Summary
Mycobacterium tuberculosis (the causal agent of TB) has co-evolved with humans for centuries. It infects via the airborne route and is a prototypic highly adapted intracellular pathogen of macrophages. Extensive sequencing of the M. tuberculosis genome along with recent molecular phylogenetic studies is enabling us to gain insight into the biologic diversity that exists among bacterial strains that impact the pathogenesis of latent infection and disease. The majority of the M. tuberculosis cell envelope is comprised of carbohydrates and lipids, and there is increasing evidence that these microbial determinants that are readily exposed to the host immune system play critical roles in disease pathogenesis. Studies from our laboratory and others have raised the possibility that M. tuberculosis is adapting to the human host by cloaking its cell envelope molecules with terminal mannosylated (i.e. Man-α-(1→2)-Man) oligosaccharides that resemble the glycoforms of mammalian mannoproteins. These mannosylated biomolecules engage the mannose receptor (MR) on macrophages during phagocytosis and dictate the intracellular fate of M. tuberculosis by regulating formation of the unique vesicular compartment in which the bacterium survives. The MR is highly expressed on alveolar macrophages (predominant C-type lectin on human cells) and functions as a scavenger receptor to maintain the healthiness of the lung by clearing foreign particles and at the same time regulating dangerous inflammatory responses. Thus M. tuberculosis exploits MR functions to gain entry into the macrophage and survive. Key biochemical pathways and mycobacterial determinants involved in the development and maintenance of the M. tuberculosis phagosome are being identified. The phylogenetic diversity observed in M. tuberculosis strains that impact its cell wall structure together with the genetic diversity observed in human populations, including those elements that affect macrophage function, may help to explain the extraordinary evolutionary adaptation of this pathogen to the human host. Major developments in these areas are the focus of this review.
Keywords: Tuberculosis, mannose-capped lipoarabinomannan, trafficking, macrophage, mannose receptor
Introduction
TB accounts for more than one quarter of all preventable adult deaths in the world1. M. tuberculosis is an intracellular pathogen that is highly adapted to its natural host; the human. Its major host cell reservoir is the mononuclear phagocyte (monocytes and macrophages). Given its potent microbicidal mechanisms, M. tuberculosis has adapted strategies to manipulate the host cell response during and after its entry into the macrophage2. The outermost components of the M. tuberculosis cell wall, predominately lipids and carbohydrates, are the first to contact host molecular constituents and play a major role in facilitating host cell recognition and modulation of host responses3.
Virtually all M. tuberculosis infections occur by airborne transmission of droplet nuclei containing a few viable organisms. The first interaction between M. tuberculosis and the human host takes place in the lung. The respiratory epithelium is actively involved in inflammation and host defense in multiple ways: providing a physical barrier, constituting the structural basis of mucociliary clearance; recognizing pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) expressed on epithelial and myeloid cells, and secreting a variety of pro- and anti-inflammatory mediators [reviewed in 4]. When M. tuberculosis bacilli reach the alveolar space, resident alveolar macrophages (AMs) within the surfactant monolayer along with recruited monocytes, neutrophils and lymphocytes represent the array of immune cells that participate in host defense.
AMs are at the interface between air and lung tissue, and represent the first line of defense against inhaled M. tuberculosis 5. In general, their primary function is the intracellular breakdown and disposal of particulate elements 6,7. AMs are uniquely positioned within the alveolar surfactant film, the latter of which is composed of lipids and proteins produced by type II epithelial cells 8. In a normal healthy individual, AMs represent more than 90% of the cells in the bronchoalveolar lavage fluid 9. Many studies have demonstrated that resident AMs can phagocytose large numbers of microbes through both opsonic and non-opsonic receptors 5,10,11. Though AMs have high phagocytic and clearance activity, their microbicidal capacity is less well-defined. Efficient microbial phagocytosis followed by slow intracellular killing may be sufficient to control infection by many routinely encountered extracellular pathogens. Intracellular pathogens like M. tuberculosis, however, appear to take advantage of the reduced microbial activity of AMs by residing and multiplying within these cells 12,13. The participation of AMs in host defense, inflammatory processes and immune mechanisms has been amply documented 14.
The importance of host and microbial glycans in health and disease
Glycans play a major role in the host response to infection. Glycosylation produces an abundant, diverse, and highly regulated repertoire of cellular glycans that are frequently attached to lipids and proteins. Decades of research on glycan function have revealed that the enzymes responsible for glycosylation (glycosyltransferases and glycosidases) are essential in the development and physiology of living organisms 15,16. Glycans participate in many key biological processes including cell adhesion, molecular trafficking and clearance, receptor activation, signal transduction, and endocytosis 17. There is increasing attention being given to the importance of surface glycosylation motifs in microbial pathogens [Reviewed in 18]. The main structure that defines a bacterium is its cell envelope. Its complexity level differs among bacteria; however, in all cases the cell envelope contains surface-exposed and solvent-extractable non-covalently bound lipids and carbohydrates associated with the peptidoglycan (in the case of gram positive and negative bacteria) or to the mycolyl-arabinogalactan-peptidoglycan complex in the case of mycobacteria 19. The cell envelope is the microbe armor conferring a relative protection by endowing the microbe with innate resistance to therapeutic agents and host defenses. In order for a microbe to survive within the host, it responds to external stimuli by modulating its metabolism and cell envelope to adapt to its surrounding environment. In addition, microbial surface components may be prone to release, shedding, and/or cleavage upon environmental stress (i.e. drug treatment). Of particular interest to us are the mannose-containing biomolecules present in the cell envelope of M. tuberculosis and how these are implicated in the intracellular survival of M. tuberculosis in the macrophage.
The mannosylated cell envelope components of M. tuberculosis
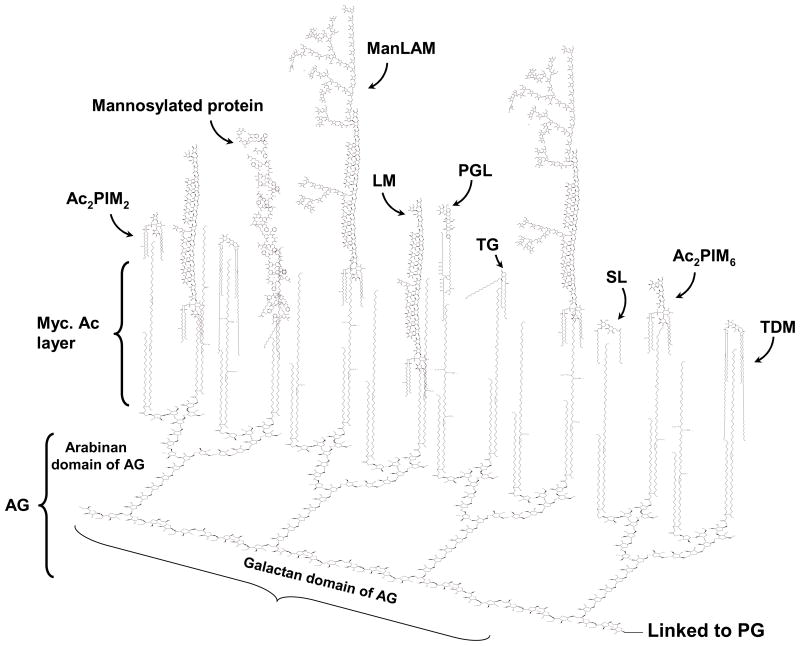
The M. tuberculosis cell envelope is characterized by the presence of a variety of unique complex lipids, constituting 60% of the bacillus total weight. This lipid-rich low permeability matrix contributes to the difficulty in combating mycobacterial diseases by endowing the organism with innate resistance to therapeutic agents and host defenses. The complex M. tuberculosis cell envelope can be divided into two major structures, the cell wall and the capsule-like outermost structures [Reviewed in detail in 20]. The outermost components are solvent-extractable non-covalently bound free lipids, carbohydrates and proteins associated with the mycolyl-arabinogalactan-peptidoglycan complex (cell wall core) 21. These surface components may be prone to release, shedding, and/or cleavage upon contact with the host cell or within an appropriate intracellular environment of the cell. The surface of M. tuberculosis is particularly rich in mannose-containing biomolecules, including mannose-capped lipoarabinomannan (ManLAM), the related lipomannan (LM), phosphatidyl-myo-inositol mannosides (PIMs), arabinomannan, mannan and manno-glycoproteins (Fig. 1). PIMs, LM and ManLAM are incorporated into the plasma membrane and also exposed on the M. tuberculosis cell surface 22,20. They act as ligands for host cell receptors and contribute to the pathogenesis of M. tuberculosis 23,24,25,26,27.
Figure 1. The cell envelope of M. tuberculosis with an emphasis on exposed mannosylated cell envelope components.
