Abstract
While many aspects of memory T-cell immunobiology have been characterized, we suggest that we know only a fraction of the effector functions that CD4 T cells can bring to bear during secondary challenges. Exploring the full impact of memory CD4 T-cell responses is key to the development of improved vaccines against many prominent pathogens, including influenza viruses, and also to a better understanding of the mechanisms of autoimmunity. Here we discuss factors regulating the generation of memory CD4 T cells during the activation of naïve cells and how the nature of the transition from highly activated effector to resting memory upon the resolution of primary responses might impact memory CD4 T-cell heterogeneity in vivo. We stress that memory CD4 T cells have unique functional attributes beyond the secretion of T helper (Th) subset-associated cytokines that can shape highly effective secondary responses through novel mechanisms. These include the recruitment of innate inflammatory responses at early phases of secondary responses as well as the action of enhanced direct effector functions at later phases, in addition to well-established helper roles for CD8 T-cell and B-cell responses.
Keywords: CD4/helper T cells (Th cells,Th0,Th1,Th2,Th17); cytokines; inflammation; memory; vaccines
Introduction
The generation of T-cell memory is a fundamental goal of vaccine strategies aimed at pathogens against which neutralizing antibody alone does not confer lasting immunity. In addition, T-cell-based vaccines offer the possibility of long-term protection against a range of seasonal and pandemic influenza virus strains as there is far less variation documented in internal viral proteins recognized by T cells compared with the antigenic shift and drift observed in coat proteins recognized by antibody.1 However, the mechanisms by which memory T cells, especially CD4 T cells, contribute to protection against influenza virus and other pathogens are not fully understood. Here we discuss aspects of the generation and maintenance of memory CD4 T cells, their heterogeneity, and the diverse functions memory CD4 T cells can carry out during secondary immune responses.
Memory CD4 T-cell generation
During primary immune responses, naïve CD4 T cells recognizing antigen expand many fold and differentiate into highly activated effector cells.2,3 Depending on the impact of several variables during priming, effectors can become polarized into distinct subsets with specialized functions and attributes. The best characterized of such subsets are T helper type 1 (Th1) and Th2, which have long been known to promote effective cell-mediated and humoral responses, respectively.4 More recently, additional subsets have been described, including Th17, follicular helper cells (TFH), and various regulatory populations5–7 (Table 1). In addition, substantial heterogeneity and plasticity, as assessed by cytokine production patterns, have been observed within subsets, especially in vivo.8,9 This impressive diversity in effector subtype underscores the tremendous potential range of T-cell functions and suggests that there is a corresponding complexity in the orchestration of optimal protective immunity. Effector CD4 T cells influence immune responses through both ‘helper’ and direct ‘effector’ functions.10 These will be discussed later in more detail.
Table 1.
Characteristics of CD4 T-cell subsets
| CD4 T-cell effector subset | Polarizing factors | Transcription factors | Signature cytokines | Susceptibility to AICD |
|---|---|---|---|---|
| Th1 | IL-12, IFN-γ | T-bet | IFN-γ, TNF, IL-2 | ++++ |
| Th2 | IL-4 | GATA-3 | IL-4, IL-5, IL-13 | + |
| Th17 | TGF-β, IL-6 | ROR-γt | IL-17, IL-21, IL-22 | ++ |
| TFh | IL-6, IL-21 | Bcl-6 | IL-4, IL-21 | ? |
| Treg | TGF-β, RA | Foxp3 | IL-10 | ? |
AICD, activation induced cell death; IFN, interferon; IL, interleukin; TGF, transforming growth factor; Th, T helper; TNF, tumour necrosis factor; Treg, regulatory T cell.
After the resolution of a primary response, the majority of effector CD4 T cells die via apoptosis, leaving a small population of memory cells. Surviving antigen-specific cells revert to a resting state but at frequencies generally much higher than those found in naïve individuals. A quantitative increase in antigen-specific T cells thus represents a well-documented advantage of the memory state.1,11–13 In at least some situations, the magnitude of the effector response has been found to correlate with the size of the resulting memory pool.14 The intracellular pathways and external signals involved in co-ordinating the contraction of effectors in vivo remain poorly understood.15 For example, signalling through the death receptor Fas has been studied extensively, but its relevance during contraction in vivo remains unclear.16 The pro-apoptotic molecule Bim (B-cell lymphoma 2 interacting mediator of cell death) seems to play a central role during the contraction of effectors and its expression within a population of responding cells limits the pool of memory-competent cells.17 Recent studies suggest that Bim expression by effector CD4 T cells is influenced during priming by the strength of T-cell receptor (TCR) stimulation,18 which may be regulated by competition between responding cells for antigen.
Signals impacting the effector-to-memory transition
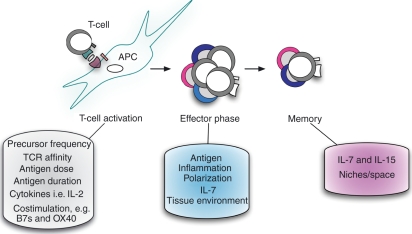
While the signals involved in the activation of naïve cells and their transition into effectors have been studied extensively,3,19,20 comparatively little is known regarding the cessation of effector responses and the effector-to-memory transition. Understanding this process in greater detail holds the potential for improving memory generation and survival (Fig. 1). After optimal priming, we and others have shown that removal of antigen and inflammatory cytokines marks a critical starting point for the generation of memory.14,21,22 In fact, few other extrinsic signals are required, although interleukin (IL)-7 signalling has been shown to support the survival of effectors during the transition to memory.23,24 The lack of signalling in this process is in contrast to the tightly regulated set of cell-surface molecules and cytokines required for the full activation of naïve CD4 T cells. This suggests that critical aspects of the effector-to-memory transition are programmed at earlier stages of the CD4 T-cell response.
