Abstract
As functional magnetic resonance imaging (fMRI) has become a driving force in cognitive neuroscience, it is crucial to understand the neural basis of the fMRI signal. Here, we discuss a novel neurophysiological correlate of the fMRI signal, the slow cortical potential (SCP), which also seems to modulate the power of higher-frequency activity, the more established neurophysiological correlate of the fMRI signal. We further propose a hypothesis for the involvement of the SCP in the emergence of consciousness, and review existing data that lend support to our proposal. This hypothesis, unlike several previous theories of consciousness, is firmly rooted in physiology and as such is entirely amenable to empirical testing.
Introduction
Since its introduction in the early 1990s, functional magnetic resonance imaging (fMRI) has become the most widely used tool in human cognitive neuroscience and has produced a formidable array of brain maps depicting both localization (as in traditional activation studies) and integration (as in more recent functional connectivity studies) of brain activity. Because the fMRI signal measures directly blood oxygenation and only indirectly neuronal activity, an important need for understanding the neural events contributing to the fMRI signal has been widely recognized. Such a need is further stressed by the inconsistencies between several human fMRI and monkey unit physiological studies employing the same tasks [1].
Responding to this need, several studies have compared the fMRI signal (reviewed in Ref. [2]) or its close relatives (including tissue oxygenation [3], blood flow [4] and optical intrinsic signals [5]) with simultaneously recorded electrophysiological signals. The convergent results from these studies suggest that the fMRI signal is contributed predominantly by synaptic activity representing inputs and local processing in an area as measured by local field potentials (LFP) [2–4,6,7]. The spiking activity, though often correlated with both the LFP and the fMRI signal, can be dissociated from the latter two in several conditions, including adaptation [8], drug modulation [9], manipulations of excitatory and inhibitory inputs [4] and a spatial separation between input and output activity [7].
Whereas multiple frequency ranges of the LFP (e.g. 5–30 Hz [10], 20–60 Hz [11,12], ~25–90 Hz [3,5,8,9,13]) have been correlated with the fMRI signal in different conditions, all of these studies have only assessed power modulations of the LFP because only the power of these frequency ranges has a comparable temporal scale to that of the fMRI signal (< 0.5 Hz). Here, we add a new dimension to this evolving story by bringing in the low-frequency end of field potentials (<4 Hz), which, with a temporal scale overlapping that of the fMRI signal, seems to correlate with the fMRI signal in its raw fluctuations. This signal, termed the ‘slow cortical potential’ (SCP) by us and others [14–16], seems optimally positioned for carrying out large-scale information integration in the brain. Because conscious experience* is always a unitary and undivided whole [17,18], segregated information processing in the brain cannot contribute to the conscious awareness of ‘I’. Hence, we propose that the SCP might contribute directly to the emergence of consciousness and review existing empirical evidence supporting this idea. As the current hypothesis is based on a well-defined, well-characterized physiological process, it is entirely amenable to empirical testing.
Evidence for a relationship between the SCP and the fMRI signal
The SCP is the slow end (mainly < 1 Hz, can extend up to ~4 Hz) of the field potential that can be recorded using either depth [19,20] or surface [15,21] electrodes (Box 1). Negative shift in surface-recorded SCP indexes increased cortical excitability (for detailed physiology please see the following section). Because the SCP frequency range is subject to artifacts due to sweating (in scalp-electroencephalography [EEG] recordings), movement and electrode drift (if polarizable electrodes are used), it has been eliminated in most animal physiology and human EEG studies by online high-pass filtering. This is unfortunate because, as was recognized in the 1970s, ‘If DC [i.e. direct-current] recording is used, virtually every stimulus-bound cortical activity is seen to be accompanied by a change in cortical steady potentials’ [19]. As a result of this methodological neglect, studies on the relationship between SCP and the fMRI signal are scarce. Nonetheless, despite the limited data available, a correlation between the SCP and the fMRI signal, no less intimate than that between higher-frequency (>5 Hz) LFP power and fMRI signal, can be observed [15,16,22].
Box 1. Is the SCP an oscillation?
The SCP frequency band has also been referred to as ‘infraslow oscillations’ [37], but is the SCP really an oscillation? EEG can be classified in three distinct groups [1]: rhythmic, arrhythmic and dysrhythmic. The first two appear in normal subjects and refer to waves of approximately constant frequency and no stable rhythms, respectively. The latter refers to pathological rhythms in patient groups. Rhythmic EEG is further subdivided into frequency bands known as δ, θ, α, β and γ, etc. The SCP frequency range does not normally contain any true rhythmic activity, except the ‘up-and-down states’ (also called the ‘slow oscillation’ by its discoverer [71]) that occurs during deep sleep (~0.8 Hz). The ‘up-and-down states’ is a distinct phenomenon that can be easily differentiated from the SCP (for detailed discussions see supplementary materials in He et al. [15]). Therefore SCP is a fluctuation rather than oscillation [72] (B.J. He et al., unpublished). The confusion between fluctuations and oscillations, or, arrhythmic and rhythmic activities, is quite common. This is largely because time-frequency analyses widely adopted create artificial rhythmic signals. However, as pointed out by T.H. Bullock [48], ‘Most of the time in most animals there is little evidence of really rhythmic oscillators in the ongoing cerebral activity, let alone that rhythms account for much of the total energy’. We here avoid using terms such as ‘delta’ or ‘infra-delta’ to describe the SCP because these terms have connotations of oscillations that are not present in an arrhythmic signal.
Investigations of the relationship between LFP power and the fMRI signal have usually showed one of the following: (i) covariation of simultaneously recorded LFP power and the fMRI signal during a task or electrical stimulation [3,5,8,9,11,12], (ii) covariation of simultaneously recorded spontaneous LFP power and the fMRI signal [23] and (iii), similar correlation patterns in the spontaneous fluctuations of LFP power and the fMRI signal measured separately [15,24,25].
