Abstract
Immunological memory is one of the features that define the adaptive immune response: by generating specific memory cells after infection or vaccination, the host provides itself with a set of cells and molecules that can prevent future infections and disease. Despite the obvious importance of memory cells, memory CD4 T cells are incompletely understood. Here we discuss recent progress in understanding which activated T cells surmount the barrier to enter into the memory pool and, once generated, what signals are important for memory cell survival. There is still, however, little understanding of how (or even whether) memory CD4 T cells are useful once they have been created; a surprising thought considering the critical role CD4 T cells play in all adaptive primary immune responses. In light of this, we will discuss how CD4 T memory T cells respond to reactivation in vivo and whether they are malleable to a re-assignment of their effector response.
Keywords: CD4, infection, memory, reactivation, T cell
The many roles of CD4 T cells in the primary response
Naïve CD4 T cells must usually interact with mature antigen-loaded dendritic cells (DCs) to be successfully activated. This interaction takes place in the T-cell areas of secondary lymphoid organs, locations that have evolved to facilitate contact between antigen-specific T cells and DCs. The ensuing response is defined by this initial interaction: the specificity of the response is controlled by the peptides presented on major histocompatibility complex (MHC) class II molecules on the DCs, while the size and type of response are determined by costimulatory molecules and cytokines expressed by the activated DCs.
The clonal proliferation that follows CD4 T-cell activation serves several purposes. First, it provides a greater number of cells that are able to help both CD8 T cells and B cells. It has long been known that CD4 T cells are essential for B-cell germinal centre formation, class switching and affinity maturation;1 such cells are now defined as T follicular helper cells.2 CD4 T cells are also required for effective CD8 T-cell responses. When the inflammation induced by immunization or infection is low, CD8 T-cell activation requires the presence of CD4 T cells responding to antigens associated with those driving the CD8 T cells. The CD4 T cells probably function by properly activating the DCs that will present antigen to the CD8 T cells and may also themselves signal directly to the CD8 T cells via expression of CD40 or cytokines such as interleukin (IL)-2.3–5 In more inflammatory environments, CD8 T cells can be activated in the absence of CD4 T cells; however, the memory cells generated from these responses respond poorly to reactivation.6 Therefore, CD4 T cells are crucial for the generation of functional and protective antigen-specific CD8 T cells and B cells.
The second reason that specific CD4 T cells must expand is so that the cells can differentiate into effector cells that can migrate to infection sites. Cytokine-producing T helper (Th) type 1 or Th2 CD4 cells can activate macrophages to expel micro-organisms, for example Mycobacterium tuberculosis in the lungs or helminthes in the gut, respectively.7–9 Similarly, the more recently described Th17 cells, which make both IL-17 and IL-22, may play important roles in defence against fungi and extracellular bacteria either by recruiting other cell types or by inducing antimicrobial factors.10–14 Th1 cells can also act in an indirect manner to protect the host. For example, Nakanishi et al.15 found that herpes simplex virus-specific CD4 T cells migrate to the vagina and produce the interferon (IFN)-γ required to induce local chemokine expression which allows effector CD8 T cells to enter the infection site. Activated T cells can also differentiate into induced regulatory T cells. These cells are important in controlling immune responses via either cell surface molecules or inhibitory cytokines;16 how these regulatory T cells relate to memory cells is a complicated issue that has been discussed elsewhere.17
More than one pathway to memory
Towards the end of the immune response, most antigen-specific T cells die, but a small percentage convert to memory cells. Considerable effort has gone into searches for markers that distinguish the cells that will become memory cells versus those that will die. Underlying these searches is the notion that conversion to memory cells is not a random event but rather predetermined in some way. The correlation of expression of the IL-7 receptor alpha chain on activated CD4 T cells with survival suggested that increased signalling via IL-7 was responsible for memory cell generation.18,19 However, increasing the number of either CD4 or CD8 T cells that received an IL-7 signals did not result in an increase in the number of cells that entered the memory pool.20,21 More recently, low-level expression of the inhibitory receptor, killer cell lectin-like receptor G1 has been found on the CD8 T cells that go on to enter the memory pool;22 whether the same is true for CD4 cells remains to be determined.
