Abstract
Human immunodeficiency virus (HIV) -specific T-cell responses are detectable in the female genital tract of HIV-infected women but little is known about their frequency or the factors that influence their detection. We investigated the feasibility of polyclonal in vitro expansion of cervical cytobrush-derived T cells to investigate HIV-specific responses in the female genital tract in HIV-infected women. Cytobrush-derived cervical cells were isolated from 22 HIV-infected women and expanded with anti-CD3 and recombinant interleukin-2. Cervical T-cell lines were investigated for Gag-specific responses by interferon-γ ELISPOT and compared with those detected in matched blood samples. Cervical T-cell lines were established from 16/22 (72·7%) participants. Although the absolute number of CD3± cells recovered after expansion was positively associated with the number of cells isolated ex vivo (P = 0·01; R = 0·62), we observed a significant negative correlation between fold expansion and ex vivo cell number (P = 0·004; R = −0·68). We show that both the magnitude (P = 0·002; R = 0·7) and specific Gag regions targeted by cervical T-cell lines (P < 0·0001; R = 0·5) correlated significantly with those detected in blood. With one exception, cervical interferon-γ T-cell responses to Gag were detected only in HIV-infected women with blood Gag-specific response > 1000 spot-forming units/106 cells. We conclude that cervical Gag-specific T-cell responses in expanded lines are most easily detectable in women who have corresponding high-magnitude Gag-specific T-cell responses in blood.
Keywords: cervical, expansion, gag, human immunodeficiency virus, mucosal, T cell
Introduction
Sexual transmission of human immunodeficiency virus (HIV) accounts for the majority of new infections worldwide.1 In women, the mucosal surface of the female genital tract is ultimately the site of HIV infection and transmission.2 Although there is clear evidence that HIV-specific cytotoxic T lymphocytes (CTLs) in blood play an important role in controlling HIV replication systemically,3–7 comparatively little is known about both CTL responses to HIV in the genital tract and the factors that govern these responses. HIV-specific mucosal CTLs have been detected in men and women during chronic HIV infection.8–14 The potential importance of such CTLs in protection from HIV is highlighted by the fact that they have also been detected in individuals who, despite frequent exposure to HIV-1, fail to become productively infected.10 In chronically HIV-infected men14 and women,13 the detection of HIV-specific T cells in the genital tract was independent of HIV shedding and viral load in genital secretions.
There is an urgent need for reliable, validated and non-invasive methods for investigating mucosal immune responses in the female genital tract. Such methods would be particularly valuable in HIV vaccine trial settings where intensive mucosal sampling is not an option. To date, however, the HIV-specific mucosal CTL literature has been dominated by biopsy approaches to isolating lymphocytes from rectal and gastrointestinal mucosal tissue.9,15–19 Fewer approaches are available for sampling mucosal tissue from the female genital tract (cervical lavages and cervical cytobrushes). The usefulness of both these methods is, however, constrained by the low numbers of ex vivo lymphocytes they yield.10,12–14
Here we investigate the feasibility of polyclonal in vitro expansion of cervical cytobrush-derived T cells to investigate HIV-specific responses in the female genital tract. We show that cervical T cells sampled from women with chronic HIV-1 infection can be expanded and that the magnitude of expansion is significantly associated with initial ex vivo cell yield and viability. Following in vitro expansion, we found that both the magnitude and breadth of HIV-specific T-cell responses at the cervix correlate significantly with those detected in blood. Cervical responses were, however, generally only detectable in women with corresponding blood HIV-specific T-cell responses above 1000 spot-forming units (SFU)/106 cells.
Materials and methods
Study population
Twenty-seven women with chronic HIV infection were enrolled. All women had CD4 counts > 300 cells/μl and were antiretroviral therapy naïve at the time of study. Samples were not collected if participants were menstruating. All women gave informed consent and the Research Ethics Committee of the University of Cape Town approved all aspects of the study.
Collection and processing of cervical and blood specimens
Cervical samples were collected using a cytobrush as previously described.13,20 Briefly, a Digene cervical cytobrush was inserted into the cervical os and rotated through 360°. The cytobrush was immediately placed in a 15-ml tube containing ice-cold transport medium or R10 (RPMI-1640 medium, supplemented with 10% heat-inactivated human AB serum, 5 mm l-glutamine, fungazone, 50 U/ml penicillin and 50 μg/ml streptomycin). The cervical samples were kept in a Bench-top cooler (Nalgene, Rochester, NY, USA) at 4° until transport to the laboratory and were processed within 4 hr of sampling. Of the 27 samples collected, five (18·5%) were discarded because they were visibly contaminated with blood. Red blood cell contamination was measured by macroscopic visual inspection of cells in suspension and following centrifugation. We have previously demonstrated that macroscopic assessment of red blood cell contamination has a threshold of sensitivity equivalent to ≤ 0·0005% peripheral blood mononuclear cell (PBMC) contamination per cytobrush sample.20
Cervical cells were isolated from the cytobrush by flushing approximately 20 times using a sterile plastic disposable pipette to dislodge mucus. The cell suspension was centrifuged at 437 g for 10 min and the pellet was resuspended in R10.