Figure 1.
Many factors can influence the generation of memory CD4 T cells. Variables present during T-cell activation, the effector phase, and after the resolution of a primary response can all impact the size and quality of the resulting memory population. APC, antigen-presenting cell; IL, interleukin; TCR, T-cell receptor.
In support of this theory, several factors associated with T-cell activation and effector generation have been reported also to impact the generation of memory. For example, IL-2 signalling during priming has been shown to enhance long-term survival of CD4 T cells,25 and a similar role for CD28-dependent signalling during priming has been reported for optimal memory generation.26 The results of several studies also support an important role for OX-40-dependent signalling in maximizing memory, at least under Th2-polarizing conditions.27–30 Finally, the level of or duration of antigen stimulation during priming can impact memory CD4 T-cell generation, as studies suggest that limiting TCR signalling can inhibit memory formation.31
Rapid effector-to-memory transition
We have recently investigated the kinetics of the effector-to-memory transition by comparing the phenotypes, functional attributes, and gene expression profiles of well-defined effector and memory CD4 T cells to those of transitional populations of effectors rested for short periods of time in the absence of antigen and inflammatory cytokines.22 Surprisingly, we found the effector-to-memory transition to be quite rapid – effectors rested for only 3 days closely resembled canonical long-term memory cells by all criteria tested. The rapid kinetics of the effector-to-memory transition seem to be part of a default program requiring few external signals, as similar results were obtained using both Th1 and Th2 lineage effectors rested both in vitro and in vivo and primed both in vitro and in vivo.32 Thus, the immune system is poised to abruptly shift from the effector mode, which can itself be pathogenic, to the more benign memory mode shortly after the resolution of a primary response.
Differential maintenance of CD4 and CD8 T-cell memory
While an important difference distinguishing the naïve from the memory state is an increase in antigen-specific cells, attrition of memory T-cell populations occurs with time. There also appear to be very different rules for how memory CD4 and CD8 T cells are maintained in the long term. As compared with memory CD8 T-cell populations, which tend to remain relatively stable, memory CD4 T-cell numbers tend to gradually decrease.33–35 Several factors could account for the differential homeostasis of antigen-experienced CD4 and CD8 T cells. A primary mechanism could involve the ability to access critical survival factors. In support of this hypothesis, long-term CD4 T-cell survival has been shown to be positively affected by IL-15 signalling,36 and IL-15 is a cytokine thought to be primarily involved in memory CD8 T-cell homeostasis. Thus, memory CD8 T cells may out-compete memory CD4 T cells for IL-15 via expression of a higher affinity receptor,37 thereby causing the latter population to be less fit for long-term survival. A larger population of antigen-experienced CD4 as compared to CD8 T cells may also require cognate interactions with antigen.38–40 Residual antigen depots have been reported to be present after the resolution of primary responses in diverse models,41–44 and their gradual waning over time might constrict survival niches. For example, following influenza infection, residual viral antigen presentation seems differentially capable of generating and perhaps maintaining CD4 and CD8 T-cell memory.41,45
Several observations support the hypothesis that niches for memory populations are indeed limited in T-cell-replete mice, suggesting an environment of intense competition amongst antigen-experienced T cells.46 It is currently unclear what defines such niches in vivo. An obvious possibility is access to major histocompatibility complex class II (MHC II)–peptide complexes recognized by TCRs above a certain threshold47 (although these need not be complexes for which the TCR is specific). This hypothesis is supported by observations showing that long-lived memory CD4 T cells can express higher affinity TCRs than naïve cells of the same specificity.43,48 A hallmark of memory CD4 T cells, however, is their ability to survive for extended periods of time upon transfer to MHC II-deficient hosts, while naïve cells rapidly disappear.49 Alternatively, survival niches may be based on the ability of memory cells to better access sources of IL-7 and/or other survival signals through interactions between tumour necrosis factor (TNF) family receptors and their ligands.50
Functional properties of memory CD4 T cells
While a quantitative increase in antigen-specific CD4 T cells represents a major advantage of the memory state, we believe that a more important feature of T-cell memory is the enhanced qualitative attributes of memory as compared with naïve and effector T cells. These changes affect homeostasis and longevity as well as functional attributes (Table 2). Most of the functional changes that distinguish memory CD4 T cells from naïve cells can be viewed as endowing memory cells with enhanced and more rapid responsiveness upon antigen recognition.
Table 2.
Properties of CD4 T-cell differentiation stages
| Functional characteristics | Naïve | Effector | Memory | Secondary effector |
|---|---|---|---|---|
| Effector function | – | ++++ | +++ | +++++ |
| IL-2:IL-10 ratio | +++ | + | +++++ | ++++ |
| AICD | – | ++++ | ++ | ? |
| Adhesion | – | ++++ | ++ | +++++ |
| Migration | Lymphoid | Non-lymphoid | Lymphoid | Non-lymphoid |
AICD, activation induced cell death; IL, interleukin.