The strength of the signal through the T-cell receptor (TCR) may also influence whether an activated CD4 T cell proceeds into the memory pool: cells that receive relatively weak signals lose out to competing T cells that have higher affinity TCRs.23 This finding contrasts with a theory that stems from recent work from the Reiner group.24 Their findings, although primarily in CD8 T cells, suggest that memory cells may be generated as a consequence of asymmetric cell division. During cell division, certain factors can be found on opposing sides of the dividing cell such that daughter cells receive an unequal share of these factors. The consequence of this is that, while one daughter proceeds down the effector pathway, the other, with the same TCR, is more likely to become a memory cell. In this case, therefore, differentiation of the memory cell is independent of TCR affinity.
In contrast to the limited progress in identifying CD4 memory cell precursors, it is now clear that memory cells can be generated from cells that have undergone effector differentiation during the primary response. In an elegant study, Harrington et al25 genetically marked IFN-γ-producing CD4 T cells during a primary response, and these cells were present in the subsequent memory pool. Similarly, Lohning et al.26 purified cytokine-producing antigen-specific T cells, and showed that these cells successfully made it into the memory pool following adoptive transfer. This is in contrast to an earlier report that used similar techniques to purify cytokine-producing CD4 T cells, where it was found that differentiated cells were not able to survive following transfer.27 This suggests that, although differentiated cells may be at a disadvantage for survival, they are certainly able to survive the contraction phase.
Memory cell survival: cytokine niches and MHC controversies
In the widely used mouse infection model lymphocytic choriomeningitis virus (LCMV), viral-specific memory CD8 T cells are maintained at an impressively steady level for many years. CD4 T cells, specific for the same virus and present in the same mouse, decline.28 This disparity in survival may be caused by the different abilities of the two cell types to bind to the survival cytokine IL-15. IL-15, a member of the common γ chain family, is central to the survival of CD8 memory T cells.29–32 Although CD4 memory cells can develop in the absence of IL-15, it is now clear that memory CD4 T cells can and do respond to IL-15 and that this contributes to the slow turnover of these cells33,34 (Fig. 1).
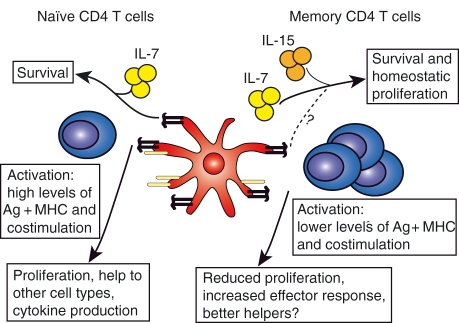
Figure 1.
Comparison of naïve and memory CD4 T cells. Naïve T cells require signals from interleukin (IL)-7 and major histocompatibility complex (MHC) class II molecules to survive. Activation of naïve T cells occurs following interactions with antigen (Ag)-loaded dendritic cells (DCs) that express costimulatory molecules at a high level. Memory CD4 T cells receive survival signals both from IL-7 and IL-15 and there may be a role for MHC II. Activation can occur in the presence of lower levels of peptide–MHC and costimulatory signals. Following activation, memory cells proliferate less than primary responding cells but can produce a greater effector response.
IL-7, however, appears to be the main survival cytokine for CD4 memory cells, as transferred cells cannot survive in its absence.18,19 This cytokine is used by all T cells, and by developing B cells, as a survival signal and therefore is not specific for CD4 memory T-cell survival. This prompts the question: how do T cells compete for this signal? Park et al.35 found that T cells that had recently received an IL-7 signal subsequently down-regulated the receptor, suggesting that T cells are not greedily consuming the cytokine unnecessarily.
