Abstract
The c-Jun N-terminal Kinase (JNK) signaling cascade has been implicated in a wide range of diseases, including cancer. It is unclear how different JNK proteins contribute to human cancer. Here, we report that JNK2 is activated in over 70% of human squamous cell carcinoma (SCC) samples and that inhibition of JNK2 pharmacologically or genetically impairs tumorigenesis of human SCC cells. Most importantly, JNK2, but not JNK1, is sufficient to couple with oncogenic Ras to transform primary human epidermal cells into malignancy with features of SCC. JNK2 prevents Ras-induced cell senescence and growth arrest by reducing the expression levels of the cell cycle inhibitor p16 and NF-κB activation. On the other hand, JNK, along with PI3K, is essential for Ras-induced glycolysis, an energy producing process known to benefit cancer growth. These data indicate that JNK2 collaborates with other oncogenes, such as Ras, at multiple molecular levels to promote tumorigenesis and hence represents a promising therapeutic target for cancer.
Keywords: JNK1, JNK2 and skin cancer
INTRODUCTION
The c-Jun N-terminal kinases (JNK) comprise one family of the mitogen activated protein kinases (MAPK), along with Erk and p38 (1). The JNK proteins, also known as SAPKs (stress activated protein kinases), include JNK1, JNK2 and JNK3 that are encoded by three different genes. JNK1 and JNK2 are ubiquitously expressed and each has four splice isoforms whose expression levels vary among different cell types and environmental conditions. In contrast, JNK3 is abundant in a limited number of tissues, including brain, testis and heart, although wider expression of JNK3 has been implicated in recent studies. JNK is highly responsive to a variety of extracellular stimuli, including a wide range of inflammatory cytokines (2). Signals transmitted from membrane receptors activate MKK4 and MKK7, which then activate JNK via dual phosphorylation on the ThrProTyr (TPY) motif. MKK7 is highly specific for JNK, whereas MKK4 also activates p38 (3). The major downstream targets of the JNK cascade are members of the AP1 family transcription factors, including Jun and Fos proteins that function as hetero- or homo-dimers (2).
The JNK signaling pathway is not only involved in cellular stress responses but also plays key roles in tissue homeostasis by regulating cell proliferation, apoptosis and differentiation in a cell type- and tissue-specific manner. Abnormal JNK signaling has been associated with a number of pathological conditions in animal models, including neurodegenerative disorders, diabetes, arthritis, atherosclerosis and cancer (4) (5) (6) (7). In line with these animal data, increased JNK activation has been detected in an array of human cancers, including glioma, prostate carcinoma, osteosarcoma and squamous cell carcinoma (SCC) (8) (9) (10, 11). SCC is the most common and invasive type of nonmelanoma skin cancer with an annual incidence of over 250,000 cases in USA alonea. It has been recently recognized as one of the most costly types of cancers that entail a huge financial burden to the health care system (12). Therapeutic strategies targeting the JNK signal transduction pathway has already been under active development for a broad spectrum of other diseases (13). It is imperative to explore their use in human SCC. Our previous studies by immuno-staining have demonstrated that the JNK is highly activated in over 75% of patient SCC samples examined. Moreover, JNK activation by expression of a constitutively active mutant of MKK7 is sufficient to couple with oncogenic Ras to promote invasive epidermal malignancy with features of SCC (11). These findings underscore a dominant role of the JNK signaling pathway in human SCC. However, the functional significance and molecular mechanisms of different JNK proteins in SCC are still unclear.
JNK proteins exhibit both redundant and differential roles in tissue homeostasis as demonstrated by recent mouse genetic studies (14). Deletion of either Jnk1 or Jnk2, together with or without Jnk3, produces viable mice with defects in T-helper cell differentiation. Compound deletion of Jnk1 and Jnk2 leads to early embryonic lethality (E10.5) due to aberrant cell death in the forebrain and hindbrain (15, 16), which indicates that JNK1 and JNK2 are functionally redundant. On the other hand, studies using isolated mouse fibroblasts or erythroblasts have demonstrated opposite roles for JNK1 and JNK2 in cell proliferation with JNK2 being recognized as a negative growth regulator (17). Similar observations have been reported for epidermal cells such that Jnk2−/- and Jnk1−/− epidermis is hyperplastic and hypoplastic, respectively (18). On the contrary to the growth phenotype, Jnk2−/− mice are resistant whereas Jnk1−/− mice are sensitive to chemically induced skin carcinogenesis (7, 19). Of further interest, only JNK1 is involved in UV-induced signal transduction in epidermis (20). Taken together, these findings underscore important functions for each JNK in murine cell growth and neoplasia. It is yet to be determined whether human cells respond to JNK in the same manner as mouse cells do, and what are the molecular mechanisms that govern JNK-effects on pathogenesis. To address these questions, we studied gain- and loss-of-function effects of JNK1 and JNK2 on epidermal neoplasia by using the recently developed human epidermal tissue models regenerated on immune-deficient mice (11, 21, 22). We found that activation of JNK2, but not JNK1, was able to act in concert with oncogenic Ras to transform normal epidermal cells to malignancy with features of spontaneous SCC. JNK2 reduced protein levels of p16 cell cycle inhibitor, blocked Ras-induced NF-κB activation, and prevented Ras-induced epidermal cell senescence and growth arrest (21). On the other hand, JNK activity is essential for Ras-induced glycolysis, a metabolic feature known to confer survival advantage to cancer (23). Moreover, JNK2 is not only required for the Ras-driven tumorigenesis but also is essential for tumorigenesis of spontaneous SCC cells. Our data indicate that JNK2 plays a dominant role in human epidermal neoplasia, and implicate that selective JNK2 inhibition promises therapeutic values.
MATERIAL AND METHODS
Cell culture and gene transfer
cDNAs encoding the dominant negative c-Jun (DNc-Jun) (24), the constitutively active fusions of MKK7-JNK1a1 (JNK1) and MKK7-JNK2a1(JNK2) (25, 26), the kinase-deficient mutants of JNK1α1 (JNK1APF) and JNK2α1 (JNK2APF) (26) were subcloned into the LZRS retroviral vector. The shRNA retroviral vectors targeting JNK1 or JNK2, and the control vector were obtained from Open Biosystems. Viral production and infection of human keratinocytes were performed as described (27). A431 cells (ATCC) were cultured in 10% FBS/DMEM and transduced in the same manner as above. For gene silencing, transduced cells were selected with 1 µg/ml puromycin for 5 days prior to cell growth and soft agar colony assays. Dural luciferase gene reporter assays were performed in 293T cells as described (28). Relative luciferase unit was obtained by normalizing the firefly luciferase reading unit to that of the internal renilla-luciferase control and then to the normalized numbers of LacZ control cells.
Animal studies
Animal studies were conducted in accordance with protocols approved by the Duke Animal Care and Use Committee. Human skin regeneration was performed using CB.17 SCID mice as described (11, 21). Skin biopsies were taken at 8 weeks post grafting. Data shown represent two or more sets of independently grafted animals with 3 to 5 animals per group.