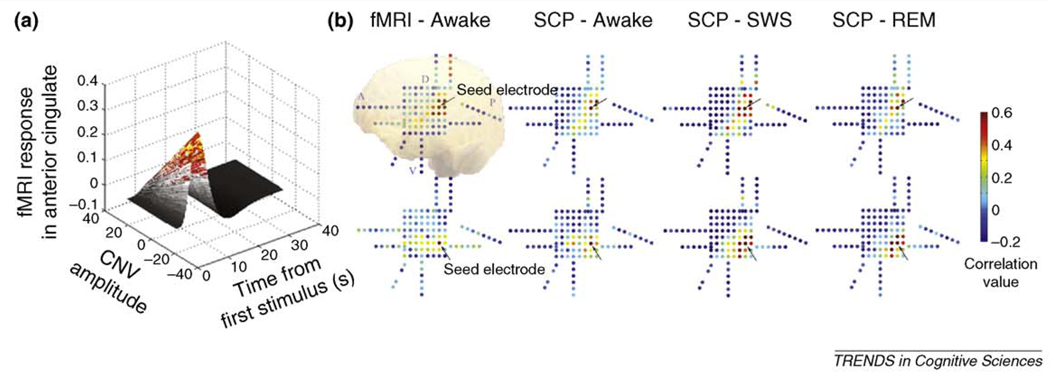
To our knowledge, the only available data that demonstrate covariation of simultaneously recorded SCP and the fMRI signal during task stimulation [akin to aforementioned type (i)] has been provided by Nagai and colleagues [22] using simultaneously recorded EEG and fMRI. These authors found a trial-by-trial correlation between the amplitude of a negative SCP response indexing expectancy (‘contingent negative variation’ [CNV]) and the fMRI signal amplitude in anterior cingulate cortex (Figure 1a). The anterior cingulate has previously been determined as a generator region of CNV [22]. Evidence for SCP–fMRI correlation of the aforementioned type (ii) is provided by Jones et al. [26], who showed that spontaneously fluctuating total hemoglobin concentration (a signal tightly linked to the fMRI signal) and low-pass filtered LFP (i.e. depth recorded SCP) are temporally correlated. Data of the aforementioned type (iii) is provided by He et al. using invasive EEG (i.e. electrocorticography, ECoG) and fMRI in neuro-surgical patients [15]. It was shown that large-scale (2–10 cm on cortical surface) correlation patterns in the spontaneous SCP and fMRI signals were similar (Figure 1b). This finding has since been extended to inter-hemispheric correlations as well (B.J. He et al.,unpublished). Taken together, all three types of evidence for the correlation between LFP power and the fMRI signal are also available for a correlation between SCP and the fMRI signal.
Figure 1. Evidence for a correlation between the slow cortical potential (SCP) and the fMRI signal.
(a) Simultaneous fMRI and EEG was used to identify fMRI signal activation correlated with trial-by-trial measurement of contingent negative variation (CNV) amplitude. CNV is a negative slow potential that relates to the anticipation of a stimulus and it is maximal over frontal midline electrodes. Trial-by-trial covariation between CNV amplitude and fMRI signal time course in anterior cingulate cortex is shown in a 3D plot. Adapted with permission, from Nagai et al. [22] (b) Correlation patterns in the spontaneous fMRI signals and spontaneous SCPs are similar. A group of 5 patients with intractable epilepsy underwent approximately a week of continuous video-monitored electrocorticography (ECoG) for the purpose of determining the epileptic focus before surgical resection. Artifact-free, spontaneous ECoG data were collected from three arousal states: wakefulness, slow-wave sleep (SWS) and rapid-eye-movement (REM) sleep, and then low-pass filtered at <0.5 Hz to yield spontaneous SCPs. In addition, patients underwent a session of resting-state fMRI before or after surgical intervention. SCP correlation maps were obtained by computing Pearson correlation coefficients between a seed electrode (arrow) and all other electrodes. For corresponding fMRI correlation maps, the fMRI signal was averaged across a group of voxels centered at each electrode, and correlation coefficients were computed between the fMRI signal associated with the seed electrode and that from all other electrodes. Representative maps from one patient are shown. A 2D representation of the electrode grid is shown with each dot representing one electrode. Color represents the correlation value between each electrode and the seed electrode (arrow). Maps in the top row seed at a same electrode, those in the bottom row seed at another electrode 2 cm apart. Note that correlation maps with the same seed electrode are similar regardless of whether the fMRI signal or the SCP was used in computing the map. Abbreviations: A, anterior; D, dorsal; P, posterior; V, ventral. Adapted, with permission, from He et al. [15]
Beyond these approaches, there is an extensive literature showing similar modulation patterns of the SCP and the fMRI signal in a wide range of cognitive tasks [14,16,21]. For example, visual working memory tasks elicit a negative-going slow potential over the parietal cortex, the amplitude of which scales with the load of working memory [27]. This pattern is very similar to that observed for the fMRI signal in posterior parietal cortex during the same task [28].
In summary, convergent results suggest that the SCP has a close correspondence to the fMRI signal in different experimental conditions. Like many advances in science, the relationship between SCP and fMRI signal is not without prescient conjecture. In 1975, H.W. Shipton wrote: ‘the work of Cooper, which showed slow rhythmic changes in brain pO2 and in blood flow (e.g. [29]), is of interest in the context of slow [cortical] potential change’ [30]. Next, we consider the physiological mechanisms underlying the SCP.