This scheme depicts the cell envelope “skeleton or core” determinants (mycolyl-arabinogalactan-peptidoglycan complex) and emphasizes the distribution of intercalated major mannosylated cell envelope components that are exposed on the M. tuberculosis surface. AG is covalently linked to PG via the galactan chain and the arabinan chain is in turn linked to the mycolic acids (Myc Ac) which are shown perpendicular to the plasma membrane. The polar groups (i.e. carbohydrate domains) of several mannosylated cell envelope components are exposed on the cell surface and their lipid domains are intercalated with the Myc Ac acid layer. These envelope components include ManLAM, LM, higher- and lower-order PIMs, and lipomannoproteins. Other known virulence factors described for M. tuberculosis that interact with the Myc Ac layer [i.e. TDM, SL; and TGs and PGL, the latter on some M. tuberculosis strains)] are also depicted. Not all Myc Ac are depicted interacting with cell surface components. Not shown are capsule-like components (i.e., arabinomannan, glucan, mannan, and xylan). In order to maintain simplicity, molecular quantities depicted (relative number of molecules) do not accurately reflect experimental data. AG (arabinogalactan); PG (peptidoglycan); Myc Ac (mycolic acids); ManLAM (mannose-capped lipoarabinomannan); LM (lipomannan); PIMs (phosphatidyl-myo-inositol mannosides); TDM (trehalose dimycolate); SL (sulfolipid); TGs (triglycerides); PGL (phenolic glycolipid).
Biological functions of M. tuberculosis mannosylated cell envelope components ManLAM
One of the most abundant mannose-containing macromolecules of the M. tuberculosis cell envelope is ManLAM 19, which is implicated as a key molecule in immunopathogenesis and virulence of the bacterium 19,28,23. ManLAM is expressed on the M. tuberculosis surface 29, and is in an ideal position to mediate interaction between M. tuberculosis and phagocytes. In the case of slow-growing mycobacteria like M. tuberculosis, M. leprae, M. bovis BCG and M. avium among others, ManLAM is an extremely heterogeneous lipoglycan with a defined tripartite structure: a carbohydrate core (i.e. D-mannan and D- arabinan), a mannosyl-phophatidyl-myo-inositol (MPI)-anchor and various mannose-capping motifs. These mannose-capping motifs are surface exposed mannooligosaccharides linked to the non-reducing end of the D-arabinan and define the characteristic ManLAM of M. tuberculosis. They are not found on LAM from fast-growing mycobacteria which have phospho-myo-inositol caps (PILAM) or are uncapped (AraLAM). The mannose caps bind to the macrophage MR and mediate phagocytosis of bacteria by human macrophages 30,24. ManLAMs from different M. tuberculosis strains vary in the degree to which they bind to the MR pointing to a potential relationship between the length and/or presentation of the mannose-caps and their affinity for the MR 25. The ManLAM caps also bind to DC-SIGN present on dendritic cells 26,27,31. Thus, terminal components of ManLAM are very important in host cell recognition. Apart from ManLAM, the outermost layer of M. tuberculosis also contains manno-proteins (i.e. 45KDa) and other major mannose-containing polysaccharides, arabinomannan and mannan, whose mannan structures appear to be identical to that of ManLAM and LM, respectively, except for absence of the lipid anchor 32.
The macrophage immunomodulatory responses to ManLAM differ from those due to AraLAM and PILAM. ManLAM reduces macrophage microbicidal activities by negatively modulating the production of nitric oxide, oxygen radicals, inflammatory cytokines and inhibits M. tuberculosis induced-apoptosis through altering Ca2+-depending signaling 28,33,34,35. In contrast, PILAM generally induces pro-inflammatory responses. Using immunoelectron microscopy, ManLAM has been shown to traffic away from the mycobacterial phagosome in dense intracellular vesicles into the membrane-trafficking network of the macrophage36; however, this study could not address if it was intact ManLAM or ManLAM metabolites derived from intracellular processing. ManLAM has been found in the MHC class II antigen loading compartment of macrophages where it is loaded onto CD1 molecules for presentation to T cells 37. It has been suggested that ManLAM undergoes intracellular processing to be accessible to the CD1 binding groove 38. Lending support to this idea is the recent discovery of a single smaller ManLAM variant with specific structural characteristics that is uniquely involved in the presentation to T cells via CD1 39.
Following phagocytosis of most bacteria, bacterial phagosomes rapidly mature to phagolysosomes via a series of fusion steps with vesicles of the endolysosomal pathway. In contrast, M. tuberculosis modifies the phagosomal environment to support its survival inside macrophages by limiting phagosomal acidification and phagosome-lysosome fusion. Recently, we demonstrated that engagement of the MR by ManLAM directs M. tuberculosis to its initial phagosomal niche enhancing its potential for survival in human macrophages 40. The biochemical mechanisms underlying phagosome-lysosome fusion inhibition and where ManLAM or its metabolite(s) appear to be directly involved are being elucidated 41,42,43. In this regard, ManLAM blocks the increase of macrophage cytosolic Ca2+ and thereby inhibits interaction of the phosphatidylinositol 3-kinase (PI3K), hVPS34, with cytosolic calmodulin, a step necessary for the production of phosphatidylinositol 3-phosphate (PI3P) which, in turn, is required for the recruitment of the Rab5 effector Early Endosome Antigen 1 protein (EEA1) to phagosomes 42. EEA1, in combination with Syntaxin 6, is necessary for the delivery of lysosomal components from the trans-Golgi network to the phagosome and regulates fusion of phagosomes with vesicles of the endosomal-lysosomal pathway 43. However, recently, a study showed that ManLAM does not induce the phagosomal maturation block through activation of p38 MAP kinase, contradicting some previous suggestions 44.
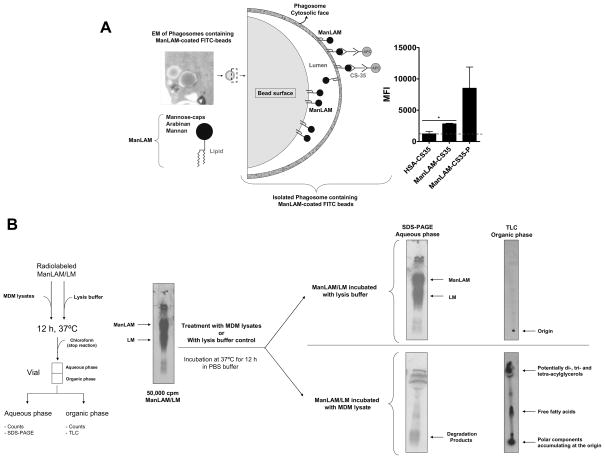
Although the effects of ManLAM on phagosome biogenesis are being defined biochemically from the host cell perspective, there is essentially nothing known about the biochemistry of ManLAM itself in this process. For example, is ManLAM or a metabolite(s) shed from M. tuberculosis in the phagosome and intercalated into the phagosomal wall via its MPI-anchor? Questions regarding the nature of ManLAM within the host and how it directly participates in regulating vesicular fusion remain unanswered. Our studies using the bead model of ManLAM uptake by human macrophages via the MR show that over time the carbohydrates of ManLAM are increasingly exposed on the cytoplasmic face of the phagosomal compartment, suggesting that ManLAM (or a metabolite) is being physically intercalated within the phagosomal membrane and flipped outside of the phagosomal compartment (Fig. 2A, unpublished results). Our findings are supported by other reports using free ManLAM in human macrophage cultures, where it was incorporated into membrane rafts of the macrophage cell membrane via its MPI-anchor and this incorporation was critical in reducing phagosomal maturation 45,44. Similarly, studies on human lymphocytes showed that ManLAM localized to membrane rafts of the lymphocyte membrane interfering with signaling pathways and subsequently affecting cytokine production 46. Thus, further work is necessary to answer the role of intact ManLAM or a metabolite(s) influencing the fluidity of the phagosome membrane and trafficking. Intracellular processing of ManLAM may be a critical step in directing the outcome of M. tuberculosis infection. Does ManLAM intracellular processing really occur? Our laboratory has begun to address this issue by incubating intact metabolically radiolabeled ManLAM with human macrophage lysates (cytosol + membranes). Our results indicate significant degradation of ManLAM by macrophage enzymatic activities, consistent with the notion that intracellular processing of mannosylated biomolecules present in the cell envelope of M. tuberculosis may occur during infection in vivo (Fig. 2B, unpublished results). Thus, an important question arises from these studies; does intact ManLAM or its metabolite(s) traffic to the cytosol and engage host cell molecules? These events are likely to influence not only phagosome biogenesis but also a number of immune and metabolic processes of the macrophage.
Figure 2. Phagosomes contain ManLAM exposed on their cytosolic face.
A. Purified phagosomes containing ManLAM-coated or human serum albumin (HSA)-coated (control) FITC positive beads were incubated with anti-ManLAM (CS-35) or mouse IgG for 20 minutes, washed, stained with secondary anti-mouse IgG -APC and analyzed on a LSRII flow cytometer. A subset of phagosomes was permeabilized (P) using reagents from BD Pharmingen (Fix/Perm kit) and stained and analyzed as described above for the non-permeabilized phagosomes. Analysis was performed using DIVA software. Gates were set around FITC positive phagosomes and MFI determined for the presence of ManLAM using anti-ManLAM antibody (IgG control antibody was subtracted out). HSA-CS-35: phagosomes-containing HSA-coated FITC beads stained with anti-ManLAM mAb (CS-35); ManLAM-CS-35: phagosomes containing ManLAM-coated FITC beads stained with CS-35; ManLAM-CS-35-P: permeabilized phagosomes containing ManLAM-coated FITC beads stained with CS-35. Mean ± SEM from 2 independent experiments, *p<0.05, T-test using GraphPad Prism v.4.01 software. The EM photomicrograph on the left shows ManLAM-coated bead phagosomes within a macrophage prior to phagosome purification (× 40,000). Based in these results, the depicted scheme shows ManLAM leaving the bead and intercalating into the phagosome inner leaflet by its lipidic domain, to later become exposed on the phagosome cytosolic leaflet through yet an unknown mechanism. B Effects of enzymatic activities derived from human macrophages on ManLAM/LM. Radiolabeled ManLAM/LM (50,000 cpm) was incubated with lysis buffer (PBS) or with human macrophage lysate (cytosol and membranes in PBS) for 12 h at 37°C. Results show that ManLAM/LM treated with lysis buffer alone remained intact [SDS-PAGE and TLC (chloroform:methanol, 96:4, v/v)]; whereas ManLAM/LM treated with MDM lysate was hydrolyzed into smaller metabolites that migrated to the organic layer after chloroform-water partition as revealed by SDS-PAGE and TLC. Shown is a representative experiment of n=3.