β-galactosidase staining
Human keratinocytes were fixed in methanol at 48 hrs post transduction and then undergone staining for the senescence-associated acidic β–Galactosidase (Cell Signaling). Blue cells and non-blue cells were counted on photographed microscopic images (n=10) taken under Olympia BX41 microscopic imaging system.
Immunoblotting and immuno-histology
Protein lysates from surgically discarded human skin and SCC samples (20 µg each) were used for immunoblotting with primary antibodies against p-JNK (Santa CruZ, SC6254), JNK1 (SC474), JNK2 (SC827) and Actin (SC1616) followed by IRdye-conjugated secondary antibodies (Odyssey). For immuno-precipitation, 400 µg of each sample were incubated with 2 µg rabbit antibodies against JNK1 (SC474), JNK2 (SC827) or GM-CSF (SC13101) overnight at 4°C. The precipitated proteins were analyzed by immunoblotting with primary antibodies against p-JNK (Promega, V993A), JNK1 (SC474) or JNK2 (Abcam, Ab76125) followed by a conformation-specific mouse secondary antibody against rabbit (Cell Signaling, L27A9), and then an HRP-conjugated goat anti-mouse antibody (Chemicon International). For cultured cells, protein lysates (20 µg each) collected two days following viral transduction were used for immunoblotting with antibodies against JNK1 (SC474), JNK2 (Ab76125), p16, p53 (Genescript) or Actin. Nuclei and cytoplasmic fractionations were processed as described (29), and 20 µg each were used for immunoblotting with antibodies against RelA (SC109), lamin A/C (SC6215) and Actin. Immuno-histochemistry and immuno-fluorescent analyses were performed as described (11).
Real-time RT-PCR
Total RNA were isolated from primary human keratinocytes transduced for expression of LacZ, JNK1 and JNK2 using Qiagen RNA isolation kit. For gene silencing, cells expressing JNK2 were transfected with 100 nM siRNA oligonucleotides targeting c-Jun or non-silencing control (Dharmacon) using RNAi Max reagents (Invitrogen) 24 hrs prior to RNA isolation. Standard real-time RT-PCR was performed with the following primers: p16 (5' GCCTTTTCACTGTGTTGGAGTTT 3' and reverse 5' CGCAAGAAATGCCCACATG 3') and 18S (forward: 5' TCTCCGGAATCGAACCCTGATT 3' and reverse: 5' CCCATTCGAACGTCTGCCCTATC 3'). Relative mRNA levels of p16 were normalized to that of 18S and presented as fold changes by comparing the normalized data to that of LacZ control cells.
Glycolysis
Primary human keratinocytes undergone gene transduction were used at 48 hrs post the final transduction for glycolysis analysis as described (30). The fold changes were obtained by normalizing the number of mM glucose per mg protein consumed in each sample to that of LacZ control cells. For kinase inhibition, cells transduced for expression of Ras were treated with 10 µM SP600125, LY294002, PD98059 or BI78D3 or 20 µM TAT-JIP peptide for 24 hrs prior to glycolysis analysis. All kinase inhibitors were obtained from Sigma.
RESULTS
JNK2 is required for the tumorigenesis of spontaneous SCC cells
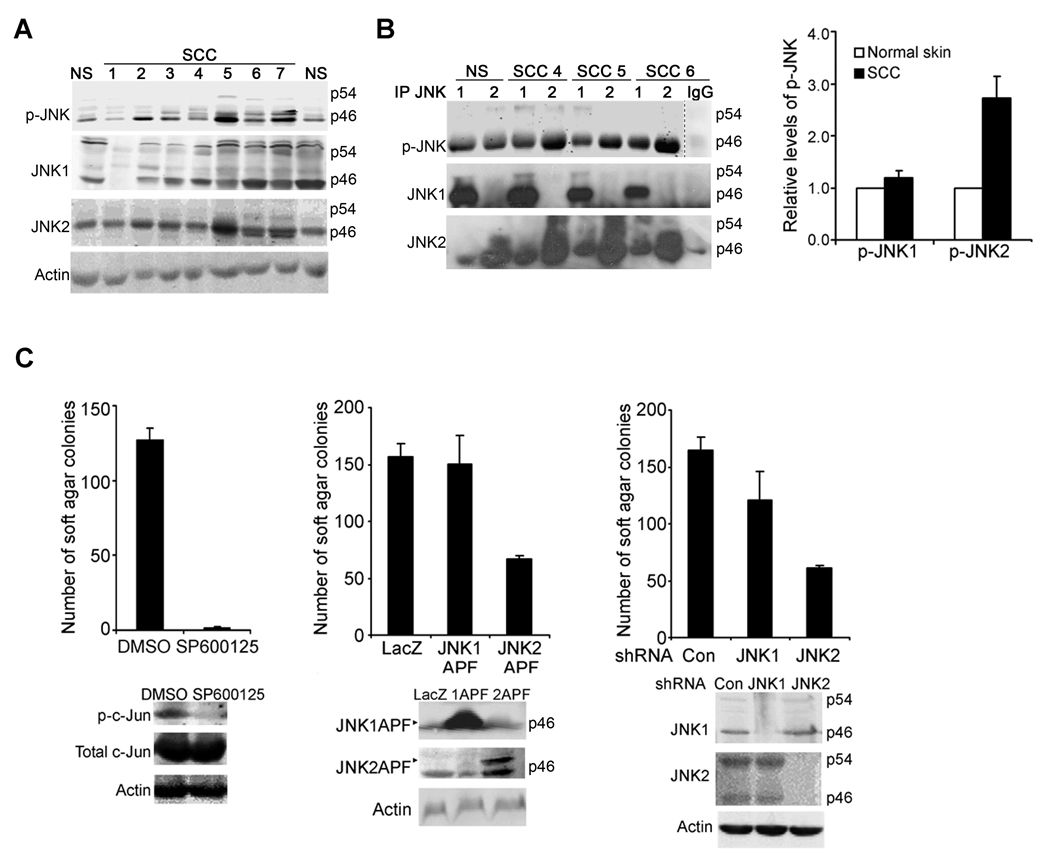
Our previous studies have shown that a majority of spontaneous human SCC samples display positive immuno-staining for phosphorylated JNK (p-JNK) (11). In this study, we performed immunoblotting, and found that p-JNK was increased in about 70% SCC samples (n=15) as compared to the normal skin, whereas the total JNK1 and JNK2 were expressed at varying levels in different SCC samples (Fig. 1A, Supplementary Fig. 1A). Antibodies specific for p-JNK1 or p-JNK2 are not available because the JNK activation motif is highly conserved. In order to determine whether JNK1 and JNK2 are differentially activated in SCC, we performed immuno-precipitation (IP) with antibodies targeting JNK1 or JNK2 and then immunoblotting for p-JNK. The validity of this approach was confirmed by the positive detection of an increased level of p-JNK pulled down specifically by the JNK1-antibody from protein lysates of UV-irradiated human keratinocytes (Supplementary Fig. 1B), a finding consistent with a previous report that JNK1 is specifically phosphorylated in response to UV-treatment (20, 31). Using this method, we found that the JNK1-antibody pulled down similar levels of p-JNK from protein lysates of normal skin and SCC tissues. In contrast, JNK2-antibody pulled down an average of 2.5-fold higher levels of p-JNK from SCC samples than the normal skin (Fig. 1B, Supplementary Fig. 1C). Although cross-reactivity was observed between JNK1- and JNK2-antibodies when used for IP with human tissue samples, the increased detection of p-JNK in SCC samples following IP with JNK2-antibody was highly significant. Thus, these data indicate that JNK2 activation is increased in human SCC.