Most strikingly, while naïve CD4 T cells primarily produce IL-2 shortly after TCR triggering, memory CD4 T cells retain the ability to produce effector-associated cytokines upon re-stimulation.51 Also, while naïve cells respond to antigenic stimulation only after a lag period, memory cells are capable of more rapid cytokine production and proliferation.52,53 The intracellular signalling components responsible for the rapid response kinetics of memory CD4 T cells are now being investigated.54 Finally, memory CD4 T cells display less stringent activation requirements, and respond optimally to lower doses of antigen and lower levels of costimulation than naïve cells.22,52,53,55–59 More recent studies, however, suggest that costimulation (in particular through CD28 and CD40L) is important in regulating certain aspects of memory responses.58,60 Combined, these observations point towards a ‘faster, bigger and better’ response of memory as compared with naïve CD4 T cells.
Additional functional qualities distinguish memory CD4 T cells from highly activated effectors (Table 2). Several groups have demonstrated that memory CD4 T cells lose susceptibility to the cytokine-induced apoptosis61 and activation-induced cell death that characterizes activated effectors.21,22,62 In several instances, effectors lose the ability to produce IL-2, while its expression is often observed in responding memory cells.12,21,22,32,63 We and others have also observed that IL-10 production is significantly reduced in memory as compared with effector CD4 T cells.22,64,65 It is interesting to speculate that production of IL-10, a factor associated with suppression of immune responses,66 is involved in a type of immune modulation acting during the peak of primary but not secondary T cell responses.65 Memory CD4 T cells also down-regulate expression of a myriad of activation and adhesion molecules highly expressed on effectors, consistent with a return to a resting state.22 However, expression of certain molecules, such as CD4467 and CD54,55 remains up-regulated on resting memory cells, and may facilitate enhanced interactions with antigen-bearing antigen-presenting cells (APCs). Finally, the in vivo migration of memory CD4 T cells differs from that of effector CD4 T cells under steady-state conditions. While highly activated effectors can traffic throughout lymphoid organs as well as the periphery, trafficking of memory cells resembles the more restricted pattern of recirculation through lymphoid organs that characterizes naïve populations,68 and can even be more restricted than that of naïve cells.69 The wider trafficking pattern of effectors correlates with their increased expression of several adhesion molecules and loss of expression of CD62L.68
Together, these observations support the theory that effector CD4 T cells progress beyond an early ‘helper’ stage, where they enhance responses of B cells and CD8 T cells, towards an end stage where they are more likely to be of a restricted subset. Memory cells, in contrast, seem to retain important effector attributes but also re-acquire others associated with naïve CD4 T cells and polyfunctional potential. Gene expression analysis confirms this, as long-term memory populations closely resemble naïve CD4 T cells.22
Heterogeneity
One of the most striking elements of T-cell memory is heterogeneity. Several groups have demonstrated a great deal of diversity, and have defined memory CD4 T-cell subsets based on functional capability, surface marker expression, and anatomical location.70–73 The root of this diversity is not well understood, nor is its long-term maintenance.74–77 The rapid kinetics of the effector-to-memory transition may help to explain some elements of heterogeneity. For example, we have previously shown that, based on dilution of carboxyfluorescein succinimidyl ester (CFSE) and surface marker expression, a wide range of heterogeneity characterizes influenza-specific effectors during a primary infection.78 Surprisingly, much of this diversity is retained in long-term memory populations.10 This suggests that, at least in terms of the number of cell divisions and diverse cell-surface markers, no obvious pre-memory effector state is required to facilitate the effector-to-memory transition (Fig. 2).
Figure 2.
Effector heterogeneity is influenced by several factors. While some effectors progress to a terminally differentiated state, the rapid kinetics of the effector-to-memory transition upon resolution of a primary immune response enables a great deal of heterogeneity to be retained in the memory phase. APC, antigen-presenting cell; TCR, T-cell receptor.
An intriguing possibility is that some elements of heterogeneity are attributable to differential kinetics of entry of naïve T cells into a dynamic immune response. Observations show that T cells respond to antigen throughout the primary response, even during its resolution, and that naïve cells responding later, when levels of antigen and inflammation are lower, can give rise to effectors that are phenotypically and functionally distinct from effectors generated at earlier times.41,45,79–81 This dynamic variable, coupled with T-cell-intrinsic variables such as TCR affinity, and T-cell-extrinsic variables such as the type of APC, could then potentially lead to a spectrum of responding antigen-specific T cells with regard to activation status, phenotype, function and longevity. It is possible that different subsets of responding T cells thus created during the primary response might represent populations of specialized cells with optimal helper, killer and regulatory functions.10,82 Whatever the mechanism, diversity in T-cell effectors seems to be fostered and may thus be important in orchestrating and affecting optimal responses. We suggest that, if CD4 T-cell effectors in different relative states of activation gain and/or lose functional and/or regulatory attributes, then it is likely that an equally diverse memory pool would provide optimal protection during secondary challenges.
Potential impact of memory CD4 T cells
How memory CD4 T cells protect during secondary challenge is not fully understood. This is in part a consequence of the fact that CD4 T cells and their products influence immune responses against different pathogens differently. For example, CD4 T-cell help is required for optimal primary CD8 T-cell responses in some but not all challenge models and interferon (IFN)-γ produced by memory CD4 T cells is required for protection against some but not all pathogens that elicit a Th1-response.83–86 Also, as already discussed, a great deal of heterogeneity characterizes memory CD4 T cells, with different subsets displaying different functions. Thus, it is increasingly difficult to ascribe protective function to memory CD4 T cells in general, rather than to discrete subsets that might not be present or play an important role in all situations. Finally, the ‘quality’ of memory CD4 T cells can vary dramatically, impacting their protective potential. For example, the ability of both mouse and human memory CD4 T cells to produce multiple cytokines has recently received considerable attention, and this phenotype has been correlated with superior functional ability and protective capacity compared with populations displaying a more restricted cytokine secretion pattern.87–92
We propose that the unique functional qualities of memory CD4 T cells have the potential to impact a secondary response in multiple ways throughout its continuum. Which aspects of CD4 T-cell responses are critical for protection will depend on the nature of the pathogen and the contribution of other arms of innate and acquired immunity. Below we briefly describe the potential for unique impacts of memory CD4 T cells throughout secondary responses (Fig. 3).