Whole blood was collected in ACD Vacutainer tubes [Becton Dickinson (BD) Biosciences, Plymouth, UK] by venepuncture. The PBMCs were isolated from whole blood by density gradient centrifugation using Ficoll–Histopaque (Sigma-Aldrich, Egham, Runnymede, UK) and LeucoSep® centrifuge tubes (Greiner Bio-one, Frickenhausen, Germany). Cell concentrations were adjusted to 1 × 106 to 2 × 106 cells/ml and incubated overnight at 37° in 5% CO2 for use in enzyme-linked immunosorbent spot-forming cell assays (ELISPOT). Mononuclear cells were counted using Trypan Blue staining to assess viability.
Expansion of cervical T cells
Polyclonal expansion of cervical cells was performed using anti-CD3 [Anti-CD3 monoclonal antibody (mAb); Clone UCHT1; Lot no MAB100; R&D Biosystems, Minneapolis, MN, USA] in the presence of recombinant human interleukin-2 (rhIL-2; NIH AIDS Research and Reference Reagent Program, Germantown, MD). UCHT1 is a well-characterized immunoglobulin G1 mouse mAb that identifies a determinant present on all human T cells and appears to share the same characteristics as OKT3.21 Freshly isolated cervical cells were plated (at 4 × 100 μl per well per cytobrush) into 96-well round-bottomed plates pre-coated with anti-CD3 mAb (10 μg/ml). Irradiated autologous PBMCs (106 cells/ml; 100 μl/well; irradiated at 40 Gy) were added to each well as feeders. Recombinant hIL-2 was added to each well at a final concentration of 100 IU/ml. Cervical cytobrush T-cell lines were incubated at 37° in 5% CO2 and cultures were supplemented every 2 days with fresh R10 containing rhIL-2 (100 IU/ml). Wells containing irradiated feeder cells alone in the presence and absence of anti-CD3 mAb and rhIL-2 were included on each plate to control for outgrowth of feeders. The T-cell lines were re-stimulated after 14 days with anti-CD3 mAb (10 μg/ml) and fresh medium supplemented with rhIL-2 (100 IU/ml). Throughout culture, all R10 was supplemented with 2 mg/ml fungazone, 50 U/ml penicillin and 50 μg/ml streptomycin and cell lines were monitored for bacterial and fungal infections, periodically counted and adjusted to 1 × 105 cells/well.
Cervical cytobrush cells and T-cell lines were analysed for expression of CD4 and CD8 T-cell markers before and after polyclonal expansion by flow cytometry. Because the number of cervical mononuclear cells available on day 0 was limited, only 10% (volume/volume) of the sample was used for determination of the CD4 : CD8 ratio on day 0. The antibodies used were allophycocyanin-conjugated CD3 (CD3-APC), fluorescein isothiocyanate-conjugated CD8 (CD8-FITC) and CD4-FITC (BD Biosciences, San Jose, CA). Cells were resuspended in 0·5 ml R10 and 50–100 μl (depending on cell count) of cell suspension was aliquoted for surface staining. Flow cytometric acquisition was performed using a four-colour FACSCalibur flow cytometer (BD Biosciences) and analysed using flowjo (Tree Star, Inc., Ashland, OR) software.
HIV-1 Gag peptides
The HIV-1 subtype C Du422 Gag overlapping peptides spanning the entire Gag sequence were kindly provided by Dr Clive Gray (National Institute for Communicable Diseases, Johannesburg, South Africa) and consisted of 66 (15-mer to 18-mer) peptides overlapping by 10 amino acids. Lyophilized stocks of each peptide were dissolved in dimethyl sulphoxide at a concentration of 20 mg/ml and stored at − 80°. The 66 Gag peptides were divided into five pools each containing 14 peptides (except for pool 5, which had 10 peptides).