The physiological basis of the SCP
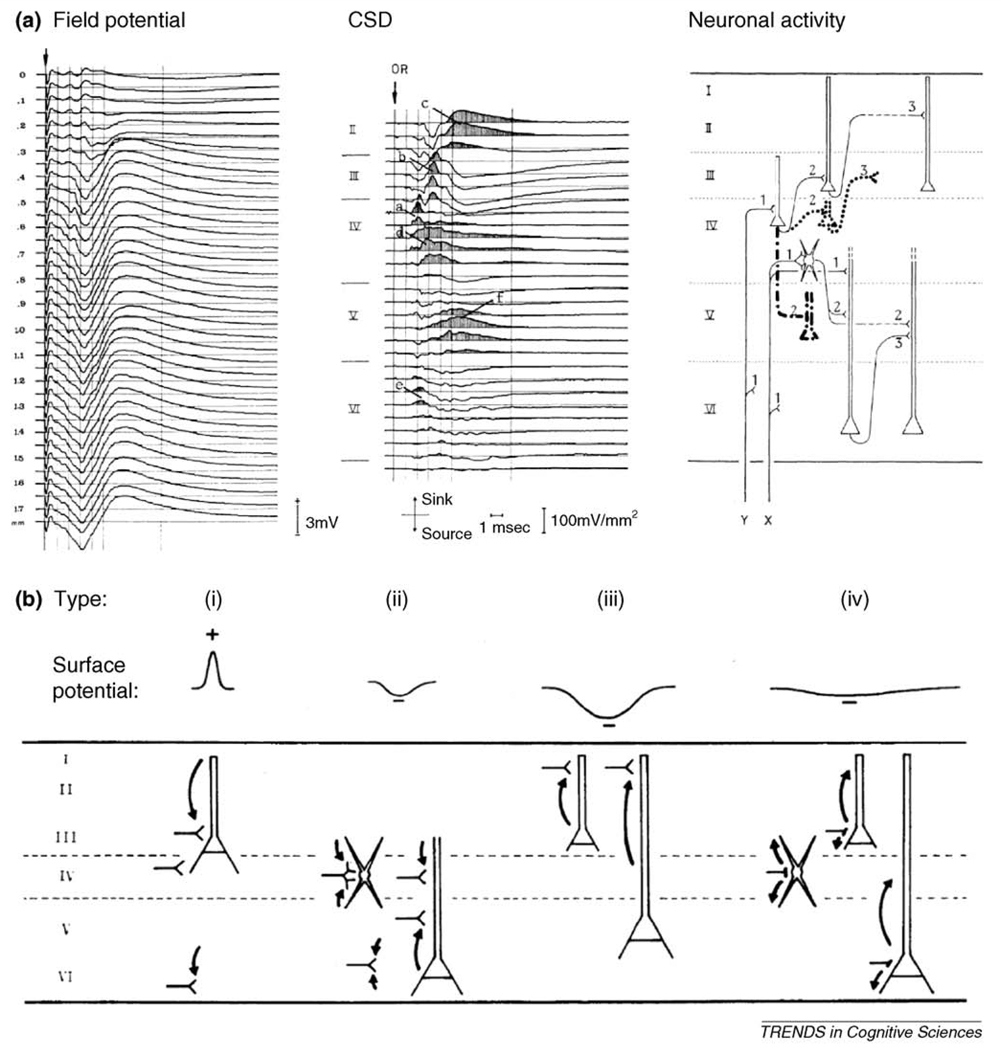
Simultaneous recordings of surface potentials, field potentials in different cortical layers and intracellular membrane potentials have clearly demonstrated that synaptic activities at apical dendrites in superficial layers are the main factor contributing to the SCP. Specifically, long-lasting excitatory postsynaptic potentials (EPSPs) at these apical dendrites underlie negative-going surface-recorded SCPs [14,19,31]. As an example, we consider the effect of visual stimulation in V1 – a standard model for the investigation of fMRI-electrophysiology correspondence [3,5,8,9,11,12]. Specific thalamic inputs terminate first on the soma of layer-IV stellate cells and layer-III pyramidal cells, and then follow one of two pathways to depolarize the apical dendrites of superficial- or deep-layer pyramidal cells [31] (Figure 2a). Given the geometry of cortical fields, the earlier processes – excitations of pyramidal cells at their soma – produce positive-going surface potentials [Figure 2b (i)]. The later processes, excitations of pyramidal cells at their apical dendrites, produce surface negative potentials [Figure 2b (ii) and (iii)]. However, EPSPs at apical dendrites of deep layer pyramidal cells create closed fields and thus have rather small influence on surface potentials [Figure 2b (ii)]. By contrast, depolarizations of superficial layer apical dendrites contribute greatly to negative SCPs (Figure 2b iii). The contribution of inhibitory interneurons to SCP or field potentials in general is also small because of the low amplitude of membrane current flow during inhibitory activity and a lack of laminar specificity [Figure 2b (iv)] [14,31]. In summary, the later component of sensory evoked potentials in EEG or ECoG recordings – a negative slow-potential shift – is primarily due to long-lasting depolarizations of superficial layer apical dendrites.
Figure 2. The physiological basis of the SCP.
(a) Left: field potential in primary visual cortex of the cat evoked by electrical stimulation of optical radiation. Each trace is the average of 20 responses. Distance between adjacent recordings is 50 µm. Middle: current source-density (CSD) distribution obtained from the potential profile on the left. Sinks, corresponding to active EPSPs, are shaded. Cortical laminae are indicated. Sinks a, b, and c reflect mono-, di-, and trisynaptic Y-type activity as shown in the right panel; sinks d and f reflect mono-, di-, and trisynaptic X-type activity shown in the right panel; sink e reflects Y-type and X-type monosynaptic activity. Sinks a, b, and e contribute to type i activity in (b); sinks d and f contribute to type ii activity in (b); sink c contributes to type iii activity in (b). Right: schematic diagram of successive intracortical excitatory relay stations as well as cell types involved. Long-range feedback connections and nonspecific thalamic inputs are not depicted. (b) Schematic diagram of 4 main types of cortical activities and their reflection in surface potential (recorded by ECoG or EEG). (i) Depolarization of pyramidal cells at their deeper extremities, which generates a surface-positive potential deflection. (ii) Depolarization of deep-layer pyramidal cells at their apical dendrites or of stellate cells. This type of activity generates a sink in the middle layers and a surface-negative potential deflection. But because of the closed-field arrangement of CSD components, its contribution to surface potential is rather small. (iii) Depolarization of superficial layer pyramidal cells at their apical dendrites. This is the main contributor to long-lasting surface-negative potentials. This type of activity involves long-distance connections and depends greatly on the general state of cortical excitability. (iv) Inhibitory activity does not usually cause significant CSD contributions because of the low amplitude of membrane currents involved and a general lack of lamina specificity. Adapted, with permission, from Mitzdorf [31].
Other than activations by specific thalamic inputs described earlier, the superficial layers are also where long-range intracortical and cortico-cortical connections preferentially terminate [31–33]. First, only in superficial layers do pyramidal cells make extensive horizontal arborizations [33]. Thus, EPSPs in superficial layers spread over a considerable spatial extent and manifest themselves as ‘depolarization fields’ (~several mm2) in optical imaging recordings [34]. Second, long-range inter-areal feedback connections also terminate mainly in superficial layers. Hence, it is not surprising that the SCP and the correlated fMRI signal reveal large-scale brain networks in their spontaneous fluctuations [15]. Moreover, superficial-layer apical dendrites are also the main target of nonspecific thalamic inputs that originate from ‘matrix cells’ spread throughout the thalamus [35]. Interestingly, the reticular thalamic nucleus, which the nonspecific thalamocortical projections must pass through, exerts a low-pass filter influence that might facilitate the emergence of slow activity [36]. In summary, long-range intracortical and feedback cortico-cortical connections, as well as the nonspecific thalamic inputs, all contribute directly and significantly to the SCP.
