin-2 and interferon alfa-2b. J Clin Oncol. 1999;17:968–75. doi: 10.1200/JCO.1999.17.3.968. [DOI] [PubMed] [Google Scholar]
- 25.O’Day SJ, Boasberg PD, Piro L, et al. Maintenance biotherapy for metastatic melanoma with interleukin-2 and granulocyte macrophage-colony stimulating factor improves survival for patients responding to induction concurrent biochemotherapy. Clin Cancer Res. 2002;8:2775–81. [PubMed] [Google Scholar]
- 26.Glaser R, Rass K, Seiter S, Hauschild A, Christophers E, Tilgen W. Detection of circulating melanoma cells by specific amplification of tyrosinase complementary DNA is not a reliable tumor marker in melanoma patients: a clinical two-center study. J Clin Oncol. 1997;15:2818–25. doi: 10.1200/JCO.1997.15.8.2818. [DOI] [PubMed] [Google Scholar]
- 27.Sarantou T, Chi DD, Garrison DA, et al. Melanoma-associated antigens as messenger RNA detection markers for melanoma. Cancer Res. 1997;57:1371–6. [PubMed] [Google Scholar]
- 28.Xi L, Nicastri DG, El-Hefnawy T, Hughes SJ, Luketich JD, Godfrey TE. Optimal markers for real-time quantitative reverse transcription PCR detection of circulating tumor cells from melanoma, breast, colon, esophageal, head and neck, and lung cancers. Clin Chem. 2007;53:1206–15. doi: 10.1373/clinchem.2006.081828. [DOI] [PubMed] [Google Scholar]
- 29.de Graaf H, Maelandsmo GM, Ruud P, et al. Ectopic expression of target genes may represent an inherent limitation of RT-PCR assays used for micrometastasis detection: studies on the epithelial glycoprotein gene EGP-2. Int J Cancer. 1997;72:191–6. doi: 10.1002/(sici)1097-0215(19970703)72:1<191::aid-ijc27>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 30.Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420–30. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 31.Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–24. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura S, Yagata H, Ohno S, et al. Multi-center study evaluating circulating tumor cells as a surrogate for response to treatment and overall survival in metastatic breast cancer. Breast Cancer. 2009 Aug 1; doi: 10.1007/s12282-009-0139-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]