Another way in which IL-7 signals can be fairly dished out is by the migration of T cells towards and then away from the stromal cells that produce the cytokine. IL-7 is produced by fibroblastic reticular cells in the T-cell zones of secondary lymphoid organs.36 These cells also produce chemokine (C-C motif) ligand 19 (CCL19), which is recognized by chemokine (C-C motif) receptor 7 (CCR7), a receptor that is expressed on naïve and some memory T cells. These cells are therefore capable of attracting T cells in order to deliver IL-7 directly to them.
IL-7 is also made in the bone marrow, and CD4 T cells can be found here in contact with IL-7-producing cells.37 It has been known for many years that memory CD4 T cells are present in the bone marrow (reviewed by Di Rosa and Pabst38) and that long-lived CD8 memory T cells that have superior homeostatic proliferation potential reside in the bone marrow.39 However, memory phenotype CD4 T cells in the bone marrow may have reduced proliferative potential compared with similar cells in other organs,37 suggesting that these cells may not be permanently maintained in the animal. Moreover, while one group has found that memory CD4 T cells can be maintained at stable levels in the bone marrow while declining in the periphery,37 others have not observed any preferential survival at this site, instead finding that most antigen-specific memory CD4 T cells are found in lymph nodes and the spleen.40
If memory CD4 T cells are able to receive survival signals from IL-7 and to some extent through IL-15, the question remains of why they decline over time. Is it simply because they are not efficient at migrating to cells that make the survival cytokines or is there some further competitive factor at play?
In view of the central role that self-peptide and MHC (pMHC) plays in the development and survival of naïve CD4 cells,41 contact with pMHC is an attractive candidate for memory survival. The finding that CD4 memory cells could survive in the absence of MHC class II molecules implied that contact with pMHC was not required for memory cell survival.42 However, the experiments that produced this finding were carried out in the absence of any other CD4 cells. In such circumstances there would be abundant IL-7, and exaggerated signalling via the IL-7 receptor may allow T cells to survive in circumstances that would normally cause their death. In the presence of a physiological number of T cells, survival signals other than IL-7, for example pMHC, may be required. A study from the Jenkins group suggested that this could be the case, as DO11.10 TCR transgenic memory cells declined at a faster rate when present at higher frequencies in adoptive transfer recipients.43 The TCR transgenic cells would presumably compete with each other for the same pMHC and hence, when their numbers are increased, they would decline more rapidly. However, the interpretation of these results has been muddied by the finding that DO11.10 cells may be rejected from BALB/c recipients as a result of minor mismatches between the strains.44
Other factors, such as the signals present during T-cell priming, can also affect the long-term survival of memory cells. For example, immunization with antigen and Toll-like receptor 2 (TLR2) agonists generated memory cells that survived in greater numbers than those generated by immunization with the same antigen and a TLR4 agonist.45 Regardless, antigen-specific CD4 memory cells have been found to decline both in mouse models and in humans.28,40,46–50 This prompts the question of whether, if the host does not actively maintain them, CD4 memory T cells are irrelevant! As the directors of other cell types during the primary response, CD4 T cells lay the groundwork for the provision of protection, enabling the generation of memory CD8 T cells, B cells and plasma cells, all of which can protect the host from subsequent infection. This does not, however, mean that CD4 T cells cannot be involved in secondary responses. To appreciate what CD4 T cells can do to offer protection, it is important to know what they do upon reactivation in vivo.
Reactivation of CD4 memory cells in vivo– what are CD4 memory cells for?
There is a wealth of knowledge about the ability of CD4 memory cells to respond to lower doses of antigen and/or to reduced levels of costimulation compared with naïve CD4 T cells51–53 (Fig. 1). A teleological explanation for the reduced requirements for activation is that it allows CD4 memory cells to respond very early following an infection, before the pathogen has had time to replicate extensively or cause substantial damage. These activated cells may then directly attack the invading organism or provide help to B cells or cytotoxic T cells.