Given that negative SCPs index increased cortical excitability, it should not come as a surprise that during the negative shift of spontaneous SCP fluctuations there are increased multi-unit activity [20], increased higher-frequency field potentials [37] (B.J. He et al., unpublished), higher amplitude of short-latency evoked potentials such as P300 [38] and better behavioral performance ([39] and see references in Ref. [14]). The recently observed phase-coding in the delta frequency range [40,41] is probably of the same origin as information carried in the SCP phase. Of particular interest in the current context, since the SCP modulates the power of higher-frequency activities, it might be a more fundamental correlate of the fMRI signal than LFP power is, as implicated in a previous study [15].
The SCP is one important and substantial contributor to the fMRI signal (but not the only one – see Box 2). In addition to advancing our understanding of the fMRI signal and bridging the fMRI field and neurophysiological fields, this observation is also of particular interest in the study of consciousness. For example, fMRI experiments and single-unit recordings often show discordant results during manipulations of consciousness; this disagreement has been most dramatic in V1 [1,10,42,43]. These puzzling results are at least partially illuminated when we bring the SCP and its underlying physiology into the picture. In the remainder of this article, we discuss these data and further propose a specific hypothesis on the involvement of the SCP in engendering conscious awareness.
Box 2. Other contributors to the fMRI signal.
The arguments we have put forward for a close correspondence between the SCP and the fMRI signal do not imply that the SCP is the sole contributor to the fMRI signal. Because excitatory synaptic activities contribute to an increased fMRI signal irrespective of cortical depth (the mechanisms of which are reviewed in details elsewhere [4,7]), the short-latency positive components of sensory evoked potentials, indexing the initial excitations of mid-layer cell bodies, should also contribute to the fMRI signal activation (e.g. [73,74]). Accordingly, fMRI signal increases were found to spread first from layer IV during sensory stimulation in anaesthetized animals [75]. The contribution of inhibitory neurons to the fMRI signal remains undetermined, even though their activity is clearly accompanied by changes in blood flow and glucose metabolism [4,76].
The SCP and consciousness – a neurophysiological hypothesis of consciousness
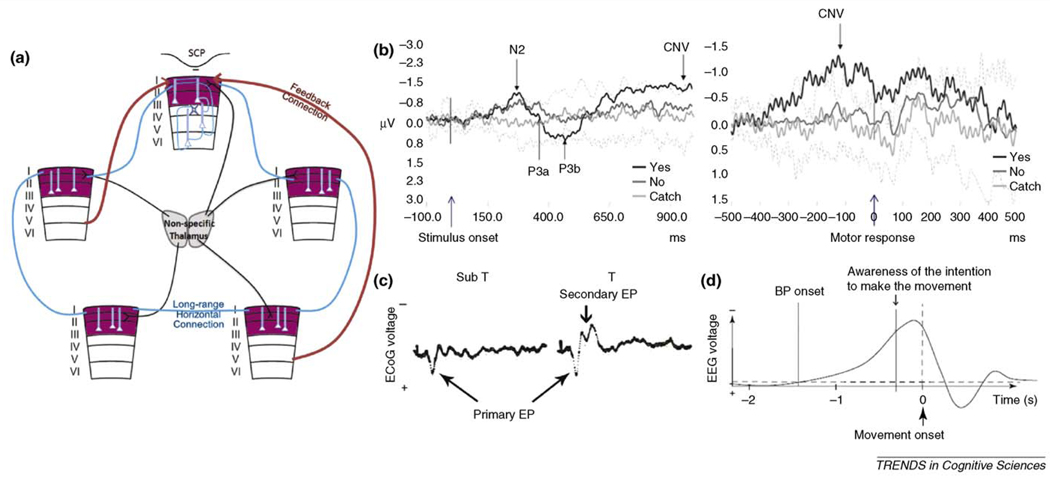
From a theoretical perspective, information has to be integrated to contribute to conscious awareness, for conscious experience is always a unitary and undivided whole [17,18]. We suggest that the SCP might be an optimal neural substrate to carry such information integration across wide cortical areas because (i) its slow time scale allows synchronization across long distance despite axonal conduction delays [15,44–46]; (ii) long-range intracortical and corticocortical connections terminate preferentially in superficial layers and thus contribute significantly to the SCP. Furthermore, for each patch of superficial-layer pyramidal neurons (for definition of ‘patch’, see Figure 1 in Ref. [33]), corresponding deep layer neurons could provide additional information through specialized local processing. These local deep-layer loops might constitute neural substrates for unconscious processes that can affect and be affected by conscious experience (for discussions on the relation between conscious and unconscious processes see Refs [18,47]). A rough schematic depicting our hypothesis is shown in Figure 3a.
Figure 3. SCP and consciousness.