Fig. 1. JNK2 is activated in human SCC and required for the tumorigenesis of A431 cells.
(A) Immunoblotting for p-JNK, JNK1, JNK2 with protein lysates from normal human skin (NS) and SCC samples. Please note: JNK1, JNK2 and p-JNK displayed varying intensities at 46 and 54 KD MW positions among different tissue samples, but the 46 KD bands were stronger than the 54 KD bands in most samples. (B) Immuno-precipitation with antibodies against JNK1, JNK2 or GM-CSF for IgG control followed by immunoblotting for p-JNK, JNK1 and JNK2. Representative images for NS and 3 SCCs were shown and additional samples were presented in (Supplementary Fig. 1); Data shown in the graph represent averages of the relative optical densitometry of the p-JNK band of 9 SCC samples examined ± standard deviations. (C) A431 soft agar colony formation in response to JNK inhibition by 10 µM SP600125, expression of JNK1(APF) or JNK2(APF), or shRNA-mediated gene silencing of JNK1 or JNK2. Data represent average numbers of colonies from triplicate experiments ± standard deviations. JNK kinase inhibition, expression of the JNK mutants and targeted gene silencing were confirmed by immunoblotting for p-c-Jun, total c-Jun, JNK1 or JNK2 with Actin for loading control, as shown below each graph.
We next asked whether JNK is directly involved in tumorigenesis. To address this question, we used pharmacological and genetic approaches to achieve targeted inhibition of JNK1 and/or JNK2 in A431, a spontaneous SCC cell line, and then performed soft agar colony formation, an assay commonly used to assess anchorage-independent growth of cancer cells. Pharmacological inhibition of JNK kinase activity with SP600125 inhibited A431 cells in soft agar colony formation (Fig. 1C). Targeted inhibition of JNK1 or JNK2 was next performed by expression of their corresponding kinase-deficient mutants, in which the JNK signature motifs ThrProTyr (TPY) have been mutated to AlaProPhe (APF) (26) (32). We found that of JNK2(APF), but not JNK1(APF), significantly reduced the number of colonies (Fig. 1C). In line with this, retroviral shRNA-mediated gene silencing of JNK2 markedly decreased the number of colonies, whereas gene silencing of JNK1 induced a minimal effect (Fig. 1C). These findings indicate that JNK2 is specifically required for the anchorage-independent growth of A431 cancer cells.
Co-activation of JNK2 and Ras transforms primary human epidermal cells into malignancy
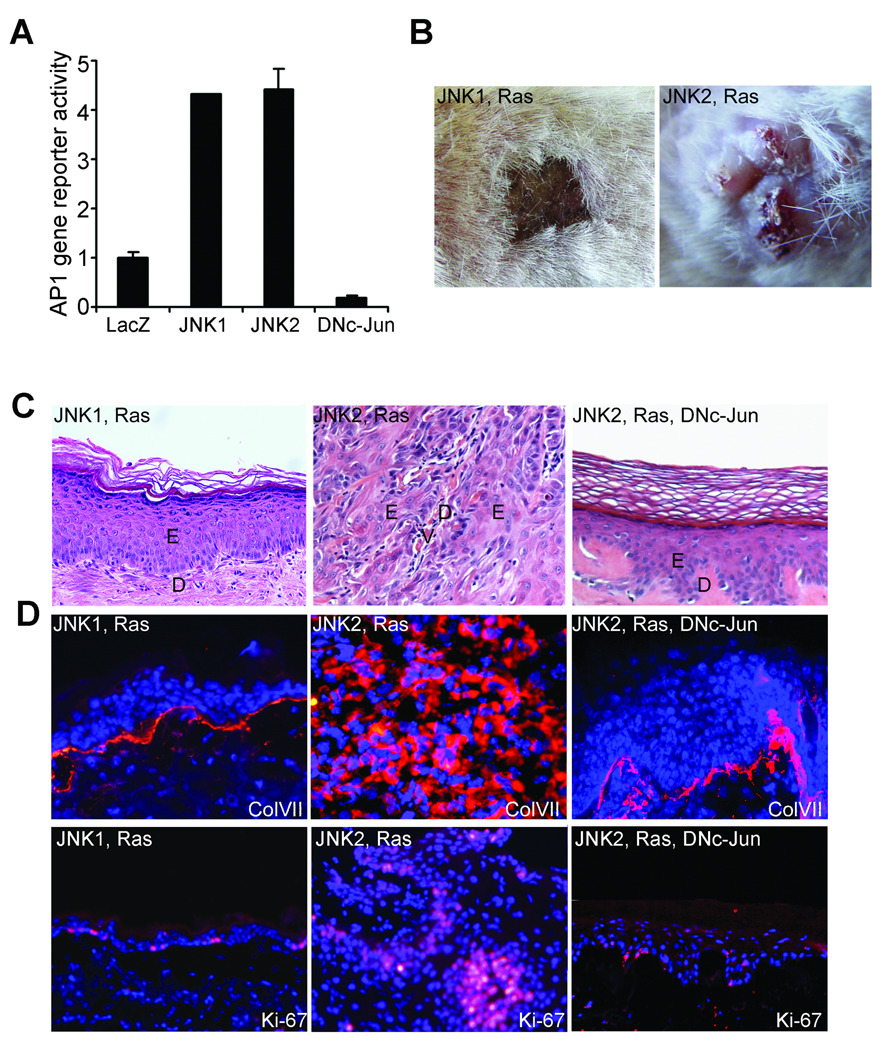
To further examine whether JNK2 plays a dominant role in skin cancer, we expressed constitutively active recombinant proteins of JNK1 or JNK2 along with the oncogenic Ha-Ras (Gly12Val) mutant in primary human keratinocytes through retroviral transduction. Functional activities of the constitutively active JNK proteins have been characterized previously (25, 26), and were further confirmed using AP1-driven gene reporter assay (Fig. 2A, Supplementary Fig. 2A). Cells transduced for expression of Ras along with JNK1 or JNK2 were seeded onto devitalized human dermis for human skin regeneration on immune-deficient SCID mice. At 6 weeks post grafting, tissues expressing JNK2 and Ras displayed vertically elevated and crusty appearance resembling clinical features of human SCC (Fig. 2B). Tissue invasion is apparent as indicated by the absence of a clear delineation between epidermis and dermis and the aberrant detection of collagen VII (ColVII), a protein component of the basement membrane structure (Fig. 2C–D). In line with the invasive growth phenotype, the epidermal tissue was hyper proliferative as indicated by the increased number of cells positive in Ki-67, a cell proliferation marker (Fig. 2D). In contrast, tissues expressing JNK1 and Ras displayed normal clinical and histological features of human skin, including the delineated staining of ColVII and the basal layer-limited cell proliferation (Fig. 2B–D). These data indicate that JNK2, but not JNK1, is sufficient to transform primary human epidermal cells into malignancy when coupled with oncogenic Ras.