Figure 3.
Memory CD4 T cells impact multiple stages of secondary responses. Memory CD4 T cells rapidly produce an array of cytokines and chemokines upon antigen recognition, and can recruit elements of the innate immune system to do likewise. As they divide and further differentiate, memory CD4 T cells can act as superior helpers for CD8 T-cell and B-cell responses compared with naïve CD4 T cells. Finally, highly activated secondary effectors impact secondary responses through direct effector functions that differ from those of primary effectors. All stages concertedly impact the kinetics of pathogen clearance.
Recruitment of innate immunity
Production of pathogen-appropriate cytokines at the early stages of immune responses represents an important contribution of memory CD4 T cells. For example, it has long been established that Th1-associated cytokines, most notably IFN-γ, can activate macrophages.93 This mode of action represents a critical protective effect of CD4 T cells during experimental tuberculosis (TB) challenge.86 IFN-γ production by memory CD4 T cells can also induce an antiviral state within infected cells in addition to other systemic effects.94 In addition, IFN-γ produced by CD4 T cells can enhance the production of an array of proinflammatory cytokines and chemokines that can act to recruit diverse innate cellular populations and CD8 T-cell effectors to sites of infection.95,96 Similarly, the Th2-associated cytokines IL-4 and IL-13 play important roles in protection against a broad range of pathogens.97
Our recent studies indicate that, during influenza infection, Th1-polarized virus-specific memory, but not naïve, CD4 T cells induce the expression of a wide array of innate inflammatory cytokines and chemokines (IICs) in an IFN-γ- and TNF-independent manner (Strutt, McKinstry, and Swain, submitted). Furthermore, we observed IIC induction by memory CD4 T cells in the absence of conserved pattern recognition receptor signalling. Importantly, memory CD4 T-cell induction of IIC responses led to early viral control. These results demonstrate that memory CD4 T-cell responses can directly recruit elements of the innate immune system to combat infection at early stages of a recall challenge. However, some elements of the inflammatory responses mediated by memory CD4 T cells may become pathogenic at later stages of infection,98 and may thus be involved in certain autoimmune conditions.
Helper functions of memory CD4 T cells
That CD4 T cells are commonly referred to as ‘helper’ T cells underscores their specialized role in the adaptive immune system. CD4 T cells are critical in helping B-cell responses, both through cognate interactions and through secreted mediators. In particular, interactions between CD40L, expressed on activated CD4 T cells, and CD40, constitutively expressed on B cells, are critical for germinal centre formation, isotype switching and affinity maturation.99 Other molecules expressed by activated CD4 T cells, such as CD28, OX40 and inducible T-cell costimulator, can also play prominent roles in the generation of optimal B-cell responses.100–102 In addition, signaling lymphocyte activation molecule (SLAM) associated protein expression by CD4 T cells is required for helper function through a mechanism distinct from ligation of CD40 and OX40L.103 Finally, Th1/Th2 cytokines produced by CD4 T cells can dramatically impact B-cell responses.4,104 More recently, IL-21 and IL-17 have been also reported to profoundly influence the quality of T-cell-dependent B-cell responses.105,106 Importantly, memory CD4 T cells have been demonstrated to be superior helpers for B-cell antibody production as compared with naïve CD4 T cells, and this is most readily explained by the ability of memory cells to reach a helper-competent activation status more quickly than naive cells, and their ability to produce more cytokines with more rapid kinetics.12,55,107
CD4 T-cell help can also be critical in the generation and maintenance of optimal cytotoxic CD8 T-cell responses.83,108 Several studies support a critical role for CD40L-CD40 signalling in this process, whether through direct interactions with CD8 T cells109 or through interactions with APCs.110–112 As for B-cell responses, recent studies have demonstrated an enhanced capability of memory CD4 T cells to help both primary113,114 and secondary115,116 CD8 T-cell responses. In at least some situations, IL-2 production by CD4 T cells plays a critical role in helping primary and secondary CD8 T-cell responses.117,118 Again, the enhanced helper capability of memory CD4 T cells in these models is presumably directly linked to their enhanced functional attributes and their ability to provide help sooner and more effectively than naïve CD4 T cells.
Secondary CD4 T-cell effectors
It is also understood that memory CD4 T cells serve as precursors to a population of activated effectors during secondary responses. Although the emergence of secondary effectors at later stages of responses may represent an important aspect of the continuing impact of memory CD4 T cells, the consequences of generating secondary CD4 T-cell effectors have not been investigated. This is in part because of the fact that primary and secondary effectors appear identical phenotypically,119 causing their discrimination to be difficult if not impossible. However, it was shown, via an adoptive transfer system, that secondary effector CD4 T cells were superior to primary effectors as well as to resting memory cells in terms of IL-4 production and providing help for immunoglobulin G (IgG) antibody responses.12 Thus, while memory cells provide an increased level of help as compared with naïve cells, their helping capacity can increase during the kinetic development of recall responses.