(a) Schematic illustration of our hypothesis. Superficial layers of the cerebral cortex (shown in purple) are the only layers containing extensive long-range horizontal connections (thick blue lines); they are also the main target for nonspecific thalamocortical inputs (black lines) in addition to long-range inter-areal feedback connections (thick red arrows). We propose that long-lasting synaptic activities in superficial layers, manifesting as SCPs in surface recording or low-frequency current source density (CSD) activity in superficial layers, carry large-scale information integration in the brain and contribute directly to conscious awareness. Neuronal circuits in deep layers (thin blue lines) provide specialized local processing that assist superficial-layer computations and send output to subcortical structures. Two specific predictions made by this hypothesis are provided in ‘concluding remarks’ (b) Subjects performed a target detection task in which a visual grating stimulus at threshold was briefly presented. Following a variable delay, the subject was prompted by an auditory cue to press one of two buttons to indicate whether they saw the stimulus. A small percentage of catch trials in which no grating was presented were randomly interleaved. EEG potential from the left parietal electrode (P3, using Laplacian derivation, which emphasizes local vertical currents underneath the electrode) was averaged around the onset of grating stimulus (left panel), or around the motor response (right panel). The evoked potentials for ‘Yes, I saw’, ‘No, I did not see’, and catch trials are shown in black, dark gray and light gray respectively. The inter-subject s.d. for catch trials are shown as dotted lines. A negative slow potential builds up between stimulus onset and motor response during ‘Yes’ trials but not catch trials nor the trials during which the stimulus was present but not perceived. Adapted, with permission, from Pins and ffytche et al. [50] (c) Average evoked-potentials (EPs) in response to single stimulus pulses at the skin, recorded from the surface of somatosensory cortex. EPs to 500 stimulus presentations were averaged for each condition. Abbreviations: Sub T, subthreshold stimuli, none of the 500 stimuli were felt by the subject; T, threshold stimuli, subject reporting feeling some of the 500 stimuli. Each recording trace is 500 ms long; Primary EP, a transient, surface-positive deflection that occurs ~30 ms after the stimulus, was present in both cases; Secondary EP, a later slower surface-negative component, only occurs when the stimulus was at times felt. Adapted, with permission, from Libet et al. [52] (d) The Bereitschaft potential (BP) is a negative SCP shift preceding the onset of a voluntary movement [56]. It was shown by Libet [57] that the onset of the BP also precedes the subject’s awareness of the intention to make the movement by a few hundred milliseconds. Adapted, with permission, from Haggard [58].
Interestingly, the cerebellum, usually considered non-essential for consciousness [18,43], is notably weak in its low-frequency activity as compared to the neocortex [48]. The cerebellar cortex also lacks the ‘crowning mystery’ – layer I, which is one important target for long-range feedback connections and nonspecific thalamic inputs [33,35].
In what follows we review existing empirical data supporting a functional role of the SCP in the emergence of conscious awareness.
Attention
Although attention and consciousness are distinct and dissociable phenomena [43], attention clearly affects which information has better access to conscious awareness. The top-down effect of attention in early sensory cortex is largely invisible to spike recordings, but is readily seen in the fMRI signal [1]. Consistent with a close correspondence between the SCP and the fMRI signal as argued here, top-down influence in V1 can be seen with measurements of the SCP using either optical imaging or field potential recordings [40,49]. In the first case, a feedback wave of depolarization was found to traverse the superficial layers from higher-order to lower-order visual areas [49]. In the second case, top-down attention was found to modulate the phase of delta-frequency activity which further modulated the power of higher frequencies [40]. Importantly, this effect was found only in superficial layers, consistent with the physiology of the SCP and with the laminar preference of feedback connections.
Perception
Many studies have investigated the neural correlates of conscious visual perception (for a recent review see Ref. [43]), however, only a handful presented data including the SCP, which we will focus on here. Pins and ffytche [50] presented visual stimulation at threshold to normal subjects, so that an identical stimulus would sometimes be perceived and at other times not. In trials during which the subject perceived the stimulus, a negative slow potential builds up over parietal electrodes between the stimulus onset and the response. This slow potential was next to nonexistent in trials during which the stimulus escaped conscious perception (Figure 3b). Using a visual illusion task and depth recording in V1, Leopold and colleagues [51] showed that perceptual suppression was only associated with changes in the lowest frequencies in upper cortical layers when the current source density (CSD) method (which has much better localizing power than raw field potentials, see Figure 2a) was used. Similar to the SCP, the fMRI signal also tracks perceptual changes, whereas spiking activity was unaffected [10]. Furthermore, momentary fluctuations in the spontaneous SCP have an effect on whether a stimulus at threshold is consciously perceived or not [39]. An active involvement of the SCP in conscious perception is also supported by early experiments in the somatosensory domain. Through a series of elegant experiments using skin stimuli, electrical stimulations applied to the subcortical pathway and the cortex itself, Libet [52] showed that the secondary evoked response (i.e. a long-lasting negative potential in surface-recordings), but not the primary response (i.e. the short-latency positive potential), was essential for conscious perception (Figure 3c). These findings have since received support from more recent studies [53,54].
Volition
If consciousness is a two-sided coin, then on one side it is occupied by perception and experience; on the other side by volition and agency [55]. Similar to perception, volition (i.e. voluntary actions) also has a long recognized association with the SCP. It was discovered more than 20 years ago that a negative SCP shift preceded voluntary movement [56] and even the subjective awareness of the intention to make the movement [57] (Figure 3d). Though the implications of these results were highly debated by philosophers, the essential findings have been replicated numerous times and extended [58,59]. Specifically, recent fMRI experiments have determined both the brain regions underlying the intention to make a movement and those underlying the awareness of such intention [58]. Important in the current context, recent results showed that the outcome of a free choice can be decoded using the fMRI signal up to 6s before the decision outcome enters conscious awareness [60]. Future experiments should illuminate whether this early fMRI signal is related to the early negative SCP shift preceding a voluntary action.
The unconscious states
One of the most dramatic experimental manipulations of consciousness comes from stimulations of intralaminar thalamic nuclei in minimally conscious patients [61] and anesthetized rats [62]. In both cases of impaired consciousness, stimulation of nonspecific thalamic pathways significantly improved behavioral responsiveness. As mentioned previously, nonspecific thalamic afferents terminate preferentially on apical dendrites in cortical superficial layers. Therefore, stimulation of these pathways would drive negative shifts of the SCP (i.e. increased excitability) and thus might restore long-range communications carried by this signal. Similarly, recovery from persistent vegetative state (PVS) was accompanied by restoration of functional connectivity between the intralaminar thalamic nuclei and prefrontal cortex [63]. These experiments lend important support for the current hypothesis of a relation between the SCP and conscious awareness. Further support comes from DC-recordings of auditory evoked potentials (AEP) in humans undergoing propofol anesthesia [64]. Whereas the early positive component of AEP was preserved during anesthesia, the later component – a negative shift in the SCP – was abolished under anesthesia and reappeared during emergence from anesthesia.
These results, however, do not suggest that the negative SCP, whenever it appears, is an index of conscious awareness. Instead, key brain regions might be required (Box 3) [43,65,66]. For example, the negative SCP can occur in primary sensory cortex under anesthesia (Figure 2a) without propagating to higher-order areas [64]. In parallel, fMRI signal activation in response to sensory stimulation is usually found in primary sensory but not higher-order areas in anesthetized or PVS patients [67,68].