Fig. 2. JNK2 promotes Ras-driven epidermal neoplasia.
(A) AP-1 gene reporter assay. Note: Expressions of the recombinant JNK proteins were confirmed by immunoblotting (Supplementary Fig. 2A). (B–F) Human skin tissues regenerated with primary human keratinocytes transduced to express genes denoted on top of each image: (B) clinical appearance, (C) histological analysis by H&E staining [E, epidermis; D, Dermis and V, blood vessel] and (D) immuno-fluorescent staining for Collagen VII (ColVII) and Ki-67 [orange]; nuclei [blue]. (C–D) Magnification (100×). Please note: no metastasis was observed during the time frame of these experiments.
AP-1 family transcription factors are the major downstream targets of JNK. To determine whether AP-1 function is required for JNK2-induced tumor growth, we examined the effect of expression of an N-terminal deletion mutant of c-Jun (DNc-Jun), which functions as a dominant negative protein to AP-1 function (24). We found that skin tissues generated with primary human keratinocytes expressing DNc-Jun along with JNK2 and Ras displayed normal histological appearances comparable to those of control tissues (Fig. 2C–D). These data indicate that JNK2-induced tumor growth is dependent on intact AP-1 function.
JNK2 prevents Ras-induced cell senescence
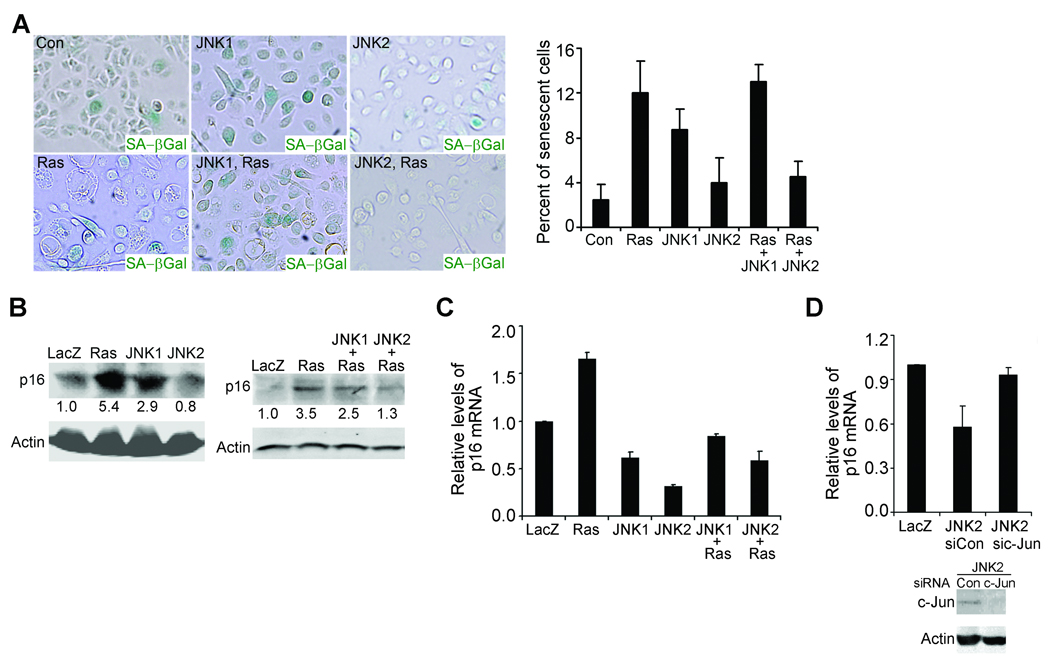
Oncogenes, including Ras, often induce cell senescence and/or cell growth arrest of primary human cells in the absence of other genetic alterations (33, 34). When expressed in human keratinocytes (Supplementary Fig. 2B), Ras induced up to a 3-fold increase in the number of cells positive in acidic β-galactosidase, a marker associated with senescent cells (Fig. 3A). Expression of JNK2 or siRNA-mediated gene silencing of p16, a cell cycle inhibitor commonly associated with cell senescence (35), prevented Ras-induction of senescence (Fig. 3A and Supplementary Fig. 3A). In contrast, expression of JNK1 increased the number of β-galactosidase positive cells by itself, and failed to inhibit Ras-induced cell senescence (Fig. 3A). These data indicate that JNK2, but not JNK1, is able to prevent Ras-induced cell senescence. Immunoblotting and quantitative RT-PCR revealed that Ras increased whereas JNK2 inhibited Ras-induction of p16 at both protein and mRNA levels (Fig. 3B–C). We predicted that JNK2 acted through AP-1 family transcription factor to regulate p16. Consistent with this idea, siRNA-mediated gene silencing of c-Jun, a predominant AP-1 subunit, diminished JNK2-mediated downregulation of p16 (Fig. 3D). Interestingly, albeit less efficient than JNK2, JNK1 also downregulated p16 mRNA level (Fig. 3C). In contrast to JNK2, JNK1 significantly increased p16 protein level (Fig. 3B), which suggests that JNK1 upregulates p16 at a post-transcriptional level. In line with the in vitro data, tissues expressing Ras and JNK2 expressed much lower levels of p16 than those expressing LacZ or Ras and JNK1 (Supplementary Fig. 3B). Taken together, these findings indicate that JNK2 is able to prevent cell senescence by downregulating p16 mRNA and protein levels in an AP-1-dependent manner.
Fig. 3. JNK2 blocks Ras-induced cell senescence.
(A) Staining for senescence-associated acidic β-galactosidase [β-Gal(+) cells, blue]. Shown on the graph were the average percentages of β-Gal(+) cells ± standard deviations. (B) Semi-quantitative immunoblotting for p16 with protein lysates from transduced primary human keratinocytes. Relative optical densitometry is shown below each p16 band. (C–D) Quantitative RT-PCR for p16 with total RNA isolated from cells transduced as in (A) with or without additional treatment with siRNA oligonucleotides targeting c-Jun (sic-Jun) or non-silencing control (sicon). Gene silencing of c-Jun was confirmed by immunoblotting for c-Jun with Actin control, as shown below the graph. Data in the graphs represent averages of triplicate experiments ± standard deviations. Note: Expressions of the exogenous proteins in (A–D) were confirmed by immunoblotting (Supplementary Fig. 2B).