We have recently investigated further functional attributes of secondary effectors utilizing an influenza infection model. Our laboratory has previously described several aspects of the responses of both naïve and of highly activated effector CD4 T cells responding to influenza virus.78,120 Our new studies show that resting memory CD4 T cells give rise to a population of secondary effectors with similar kinetics to those of primary effectors arising from naïve precursors and that the two populations express similar levels of multiple adhesion and activation markers. Functionally, however, secondary effectors are superior to primary effectors and exhibit a pronounced multifunctional phenotype both at the per-cell and at the population level, producing higher concentrations of more cytokines.
We tested the impact of secondary CD4 T-cell effectors on survival following lethal influenza challenge by adoptively transferring memory populations into host mice deficient in CD8 T cells or B cells. These transfers allowed a focus on helper-independent functions of memory CD4 T cells. We found that secondary effectors could protect otherwise naïve mice devoid of CD8 T cells or B cells from lethal challenges of influenza via several distinct mechanisms while primary effectors, either generated in vivo from naïve precursors or transferred directly, could not. These results highlight the substantial helper-independent protective impact of memory CD4 T cells occurring at later stages of recall responses after their re-expansion and transition into a population of secondary effector cells.
Concluding remarks
A more complete understanding of memory CD4 T-cell immunobiology has the potential to significantly impact diverse fields, including vaccine design. Although many central questions about how memory CD4 T cells are generated and even how they protect remain unanswered, it is clear that memory CD4 T cells have the potential to impact secondary immune responses in unique and important ways (Fig. 4). A complicating factor in the study of CD4 T-cell memory is potential. Depending on the model system employed or on variables present during priming, the number and functional quality of resulting memory cells can vary greatly. Furthermore, the importance of memory CD4 T cells in orchestrating immune responses can vary depending on the pathogen challenge, and on the quality of memory CD8 T cells and B cells. Thus, the continued integration of observations from a variety of experimental models will be required to further understand and utilize the full potential of memory CD4 T cells.
Figure 4.
Both through cell-to-cell contact and through cytokine and chemokine production, memory CD4 T cells have the potential to impact a broad range of both innate and adaptive immune functions during a secondary response. DC, dendritic cell.
Acknowledgments
This work was supported by the National Institutes of Health (grants P01AI04630 and P01AI04566 to S.L.S.), the Department of Defense (HR#3222), and the Trudeau Institute.
Disclosures
The authors have no competing financial interests nor conflicts of interest to disclose.
References
- 1.Powell TJ, Strutt T, Reome J, Hollenbaugh JA, Roberts AD, Woodland DL, Swain SL, Dutton RW. Priming with cold-adapted influenza a does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J Immunol. 2007;178:1030–8. doi: 10.4049/jimmunol.178.2.1030. [DOI] [PubMed] [Google Scholar]
- 2.Garcia S, DiSanto J, Stockinger B. Following the development of a CD4 T cell response in vivo: from activation to memory formation. Immunity. 1999;11:163–71. doi: 10.1016/s1074-7613(00)80091-6. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 4.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 5.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–6. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–35. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Openshaw P, Murphy EE, Hosken NA, Maino V, Davis K, Murphy K, O’Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–67. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–55. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Swain SL, Agrewala JN, Brown DM, et al. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol Rev. 2006;211:8–22. doi: 10.1111/j.0105-2896.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varga SM, Welsh RM. Stability of virus-specific CD4+ T cell frequencies from acute infection into long term memory. J Immunol. 1998;161:367–74. [PubMed] [Google Scholar]
- 12.Bradley LM, Duncan DD, Yoshimoto K, Swain SL. Memory effectors: a potent, IL-4-secreting helper T cell population that develops in vivo after restimulation with antigen. J Immunol. 1993;150(8 Pt 1):3119–30. [PubMed] [Google Scholar]
- 13.Topham DJ, Doherty PC. Longitudinal analysis of the acute Sendai virus-specific CD4+ T cell response and memory. J Immunol. 1998;161:4530–5. [PubMed] [Google Scholar]
- 14.Hu H, Huston G, Duso D, Lepak N, Roman E, Swain SL. CD4(+) T cell effectors can become memory cells with high efficiency and without further division. Nat Immunol. 2001;2:705–10. doi: 10.1038/90643. [DOI] [PubMed] [Google Scholar]
- 15.Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–87. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 16.Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25:610–5. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Wojciechowski S, Jordan MB, Zhu Y, White J, Zajac AJ, Hildeman DA. Bim mediates apoptosis of CD127(lo) effector T cells and limits T cell memory. Eur J Immunol. 2006;36:1694–706. doi: 10.1002/eji.200635897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–45. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swain SL, Croft M, Dubey C, Haynes L, Rogers P, Zhang X, Bradley LM. From naive to memory T cells. Immunol Rev. 1996;150:143–67. doi: 10.1111/j.1600-065x.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 20.Jelley-Gibbs DM, Lepak NM, Yen M, Swain SL. Two distinct stages in the transition from naive CD4 T cells to effectors, early antigen-dependent and late cytokine-driven expansion and differentiation. J Immunol. 2000;165:5017–26. doi: 10.4049/jimmunol.165.9.5017. [DOI] [PubMed] [Google Scholar]