Box 3. Brain networks, information integration and consciousness.
Spontaneous SCP and fMRI signals are temporally correlated within a set of large-scale functional brain networks such as those associated with visual, auditory, somato-sensory and motor, language, attention, executive and self-reflective functions (see Ref. [15] and the references therein). The temporal correlation or independent component maps normally presented to describe these networks tend to leave an impression that these networks are separate entities. This impression overlooks the cross-network interactions that are constantly taking place. If one watches the raw fluctuations of the spontaneous fMRI signal, the signal increases seem to move from one network to another with different time-lags in different nodes within a network (see ftp://imaging.wustl.edu/pub/raichlab/Spontaneous_fMRI_signal_movies/). The exact physiology underlying cross-network interaction is yet unclear, though one specific example might be the anti-correlation between the attention and cognitive-control networks and the default network [77]. Interestingly, though both sets of networks were present in anesthetized monkeys [69], this anti-correlation between networks was gone (our unpublished observation). Both cross-network and within-network interactions should have a role in large-scale information integration that contributes to conscious awareness.
Not all brain networks contribute to consciousness equally, as discussed in the text. We speculate that the anterior cingulate and anterior insular cortices, in addition to the default network, might be more pivotal than the sensory and motor networks and maybe even the dorsal attention network (including the dorsal visual stream and frontal eye field) in the emergence of consciousness. This conjecture mainly comes from a thought experiment comparing the largely unconscious state – slow-wave sleep (SWS), with the conscious states including wakefulness and rapid-eye-movement (REM) sleep. Whereas the sensory and motor regions and the dorsal attention network are as active in SWS as in wakefulness; the anterior cingulate, anterior insular and the midline regions of the default network are deactivated in SWS and reactivated in both REM sleep and wakefulness [78,79]. To the best of our knowledge, this conjecture is also consistent with existing data from persistent vegetative patients [68], blindsight patients [80] and from manipulations of momentary conscious perception [65,66].
Finally, a corollary prediction of the present hypothesis is that in most invertebrates (except octopus which does not conform to the following characterization), consciousness, if present, might be very different from that in vertebrates because the invertebrate nervous systems are similar to the vertebrate cerebellum, spinal cord or brain stem, but distinct from the vertebrate cerebrum, in two aspects: (i) with much pronounced fast activity but notably weak slow activity; (ii) seems to consist of a population of relatively independent neurons with little integration across the population [81]. This prediction is not formalized herein because we consider it to be non-testable by current empirical means, for a human being cannot be an invertebrate and experience what it experiences from inside, therefore to judge what a fruit fly experiences by observing its exhibited behaviors from outside is ill-defined by empirical standards.
Large-scale coherent structures in the spontaneous fluctuations of both the SCP and the fMRI signal (Box 3) have been described under unconscious states – slow-wave sleep (SWS) and deep anesthesia, respectively [15,69]. Do these findings contradict the current hypothesis? We suggest not. These coherent fluctuations could reflect spontaneous synaptic activity constrained by anatomical connections that continue to maintain homeostasis in the brain [70], but which lack sufficient information content (such as the bistable dynamics of ‘up-and-down states’ [18]) or the integration necessary for the emergence of consciousness. In fact, in both cases, the patterns of SCP or fMRI signal coherence were weaker in the unconscious state as compared to the conscious states [15,69]. A decreased baseline level of cortical excitability (not assessed by temporal correlation measurements) could also contribute to loss of consciousness, as supported by aforementioned experiments in which stimulation of nonspecific thalamic pathways restored responsiveness in subjects with impaired consciousness [61,62].
Concluding remarks
Studies on the neural basis of the fMRI signal have focused on the LFP power. Here, we present evidence for another neurophysiological signal underlying the fMRI signal that has received much less attention – the SCP. The linkage between the SCP and the fMRI signal not only advances our understanding of cortical physiology but also provides a different vantage point to many experimental results. We further propose that the SCP might carry large-scale information integration in the neocortex that contributes to the emergence of conscious awareness. Experiments on consciousness have seldom included the SCP, but whenever it was included, the results seem to be consistent with this hypothesis. Given that our hypothesis involves a specific physiological process, it is clearly amenable to empirical testing.
Specifically, the hypothesis put forward makes two testable predictions:
Whenever there is a change in the content of conscious awareness, there should also be a concurrent change in the SCP in corresponding essential brain regions (which yet need to be determined for different forms of conscious awareness – we offer a speculation on this in Box 3).
In altered states of consciousness, such as deep slow-wave sleep, under anesthesia, or in patients with severe brain injury, the spontaneous organization of the SCP that is important for large-scale information integration should be altered compared to the fully conscious state. This should also apply to systems with reduced consciousness, such as the cerebellum (in comparison to the cerebrum), and perhaps organisms lower on the evolutionary tree (Box 3).
We look forward to future work that confirms or falsifies our hypothesis.
Acknowledgements
We are grateful to Giulio Tononi, Bruno van Swinderen, Avi Snyder, John Zempel, and Harold Burton for insightful discussions. B.J.H. would also like to thank the U.S. Immigration Service under the Bush administration, whose visa background security check forced her to spend two months (following an international conference) in a third country, free of routine obligations—it was during this time that the hypothesis presented herein was initially conjectured. This research was supported by NIH grant 06833.
Footnotes
In this article, we use ‘consciousness’ or ‘conscious awareness’ synonymously as ‘subjective awareness’. We use ‘conscious experience’ to refer to the experience of subjective awareness. Lastly, ‘conscious state’ refers to the physiological states under which conscious awareness is present..