LM and the PIMs
Other important mannose-containing biomolecules present on the M. tuberculosis surface are LM and PIMs. LM is present in all mycobacterial species with structural differences among some pathogenic mycobacterial strains 47,48,49. Both LM and PIMs regulate cytokine, oxidant and T cell responses 50,51,52. LM associates with DC-SIGN but not with the MR 53 and induces apoptosis and a pro-inflammatory response through TLR2 49,54,55,56,57. PIMs are divided in two distinct groups depending on their number of mannoses. Lower- and higher-order PIMs contain 1 to 4 mannoses and 5 to 6 mannoses, respectively 58. Lower order PIMs [PIM2 to 4] have a terminal α(1→6)-mannose and participate in phagocytosis events through complement receptor (CR) 3 and also facilitate fusion with early endosomal compartments 59,60,61. Conversely, higher-order PIMs [PIM5 to 6] have the same α(1→2)-mono or dimannoside termini characteristic of the mannose-caps of ManLAM and participate in phagocytosis events through the MR limiting phagosome-lysosome fusion events 53. Importantly, for higher-order PIMs, the degree of acylation is critical for host recognition via the MR, where only triacylated forms of PIM6 [i.e. Ac1PIM6] efficiently bind to the MR 53. Additional studies have shown that processing for antigen presentation via CD1 to T cells 62. This intracellular sCD1e is involved in PIM6 is in line with previous studies showing that CD1 loading of biomolecules containing terminal α(1→2)-mannose occurs via the MR 38 and thus highlights the critical importance of both the ManLAM/MR and higher-order PIMs/MR phagocytic pathways in limiting phagosome-lysosome fusion and antigen presentation. Additional studies using different PIM sources and mammalian cell lines expressing DC-SIGN also showed differences in the degree of PIM recognition by DC-SIGN 53,63.
Mannan and arabinomannan
Daffe and colleagues showed that the mannose-containing biomolecules mannan and arabinomannan are exposed on the surface of M. tuberculosis forming part of the so called outer material or capsule 64,65. Although there is no direct evidence for it, capsular mannan and arabinomannan are thought to relate to LM and ManLAM, respectively, following loss of their MPI anchor due to their similar glycosidic structure 33. Based on antibody recognition assays, the surface expression of arabinomannan appears to change with culture age during bacterial growth in vitro but this phenotype seems to be strain dependent 66. In the same study arabinomannan was shown to be produced by bacteria grown in vivo, where the amount of in vivo-detected arabinomannan depended on the number of bacteria in the infected organ 66. In general, arabinomannans appear to be immunosupressive components that can affect macrophage-dependent antigen-induced TH1 cytokine production by human and murine lymphocytes 67,68,69. Interestingly, a recent study using purified arabinomannans from virulent and non-virulent M. avium strains showed that the degree of acylation of arabinomannan (additional acylation independent of the MGI-anchor) is a prerequisite for the effective stimulation of antigen presenting cells 70.
Mannosylated proteins
M. tuberculosis is reported to produce both acylated and glycosylated proteins 71,72,73,74,75,76. Among the mannosylated proteins, the most intensely studied are the 19 KDa and 45 KDa proteins. The 19 KDa protein is an abundantly expressed cell wall-associated and secreted glycolipoprotein that has biological activity attributable to its interaction with mammalian Toll-like receptors, especially with TLR2 77,78. However, the importance of the glycosylation units on the 19 KDa protein in M. tuberculosis pathogenesis is still not clear, since recombinant 19 KDa lacking posttranslational modifications is still capable of generating the pro-inflammatory response described for the native protein 79. The 45 KDa glycoprotein was first identified as having three distinct glycoforms of 55, 50 and 38 KDa within the culture filtrate proteins of M. tuberculosis 71. Later it was demonstrated that these three bands represented the same 45 KDa protein 80,81. A clear structure-function relationship for the glycosylation of M. tuberculosis proteins is still largely unknown. It is speculated that glycosylation may be involved in protein export through the mycobacterial membrane 82 or that it increases the stability of glycosylated proteins in the intracellular environment in which M. tuberculosis normally resides 76 due to the fact that O-glycosylated proteins are more resistant to intracellular proteolytic activities 83,84. The direct contribution of the glycosyl units to the immunoreactivity of M. tuberculosis glycoproteins and the role of these glycoproteins in pathogenesis was shown in one study where M. tuberculosis recombinant proteins with significant changes in their mannosylation types had little or no ability to elicit a DTH reaction in BCG pre-immunized guinea pigs85.
Thus, M. tuberculosis uses its mannosylated biomolecules to enter macrophages through defined receptor-mediated pathways [reviewed in 86,87], signals the cell during and potentially after entry, and regulates a number of immune processes. Interestingly, these mannosylated biomolecules are apparently not transported out of the cell like some mycobacterial lipids 88 suggesting that the terminal fate of these mannosylated biomolecules is within the macrophage. However, a recent study using immunoblotting suggested that M. tuberculosis and/or M. bovis BCG infected macrophages or monocytic cell lines released the 19 KDa protein and ManLAM into the media via exosomes 89. Such blotting techniques cannot distinguish between intact molecules or those that are processed. A more recent study by the same group showed that the antigen 85 protein is the major protein antigen in the exosomes 90.
Microbial carbohydrates and immunity: The importance of carbohydrate modifications in the host
Several microbes contain surface carbohydrates that are similar in structure to mammalian surface glycoproteins and glycolipids. As a result, these pathogens can evade immune surveillance due to a tolerance towards those structures in their niche 91,92. The mannose capping of several M. tuberculosis surface structures fits this concept. Important conceptually, however, is whether the mannosylation motifs produced on the M. tuberculosis surface are different within the macrophage as well as during different stages of tuberculosis. It is well established that interactions with host intracellular enzymes and carbohydrates can lead to modification(s) of other microbial surface carbohydrates 93.
Immunologically active complex biomolecules like the ones present on the M. tuberculosis surface are also present on the surface of many other pathogens. Two examples of such molecules are gram-negative bacterial LPS and the lipophosphoglycan (LPG) of Leishmania spp. Evidence for intracellular modifications has been reported for both. A unique macrophage acyloxyacyl hydrolase (AOAH) detoxifies LPS by selectively removing two of its six fatty acyl chains 94. Enzymatically deacylated LPS is about 0.2–1% as potent as an endotoxin as fully acylated LPS, as demonstrated in cell activation assays in vitro 95 and in an assay of tissue toxicity in vivo 96. LPS deacylation, which occurs over several hours post internalization, is inhibited by agents that reduce lysosomal (endosomal) acidification, suggesting that LPS moves at least transiently into an acidic intracellular compartment 97. Thus, enzymatic deacylation of LPS by AOAH is an intrinsic, regulated mechanism by which the macrophage may modulate host responses to this potent bacterial agonist 98. This is not the case for the M. tuberculosis mannose-containing biomolecules like ManLAM, LM and PIMs, where their MPI-anchor is resistant to the action of AOAH (unpublished results). However, this does not preclude the involvement of other intracellular hydrolases in the deacylation of these molecules.
LPG is a key determinant of Leishmania invasion into macrophages and survival in vertebrate and invertebrate environments 99. LPG undergoes specific trafficking inside infected macrophages 99. Antibodies against LPG display a broad band of 50 to 110 KDa, however, in lysates of macrophages infected for 24 h with Leishmania major, an extra band of 21 KDa is detected indicating LPG processing within the host cell 99. In addition to LPS and LPG, zwitterionic capsular polysaccharides from B. fragilis are processed and presented in the context of MHC class II proteins without a protein or peptide carrier and these metabolized polysaccharides activate CD4+ T cells 100. The requirement for processing glycolipid antigens in T cell recognition has been shown for the disaccharide glycosphingolipid Gal-(α1→2)-GalCer, which involves a lysosomal α-galactosidase and lipid transfer proteins known as saposins 101,102. Glycosphingolipid intracellular processing was later shown to be further enhanced by the addition of anionic lipids into substrate carrying liposomes 103. Thus, macrophages not only process phospholipids, protein and RNA, but they can also process various carbohydrates including mannosylated biomolecules and this processing appears to be important in M. tuberculosis pathogenesis although more work needs to be done in this area.