We found that, although CD4 memory cells proliferated in response to restimulation, they divided for a shorter period of time than primary responding cells.49 This reduced proliferation was a consequence of the reactivated memory cells producing a different cytokine response compared with primary responding cells: both their increased IFN-γ and decreased IL-2 production reduced the proliferation of the memory cells. Thus, CD4 memory cells do not expand as exuberantly as memory CD8 T cells.54 Such reduced expansion might suggest that CD4 cells are not involved in the direct attack of invaders but rather act primarily as catalysts, helping the responses of other cells. However, cytokine-producing memory CD4 T cells can provide protection against some infections, the most studied example being IFN-γ-producing CD4 T cells generated by vaccination with bacillus Calmette–Guérin (BCG) which protect against the worse forms of disease caused by Mycobacterium tuberculosis.8,9 Therefore, migration to, and cytokine production at, infection sites may be the most effective response that memory CD4 T cells can offer.
If it assumed that a primary response has resulted in the development of memory CD8 T cells, B cells and plasma cells, then there is little need for a large number of specific CD4 T cells to help other cell types. Memory B cells probably do not require the help of antigen-specific memory CD4 cells to respond to an infection and, once generated, CD8 memory T cells, which can also respond to lower levels of antigen and costimulation, should not require antigen-specific memory CD4 help.52,55–58 However, it is interesting, and potentially relevant, to ask whether memory CD4 T cells can provide enhanced help to primary responding B cells and CD8 T cells. This may occur under circumstances in which pathogens mutate their antibody and/or CD8 T-cell epitopes to escape from immune attack but leave their CD4 epitopes intact. A prime example of this is influenza virus; annual infections are a consequence of mutations of the cell surface molecules, haemagglutinin and neuraminidase, that are the main antibody targets.59,60
In the case of influenza, epitopes recognized by cytotoxic T lymphocytes (CTLs) have also been found to mutate,61,62 suggesting that viruses capable of escaping either or both antibodies and CTLs are likely to propagate. CD4 epitopes have been found to be conserved within different subtypes of influenza virus, and individuals have been shown to have CD4 T cells that cross-react with emerging stains, such as H5N1, that have developed as a result of re-assortment of different virus subtypes.63–66 Whether these cells can provide protection against infection has not been adequately tested, although there is evidence that this is a possibility. For example, mice primed with a DNA vaccine containing a number of CD4 epitopes were protected from lethal influenza challenge63 and large numbers of in vitro activated TCR transgenic CD4 T cells were found to be able to provide protection, which was at least partly mediated by providing help to B cells.67 However, in one study, although CD4 memory cells accelerated clearance of the virus, the cells caused immunopathology that increased weight loss in the mice that contained memory cells.68 Memory CD4 T cells may also be able to speed up primary CD8 T-cell responses; for example, the CD8-mediated clearance of Listeria moncytogenes was increased in mice that contained antigen-specific CD4 memory cells.69
Re-assigning memory cells
The negative side of reduced requirements for reactivation is that it is very difficult to tolerize memory CD4 T cells. This is an important issue when considering treatments for autoimmune conditions or in transplantation. While general suppression of the immune system can alleviate autoimmunity or transplant rejection, specific tolerization is a much more attractive process. Many studies have been focused on generating regulatory T cells that can inhibit immune responses to self or transplant antigens.70 However, if regulatory T cells are unable to constrain the proliferation and effector functions of self or transplant reactive memory cells, such strategies are unlikely to be successful on their own. Indeed, Yang et al.71 found that memory cells were not inhibited by regulatory T cells in vivo, allowing the memory cells to reject a transplant.