References
- 1.Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 3.Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat. Neurosci. 2007;10:1308–1312. doi: 10.1038/nn1977. [DOI] [PubMed] [Google Scholar]
- 4.Lauritzen M, Gold L. Brain function and neurophysiological correlates of signals used in functional neuroimaging. J. Neurosci. 2003;23:3972–3980. doi: 10.1523/JNEUROSCI.23-10-03972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niessing J, et al. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- 6.Arthurs OJ, Boniface S. How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci. 2002;25:27–31. doi: 10.1016/s0166-2236(00)01995-0. [DOI] [PubMed] [Google Scholar]
- 7.Raichle ME, Mintun MA. Brain work and brain imaging. Annu. Rev. Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 8.Logothetis NK, et al. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 9.Rauch A, et al. The effect of a serotonin-induced dissociation between spiking and perisynaptic activity on BOLD functional MRI. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6759–6764. doi: 10.1073/pnas.0800312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maier A, et al. Divergence of fMRI and neural signals in V1 during perceptual suppression in the awake monkey. Nat. Neurosci. 2008;11:1193–1200. doi: 10.1038/nn.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goense JB, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr. Biol. 2008;18:631–640. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 12.Kayser C, et al. A comparison of hemodynamic and neural responses in cat visual cortex using complex stimuli. Cereb. Cortex. 2004;14:881–891. doi: 10.1093/cercor/bhh047. [DOI] [PubMed] [Google Scholar]
- 13.Mukamel R, et al. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- 14.Birbaumer N, et al. Slow potentials of the cerebral cortex and behavior. Physiol. Rev. 1990;70:1–41. doi: 10.1152/physrev.1990.70.1.1. [DOI] [PubMed] [Google Scholar]
- 15.He BJ, et al. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16039–16044. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khader P, et al. On the relationship between slow cortical potentials and BOLD signal changes in humans. Int. J. Psychophysiol. 2008;67:252–261. doi: 10.1016/j.ijpsycho.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Searle JR. Consciousness. Annu. Rev. Neurosci. 2000;23:557–578. doi: 10.1146/annurev.neuro.23.1.557. [DOI] [PubMed] [Google Scholar]
- 18.Tononi G. Consciousness as integrated information: a provisional manifesto. Biol. Bull. 2008;215:216–242. doi: 10.2307/25470707. [DOI] [PubMed] [Google Scholar]
- 19.Goldring S. DC Shifts Released by Direct and Affrent Stimulation. In: Remond A, editor. Handbook of Electroencephalography and Clinical Neurophysiology. Elsevier; 1974. pp. 12–24. [Google Scholar]
- 20.Rebert CS. Slow potential correlates of neuronal population responses in the cat’s lateral geniculate nucleus. Electroencephalogr. Clin. Neurophysiol. 1973;35:511–515. doi: 10.1016/0013-4694(73)90027-8. [DOI] [PubMed] [Google Scholar]
- 21.Rosler F, et al. Slow negative brain potentials as reflections of specific modular resources of cognition. Biol. Psychol. 1997;45:109–141. doi: 10.1016/s0301-0511(96)05225-8. [DOI] [PubMed] [Google Scholar]
- 22.Nagai Y, et al. Brain activity relating to the contingent negative variation: an fMRI investigation. Neuroimage. 2004;21:1232–1241. doi: 10.1016/j.neuroimage.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 23.Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum. Brain Mapp. 2008;29:751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu H, et al. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18265–18269. doi: 10.1073/pnas.0705791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nir Y, et al. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat. Neurosci. 2008;11:1100–1108. doi: 10.1038/nn.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones MO, et al. Interactions between evoked and spontaneous hemodynamic fluctuations in rodent brain. 37th Annual Meeting of Society for Neuroscience; San Diego.2007. [Google Scholar]
- 27.Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- 28.Song JH, Jiang Y. Visual working memory for simple and complex features: an fMRI study. Neuroimage. 2006;30:963–972. doi: 10.1016/j.neuroimage.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Cooper R, et al. Regional control of cerebral vascular reactivity and oxygen supply in man. Brain Res. 1966;3:174–191. doi: 10.1016/0006-8993(66)90075-8. [DOI] [PubMed] [Google Scholar]
- 30.Shipton HW. EGG analysis: a history and a prospectus. Annu Rev. Biophys. Bioeng. 1975;4:1–13. doi: 10.1146/annurev.bb.04.060175.000245. [DOI] [PubMed] [Google Scholar]
- 31.Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol. Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- 32.Braitenberg V, Schuz A. Cortex: Statistics and Geometry of Neuronal Connectivity. 2nd edn. Springer; 1998. [Google Scholar]
- 33.Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu. Rev. Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- 34.Roland PE. Dynamic depolarization fields in the cerebral cortex. Trends Neurosci. 2002;25:183–190. doi: 10.1016/s0166-2236(00)02125-1. [DOI] [PubMed] [Google Scholar]
- 35.Jones EG. Viewpoint: the core and matrix of thalamic organization. Neuroscience. 1998;85:331–345. doi: 10.1016/s0306-4522(97)00581-2. [DOI] [PubMed] [Google Scholar]
- 36.Scheibel ME, Scheibel AB. Structural organization of nonspecific thalamic nuclei and their projection toward cortex. Brain Res. 1967;6:60–94. doi: 10.1016/0006-8993(67)90183-7. [DOI] [PubMed] [Google Scholar]
- 37.Vanhatalo S, et al. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5053–5057. doi: 10.1073/pnas.0305375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ergenoglu T, et al. Slow cortical potential shifts modulate P300 amplitude and topography in humans. Neurosci. Lett. 1998;251:61–64. doi: 10.1016/s0304-3940(98)00498-4. [DOI] [PubMed] [Google Scholar]
- 39.Devrim M, et al. Slow cortical potential shifts modulate the sensory threshold in human visual system. Neurosci. Lett. 1999;270:17–20. doi: 10.1016/s0304-3940(99)00456-5. [DOI] [PubMed] [Google Scholar]
- 40.Lakatos P, et al. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- 41.Montemurro MA, et al. Phase-of-firing coding of natural visual stimuli in primary visual cortex. Curr. Biol. 2008;18:375–380. doi: 10.1016/j.cub.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 42.Tong F. Primary visual cortex and visual awareness. Nat. Rev. Neurosci. 2003;4:219–229. doi: 10.1038/nrn1055. [DOI] [PubMed] [Google Scholar]
- 43.Tononi G, Koch C. The neural correlates of consciousness: an update. Ann. N. Y. Acad. Sci. 2008;1124:239–261. doi: 10.1196/annals.1440.004. [DOI] [PubMed] [Google Scholar]