Little is known regarding the potential of macrophages to modify the covalent structure of mycobacterial lipid antigens. Current evidence suggests that either exogenous (i.e. taken up by phagocytosis or endocytosis) or endogenous (i.e. produced by intracellular M. tuberculosis) lipid antigens can enter the CD1 antigen metabolic processing route and be presented to CD1-restricted T cells. A recent study showed that soluble CD1e allows for the intracellular processing of M. tuberculosis glycolipids with a large carbohydrate component 62. This study provides evidence that mannosylated-glycoconjugates biosynthetically related to ManLAM (i.e. PIM6) are processed intracellularly to smaller-size components (i.e. PIM2) prior to CD1 presentation to T cells, indicating that carbohydrate degradation or editing of M. tuberculosis glycolipids and lipoglycans is important in enhancing immunogenicity. Whereas for some mycobacterial lipid antigens (i.e. trehalose-6,6′-dimycolate) intracellular processing appears to be less important for antigen loading to CD1 104, for ManLAM, which has a very large, bulky polysaccharide component, enzymatic processing in the macrophage appears to be critical in order to reduce the antigen to a smaller core structure for optimizing antigen presentation 37,38. It is unknown whether intracellular processing plays a role in enabling two different M. tuberculosis antigens to limit phagosome-lysosome fusion events (i.e. ManLAM and Ac1PIM6) 40,53. Even less is known about M. tuberculosis carbohydrates and lipids produced endogenously, i.e. within the phagosome, however, interaction between host and pathogen metabolic pathways provides a mechanism for the immune response to pathogenic mycobacteria that have productively infected tissues 105.
Importance of mannose on the surface of the M. tuberculosis cell envelope: A concept
M. tuberculosis mannosylated biomolecules like ManLAM are key microbial virulence determinants in the M. tuberculosis-macrophage interaction. Great efforts are being made by several laboratories to resolve the complicated biosynthetic pathways that involve ManLAM, LM and PIMs production [reviewed in 106]. Several studies, including ours, have shown that altering the presence of mannose on the surface of M. tuberculosis has relevant biological consequences. This is the case for PimB since disruption of this mannosyltransferase decreases surface exposed ManLAM and LM by ~60% and results in faster intracellular replication and increased macrophage death 107. Conversely, increasing surface mannosylation of M. smegmatis by over-expressing ManB, a phosphomannomutase involved in the biosynthesis of GDP-mannose (a major mannose donor in ManLAM biosynthesis), results in a greater association of mycobacteria with human macrophages in a mannan-inhibitable fashion 108. The essentiality of mannose on the mycobacterial cell envelope is also supported by other studies where it is speculated that mannose-containing biomolecules have a critical role in regulating septation and cell division without perturbing other pathways of lipid biosynthesis 109. Although these studies support the importance of mannose on the surface of M. tuberculosis for host recognition, other studies have pointed out that this may not be the case for other mycobacterial species. For example, using in vitro and in vivo models, Dinadayala et al. reported that a mutation in Rv1635c, the gene responsible for the mannose capping of ManLAM in M. tuberculosis 110, did not attenuate M. bovis BCG 111. Similarly, another study showed that M. bovis BCG lacking surface exposed mannose did not influence the immune response 63. Thus, these studies indicate that the relative importance of surface mannose varies among mycobacterial species. In the case of M. tuberculosis there is evidence that surface mannosylated biomolecules play a critical role in the recognition, intracellular survival and nature of the immune response of the bacillus in the host. In this context, we recently showed that clinical isolates of M. tuberculosis deficient in surface mannosylation were defective in phagocytosis by primary human macrophages when compared to the heavily mannosylated standard laboratory strains (i.e. M. tuberculosis H37Rv and Erdman strains) although those bacteria that did enter macrophages had a short doubling time under some conditions 112.
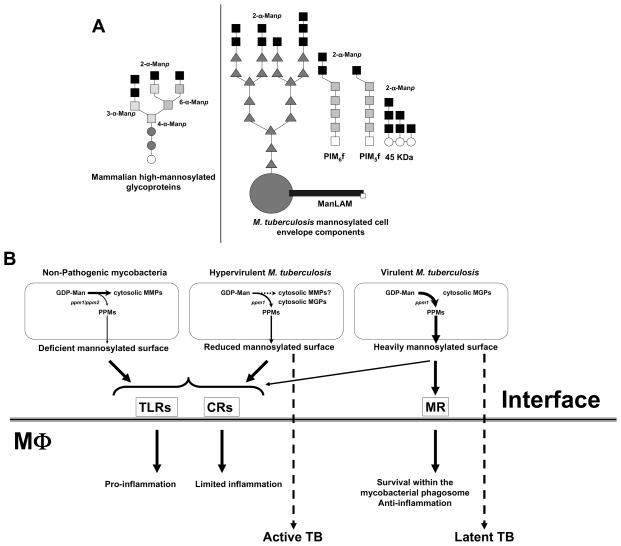
Our recent studies 40,53 have led to the conclusion that M. tuberculosis is adapting to the human host by cloaking its cell wall molecules with terminal Man-α [1→2]-Man oligosaccharides that resemble the glycoforms of mammalian mannoproteins 113 (Fig. 3A). Continued efforts to define the molecular events in the early interaction between M. tuberculosis and the human macrophage are necessary to further our understanding of the immunopathogenesis of TB and disease outcome. To identify a relationship between a group of clinical isolates of a distinct genetic lineage of M. tuberculosis and their phenotype with regard to cellular interactions is a first step to understanding how M. tuberculosis is evolving to adapt to the human host. Phylogenetic studies have grouped clinical isolates that were found associated with large cluster outbreaks 114 in geographical areas of high TB incidence 115. Some of these clinical isolates are hypervirulent in animal models 116,117,118 and better able to bypass the protection afforded by the BCG vaccine 119. They may represent “ancestor strains” 120 within distinct phylogenetic lineages that have evolved in genetic isolation with little effective horizontal gene transfer 114,115. We have studied a few strains from these phylogenetic groups and found that some within the principal genetic group (PPG)-1 120 have a marked reduction in macrophage phagocytosis. This reduction resulted from significant alterations in M. tuberculosis cell envelope components as determined at the molecular level (limited exposed mannose and the presence of phenolic glycolipid and triacylglycerols) that impacted recognition by macrophage receptors and bacterial intracellular survival 112. We have speculated that these clinical isolates may be less adapted to the human host (more prone to disease development following infection). We propose a new model for the phagocytosis and host response of M. tuberculosis strains, where the amount and nature of mannose exposed on the surface are major determinants (Fig. 3). M. tuberculosis strains with less surface mannosylation do not use the MR during phagocytosis by the human macrophage. Such strains have reduced phagocytosis, relying primarily on C3 opsonization and the more primitive CR3 pathway for entry. These strains are “hypervirulent” in part due to the presence of other surface exposed cell envelope components [i.e. phenolic glycolipid and triacylglycerols] 121,122, which regulate the cytokine response, and demonstrate rapid intracellular growth and marked tissue damage 116,117. Conversely, M. tuberculosis strains with abundant mannose on their surface have become more host-adapted in part by increasing surface mannosylation with mannans that resemble the glycoforms of eukaryotic mannoproteins that are normally removed from circulation by the homeostatic macrophage MR to maintain a healthy state 113. In support of this concept, M. tuberculosis was recently found to contain a mammalian mannosyltransferase homologue 123. Thus, more host-adapted M. tuberculosis strains may expose a large and heavily mannosylated ManLAM and greater amounts of higher-order PIMs that bind to the MR and other C-type lectins. Such strains are optimized in phagocytosis by cooperatively engaging the MR and complement receptors. Use of the mannose-containing biomolecule/MR pathway provides a safe portal for M. tuberculosis within the macrophage by regulating the trafficking of bacteria and cytokine response. These strains grow more slowly in the macrophage and cause less tissue damage during infection. We speculate that such host-adapted strains would be highly successful in establishing an infection in humans but would more likely lead to the latent state rather than to an active disease state following infection. The concept that M. tuberculosis has evolved by increasing surface mannosylation as an adaptation to the host has support, but will require additional investigation. However, using biochemical and immunologic approaches, it is now possible to categorize genetically defined groups of M. tuberculosis for the potential relationship between strain genotype and disease phenotype.
Figure 3. Mannosylated biomolecules present on the surface of M. tuberculosis and their contribution to pathogenesis.
A. M. tuberculosis strains decorate their surface with various amounts of α (1→2)-Man oligosaccharides mimicking eukaryotic glycoforms that are normally removed from circulation by the homeostatic macrophage MR to maintain a healthy state. Among these bacterial mannose-containing biomolecules are ManLAM, LM, PIMs, arabinomannan, mannan, and several glycoproteins (i.e. 19 KDa and 45 KDa among others). B. A scheme depicting the contribution of M. tuberculosis surface mannosylation in directing M. tuberculosis to a pathway/niche for intracellular survival within human macrophages by limiting phagosome-lysosome fusion events and down-regulating the inflammatory response of the host. Details are found in the text. It is unknown whether the M. tuberculosis hypervirulent strains produce MMPs. CRs (complement receptors); MGPs (methyl glucose polysaccharides); MMPs (methylated mannose polysaccharides); MR (mannose receptor); MTs (mannosyltransferases) PPMs (polyprenol-phosphate-mannoses); ppm1/ppm2 (polyprenol phosphate mannose syntheses); TLRs (Toll-like receptors).