Interferon-γ ELISPOT
The production of interferon-γ (IFN-γ) by HIV Gag-specific T cells was detected by ELISPOT. Briefly, 96-well nitrocellulose plates were pre-coated with IFN-γ mAb (clone 1-D1K; Mabtech, Stockholm, Sweden). Cervical mononuclear cells were washed twice, counted and plated into wells either with or without HIV-1 subtype C Gag peptides (1 μg/ml) or in phytohaemagglutinin. Fresh PBMCs were plated in triplicate at 1 × 105 cells/well and expanded mucosal cells were plated at 0·5 × 105 cells/ml. Plates were incubated overnight at 37° in a 5% CO2 humidified incubator. After washing, bound IFN-γ was detected by a second biotinylated IFN-γ mAb (clone 7-B6-1; Mabtech), followed by streptavidin-bound horseradish peroxidase and visualization was performed using a Nova Red substrate kit (Vector Laboratories, Burlingame, CA). Finally, individual cytokine-producing cells (spots) were counted using a CTL ELISpot reader (Cellular Technology, Jessup, MD). Totals for plated wells were averaged and normalized to numbers of IFN-γ spot-forming cells per 1 × 106 PBMCs (SFU/106 PBMCs). Mean values for negative media control wells were subtracted from the mean values of antigen-stimulated wells to give net SFU/106 cells. Assays with a negative control background mean of < 100 SFU/106 cells were considered valid and a positive response was defined as exceeding the mean + 2 standard deviations.12
Intracellular cytokine staining of PBMCs following HIV Gag stimulation
To define which T-cell subset was contributing to the HIV Gag-specific IFN-γ responses detected by ELISPOT, we also assessed IFN-γ production by PBMCs using intracellular cytokine staining. The PBMCs (2 × 106 to 3 × 106 cell/ml) were stimulated with HIV Gag peptides (1 μg/ml), staphylococcal enterotoxin-B (10 μg/ml; Sigma-Aldrich; positive control) or not stimulated (negative controls) for 6 hr at 37° in 5% CO2. Brefeldin A (10 μg/ml; Sigma, St Louis, MO) was added after the first hour. After 6 hr, cells were washed with fluorescence-activated cell sorting (FACS) wash buffer [10% fetal calf serum–phosphate-buffered saline (FCS-PBS) containing 0·01% NaN3] and centrifuged at 200 g for 5 min. The cells were fixed and permeabilized with 2 ml BD cytofix/cytoperm solution (BD Biosciences-Pharmingen, San Diego, CA) for 10 min at room temperature. Cells were then washed once with 0·1% weight/volume Saponin (Fluka Biochemica, Mulhouse, France) with PBS (containing 5% FCS and 0·01% NaN3) at 200 g for 5 min. Cells were then stained with phenotypic markers APC-labelled anti-CD3, peridinin chlorophyll protein (PerCP) -labelled CD4, and FITC-labelled anti-CD8 (BD Biosciences). To detect IFN-γ, cells were simultaneously stained with phycoerythrin (PE) -labelled anti-IFN-γ (BD Biosciences-Pharmingen) for 30 min on ice. Cells were washed with 2 ml FACS wash buffer, centrifuged at 200 g for 5 min and fixed with 1% paraformaldyde in PBS. Stained PBMCs were acquired on a FACSCalibur flow cytometer (BD Biosciences) and data were analysed using either Cellquest (BD Biosciences) version 4 or flowjo (Tree Star, Inc) software version 6. To define a cut-off for positive HIV Gag responses by either CD8+ or CD4+ T cells, IFN-γ responses to Gag peptides were initially evaluated in a panel of 10 HIV-negative women [Western blood Transfusion Service (WBTS), Cape Town, South Africa]. CD8+ or CD4+ T-cell responses to Gag greater than threefold above background were considered positive.
Impact of polyclonal expansion on Vβ T-cell receptor usage by PBMCs
The impact of polyclonal expansion on Vβ T-cell receptor (TCR) usage by PBMCs was evaluated using the IOTest Beta Mark flow cytometry kit according to the manufacturer’s protocol (Immunotech, Marseille, France). The kit contains eight distinct combinations of conjugated TCR Vβ antibodies corresponding to 24 different specificities (Vβ1, Vβ2, Vβ3, Vβ4, Vβ5.1, Vβ5.2, Vβ5.3, Vβ7.1, Vβ7.2, Vβ8, Vβ9, Vβ11, Vβ12, Vβ13.1, Vβ13.2, Vβ13.6, Vβ14, Vβ16, Vβ17, Vβ18, Vβ20, Vβ21.3, Vβ22, Vβ23). Briefly, unexpanded (day 0) or polyclonally expanded (day 28) PBMCs (2 × 106 to 3 × 106 cells/ml) from four individuals [patient identities (PID) 23–26] were each divided into eight BD FACS tubes. The eight tubes were then stained with a panel of 24 Vβ family-specific antibodies (three Vβ specificities/tube). Antibodies directed against Vβ specificities were restricted to FITC, PE and APC, so we added anti-CD3 PerCP (BD Biosciences, San Diego) to differentiate T-cell subsets. All tubes were incubated at 4° for 30 min, then washed twice and fixed by the addition of 500 μl BD Cell fix. Cells were acquired using a FACSCalibur flow cytometer (BD Biosciences) and were analysed using flowjo (Tree Star, Inc) software.