- 21.Harbertson J, Biederman E, Bennett KE, Kondrack RM, Bradley LM. Withdrawal of stimulation may initiate the transition of effector to memory CD4 cells. J Immunol. 2002;168:1095–102. doi: 10.4049/jimmunol.168.3.1095. [DOI] [PubMed] [Google Scholar]
- 22.McKinstry KK, Golech S, Lee WH, Huston G, Weng NP, Swain SL. Rapid default transition of CD4 T cell effectors to functional memory cells. J Exp Med. 2007;204:2199–211. doi: 10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–15. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J Exp Med. 2007;204:547–57. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dooms H, Abbas AK. Control of CD4+ T-cell memory by cytokines and costimulators. Immunol Rev. 2006;211:23–38. doi: 10.1111/j.0105-2896.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 27.Soroosh P, Ine S, Sugamura K, Ishii N. OX40-OX40 ligand interaction through T cell-T cell contact contributes to CD4 T cell longevity. J Immunol. 2006;176:5975–87. doi: 10.4049/jimmunol.176.10.5975. [DOI] [PubMed] [Google Scholar]
- 28.Salek-Ardakani S, Croft M. Regulation of CD4 T cell memory by OX40 (CD134) Vaccine. 2006;24:872–83. doi: 10.1016/j.vaccine.2005.07.108. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg AD, Evans DE, Thalhofer C, Shi T, Prell RA. The generation of T cell memory: a review describing the molecular and cellular events following OX40 (CD134) engagement. J Leukoc Biol. 2004;75:962–72. doi: 10.1189/jlb.1103586. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins SJ, Perona-Wright G, Worsley AG, Ishii N, MacDonald AS. Dendritic cell expression of OX40 ligand acts as a costimulatory, not polarizing, signal for optimal Th2 priming and memory induction in vivo. J Immunol. 2007;179:3515–23. doi: 10.4049/jimmunol.179.6.3515. [DOI] [PubMed] [Google Scholar]
- 31.Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci U S A. 2007;104:15045–50. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKinstry KK, Strutt TM, Swain SL. The effector to memory transition of CD4 T cells. Immunol Res. 2008;40:114–27. doi: 10.1007/s12026-007-8004-y. [DOI] [PubMed] [Google Scholar]
- 33.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–42. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 34.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–9. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 35.De Boer RJ, Homann D, Perelson AS. Different dynamics of CD4+ and CD8+ T cell responses during and after acute lymphocytic choriomeningitis virus infection. J Immunol. 2003;171:3928–35. doi: 10.4049/jimmunol.171.8.3928. [DOI] [PubMed] [Google Scholar]
- 36.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–61. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J Exp Med. 2002;196:935–46. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson JM, MacLeod M, Marsden VS, Kappler JW, Marrack P. Not all CD4+ memory T cells are long lived. Immunol Rev. 2006;211:49–57. doi: 10.1111/j.0105-2896.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 39.Gray D. A role for antigen in the maintenance of immunological memory. Nat Rev Immunol. 2002;2:60–5. doi: 10.1038/nri706. [DOI] [PubMed] [Google Scholar]
- 40.Gray D, Matzinger P. T cell memory is short-lived in the absence of antigen. J Exp Med. 1991;174:969–74. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gray PM, Reiner SL, Smith DF, Kaye PM, Scott P. Antigen-experienced T cells limit the priming of naive T cells during infection with Leishmania major. J Immunol. 2006;177:925–33. doi: 10.4049/jimmunol.177.2.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fazilleau N, Eisenbraun MD, Malherbe L, Ebright JN, Pogue-Caley RR, McHeyzer-Williams LJ, McHeyzer-Williams MG. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat Immunol. 2007;8:753–61. doi: 10.1038/ni1472. [DOI] [PubMed] [Google Scholar]
- 44.Turner DL, Cauley LS, Khanna KM, Lefrancois L. Persistent antigen presentation after acute vesicular stomatitis virus infection. J Virol. 2007;81:2039–46. doi: 10.1128/JVI.02167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jelley-Gibbs DM, Dibble JP, Brown DM, Strutt TM, McKinstry KK, Swain SL. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. J Immunol. 2007;178:7563–70. doi: 10.4049/jimmunol.178.12.7563. [DOI] [PubMed] [Google Scholar]
- 46.Freitas AA, Rocha B. Population biology of lymphocytes: the flight for survival. Annu Rev Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- 47.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–6. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 48.Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J Exp Med. 1999;189:701–10. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swain SL. CD4 T-cell memory can persist in the absence of class II. Philos Trans R Soc Lond B Biol Sci. 2000;355:407–11. doi: 10.1098/rstb.2000.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabbagh L, Snell LM, Watts TH. TNF family ligands define niches for T cell memory. Trends Immunol. 2007;28:333–9. doi: 10.1016/j.it.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Swain SL. Generation and in vivo persistence of polarized Th1 and Th2 memory cells. Immunity. 1994;1:543–52. doi: 10.1016/1074-7613(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 52.Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J Immunol. 2000;164:2338–46. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 53.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164:265–72. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 54.Chandok MR, Okoye FI, Ndejembi MP, Farber DL. A biochemical signature for rapid recall of memory CD4 T cells. J Immunol. 2007;179:3689–98. doi: 10.4049/jimmunol.179.6.3689. [DOI] [PubMed] [Google Scholar]