A final intriguing concept is the potential importance of the content and location of mannose present in different mycobacterial species. GDP-mannose has been described as the universal donor for the biosynthesis of mannose-containing biomolecules in mycobacterial species. Interesting to us is the knowledge that fast-growing, non-pathogenic mycobacteria contain cytosolic methylated-mannose polysaccharides (MMPs). Conversely, to date, the production of these polysaccharides in slow-growing mycobacteria like M. tuberculosis has not been reported [reviewed in 124]. Rather, pathogenic mycobacteria contain cytosolic methylated-glucose polysaccharides (MGPs) 125,126. Thus, the relative amount of mannose retained and/or sequestered in the cytosol versus that available for building mannosylated biomolecules in the mycobacterial cell envelope may also be an important evolutionary attribute. How GDP-mannose is used is likely to be linked to the species-specific expression of carbohydrate biosynthetic enzymes in different environments. In this context, there are still many unanswered questions about the location and functionality of the mannose-containing biomolecules produced during infection in vivo.
Based on the ideas raised in this review, we propose a model (Fig. 3B) whereby mycobacterial strains differentially mannosylate their surface with α (1→2)-Manp oligosaccharides mimicking eukaryotic glycosidic forms. This mannosylation depends on the amounts of cytosolic GDP-Man and polyprenol-phosphate-mannose (PPM) and the presence of the required glycosyltransferases to construct the mannose-containing biomolecules located on the surface of pathogenic mycobacterial strains. Only non-pathogenic mycobacterial species produce cytosolic MMPs. However, the role of these biomolecules is still unclear. It is possible that GDP-mannose produced by non-pathogenic mycobacteria is used mainly by its mannosyltransferases to produce lower-order PIMs and MMPs, the latter serving to store/sequester cytosolic mannose. This storage may limit the amount of PPM produced which serves as the mannose donor for mannosyltransferases involved in the production of cell envelope mannosylated biomolecules (i.e. higher-order PIMs, LM and ManLAM). The accumulation of lower-order PIMs and MMPs in non-pathogenic mycobacteria may also be related to the efficiency of their ppm1/ppm2 complex to generate PPM. Heavily mannosylated strains are optimally phagocytosed by human macrophages using both the MR and complement receptors resulting in an intracellular bacterial survival program that favors latency. In contrast, other M. tuberculosis strains have a cell envelope characterized by poor surface mannosylation and the presence of other virulence determinants such as phenolic glycolipid and triacylglycerols. These strains do not associate with the MR, but instead with CR3 and other receptors that lead to the induction of progressive lung pathology and a poor protective TH1 response 118. These strains have a “hypervirulent” phenotype which favors progression from latency to active TB disease (Fig. 3B).
Despite numerous reports regarding the effects of mannose-containing biomolecules on the immune response of macrophages, the precise structural motifs that mediate these responses remain largely unclear. These molecules have been studied mostly with in vitro systems, particularly with rodent cells, which differ in many respects from human cells. Further biochemical characterization of these molecules, including their production and metabolism inside macrophages and tissues, will further our understanding of their interactions with macrophages and their role in immunopathogenesis. Further definition of carbohydrate production and processing pathways in vivo that impact the immune response will also aid in the development of new molecular targets for diagnosis, therapy and vaccine development.
Acknowledgments
We thank Drs. Joanne Turner and Bridget Vesosky for their assistance with the flow cytometry experiments described. We thank Dr. Robert Munford for kindly providing us with the acyloxyacyl hydrolase. We also thank Dr. Dan Wozniak for his careful reading of the manuscript and helpful suggestions with the text. Work reported in this review was supported in part by National Institutes of Health [AI33004, AI52458] to LSS; [AI068846] to LSS and JBT; and [AI073856] and the Parker B. Francis fellowship to JBT.
Footnotes
Conflict of Interest
The authors have not financial and personal conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Global tuberculosis control - epidemiology, strategy, financing. World Health Organization, WHO Press; Geneva, Switzerland: 2009. WHO/HTM/TB/2009.411. [Google Scholar]
- 2.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 3.Schlesinger LS, Azad AK, Torrelles JB, Roberts E, Vergne I, Deretic V. Determinants of Phagocytosis, Phagosome Biogenesis and Autophagy for Mycobacterium tuberculosis. In: Kaufmann SHE, Britton WJ, editors. Handbook of Tuberculosis. Immunology and Cell Biology. Wiley-VCH Verlag GmbH&Co. KGaA; Weinheim, Germany: 2008. pp. 1–22. [Google Scholar]
- 4.Fenton MJ, Riley LW, Schlesinger LS. Receptor-Mediated Recognition of Mycobacterium tuberculosis by Host Cells. In: Cole ST, Eisenach KD, McMurray DN, Jacobs WR Jr, editors. Tuberculosis and the Tubercle Bacillus. ASM Press; New YOrk: 2005. pp. 405–426. [Google Scholar]
- 5.Lohmann-Matthes ML, Steinmuller C, Franke-Ullmann G. Pulmonary macrophages. Eur Respir J. 1994;7:1678–1689. [PubMed] [Google Scholar]
- 6.Cohn ZA, Wiener E. The particulate hydrolases of macrophages. I. Comparative enzymology, isolation and properties. J Exp Med. 1963;118:991–1008. doi: 10.1084/jem.118.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorber WA, Leake ES, Myrvik QN. Comparative densities of hydrolase-containing granules from normal and BCG-induced alveolar macrophages. Infect Immun. 1973;7:86–92. doi: 10.1128/iai.7.1.86-92.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonsson S, Musher DM, Goree A, Lawrence EC. Human alveolar lining material and antibacterial defenses. Am Rev Respir Dis. 1986;133:136–140. doi: 10.1164/arrd.1986.133.1.136. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds HY. Bronchoalveolar lavage. Am Rev Respir Dis. 1987;135:250–263. doi: 10.1164/arrd.1987.135.1.250. [DOI] [PubMed] [Google Scholar]
- 10.Stephenson JD, Shepherd VL. Purification of the human alveolar macrophage mannose receptor. Biochem Biophys Res Commun. 1987;148:883–889. doi: 10.1016/0006-291x(87)90958-2. [DOI] [PubMed] [Google Scholar]
- 11.Palecanda A, Kobzik L. Receptors for unopsonized particles: the role of alveolar macrophage scavenger receptors. Curr Mol Med. 2001;1:589–595. doi: 10.2174/1566524013363384. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson JS, Schlesinger LS. Pulmonary surfactant in innate immunity and the pathogenesis of tuberculosis. Tubercle Lung Dis. 2000;80:173–184. doi: 10.1054/tuld.2000.0242. [DOI] [PubMed] [Google Scholar]
- 13.Day J, Friedman A, Schlesinger LS. Modeling the immune rheostat of macrophages in the lung in response to infection. Proc Natl Acad Sci U S A. 2009;106:11246–11251. doi: 10.1073/pnas.0904846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlesinger LS. The role of mononuclear phagocytes in tuberculosis. 1997. pp. 437–480. [PubMed] [Google Scholar]
- 15.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Coutinho PM, Stam M, Blanc E, Henrissat B. Why are there so many carbohydrate-active enzyme-related genes in plants? Trends Plant Sci. 2003;8:563–565. doi: 10.1016/j.tplants.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Varki A. Nothing in glycobiology makes sense, except in the light of evolution. Cell. 2006;126:841–845. doi: 10.1016/j.cell.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Moran A, Holst O, Brennan P, von Itzstein M. Microbial Glycobiology: Structure, Relevance and Applications. Academic Press; US: 2009. pp. 1–1020. [Google Scholar]
- 19.Brennan PJ, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 20.Crick DC, Brennan PJ, McNeil MR. The cell wall of Mycobacterium tuberculosis. In: Rom WM, Garay SM, editors. Tuberculosis. Lippincott Williams and Wilkins; Philadelphia: 2003. pp. 115–134. [Google Scholar]
- 21.Kolattukudy PE, Fernandes ND, Azad AK, Fitzmaurice AM, Sirakova TD. Biochemistry and molecular genetics of cell-wall lipid biosynthesis in mycobacteria. Mol Microbiol. 1997;24:263–270. doi: 10.1046/j.1365-2958.1997.3361705.x. [DOI] [PubMed] [Google Scholar]