JNK prevents Ras-induced NF-κB activation
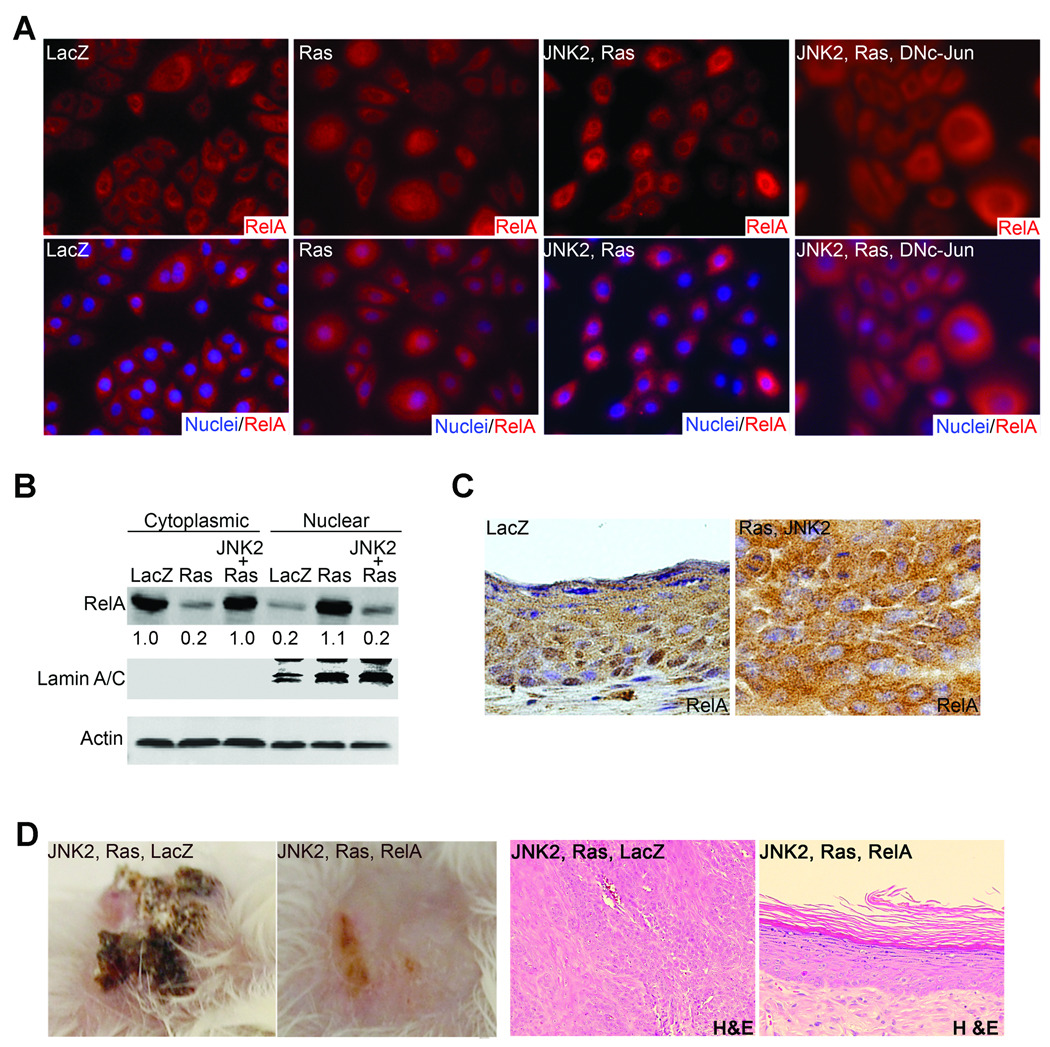
NF-κB has been recognized as a major mediator in Ras-induced epidermal cell growth inhibition and senescence (21). We thus asked whether JNK2 would be able to suppress Ras-induced NF-κB activation. To address this, we examined the sub-cellular localization of RelA, a predominant NF-κB subunit which is well-known to translocate from cytoplasm to nucleus upon activation (36). As expected, RelA was mostly localized in the cytoplasm in control cells as demonstrated by immuno-fluorescence staining. In contrast, strong nuclear detection of RelA was seen in cells expressing Ras alone or Ras along with JNK2 and DNc-Jun but not in those expressing Ras along with JNK2 (Fig. 4A). Quantitative immunoblotting with nuclear protein extracts revealed that Ras induced up to a 4-fold increase of nuclear RelA, while expression of JNK2 diminished this increase (Fig. 4B). Of note, neither Ras nor JNK proteins changed the expression levels of the total RelA (Supplementary Fig. 4A). Similar to the findings obtained in the in vitro setting, tumor tissues expressing JNK2 and Ras displayed strong cytoplasmic RelA-staining (Fig. 4C), whereas LacZ control tissues had strong nuclei staining especially in the super basal layer where NF-κB has been associated with growth arrest during terminal differentiation (37). These findings indicate that JNK2 inhibits Ras-induced NF-κB activation, and suggest that JNK2 activation might provide a mechanism for the cytoplasmic localization of RelA in human SCC as observed in our previous studies (21).
Fig. 4. JNK2 blocks Ras-induced NF-κB activation.
(A) Immuno-fluorescence staining of RelA in human keratinocytes expressing genes noted on top of each image. [RelA, orange; nuclei, blue]. Magnification (120×). (B) Semi-quantitative immunoblotting with cytoplasmic and nuclear protein extracts for RelA with Actin and nuclear marker Lamin A/C for controls. Relative optical densitometry was shown below each RelA band. (C) Immuno-staining of RelA on regenerated human skin tissues expressing LacZ or JNK2 and Ras. [RelA, brown; nuclei, blue]. (D) Clinical and histological appearances [magnification (50×)] of regenerated human skin grafts. Note: Expressions of the exogenous proteins in (A–D) were confirmed by immunoblotting (Supplementary Fig. 4A).