- 44.Buzsaki G. Rhythms of the Brain. Oxford University Press; 2006. pp. 119–135. [Google Scholar]
- 45.Leopold DA, et al. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb. Cortex. 2003;13:422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- 46.von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol. 2000;38:301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 47.Baars BJ. Global workspace theory of consciousness: toward a cognitive neuroscience of human experience. Prog. Brain Res. 2005;150:45–53. doi: 10.1016/S0079-6123(05)50004-9. [DOI] [PubMed] [Google Scholar]
- 48.Bullock TH. Signals and signs in the nervous system: the dynamic anatomy of electrical activity is probably information-rich. Proc. Natl. Acad. Sci. U. S. A. 1997;94:1–6. doi: 10.1073/pnas.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roland PE, et al. Cortical feedback depolarization waves: a mechanism of top-down influence on early visual areas. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12586–12591. doi: 10.1073/pnas.0604925103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pins D, Ffytche D. The neural correlates of conscious vision. Cereb. Cortex. 2003;13:461–474. doi: 10.1093/cercor/13.5.461. [DOI] [PubMed] [Google Scholar]
- 51.Leopold DA, et al. Laminar analysis of local field and current source density during physical and perceptual events in monkey V1. 38th Annual Meeting of Society for Neuroscience; Washington D.C.. 2008. [Google Scholar]
- 52.Libet B, et al. Responses of human somatosensory cortex to stimuli below threshold for conscious sensation. Science. 1967;158:1597–1600. doi: 10.1126/science.158.3808.1597. [DOI] [PubMed] [Google Scholar]
- 53.Sergent C, et al. Timing of the brain events underlying access to consciousness during the attentional blink. Nat. Neurosci. 2005;8:1391–1400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- 54.Rossion B, et al. Early extrastriate activity without primary visual cortex in humans. Neurosci. Lett. 2000;279:25–28. doi: 10.1016/s0304-3940(99)00926-x. [DOI] [PubMed] [Google Scholar]
- 55.Gray HM, et al. Dimensions of mind perception. Science. 2007;315:619. doi: 10.1126/science.1134475. [DOI] [PubMed] [Google Scholar]
- 56.Kornhuber HH, Deecke L. Pflugers Arch. Gesamte Physiol. Menschen Tiere. 1965;284:1–17. [PubMed] [Google Scholar]
- 57.Libet B, et al. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain. 1983;106:623–642. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- 58.Haggard P. Human volition: towards a neuroscience of will. Nat. Rev. Neurosci. 2008;9:934–946. doi: 10.1038/nrn2497. [DOI] [PubMed] [Google Scholar]
- 59.Libet B. Time factors in conscious processes: reply to Gilberto Gomes. Conscious. Cogn. 2000;9:1–12. doi: 10.1006/ccog.1999.0408. [DOI] [PubMed] [Google Scholar]
- 60.Soon CS, et al. Unconscious determinants of free decisions in the human brain. Nat. Neurosci. 2008;11:543–545. doi: 10.1038/nn.2112. [DOI] [PubMed] [Google Scholar]
- 61.Schiff ND, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 62.Alkire MT, et al. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007;107:264–272. doi: 10.1097/01.anes.0000270741.33766.24. [DOI] [PubMed] [Google Scholar]
- 63.Laureys S, et al. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet. 2000;355:1790–1791. doi: 10.1016/s0140-6736(00)02271-6. [DOI] [PubMed] [Google Scholar]
- 64.Fitzgerald RD, et al. Direct current auditory evoked potentials during wakefulness, anesthesia, and emergence from anesthesia. Anesth. Analg. 2001;92:154–160. doi: 10.1097/00000539-200101000-00030. [DOI] [PubMed] [Google Scholar]
- 65.Craig AD. How do you feel-now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 66.Dehaene S, et al. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn. Sci. 2006;10:204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 67.Heinke W, et al. Sequential effects of propofol on functional brain activation induced by auditory language processing: an event-related functional magnetic resonance imaging study. Br. J. Anaesth. 2004;92:641–650. doi: 10.1093/bja/aeh133. [DOI] [PubMed] [Google Scholar]
- 68.Laureys S, et al. Residual cognitive function in comatose, vegetative and minimally conscious states. Curr. Opin. Neurol. 2005;18:726–733. doi: 10.1097/01.wco.0000189874.92362.12. [DOI] [PubMed] [Google Scholar]
- 69.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 70.Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat. Rev. Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- 71.Steriade M, et al. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J. Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Monto S, et al. Very slow EEG fluctuations predict the dynamics of stimulus detection and oscillation amplitudes in humans. J. Neurosci. 2008;28:8268–8272. doi: 10.1523/JNEUROSCI.1910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eichele T, et al. Assessing the spatiotemporal evolution of neuronal activation with single-trial event-related potentials and functional MRI. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17798–17803. doi: 10.1073/pnas.0505508102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mobascher A, et al. Laser-evoked potential P2 single-trial amplitudes covary with the fMRI BOLD response in the medial pain system and interconnected subcortical structures. Neuroimage. 2009;45:917–926. doi: 10.1016/j.neuroimage.2008.12.051. [DOI] [PubMed] [Google Scholar]
- 75.Silva AC, Koretsky AP. Laminar specificity of functional MRI onset times during somatosensory stimulation in rat. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15182–15187. doi: 10.1073/pnas.222561899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buzsaki G, et al. Inhibition and brain work. Neuron. 2007;56:771–783. doi: 10.1016/j.neuron.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fox MD, et al. The Global Signal and Observed Anticorrelated Resting State Brain Networks. J Neurophysiol. 2009 doi: 10.1152/jn.90777.2008. DOI: 10.1152/ jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Braun AR, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120:1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 79.Maquet P, et al. Human cognition during REM sleep and the activity profile within frontal and parietal cortices: a reappraisal of functional neuroimaging data. Prog. Brain Res. 2005;150:219–227. doi: 10.1016/S0079-6123(05)50016-5. [DOI] [PubMed] [Google Scholar]
- 80.Milner AD, Goodale MA. Two visual systems re-viewed. Neuropsychologia. 2008;46:774–785. doi: 10.1016/j.neuropsychologia.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 81.Bullock TH, Basar E. Comparison of ongoing compound field potentials in the brains of invertebrates and vertebrates. Brain Res. 1988;472:57–75. doi: 10.1016/0165-0173(88)90005-7. [DOI] [PubMed] [Google Scholar]