- 22.Venisse A, Rivière M, Vercauteren J, Puzo G. Structural analysis of the mannan region of lipoarabinomannan from Mycobacterium bovis BCG. Heterogeneity in phosphorylation state. J Biol Chem. 1995;270:15012–15021. doi: 10.1074/jbc.270.25.15012. [DOI] [PubMed] [Google Scholar]
- 23.Nigou J, Gilleron M, Puzo G. Lipoarabinomannans: from structure to biosynthesis. Biochimie. 2003;85:153–166. doi: 10.1016/s0300-9084(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 24.Schlesinger LS, Hull SR, Kaufman TM. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- 25.Schlesinger LS, Kaufman TM, Iyer S, Hull SR, Marciando LK. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol. 1996;157:4568–4575. [PubMed] [Google Scholar]
- 26.Maeda N, Nigou J, Herrmann JL, Jackson M, Amara A, Lagrange PH, Puzo G, Gicquel B, Neyrolles O. The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J Biol Chem. 2003;278:5513–5516. doi: 10.1074/jbc.C200586200. [DOI] [PubMed] [Google Scholar]
- 27.Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strohmeier GR, Fenton MJ. Roles of lipoarabinomannan in the pathogenesis of tuberculosis. Microbes Infect. 1999;1:709–717. doi: 10.1016/s1286-4579(99)80072-0. [DOI] [PubMed] [Google Scholar]
- 29.Hunter SW, Brennan PJ. Evidence for the presence of a phosphatidylinositol anchor on the lipoarabinomannan and lipomannan of Mycobacterium tuberculosis. J Biol Chem. 1990;265:9272–9279. [PubMed] [Google Scholar]
- 30.Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 31.Azad AK, Torrelles JB, Schlesinger LS. Mutation in the DC-SIGN cytoplasmic triacidic cluster motif markedly attenuates receptor activity for phagocytosis and endocytosis of mannose-containing ligands by human myeloid cells. J Leukoc Biol. 2008 doi: 10.1189/jlb.0308192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemassu A, Daffe M. Structural features of the exocellular polysaccharides of Mycobacterium tuberculosis. Biochem J. 1994;297 ( Pt 2):351–357. doi: 10.1042/bj2970351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chatterjee D, Khoo K-H. Mycobcterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology. 1998;8:113–120. doi: 10.1093/glycob/8.2.113. [DOI] [PubMed] [Google Scholar]
- 34.Rojas M, Garcia LF, Nigou J, Puzo G, Olivier M. Mannosylated lipoarabinomannan antagonizes Mycobacterium tuberculosis-induced macrophage apoptosis by altering Ca+2-dependent cell signaling. J Infect Dis. 2000;182:240–251. doi: 10.1086/315676. [DOI] [PubMed] [Google Scholar]
- 35.Nigou J, Gilleron M, Rojas M, Garcia LF, Thurnher M, Puzo G. Mycobacterial lipoarabinomannans: Modulators of dendritic cell function and the apoptotic response. Microbes Infect. 2002;4:945–953. doi: 10.1016/s1286-4579(02)01621-0. [DOI] [PubMed] [Google Scholar]
- 36.Xu S, Cooper A, Sturgill-Koszycki S, Van Heyningen T, Chatterjee D, Orme I, Allen P, Russell DG. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994;153:2568–2578. [PubMed] [Google Scholar]
- 37.Prigozy TI, Sieling PA, Clemens D, Stewart PL, Behar SM, Porcelli SA, Brenner MB, Modlin RL, Kronenberg M. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity. 1997;6:187–197. doi: 10.1016/s1074-7613(00)80425-2. [DOI] [PubMed] [Google Scholar]
- 38.Sieling PA, Chatterjee D, Porcelli SA, Prigozy TI, Mazzaccaro RJ, Soriano T, Bloom BR, Brenner MB, Kronenberg M, Brennan PJ, Modlin RL. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 39.Torrelles JB, Sieling PA, Brennan PJ, Modlin RL, Chatterjee D. Characterization of Lipoarabinomannan Isoforms of Mycobacterium tuberculosis responsible for precise recognition by CD1-restricted T cells. Tuberculosis: Integrating host and pathogen biology, Keystone symposia; Taos, New Mexico. 2003. Abstract. [Google Scholar]
- 40.Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, Desjardin LE, Schlesinger LS. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kusner DJ. Mechanisms of mycobacterial persistence in tuberculosis. Clin Immunol. 2005;114:239–247. doi: 10.1016/j.clim.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 42.Vergne I, Chua J, Deretic V. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J Exp Med. 2003;198:653–659. doi: 10.1084/jem.20030527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fratti RA, Chua J, Vergne I, Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc Natl Acad Sci U S A. 2003;100:5437–5442. doi: 10.1073/pnas.0737613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welin A, Winberg ME, Abdalla H, Sarndahl E, Rasmusson B, Stendahl O, Lerm M. Incorporation of Mycobacterium tuberculosis lipoarabinomannan into macrophage membrane rafts is a prerequisite for the phagosomal maturation block. Infect Immun. 2008;76:2882–2887. doi: 10.1128/IAI.01549-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ilangumaran S, Arni S, Poincelet M, Theler JM, Brennan PJ, Nasir ud Din, Hoessli DC. Integration of mycobacterial lipoarabinomannans into glycosylphosphatidylinositol-rich domains of lymphomonocytic cell plasma membranes. J Immunol. 1995;155:1334–1342. [PubMed] [Google Scholar]
- 46.Shabaana AK, Kulangara K, Semac I, Parel Y, Ilangumaran S, Dharmalingam K, Chizzolini C, Hoessli DC. Mycobacterial lipoarabinomannans modulate cytokine production in human T helper cells by interfering with raft/microdomain signalling. Cell Mol Life Sci. 2005;62:179–187. doi: 10.1007/s00018-004-4404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilleron M, Nigou J, Cahuzac B, Puzo G. Structural study of the lipomannans from Mycobacterium bovis BCG: Characterisation of multiacylated forms of the phosphatidyl-myo-inositol anchor. J Mol Biol. 1999;285:2147–2160. doi: 10.1006/jmbi.1998.2438. [DOI] [PubMed] [Google Scholar]
- 48.Guerardel Y, Maes E, Elass E, Leroy Y, Timmerman P, Besra GS, Locht C, Strecker G, Kremer L. Structural study of lipomannan and lipoarabinomannan from Mycobacterium chelonae. Presence of unusual components with alpha 1,3-mannopyranose side chains. J Biol Chem. 2002;277:30635–30648. doi: 10.1074/jbc.M204398200. [DOI] [PubMed] [Google Scholar]
- 49.Guerardel Y, Maes E, Briken V, Chirat F, Leroy Y, Locht C, Strecker G, Kremer L. Lipomannan and lipoarabinomannan from a clinical isolate of Mycobacterium kansasii: Novel structural features and apoptosis-inducing properties. J Biol Chem. 2003;278:36637–36651. doi: 10.1074/jbc.M305427200. [DOI] [PubMed] [Google Scholar]
- 50.Barnes PF, Chatterjee D, Abrams JS, Lu S, Wang E, Yamamura M, Brennan PJ, Modlin RL. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992;149:541–547. [PubMed] [Google Scholar]
- 51.Chan ED, Morris KR, Belisle JT, Hill P, Remigio LK, Brennan PJ, Riches DWH. Induction of inducible nitric oxide synthase-NO• by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-κB signaling pathways. Infect Immun. 2001;69:2001–2010. doi: 10.1128/IAI.69.4.2001-2010.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilleron M, Ronet C, Mempel M, Monsarrat B, Gachelin G, Puzo G. Acylation state of the phosphatidylinositol mannosides from Mycobcterium bovis bacillus Calmette Guerin and ability to induce granuloma and recruit natural killer T cells. J Biol Chem. 2001;276:34896–34904. doi: 10.1074/jbc.M103908200. [DOI] [PubMed] [Google Scholar]
- 53.Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individula species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol. 2006;177:1805–1816. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- 54.Vignal C, Guerardel Y, Kremer L, Masson M, Legrand D, Mazurier J, Elass E. Lipomannans, but not lipoarabinomannans, purified from Mycobacterium chelonae and Mycobacterium kansasii induce TNF-alpha and IL-8 secretion by a CD14-toll-like receptor 2-dependent mechanism. J Immunol. 2003;171:2014–2023. doi: 10.4049/jimmunol.171.4.2014. [DOI] [PubMed] [Google Scholar]
- 55.Dao DN, Kremer L, Guerardel Y, Molano A, Jacobs WR, Jr, Porcelli SA, Briken V. Mycobacterium tuberculosis lipomannan induces apoptosis and interleukin-12 production in macrophages. Infect Immun. 2004;72:2067–2074. doi: 10.1128/IAI.72.4.2067-2074.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilleron M, Nigou J, Nicolle D, Quesniaux V, Puzo G. The acylation state of mycobacterial lipomannans modulates innate immunity response through toll-like receptor 2. Chem Biol. 2006;13:39–47. doi: 10.1016/j.chembiol.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 57.Nigou J, Vasselon T, Ray A, Constant P, Gilleron M, Besra GS, Sutcliffe I, Tiraby G, Puzo G. Mannan chain length controls lipoglycans signaling via and binding to TLR2. J Immunol. 2008;180:6696–6702. doi: 10.4049/jimmunol.180.10.6696. [DOI] [PubMed] [Google Scholar]