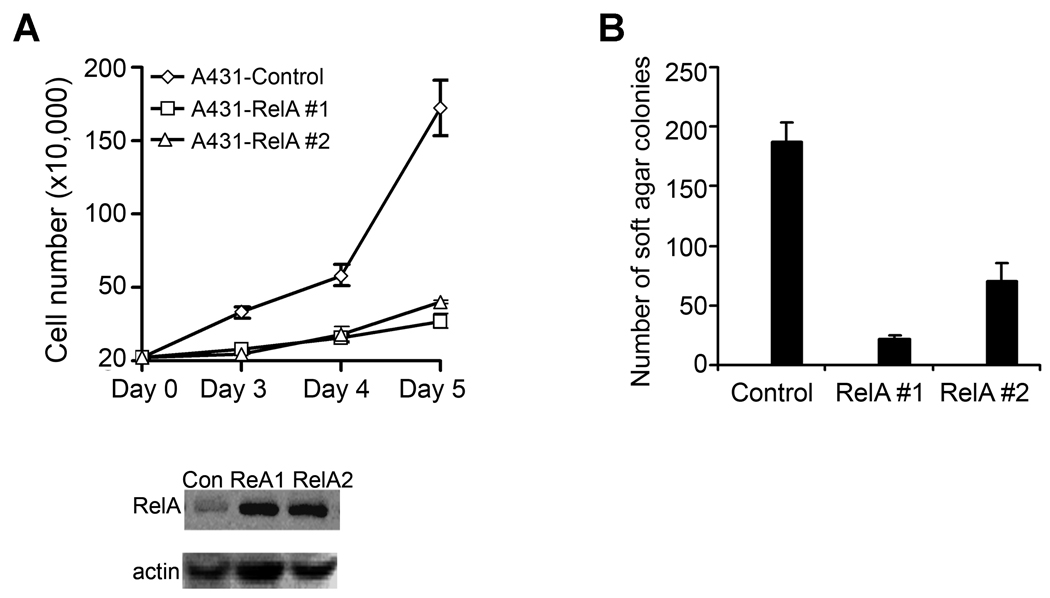
To confirm the functional significance of JNK2-mediated NF-κB blockade, we expressed RelA along with JNK2 and Ras in primary human keratinocytes through sequential retroviral transduction, and then used these cells for skin regeneration on immune-deficient mice. Increased expression of RelA inhibited tumorigenesis driven by Ras and JNK2 as demonstrated by the normal appearance of both clinical and histological features of the regenerated human skin tissue (Fig. 4D). This result indicates that JNK2-inhibition of NF-κB is essential for the Ras-driven epidermal tumorigenesis. To test whether the inhibitory effect of NF-κB is limited to the Ras-driven tumorigenesis, we expressed RelA in A431, which harbors a genetic deletion of one p53 allele but has no known abnormalities in the Ras signaling pathway (38). We found that exogenous RelA expression reduced cell growth rate of A431 cells (Fig. 5A). Moreover, when assessed by soft agar colony formation, RelA induced over 10-fold reduction in the number of A431 colonies (Fig. 5B). These findings indicate that NF-κB tumor suppressor function is relevant not only to the Ras-driven epidermal tumorigenesis but also to those induced by other genetic changes, such as p53 haploinsufficiency.
Fig. 5. NF-κB RelA decreases cell growth rate and soft agar colony formation of A431 cells.
(A) Cell growth analysis. Shown on the graph were average growth rates of two representative colonies of A431 cells transfected to express RelA along with that of control cells. RelA expression was confirmed by immunoblotting, as shown below the graph. (B) Soft agar colony assay of A431 cells transfected as above. Average numbers of colonies ± standard deviations from triplicate experiments were shown on the graph.
JNK is essential for Ras-induced glycolysis
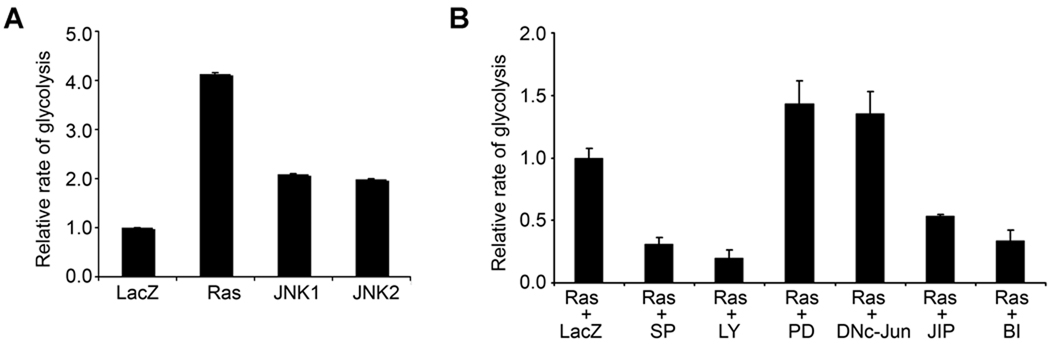
It is intriguing to observe that JNK2 but not JNK1 is able to block oncogenic Ras-induction of epidermal growth arrest and senescence. We asked whether JNK1 and JNK2 affected other hallmark processes of cancer. Among these is glycolysis, namely the Warburg effect or the energy producing process preferentially used by cancer cells in both aerobic and anaerobic conditions (39, 40). Glycolysis confers a cell survival advantage by providing not only energy but also precursors for DNA and lipid synthesis for the rapidly dividing cancer cells (41). Most importantly, increased glycolysis has been recently indentified as a consequence of Ras activation in rat fibroblasts (42). Thus, we performed glycolytic analysis in human keratinocytes. We found that Ras induced up to a 4-fold increase in the rate of glycolysis as compared to the LacZ control, whereas JNK1 and JNK2 each induced about a 2-fold increase (Fig. 6A). JNK inhibition with SP600125, BI78D3 or TAT-JIP and PI3K inhibition with LY294002 each abolished Ras-induction of glycolysis (Fig. 6B). In contrast, MEK inhibition with PD98059 or AP-1 inhibition with DN-c-Jun did not induce any inhibitory effect on this process (Fig. 6B). These data indicate that JNK, along with PI3K, are essential for Ras-induced glycolysis. Overall, our findings suggest that JNK2 blocks Ras-induced processes that are inhibitory to tumorigenesis while promoting other oncogenic effects of Ras.
Fig. 6. JNK is essential for Ras-induction of glycolysis.
(A) Ras increases glycolysis in primary human keratinocytes. Cells transduced for expression of LacZ, Ras, JNK1 or JNK2 were used for glycolytic analysis. (B) Ras-induction of glycolysis is dependent on PI3K and JNK. Cells transduced to express Ras were treated with DMSO control or pharmacological inhibitors targeting JNK (SP: SP600125; BI: BI783D or JIP: TAT-JIP), PI3K (LY: LY294002) or MEK (PD: PD98059), or transduced for expression of DNc-Jun prior to glycolytic analysis. Note: Protein expression and kinase inhibition were confirmed by immunoblotting (Supplementary Fig. 4).