Memory cells are resistant to the induction of tolerance by treatments such as CD40L blockade that render naïve T cells unable to respond to donor tissues.72 However, memory cells are not costimulation-independent, as cytotoxic T-lymphocyte antigen 4 (CTLA-4) treatment inhibited the proliferation of memory cells by reducing their ability to make IL-2 compared with cells stimulated under normal conditions.73 Moreover, while memory cells could proliferate in the absence of CD40-CD40L signals, they were unable to make IFN-γ.48
While it has been difficult to tolerize memory cells, it may be possible to change their function, or to assign a new identity to an uncommitted T central memory cell. Recently there has been a growing appreciation of the flexibility of Th cell subsets.74 Th differentiation is driven by the milieu of cytokines present during T-cell activation resulting in the expression of signature cytokines that are controlled by specific transcription factors. Wei et al.75 examined histone modifications in CD4 T cells differentiated in vitro. The authors found that, while the signature genes associated with a particular Th subset, such as Tbet and IFN-γ in Th1 cells, were marked by histone modifications that enhance transcription, genes associated with other subsets were less likely to be marked by inhibitory histone modifications. This was particularly true of Th17 cells, which were shown to re-differentiate into Th1 cells when transforming growth factor (TGF)-β was removed and IL-12 provided.76 These data suggest that it may be possible to re-educate memory CD4 T cells to produce a less harmful response. However, T cells primed in either a type 1 or type 2 environment, and then restimulated in the opposing environment, were found to make both IFN-γ and IL-4,77 suggesting that, while memory T cells can be persuaded to secrete additional cytokines, they nevertheless remain true to their original activating environment.
Concluding remarks
In summary, CD4 memory cells remain somewhat elusive in the sense that is it still difficult to appreciate how they are generated and maintained, and what they are capable of doing once reactivated. However, with the use of advancing technology, such as MHC class II tetramers,40,48,49 cytokine reporter mice,25,78 and a widening interest in CD4 T cells and infectious disease,15,67,68 a greater understanding of the in vivo roles that CD4 T cells can play will be possible.
Acknowledgments
This work was supported by NIH grants NIH-AI-18785, AI-22295 and AI-52225.
Glossary
Abbreviations:
- Ag
antigen
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- IFN-γ
interferon gamma
- IL
interleukin
- LCMV
lymphocytic choriomeningitis virus
- pMHC
self-peptide + major histocompatibility complex
- TCR
T-cell receptor
- Th
T helper
Conflict of interest
The authors have no financial or commercial conflict of interest.
References
- 1.MacLennan IC, Gulbranson-Judge A, Toellner KM, Casamayor-Palleja M, Chan E, Sze DM, Luther SA, Orbea HA. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev. 1997;156:53–66. doi: 10.1111/j.1600-065x.1997.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 2.Fazilleau N, McHeyzer-Williams LJ, McHeyzer-Williams MG. Local development of effector and memory T helper cells. Curr Opin Immunol. 2007;19:259–67. doi: 10.1016/j.coi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–3. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–3. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 5.Janssen EM, Droin NM, Lemmens EE, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 6.Williams MA, Bevan MJ. Effector and Memory CTL Differentiation. Annu Rev Immunol. 2007;25:171–92. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 7.Anthony RM, Urban JF, Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–60. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 11.Glocker EO, Hennigs A, Nabavi M, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–35. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 14.Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–3. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21:281–5. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–15. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun JC, Lehar SM, Bevan MJ. Augmented IL-7 Signaling during Viral Infection Drives Greater Expansion of Effector T Cells but Does Not Enhance Memory. J Immunol. 2006;177:4458–63. doi: 10.4049/jimmunol.177.7.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tripathi P, Mitchell TC, Finkelman F, Hildeman DA. Cutting Edge: limiting amounts of IL-7 do not control contraction of CD4+ T cell responses. J Immunol. 2007;178:4027–31. doi: 10.4049/jimmunol.178.7.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–95. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–45. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang JT, Palanivel VR, Kinjyo I, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–91. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 25.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–60. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- 26.Lohning M, Hegazy AN, Pinschewer DD, et al. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J Exp Med. 2008;205:53–61. doi: 10.1084/jem.20071855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu CY, Kirman JR, Rotte MJ, et al. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol. 2002;3:852–8. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 28.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–9. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 29.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J Exp Med. 2002;196:935–46. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–8. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 31.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–8. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–9. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 33.Lenz DC, Kurz SK, Lemmens E, Schoenberger SP, Sprent J, Oldstone MB, Homann D. IL-7 regulates basal homeostatic proliferation of antiviral CD4+T cell memory. Proc Natl Acad Sci U S A. 2004;101:9357–62. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–61. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 36.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–65. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 37.Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grun JR, Lohning M, Radbruch A. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30:721–30. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 38.Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005;26:360–6. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Parretta E, Cassese G, Barba P, Santoni A, Guardiola J, Di Rosa F. CD8 cell division maintaining cytotoxic memory occurs predominantly in the bone marrow. J Immunol. 2005;174:7654–64. doi: 10.4049/jimmunol.174.12.7654. [DOI] [PubMed] [Google Scholar]
- 40.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived T(H)1 memory cells and short-lived T(H)17 cells. Nat Immunol. 2010;11:83–9. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–62. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 42.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–3. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 43.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–6. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 44.Duffy D, Sparshott SM, Yang CP, Bell EB. Transgenic CD4 T Cells (DO11.10) Are Destroyed in MHC-Compatible Hosts by NK Cells and CD8 T Cells. J Immunol. 2008;180:747–53. doi: 10.4049/jimmunol.180.2.747. [DOI] [PubMed] [Google Scholar]
- 45.Chandran SS, Verhoeven D, Teijaro JR, Fenton MJ, Farber DL. TLR2 engagement on dendritic cells promotes high frequency effector and memory CD4 T cell responses. J Immunol. 2009;183:7832–41. doi: 10.4049/jimmunol.0901683. [DOI] [PubMed] [Google Scholar]
- 46.Cauley LS, Cookenham T, Miller TB, Adams PS, Vignali KM, Vignali DA, Woodland DL. Cutting edge: virus-specific CD4+ memory T cells in nonlymphoid tissues express a highly activated phenotype. J Immunol. 2002;169:6655–8. doi: 10.4049/jimmunol.169.12.6655. [DOI] [PubMed] [Google Scholar]
- 47.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 48.MacLeod M, Kwakkenbos MJ, Crawford A, Brown S, Stockinger B, Schepers K, Schumacher T, Gray D. CD4 memory T cells survive and proliferate but fail to differentiate in the absence of CD40. J Exp Med. 2006;203:897–906. doi: 10.1084/jem.20050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacLeod MK, McKee A, Crawford F, White J, Kappler J, Marrack P. CD4 memory T cells divide poorly in response to antigen because of their cytokine profile. Proc Natl Acad Sci U S A. 2008;105:14521–6. doi: 10.1073/pnas.0807449105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naniche D, Garenne M, Rae C, Manchester M, Buchta R, Brodine SK, Oldstone MB. Decrease in measles virus-specific CD4 T cell memory in vaccinated subjects. J Infect Dis. 2004;190:1387–95. doi: 10.1086/424571. [DOI] [PubMed] [Google Scholar]