- 58.Khoo KH, Dell A, Morris HR, Brennan PJ, Chatterjee D. Structural definition of acylated phosphatidylinositol mannosides from Mycobacterium tuberculosis: Definition of a common anchor for lipomannan and lipoarabinomannan. Glycobiology. 1995;5:117–127. doi: 10.1093/glycob/5.1.117. [DOI] [PubMed] [Google Scholar]
- 59.Vergne I, Fratti RA, Hill PJ, Chua J, Belisle J, Deretic V. Mycobacterium tuberculosis phagosome maturation arrest: Mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol Biol Cell. 2004;15:751–760. doi: 10.1091/mbc.E03-05-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chua J, Vergne I, Master S, Deretic V. A tale of two lipids: Mycobacterium tuberculosis phagosome maturation arrest. Curr Opin Microbiol. 2004;7:71–77. doi: 10.1016/j.mib.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 61.Villeneuve C, Gilleron M, Maridonneau-Parini I, Daffe M, Astarie-Dequeker C, Etienne G. Mycobacteria use their surface-exposed glycolipids to infect human macrophages through a receptor-dependent process. J Lipid Res. 2005;46:475–483. doi: 10.1194/jlr.M400308-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.de la Salle H, Mariotti S, Angenieux C, Gilleron M, Garcia-Alles LF, Malm D, Berg T, Paoletti S, Maitre B, Mourey L, Salamero J, Cazenave JP, Hanau D, Mori L, Puzo G, De Libero G. Assistance of microbial glycolipid antigen processing by CD1e. Science. 2005;310:1321–1324. doi: 10.1126/science.1115301. [DOI] [PubMed] [Google Scholar]
- 63.Driessen NN, Ummels R, Maaskant JJ, Gurcha SS, Besra GS, Ainge GD, Larsen DS, Painter GF, Vandenbroucke-Grauls CM, Geurtsen J, Appelmelk BJ. Role of phosphatidylinositol mannosides in the interaction between mycobacteria and DC-SIGN. Infect Immun. 2009;77:4538–4547. doi: 10.1128/IAI.01256-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ortalo-Magne A, Dupont MA, Lemassu A, Andersen AB, Gounon P, Daffe M. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiology. 1995;141 ( Pt 7):1609–1620. doi: 10.1099/13500872-141-7-1609. [DOI] [PubMed] [Google Scholar]
- 65.Daffe M, Draper P. The envelope layers of mycobacteria with reference to their pathogenicity. Adv Microb Physiol. 1998;39:131–203. doi: 10.1016/s0065-2911(08)60016-8. [DOI] [PubMed] [Google Scholar]
- 66.Schwebach JR, Casadevall A, Schneerson R, Dai Z, Wang X, Robbins JB, Glatman-Freedman A. Expression of a Mycobacterium tuberculosis arabinomannan antigen in vitro and in vivo. Infect Immun. 2001;69:5671–5678. doi: 10.1128/IAI.69.9.5671-5678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ellner JJ, Daniel TM. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979;35:250–257. [PMC free article] [PubMed] [Google Scholar]
- 68.Oka H, Shiraishi Y, Sasaki H, Yoshinaga K, Emori Y, Takei M. Antimetastatic effect of an immunomodulatory arabinomannan extracted from Mycobacterium tuberculosis strain Aoyama B, Z-100, through the production of interleukin-12. Biol Pharm Bull. 2003;26:1336–1341. doi: 10.1248/bpb.26.1336. [DOI] [PubMed] [Google Scholar]
- 69.Oka H, Sasaki H, Shiraishi Y, Emori Y, Yoshinaga K, Takei M. Z-100, an immunomodulatory arabinomannan extracted from Mycobacterium tuberculosis strain Aoyama B, augments anti-tumor activities of X-ray irradiation against B16 melanoma in association with the improvement of type 1T cell responses. Biol Pharm Bull. 2004;27:82–88. doi: 10.1248/bpb.27.82. [DOI] [PubMed] [Google Scholar]
- 70.Wittkowski M, Mittelstadt J, Brandau S, Reiling N, Lindner B, Torrelles J, Brennan PJ, Holst O. Capsular arabinomannans from Mycobacterium avium with morphotype-specific structural differences but identical biological activity. J Biol Chem. 2007;282:19103–19112. doi: 10.1074/jbc.M611551200. [DOI] [PubMed] [Google Scholar]
- 71.Espitia C, Mancilla R. Identification, isolation and partial characterization of Mycobacterium tuberculosis glycoprotein antigens. Clin Exp Immunol. 1989;77:378–383. [PMC free article] [PubMed] [Google Scholar]
- 72.Fifis T, Costopoulos C, Radford AJ, Bacic A, Wood PR. Purification and characterization of major antigens from a Mycobacterium bovis culture filtrate. Infect Immun. 1991;59:800–807. doi: 10.1128/iai.59.3.800-807.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garbe T, Harris D, Vordermeier M, Lathigra R, Ivanyi J, Young D. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: Immunological analysis and evidence of glycosylation. Infect Immun. 1993;61:260–267. doi: 10.1128/iai.61.1.260-267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Young DB, Garbe TR. Lipoprotein antigens of Mycobacterium tuberculosis. Res Microbiol. 1991;142:55–65. doi: 10.1016/0923-2508(91)90097-t. [DOI] [PubMed] [Google Scholar]
- 75.Dobos KM, Swiderek K, Khoo KH, Brennan PJ, Belisle JT. Evidence for glycosylation sites on the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. Infect Immun. 1995;63:2846–2853. doi: 10.1128/iai.63.8.2846-2853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sartain MJ, Belisle JT. N-Terminal clustering of the O-glycosylation sites in the Mycobacterium tuberculosis lipoprotein SodC. Glycobiology. 2009;19:38–51. doi: 10.1093/glycob/cwn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa M, Engele M, Sieling P, Barnes P, Rollinghoff M, Bolcskei P, Wagner M, Akira S, Norgard M, Belisle J, Godowski P, Bloom B, Modlin R. Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science. 2001;291:1544–1547. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 78.Noss EH, Pai RK, Sellati TJ, Radolf JD, Belisle J, Golenbock DT, Boom WH, Harding CV. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J Immunol. 2001;167:910–918. doi: 10.4049/jimmunol.167.2.910. [DOI] [PubMed] [Google Scholar]
- 79.Lopez M, Sly LM, Luu Y, Young D, Cooper H, Reiner NE. The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J Immunol. 2003;170:2409–2416. doi: 10.4049/jimmunol.170.5.2409. [DOI] [PubMed] [Google Scholar]
- 80.Espitia C, Espinosa R, Saavedra R, Mancilla R, Romain F, Laqueyrerie A, Moreno C. Antigenic and structural similarities between Mycobacterium tuberculosis 50- to 55-kilodalton and Mycobacterium bovis BCG 45- to 47-kilodalton antigens. Infect Immun. 1995;63:580–584. doi: 10.1128/iai.63.2.580-584.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nagai S, Wiker HG, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991;59:372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dobos KM, Khoo KH, Swiderek KM, Brennan PJ, Belisle JT. Definition of the full extent of glycosylation of the 45- kilodalton glycoprotein of Mycobacterium tuberculosis. J Bacteriol. 1996;178:2498–2506. doi: 10.1128/jb.178.9.2498-2506.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990;15:291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- 84.Herrmann JL, O’Gaora P, Gallagher A, Thole JE, Young DB. Bacterial glycoproteins: A link between glycosylation and proteolytic cleavage of a 19 kDa antigen from Mycobacterium tuberculosis. EMBO J. 1996;15:3547–3554. [PMC free article] [PubMed] [Google Scholar]
- 85.Horn C, Namane A, Pescher P, Riviere M, Romain F, Puzo G, Barzu O, Marchal G. Decreased capacity of recombinant 45/47-kDa molecules (Apa) of Mycobacterium tuberculosis to stimulate T lymphocyte responses related to changes in their mannosylation pattern. J Biol Chem. 1999;274:32023–32030. doi: 10.1074/jbc.274.45.32023. [DOI] [PubMed] [Google Scholar]
- 86.Torrelles JB, Azad AK, Henning LN, Carlson TK, Schlesinger LS. Role of C-type lectins in mycobacterial infections. Curr Drug Targets. 2008;9:102–112. doi: 10.2174/138945008783502467. [DOI] [PubMed] [Google Scholar]
- 87.Ehlers S. DC-SIGN and mannosylated surface structures of Mycobacterium tuberculosis: a deceptive liaison. Eur J Cell Biol. 2009 doi: 10.1016/j.ejcb.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 88.Beatty WL, Rhoades ER, Ullrich HJ, Chatterjee D, Heuser JE, Russell DG. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 2000;1:235–247. doi: 10.1034/j.1600-0854.2000.010306.x. [DOI] [PubMed] [Google Scholar]
- 89.Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giri PK, Schorey JS. Exosomes derived from M. bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS One. 2008;3:e2461. doi: 10.1371/journal.pone.0002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cobb BA, Kasper DL. Coming of age: Carbohydrates and immunity. Eur J Immunol. 2005;35:352–356. doi: 10.1002/eji.200425889. [DOI] [PubMed] [Google Scholar]
- 92.Coyne MJ, Reinap B, Lee MM, Comstock LE. Human symbionts use a host-like pathway for surface fucosylation. Science. 2005;307:1778–1781. doi: 10.1126/science.1106469. [DOI] [PubMed] [Google Scholar]