DISCUSSION
The conversion of normal cells to malignancies typically involves multiple genetic or epigenetic perturbations, and requires collaborative actions of multiple signaling pathways leading to cell growth autonomy and evasion of cell apoptosis and senescence. In response to a single mitogenic stimulus, normal human cells often initiate cell growth arrest and programmed cell death, which presumably serve as frontline defenses against cancer. Fitting into this scenario is the response of human epidermal cells to oncogenic Ras which acts in part through NF-κB to induce cell growth arrest and senescence (21). Thus, the oncogenic activity of JNK2 is manifested by downregulation of cell cycle inhibitor p16 and inhibition of NF-κB activation, while permitting many other Ras-induced molecular changes that support cancer development, including increased levels of EGF and VEGF growth factors and glycolysis (11).
p16 loss-of-function occurs in over 50% of SCC samples due to either genetic or epigenetic changes or transcriptional deregulations (43). Our findings suggest that JNK2-mediated downregulation of p16 might be clinically relevant in human SCC. Interestingly, it has been recently reported that p16 physically interacts with JNK1 and JNK3, and inhibits their kinase activity towards c-Jun in various cell lines (44). In line with these reports, we found that JNK1 was less efficient than JNK2 in inducing c-Jun activation in human keratinocyte (Supplementary Fig. 4A). Of further interest, p16 mRNA level was decreased while its protein level was increased by JNK1, indicating that JNK1 increases p16 at a posttranscriptional level. It will be interesting to examine whether JNK1-p16 interaction enhances p16 protein stability while inhibiting JNK1 kinases activity.
With regards to the crosstalk between NF-κB and JNK, it has been well-established that NF-κB can suppress JNK pathway (45), less is clear whether JNK affects NF-κB function. We demonstrated that JNK2 suppressed NF-κB activation in a manner that was presumably dependent on AP-1 target genes. Of note, we found that expression of RelA also increased the expression of p16 at both mRNA and protein levels (data not shown), which suggests that Ras acts in part through NF-κB to upregulate p16 and cell senescence. However, sequence analysis of the 5 kb proximal region of the human p16 promoter revealed two responsive elements for AP-1 but none for NF-κB (Accession# NG_007485). It is possible that NF-κB increases p16 via suppression of the JNK/AP-1 or other signaling pathways. Overall, findings to date indicate that JNK2 and NF-κB function antagonistically via cross-talks at multiple molecular levels, and that their functional balance is a critical determinant to cell growth or senescence. Regarding glycolysis, it has been shown that Ras acts through PI3K-Akt signaling pathway to increase glucose uptake and to activate glycolytic enzymes in rat fibroblasts (42). Similarly, a recent study has indicated a direct role for JNK1 in activating phosphofurctokinase-1 in mouse embryonic fibroblasts (46). It is yet to be determined which glycolytic enzymes are directly regulated by JNK and/or PI3K in human keratinocytes.
The positive role for JNK2 in human epidermal neoplasia is consistent with the mouse data demonstrating that Jnk2−/− mice are resistant to skin carcinogenesis (7, 19). However, we observed species-specific responses at two different levels. First, JNK1 was implicated as a tumor suppressor in mouse (19); in human SCC, JNK1 loss-of-function induced a minimal effect on the tumorigenesis of A431 cells. We speculate that the insufficiency of JNK1 in promoting Ras-driven human epidermal neoplasia is due to the weaker effects of JNK1 on p16 and NF-κB than those of JNK2. Nevertheless, it is worth noting that cells expressing JNK1 and Ras were able to produce viable skin grafts as opposed to the complete graft failure of cells expressing Ras alone (22). This suggests that JNK1 is able to confer a survival and growth advantage to cells expressing Ras, albeit not sufficient to promote invasive tumor growth. Second, the opposite responses between Jnk1−/− and Jnk2−/− mice were attributed to the lower levels of Erk, Akt and AP-1 activities in Jnk2−/− skin (19). In contrast, human keratinocytes expressing active JNK1 or JNK2 did not show significant differences in Akt or Erk activation (Supplementary Fig. 4A). Interestingly, both JNK1 and JNK2 decreased proteins levels of p53 in a post-translational manner (Supplementary Fig. 5), which is similar to the findings obtained in mouse cells (47). These data indicate that JNK proteins induce differential molecular responses between mouse and human cells. Overall, our findings establish that JNK2 is a predominant oncogene in human epidermal neoplasia, and may represent a potential therapeutic target for human cancer.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by K01AR051470 from NIH/NIAMS to Jennifer Y. Zhang and RO1CA123350 from NIH/NCI to Jeffrey C. Rathmell. We thank Christopher M Counter and Anthony R. Means (Duke University), and Paul A Khavari (Stanford University) for their insightful suggestions, members of Duke Children and neonatal clinics, Jonathan Cook and Stephen Ray in Duke Dermatology for providing surgically discarded skin tissues, as well as Lynn Heasley (Colorado University) for the JNK and Dirk Bohmann (University of Rochester Medical Center) for the c-Jun expression constructs.
REFERENCE
- 1.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 2.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 3.Tournier C, Dong C, Turner TK, Jones SN, Flavell RA, Davis RJ. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 2001;15:1419–1426. doi: 10.1101/gad.888501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borsello T, Forloni G. JNK signalling: a possible target to prevent neurodegeneration. Curr Pharm Des. 2007;13:1875–1886. doi: 10.2174/138161207780858384. [DOI] [PubMed] [Google Scholar]
- 5.Kaneto H, Matsuoka TA, Nakatani Y, Kawamori D, Matsuhisa M, Yamasaki Y. Oxidative stress and the JNK pathway in diabetes. Curr Diabetes Rev. 2005;1:65–72. doi: 10.2174/1573399052952613. [DOI] [PubMed] [Google Scholar]
- 6.Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology (Oxford) 2008;47:409–414. doi: 10.1093/rheumatology/kem297. [DOI] [PubMed] [Google Scholar]
- 7.Chen N, Nomura M, She QB, et al. Suppression of skin tumorigenesis in c-Jun NH(2)-terminal kinase-2-deficient mice. Cancer Res. 2001;61:3908–3912. [PubMed] [Google Scholar]
- 8.Tsuiki H, Tnani M, Okamoto I, et al. Constitutively Active Forms of c-Jun NH2-terminal Kinase Are Expressed in Primary Glial Tumors. Cancer Res. 2003;63:250–255. [PubMed] [Google Scholar]
- 9.Yang YM, Bost F, Charbono W, et al. C-Jun NH(2)-terminal kinase mediates proliferation and tumor growth of human prostate carcinoma. Clin Cancer Res. 2003;9:391–401. [PubMed] [Google Scholar]