- 55.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152:2675–85. [PubMed] [Google Scholar]
- 56.London CA, Perez VL, Abbas AK. Functional characteristics and survival requirements of memory CD4+ T lymphocytes in vivo. J Immunol. 1999;162:766–73. [PubMed] [Google Scholar]
- 57.Bates JT, Bucy RP. Enhanced responsiveness to antigen contributes more to immunological memory in CD4 T cells than increases in the number of cells. Immunology. 2005;116:318–27. doi: 10.1111/j.1365-2567.2005.02227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacLeod M, Kwakkenbos MJ, Crawford A, Brown S, Stockinger B, Schepers K, Schumacher T, Gray D. CD4 memory T cells survive and proliferate but fail to differentiate in the absence of CD40. J Exp Med. 2006;203:897–906. doi: 10.1084/jem.20050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta M, Satyaraj E, Durdik JM, Rath S, Bal V. Differential regulation of T cell activation for primary versus secondary proliferative responses. J Immunol. 1997;158:4113–21. [PubMed] [Google Scholar]
- 60.Ndejembi MP, Teijaro JR, Patke DS, Bingaman AW, Chandok MR, Azimzadeh A, Nadler SG, Farber DL. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol. 2006;177:7698–706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 61.Jaleco S, Swainson L, Dardalhon V, Burjanadze M, Kinet S, Taylor N. Homeostasis of naive and memory CD4+ T cells: IL-2 and IL-7 differentially regulate the balance between proliferation and Fas-mediated apoptosis. J Immunol. 2003;171:61–8. doi: 10.4049/jimmunol.171.1.61. [DOI] [PubMed] [Google Scholar]
- 62.Inaba M, Kurasawa K, Mamura M, Kumano K, Saito Y, Iwamoto I. Primed T cells are more resistant to Fas-mediated activation-induced cell death than naive T cells. J Immunol. 1999;163:1315–20. [PubMed] [Google Scholar]
- 63.Bradley LM, Atkins GG, Swain SL. Long-term CD4+ memory T cells from the spleen lack MEL-14, the lymph node homing receptor. J Immunol. 1992;148:324–31. [PubMed] [Google Scholar]
- 64.Dong J, Ivascu C, Chang HD, et al. IL-10 is excluded from the functional cytokine memory of human CD4+ memory T lymphocytes. J Immunol. 2007;179:2389–96. doi: 10.4049/jimmunol.179.4.2389. [DOI] [PubMed] [Google Scholar]
- 65.McKinstry KK, Strutt TM, Buck A, et al. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–63. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moore KW, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 67.Budd RC, Cerottini JC, MacDonald HR. Phenotypic identification of memory cytolytic T lymphocytes in a subset of Lyt-2+ cells. J Immunol. 1987;138:1009–13. [PubMed] [Google Scholar]
- 68.Agrewala JN, Brown DM, Lepak NM, Duso D, Huston G, Swain SL. Unique ability of activated CD4+ T cells but not rested effectors to migrate to non-lymphoid sites in the absence of inflammation. J Biol Chem. 2007;282:6106–15. doi: 10.1074/jbc.M608266200. [DOI] [PubMed] [Google Scholar]
- 69.Bradley LM, Harbertson J, Watson SR. Memory CD4 cells do not migrate into peripheral lymphnodes in the absence of antigen. Eur J Immunol. 1999;29:3273–84. doi: 10.1002/(SICI)1521-4141(199910)29:10<3273::AID-IMMU3273>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 70.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 71.Kassiotis G, Stockinger B. Anatomical heterogeneity of memory CD4+ T cells due to reversible adaptation to the microenvironment. J Immunol. 2004;173:7292–8. doi: 10.4049/jimmunol.173.12.7292. [DOI] [PubMed] [Google Scholar]
- 72.Bingaman AW, Patke DS, Mane VR, Ahmadzadeh M, Ndejembi M, Bartlett ST, Farber DL. Novel phenotypes and migratory properties distinguish memory CD4 T cell subsets in lymphoid and lung tissue. Eur J Immunol. 2005;35:3173–86. doi: 10.1002/eji.200526004. [DOI] [PubMed] [Google Scholar]
- 73.Kassiotis G, Gray D, Kiafard Z, Zwirner J, Stockinger B. Functional specialization of memory Th cells revealed by expression of integrin CD49b. J Immunol. 2006;177:968–75. doi: 10.4049/jimmunol.177.2.968. [DOI] [PubMed] [Google Scholar]
- 74.Kalia V, Sarkar S, Ahmed R. Fine-tuning CD4(+) central memory T cell heterogeneity by strength of stimulation. Eur J Immunol. 2008;38:15–9. doi: 10.1002/eji.200738044. [DOI] [PubMed] [Google Scholar]
- 75.Moulton VR, Farber DL. Committed to memory: lineage choices for activated T cells. Trends Immunol. 2006;27:261–7. doi: 10.1016/j.it.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Kalia V, Sarkar S, Gourley TS, Rouse BT, Ahmed R. Differentiation of memory B and T cells. Curr Opin Immunol. 2006;18:255–64. doi: 10.1016/j.coi.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 77.Reiner SL, Sallusto F, Lanzavecchia A. Division of labor with a workforce of one: challenges in specifying effector and memory T cell fate. Science. 2007;317:622–5. doi: 10.1126/science.1143775. [DOI] [PubMed] [Google Scholar]
- 78.Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med. 2002;196:957–68. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.D’Souza WN, Hedrick SM. Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J Immunol. 2006;177:777–81. doi: 10.4049/jimmunol.177.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peixoto A, Evaristo C, Munitic I, Monteiro M, Charbit A, Rocha B, Veiga-Fernandes H. CD8 single-cell gene coexpression reveals three different effector types present at distinct phases of the immune response. J Exp Med. 2007;204:1193–205. doi: 10.1084/jem.20062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J Exp Med. 2006;203:1045–54. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–7. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 83.Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. Compromised influenza virus-specific CD8(+)-T-cell memory in CD4(+)-T-cell-deficient mice. J Virol. 2002;76:12388–93. doi: 10.1128/JVI.76.23.12388-12393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Livingstone AM, Wilson EB, Ontiveros F, Wang JC. Unravelling the mechanisms of help for CD8+ T cell responses. Immunol Res. 2009;45:209–217. doi: 10.1007/s12026-009-8102-0. [DOI] [PubMed] [Google Scholar]