- 93.Vimr ER, Kalivoda KA, Deszo EL, Steenbergen SM. Diversity of microbial sialic acid metabolism. Microbiol Mol Biol Rev. 2004;68:132–53. doi: 10.1128/MMBR.68.1.132-153.2004. table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Munford RS, Hall CL. Purification of acyloxyacyl hydrolase, a leukocyte enzyme that removes secondary acyl chains from bacterial lipopolysaccharides. J Biol Chem. 1989;264:15613–15619. [PubMed] [Google Scholar]
- 95.Kitchens RL, Ulevitch RJ, Munford RS. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J Exp Med. 1992;176:485–494. doi: 10.1084/jem.176.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Munford RS, Hall CL. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986;234:203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- 97.Luchi M, Munford RS. Binding, internalization, and deacylation of bacterial lipopolysaccharide by human neutrophils. J Immunol. 1993;151:959–969. [PubMed] [Google Scholar]
- 98.Katz SS, Weinrauch Y, Munford RS, Elsbach P, Weiss J. Deacylation of lipopolysaccharide in whole Escherichia coli during destruction by cellular and extracellular components of a rabbit peritoneal inflammatory exudate. J Biol Chem. 1999;274:36579–36584. doi: 10.1074/jbc.274.51.36579. [DOI] [PubMed] [Google Scholar]
- 99.Duque-de-Mello M, Ho JL, Vannier-Santos MA, Pimenta PFP. Lipophosphoglycan traffic and degradation in Leishmania-infected macrophages. Mem Inst Oswaldo Cruz. 1999;94:55–99. [Google Scholar]
- 100.Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117:677–687. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prigozy TI, Naidenko O, Qasba P, Elewaut D, Brossay L, Khurana A, Natori T, Koezuka Y, Kulkarni A, Kronenberg M. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001;291:664–667. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 102.Kolter T, Sandhoff K. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu Rev Cell Dev Biol. 2005;21:81–103. doi: 10.1146/annurev.cellbio.21.122303.120013. [DOI] [PubMed] [Google Scholar]
- 103.Wilkening G, Linke T, Sandhoff K. Lysosomal degradation on vesicular membrane surfaces. Enhanced glucosylceramide degradation by lysosomal anionic lipids and activators. J Biol Chem. 1998;273:30271–30278. doi: 10.1074/jbc.273.46.30271. [DOI] [PubMed] [Google Scholar]
- 104.Moody DB, Reinhold BB, Reinhold VN, Besra GS, Porcelli SA. Uptake and processing of glycosylated mycolates for presentation to CD1b-restricted T cells. Immunol Lett. 1999;65:85–91. doi: 10.1016/s0165-2478(98)00129-1. [DOI] [PubMed] [Google Scholar]
- 105.Moody DB, Guy MR, Grant E, Cheng TY, Brenner MB, Besra GS, Porcelli SA. CD1b-mediated T cell recognition of a glycolipid antigen generated from mycobacterial lipid and host carbohydrate during infection. J Exp Med. 2000;192:965–976. doi: 10.1084/jem.192.7.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaur D, Guerin ME, Škovierová H, Brennan PJ, Jackson M. Biogenesis of the cell wall and other glycoconjugates of Mycobacterium tuberculosis. In: Laskin AI, Sariaslani S, Gadd GM, editors. Advances in Applied Microbiology. Acedemic Press; Burlington, US: 2009. pp. 23–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Torrelles JB, Desjardin LE, MacNeil J, Kaufman TM, Kutzbach B, Knaup R, McCarthy TR, Gurcha SS, Besra GS, Clegg S, Schlesinger LS. Inactivation of Mycobacterium tuberculosis mannosyltransferase pimB reduces the cell wall lipoarabinomannan and lipomannan content and increases the rate of bacterial-induced human macrophage cell death. Glycobiology. 2009;19:743–755. doi: 10.1093/glycob/cwp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McCarthy TR, Torrelles JB, Macfarlane AS, Katawczik M, Kutzbach B, Desjardin LE, Clegg S, Goldberg JB, Schlesinger LS. Overexpression of Mycobacterium tuberculosis manB, a phosphomannomutase that increases phosphatidylinositol mannoside biosynthesis in Mycobacterium smegmatis and mycobacterial association with human macrophages. Mol Microbiol. 2005;58:774–790. doi: 10.1111/j.1365-2958.2005.04862.x. [DOI] [PubMed] [Google Scholar]
- 109.Patterson JH, Waller RF, Jeevarajah D, Billman-Jacobe H, McConville MJ. Mannose metabolism is required for mycobacterial growth. Biochem J. 2003;372:77–86. doi: 10.1042/BJ20021700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dinadayala P, Kaur D, Berg S, Amin AG, Vissa VD, Chatterjee D, Brennan PJ, Crick DC. Genetic basis for the synthesis of the immunomodulatory mannose caps of lippoarabinomannan in Mycobacterium tuberculosis. J Biol Chem. 2006;281:20027–20035. doi: 10.1074/jbc.M603395200. [DOI] [PubMed] [Google Scholar]
- 111.Appelmelk BJ, den Dunnen J, Driessen NN, Ummels R, Pak M, Nigou J, Larrouy-Maumus G, Gurcha SS, Movahedzadeh F, Geurtsen J, Brown EJ, Eysink Smeets MM, Besra GS, Willemsen PT, Lowary TL, van Kooyk Y, Maaskant JJ, Stoker NG, van der Ley P, Puzo G, Vandenbroucke-Grauls CM, Wieland CW, Van Der Poll T, Geijtenbeek TB, van der Sar AM, Bitter W. The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium-host interaction. Cell Microbiol. 2008;10:930–944. doi: 10.1111/j.1462-5822.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 112.Torrelles JB, Knaup R, Kolareth A, Slepushkina T, Kaufman TM, Kang PB, Hill P, Brennan PJ, Chatterjee D, Belisle JT, Musser JM, Schlesinger LS. Identification of Mycobacterium tuberculosis clinical isolates with altered phagocytosis by human macrophages due to a truncated lipoarabinomannan. J Biol Chem. 2008;283:31417–31428. doi: 10.1074/jbc.M806350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martinez-Pomares L, Linehan SA, Taylor PR, Gordon S. Binding properties of the mannose receptor. Immunobiology. 2001;204:527–535. doi: 10.1078/0171-2985-00089. [DOI] [PubMed] [Google Scholar]
- 114.Gutacker MM, Mathema B, Soini H, Shashkina E, Kreiswirth BN, Graviss EA, Musser JM. Single-nucleotide polymorphism-based population genetic analysis of Mycobacterium tuberculosis strains from 4 geographic sites. J Infect Dis. 2006;193:121–128. doi: 10.1086/498574. [DOI] [PubMed] [Google Scholar]
- 115.Gagneux S, Small PM. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis. 2007;7:328–337. doi: 10.1016/S1473-3099(07)70108-1. [DOI] [PubMed] [Google Scholar]
- 116.Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, Barry CE, III, Freedman VH, Kaplan G. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-α/B. Proc Natl Acad Sci USA. 2001;98:5752–5757. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsenova L, Ellison E, Harbacheuski R, Moreira AL, Kurepina N, Reed MB, Mathema B, Barry CE, III, Kaplan G. Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J Infect Dis. 2005;192:98–106. doi: 10.1086/430614. [DOI] [PubMed] [Google Scholar]
- 118.Ordway D, Henao-Tamayo M, Harton M, Palanisamy G, Troudt J, Shanley C, Basaraba RJ, Orme IM. The Hypervirulent Mycobacterium tuberculosis Strain HN878 Induces a Potent TH1 Response followed by Rapid Down-Regulation. J Immunol. 2007;179:522–531. doi: 10.4049/jimmunol.179.1.522. [DOI] [PubMed] [Google Scholar]
- 119.Tsenova L, Harbacheuski R, Sung N, Ellison E, Fallows D, Kaplan G. BCG vaccination confers poor protection against M. tuberculosis HN878-induced central nervous system disease. Vaccine. 2007;25:5126–5132. doi: 10.1016/j.vaccine.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sreevatsan S, Pan X, Stockbauer KE, Connell ND, Kreiswirth BN, Whittam TS, Musser JM. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc Natl Acad Sci USA. 1997;94:9869–9874. doi: 10.1073/pnas.94.18.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reed MB, Domenech P, Manca C, Su H, Barczak AK, Kreiswirth BN, Kaplan G, Barry CE., III A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature. 2004;431:84–87. doi: 10.1038/nature02837. [DOI] [PubMed] [Google Scholar]
- 122.Reed MB, Gagneux S, Deriemer K, Small PM, Barry CE., III The W-Beijing lineage of Mycobacterium tuberculosis overproduces triglycerides and has the DosR dormancy regulon constitutively upregulated. J Bacteriol. 2007;189:2583–2589. doi: 10.1128/JB.01670-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.VanderVen BC, Harder JD, Crick DC, Belisle JT. Export-mediated assembly of mycobacterial glycoproteins parallels eukaryotic pathways. Science. 2005;309:941–943. doi: 10.1126/science.1114347. [DOI] [PubMed] [Google Scholar]
- 124.Jackson M, Brennan PJ. Polymethylated polysaccharides from Mycobacterium species revisited. J Biol Chem. 2009;284:1949–1953. doi: 10.1074/jbc.R800047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee YC, Ballou CE. 6-O-Methyl-D-Glucose from mycobacteria. J Biol Chem. 1964;239:C3602–C3603. [PubMed] [Google Scholar]
- 126.Lee YC. Isolation and characterization of lipopolysaccharides containing 6-O-methyl-D-glucose from Mycobacterium species. J Biol Chem. 1966;241:1899–1908. [PubMed] [Google Scholar]