- 10.Papachristou DJ, Batistatou A, Sykiotis GP, Varakis I, Papavassiliou AG. Activation of the JNK-AP-1 signal transduction pathway is associated with pathogenesis and progression of human osteosarcomas. Bone. 2003;32:364–371. doi: 10.1016/s8756-3282(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JY, Adams AE, Ridky TW, Tao S, Khavari PA. Tumor necrosis factor receptor 1/c-Jun-NH2-kinase signaling promotes human neoplasia. Cancer Res. 2007;67:3827–3834. doi: 10.1158/0008-5472.CAN-06-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Housman TS, Feldman SR, Williford PM, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003;48:425–429. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 13.Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554–565. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- 14.Bogoyevitch MA. The isoform-specific functions of the c-Jun N-terminal Kinases (JNKs): differences revealed by gene targeting. Bioessays. 2006;28:923–934. doi: 10.1002/bies.20458. [DOI] [PubMed] [Google Scholar]
- 15.Sabapathy K, Jochum W, Hochedlinger K, Chang L, Karin M, Wagner EF. Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech Dev. 1999;89:115–124. doi: 10.1016/s0925-4773(99)00213-0. [DOI] [PubMed] [Google Scholar]
- 16.Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 17.Sabapathy K, Wagner EF. JNK2: a negative regulator of cellular proliferation. Cell Cycle. 2004;3:1520–1523. doi: 10.4161/cc.3.12.1315. [DOI] [PubMed] [Google Scholar]
- 18.Weston CR, Wong A, Hall JP, Goad ME, Flavell RA, Davis RJ. The c-Jun NH2-terminal kinase is essential for epidermal growth factor expression during epidermal morphogenesis. Proc Natl Acad Sci U S A. 2004;101:14114–14119. doi: 10.1073/pnas.0406061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.She Q-B, Chen N, Bode AM, Flavell RA, Dong Z. Deficiency of c-Jun-NH2-terminal Kinase-1 in Mice Enhances Skin Tumor Development by 12-O-Tetradecanoylphorbol-13-Acetate. Cancer Res. 2002;62:1343–1348. [PubMed] [Google Scholar]
- 20.Katagiri C, Negishi K, Hibino T. c-JUN N-terminal kinase-1 (JNK1) but not JNK2 or JNK3 is involved in UV signal transduction in human epidermis. J Dermatol Sci. 2006;43:171–179. doi: 10.1016/j.jdermsci.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Dajee M, Lazarov M, Zhang JY, et al. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–643. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- 22.Mirella Lazarov YK, Cai Ti, Dajee Maya, Tarutani Masahito, Lin Qun, Fang Min, Tao Shiying, Green Cheryl L, Khavari Paul A. CDK4 coexpression with Ras generates malignant human epidermal tumorigenesis. Nature Medicine. 2002;8:1105–1114. doi: 10.1038/nm779. [DOI] [PubMed] [Google Scholar]
- 23.Ortega AD, Sanchez-Arago M, Giner-Sanchez D, Sanchez-Cenizo L, Willers I, Cuezva JM. Glucose avidity of carcinomas. Cancer Lett. 2009;276:125–135. doi: 10.1016/j.canlet.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Treier M, Bohmann D, Mlodzik M. JUN cooperates with the ETS domain protein pointed to induce photoreceptor R7 fate in the Drosophila eye. Cell. 1995;83:753–760. doi: 10.1016/0092-8674(95)90188-4. [DOI] [PubMed] [Google Scholar]
- 25.Han S-Y, Kim S-H, Heasley LE. Differential Gene Regulation by Specific Gain-of-function JNK1 Proteins Expressed in Swiss 3T3 Fibroblasts. J Biol Chem. 2002;277:47167–47174. doi: 10.1074/jbc.M204270200. [DOI] [PubMed] [Google Scholar]
- 26.Derijard B, Hibi M, Wu IH, et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 27.Robbins PB, Lin Q, Goodnough JB, Tian H, Chen X, Khavari PA. In vivo restoration of laminin 5 beta 3 expression and function in junctional epidermolysis bullosa. Proc Natl Acad Sci U S A. 2001;98:5193–5198. doi: 10.1073/pnas.091484998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang JY, Tao S, Kimmel R, Khavari PA. CDK4 regulation by TNFR1 and JNK is required for NF-kappaB-mediated epidermal growth control. J Cell Biol. 2005;168:561–566. doi: 10.1083/jcb.200411060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caruccio L, Banerjee R. An efficient method for simultaneous isolation of biologically active transcription factors and DNA. J Immunol Methods. 1999;230:1–10. doi: 10.1016/s0022-1759(99)00100-3. [DOI] [PubMed] [Google Scholar]
- 30.Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J Biol Chem. 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Minemoto Y, Lin A. c-Jun N-terminal protein kinase 1 (JNK1), but not JNK2, is essential for tumor necrosis factor alpha-induced c-Jun kinase activation and apoptosis. Mol Cell Biol. 2004;24:10844–10856. doi: 10.1128/MCB.24.24.10844-10856.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wojtaszek PA, Heasley LE, Siriwardana G, Berl T. Dominant-negative c-Jun NH2-terminal kinase 2 sensitizes renal inner medullary collecting duct cells to hypertonicity-induced lethality independent of organic osmolyte transport. J Biol Chem. 1998;273:800–804. doi: 10.1074/jbc.273.2.800. [DOI] [PubMed] [Google Scholar]
- 33.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 34.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharpless NE. Ink4a/Arf links senescence and aging. Exp Gerontol. 2004;39:1751–1759. doi: 10.1016/j.exger.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 37.Seitz CS, Freiberg RA, Hinata K, Khavari PA. NF-kappaB determines localization and features of cell death in epidermis. J Clin Invest. 2000;105:253–260. doi: 10.1172/JCI7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reiss M, Brash DE, Munoz-Antonia T, et al. Status of the p53 tumor suppressor gene in human squamous carcinoma cell lines. Oncol Res. 1992;4:349–357. [PubMed] [Google Scholar]
- 39.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 40.Matoba S, Kang JG, Patino WD, et al. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 41.Ortega AD, Sanchez-Arago M, Giner-Sanchez D, Sanchez-Cenizo L, Willers I, Cuezva JM. Glucose avidity of carcinomas. Cancer Lett. 2008 doi: 10.1016/j.canlet.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Wallace DC. Mitochondria and cancer: Warburg addressed. Cold Spring Harb Symp Quant Biol. 2005;70:363–374. doi: 10.1101/sqb.2005.70.035. [DOI] [PubMed] [Google Scholar]
- 43.Lang JC, Tobin EJ, Knobloch TJ, et al. Frequent mutation of p16 in squamous cell carcinoma of the head and neck. Laryngoscope. 1998;108:923–928. doi: 10.1097/00005537-199806000-00024. [DOI] [PubMed] [Google Scholar]
- 44.Choi BY, Choi HS, Ko K, et al. The tumor suppressor p16(INK4a) prevents cell transformation through inhibition of c-Jun phosphorylation and AP-1 activity. Nat Struct Mol Biol. 2005;12:699–707. doi: 10.1038/nsmb960. [DOI] [PubMed] [Google Scholar]
- 45.Tang G, Minemoto Y, Dibling B, et al. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 46.Deng H, Yu F, Chen J, Zhao Y, Xiang J, Lin A. Phosphorylation of Bad at Thr-201 by JNK1 promotes glycolysis through activation of phosphofructokinase-1. J Biol Chem. 2008;283:20754–20760. doi: 10.1074/jbc.M800024200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Das M, Jiang F, Sluss HK, et al. Suppression of p53-dependent senescence by the JNK signal transduction pathway. Proc Natl Acad Sci U S A. 2007;104:15759–15764. doi: 10.1073/pnas.0707782104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.