- 85.Brown DM, Roman E, Swain SL. CD4 T cell responses to influenza infection. Semin Immunol. 2004;16:171–7. doi: 10.1016/j.smim.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 86.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 87.Panus JF, McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific T helper cell function: differential cytokine expression in primary and memory responses. J Exp Med. 2000;192:1301–16. doi: 10.1084/jem.192.9.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 89.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468–76. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harari A, Bart PA, Stohr W, et al. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;205:63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duvall MG, Precopio ML, Ambrozak DA, et al. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol. 2008;38:350–63. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aagaard CS, Hoang TT, Vingsbo-Lundberg C, Dietrich J, Andersen P. Quality and vaccine efficacy of CD4+ T cell responses directed to dominant and subdominant epitopes in ESAT-6 from Mycobacterium tuberculosis. J Immunol. 2009;183:2659–68. doi: 10.4049/jimmunol.0900947. [DOI] [PubMed] [Google Scholar]
- 93.Stout RD, Bottomly K. Antigen-specific activation of effector macrophages by IFN-gamma producing (TH1) T cell clones. Failure of IL-4-producing (TH2) T cell clones to activate effector function in macrophages. J Immunol. 1989;142:760–5. [PubMed] [Google Scholar]
- 94.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 95.Lin AA, Tripathi PK, Sholl A, Jordan MB, Hildeman DA. Gamma interferon signaling in macrophage lineage cells regulates central nervous system inflammation and chemokine production. J Virol. 2009;83:8604–15. doi: 10.1128/JVI.02477-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–55. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 98.Teijaro JR, Njau MN, Verhoeven D, Chandran S, Nadler SG, Hasday J, Farber DL. Costimulation modulation uncouples protection from immunopathology in memory T cell responses to influenza virus. J Immunol. 2009;182:6834–43. doi: 10.4049/jimmunol.0803860. [DOI] [PubMed] [Google Scholar]
- 99.Banchereau J, Bazan F, Blanchard D, et al. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 100.McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–5. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 101.Murata K, Ishii N, Takano H, Miura S, Ndhlovu LC, Nose M, Noda T, Sugamura K. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J Exp Med. 2000;191:365–74. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferguson SE, Han S, Kelsoe G, Thompson CB. CD28 is required for germinal center formation. J Immunol. 1996;156:4576–81. [PubMed] [Google Scholar]
- 103.Kamperschroer C, Roberts DM, Zhang Y, Weng NP, Swain SL. SAP enables T cells to help B cells by a mechanism distinct from Th cell programming or CD40 ligand regulation. J Immunol. 2008;181:3994–4003. doi: 10.4049/jimmunol.181.6.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abbas AK, Burstein HJ, Bogen SA. Determinants of helper T cell-dependent antibody production. Semin Immunol. 1993;5:441–7. doi: 10.1006/smim.1993.1050. [DOI] [PubMed] [Google Scholar]
- 105.Ozaki K, Spolski R, Feng CG, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–4. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 106.Hsu HC, Yang P, Wang J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–75. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 107.Croft M, Swain SL. Recently activated naive CD4 T cells can help resting B cells, and can produce sufficient autocrine IL-4 to drive differentiation to secretion of T helper 2-type cytokines. J Immunol. 1995;154:4269–82. [PubMed] [Google Scholar]
- 108.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 109.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–3. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 110.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 111.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 112.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 113.Kumaraguru U, Banerjee K, Rouse BT. In vivo rescue of defective memory CD8+ T cells by cognate helper T cells. J Leukoc Biol. 2005;78:879–87. doi: 10.1189/jlb.0105007. [DOI] [PubMed] [Google Scholar]
- 114.Krawczyk CM, Shen H, Pearce EJ. Memory CD4 T cells enhance primary CD8 T-cell responses. Infect Immun. 2007;75:3556–60. doi: 10.1128/IAI.00086-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hwang ML, Lukens JR, Bullock TN. Cognate memory CD4+ T cells generated with dendritic cell priming influence the expansion, trafficking, and differentiation of secondary CD8+ T cells and enhance tumor control. J Immunol. 2007;179:5829–38. doi: 10.4049/jimmunol.179.9.5829. [DOI] [PubMed] [Google Scholar]
- 116.Gao FG, Khammanivong V, Liu WJ, Leggatt GR, Frazer IH, Fernando GJ. Antigen-specific CD4+ T-cell help is required to activate a memory CD8+ T cell to a fully functional tumor killer cell. Cancer Res. 2002;62:6438–41. [PubMed] [Google Scholar]
- 117.Wilson EB, Livingstone AM. Cutting edge: CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. J Immunol. 2008;181:7445–8. doi: 10.4049/jimmunol.181.11.7445. [DOI] [PubMed] [Google Scholar]
- 118.Blachere NE, Morris HK, Braun D, Saklani H, Di Santo JP, Darnell RB, Albert ML. IL-2 is required for the activation of memory CD8+ T cells via antigen cross-presentation. J Immunol. 2006;176:7288–300. doi: 10.4049/jimmunol.176.12.7288. [DOI] [PubMed] [Google Scholar]
- 119.Ahmadzadeh M, Hussain SF, Farber DL. Effector CD4 T cells are biochemically distinct from the memory subset: evidence for long-term persistence of effectors in vivo. J Immunol. 1999;163:3053–63. [PubMed] [Google Scholar]
- 120.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–98. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]