- 51.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164:265–72. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 52.Berard M, Tough DF. Qualitative differences between naive and memory T cells. Immunology. 2002;106:127–38. doi: 10.1046/j.1365-2567.2002.01447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J Immunol. 2000;164:2338–46. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 54.Ravkov EV, Williams MA. The magnitude of CD4+ T cell recall responses is controlled by the duration of the secondary stimulus. J Immunol. 2009;183:2382–9. doi: 10.4049/jimmunol.0900319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hebeis BJ, Klenovsek K, Rohwer P, Ritter U, Schneider A, Mach M, Winkler TH. Activation of virus-specific memory B cells in the absence of T cell help. J Exp Med. 2004;199:593–602. doi: 10.1084/jem.20030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leclerc C, Sedlik C, Lo-Man R, Charlot B, Rojas M, Deriaud E. Stimulation of a memory B cell response does not require primed helper T cells. Eur J Immunol. 1995;25:2533–8. doi: 10.1002/eji.1830250919. [DOI] [PubMed] [Google Scholar]
- 57.MacLeod MK, Clambey ET, Kappler JW, Marrack P. CD4 memory T cells: what are they and what can they do? Semin Immunol. 2009;21:53–61. doi: 10.1016/j.smim.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–33. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doherty PC, Turner SJ, Webby RG, Thomas PG. Influenza and the challenge for immunology. Nat Immunol. 2006;7:449–55. doi: 10.1038/ni1343. [DOI] [PubMed] [Google Scholar]
- 60.Brown LE, Kelso A. Prospects for an influenza vaccine that induces cross-protective cytotoxic T lymphocytes. Immunol Cell Biol. 2009;87:300–8. doi: 10.1038/icb.2009.16. [DOI] [PubMed] [Google Scholar]
- 61.Moskophidis D, Kioussis D. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J Exp Med. 1998;188:223–32. doi: 10.1084/jem.188.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boon AC, de Mutsert G, Graus YM, Fouchier RA, Sintnicolaas K, Osterhaus AD, Rimmelzwaan GF. Sequence variation in a newly identified HLA-B35-restricted epitope in the influenza A virus nucleoprotein associated with escape from cytotoxic T lymphocytes. J Virol. 2002;76:2567–72. doi: 10.1128/jvi.76.5.2567-2572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alexander J, Bilsel P, del Guercio MF, et al. Universal influenza DNA vaccine encoding conserved CD4+ T cell epitopes protects against lethal viral challenge in HLA-DR transgenic mice. Vaccine. 2010;28:664–72. doi: 10.1016/j.vaccine.2009.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee LY, Ha do LA, Simmons C, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008;118:3478–90. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richards KA, Chaves FA, Sant AJ. Infection of HLA-DR1 transgenic mice with a human isolate of influenza a virus (H1N1) primes a diverse CD4 T-cell repertoire that includes CD4 T cells with heterosubtypic cross-reactivity to avian (H5N1) influenza virus. J Virol. 2009;83:6566–77. doi: 10.1128/JVI.00302-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greenbaum JA, Kotturi MF, Kim Y, et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc Natl Acad Sci U S A. 2009;106:20365–70. doi: 10.1073/pnas.0911580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–98. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 68.Teijaro JR, Njau MN, Verhoeven D, Chandran S, Nadler SG, Hasday J, Farber DL. Costimulation modulation uncouples protection from immunopathology in memory T cell responses to influenza virus. J Immunol. 2009;182:6834–43. doi: 10.4049/jimmunol.0803860. [DOI] [PubMed] [Google Scholar]
- 69.Krawczyk CM, Shen H, Pearce EJ. Memory CD4 T cells enhance primary CD8 T-cell responses. Infect Immun. 2007;75:3556–60. doi: 10.1128/IAI.00086-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waldmann H, Adams E, Cobbold S. Reprogramming the immune system: co-receptor blockade as a paradigm for harnessing tolerance mechanisms. Immunol Rev. 2008;223:361–70. doi: 10.1111/j.1600-065X.2008.00632.x. [DOI] [PubMed] [Google Scholar]
- 71.Yang J, Brook MO, Carvalho-Gaspar M, et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci U S A. 2007;104:19954–9. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol. 2004;172:5456–66. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 73.Ndejembi MP, Teijaro JR, Patke DS, Bingaman AW, Chandok MR, Azimzadeh A, Nadler SG, Farber DL. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol. 2006;177:7698–706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 74.Locksley RM. Nine lives: plasticity among T helper cell subsets. J Exp Med. 2009;206:1643–6. doi: 10.1084/jem.20091442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei G, Wei L, Zhu J, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–67. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krawczyk CM, Shen H, Pearce EJ. Functional plasticity in memory T helper cell responses. J Immunol. 2007;178:4080–8. doi: 10.4049/jimmunol.178.7.4080. [DOI] [PubMed] [Google Scholar]
- 78.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–29. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]