Statistical analysis
Statistical analyses were performed using graphpad prism 5 (Graph Pad Software, San Diego). The Mann–Whitney U-test was applied for independent sample comparison, the Wilcoxon Ranks Test was used for matched non-parametric comparisons and Spearman Ranks or Pearson correlation was applied for correlation comparisons. A P-value of ≤0·05 was considered significant.
Results
Twenty-two women with chronic HIV infection were investigated for HIV Gag-specific cervical cytobrush-derived T-cell responses following in vitro expansion (Table 1). All the study subjects were under the age of 40 years (median age 31 years, range 22–39), had a median blood CD4 count of 451 cells/μl (range 300–1300) and plasma viral load of 10 097 (range 970–1 300 000). Five of the 22 (22·7%) women had visible vaginal discharge or yeast infections at the time the cytobrush was taken.
Table 1.
Description of human immunodeficiency virus (HIV) -infected women included in the study
| Patient ID | Age | CD4 count (cell/μl) | HIV viral load (RNA copies/ml) | Macroscopic findings | Day 0 cervical cell counts | Day 14 cervical cell counts | Day 28 cervical cell counts | Fold expansion (day 28) |
|---|---|---|---|---|---|---|---|---|
| 1 | 33 | 1396 | < 501 | Vaginal discharge | 20 000 | 100 000 | 1 600 000 | 80 |
| 2 | 26 | 414 | 9500 | Nil | 10 000 | 100 000 | 680 000 | 68 |
| 3 | 23 | 341 | 14 500 | Nil | 10 000 | 310 000 | 480 000 | 48 |
| 4 | 30 | 428 | < 501 | Nil | 14 278 | Contaminated | Contaminated | – |
| 5 | 28 | 698 | 1800 | Vaginal discharge | 18 520 | Contaminated | Contaminated | – |
| 6 | 28 | 341 | 100 573 | Nil | 40 000 | 130 000 | 560 000 | 14 |
| 7 | 36 | 572 | 51 325 | Nil | 68 640 | 125 600 | 1 650 000 | 24 |
| 8 | 26 | 607 | 4013 | Nil | 75 800 | 114 000 | Contaminated | – |
| 9 | 31 | 482 | 27 250 | Nil | 80 000 | 140 000 | 990 890 | 12·4 |
| 10 | 30 | 545 | 15 800 | Nil | 90 000 | 440 000 | 1 780 000 | 19·8 |
| 11 | 32 | 400 | 1 300 000 | Cervix inflamed | 90 860 | Contaminated | Contaminated | – |
| 12 | 31 | 393 | 41 700 | Nil | 100 000 | 610 000 | 850 000 | 8·5 |
| 13 | 22 | 301 | 2200 | Nil | 100 000 | 270 000 | 1 750 000 | 17·5 |
| 14 | 32 | 461 | 7100 | Nil | 110 000 | 490 000 | 1 040 000 | 9·5 |
| 15 | 31 | 788 | 1926 | Nil | 120 000 | 790 000 | 1 200 000 | 10 |
| 16 | 33 | 451 | 30 000 | Nil | 120 000 | 260 000 | 1 900 000 | 15·8 |
| 17 | 24 | ND | 3649 | Nil | 135 721 | 617 600 | 1 460 000 | 10·8 |
| 18 | 39 | 422 | 5450 | Nil | 138 040 | 652 000 | Contaminated | – |
| 19 | 39 | 338 | < 501 | Nil | 180 000 | 240 000 | 1 200 000 | 6·7 |
| 20 | 43 | 467 | 88 000 | Yeast | 218 064 | Contaminated | Contaminated | – |
| 21 | 37 | 300 | 10 097 | Vaginal discharge | 220 000 | 740 000 | 2 120 000 | 9·6 |
| 22 | 38 | 482 | 970 | Nil | 240 000 | 530 000 | 2 850 000 | 11·9 |
| Median | 31 | 451 | 10 097 | 5/22 (22·7%) | 100 000 | 290 000 | 1 330 000 | 13·2 |
| Range | 22–39 | 300–1300 | 970–1 300 000 | 10 000–240 000 | 132 500–590 000 | 480 000–2 850 000 | 6·0–80·0 |
Less than detectable Limit.
Expansion of cervical cytobrush-derived T cells from women with chronic HIV infection
We obtained a median of 0·1 × 106 cells (range 0·01 × 106 to 0·2 × 106 cells) per cytobrush ex vivo (Table 1). We initially investigated the association between ex vivo cervical cell yields and both age and clinical HIV disease status (CD4 counts and viral load). We observed a significant positive association between the age of women in the study and the yield of cervical cells obtained (P = 0·0008; Pearson R = 0·66) indicating that older age was associated with increased yield of cervical cells. We found no association between markers of disease status (CD4 count and viral load) and cervical yield (data not shown). However, all women included in this study were recruited during the chronic phase of infection with CD4 counts ≥ 300 cell/μl (CD4 counts ranging from 300 to 1300 cells/μl; Table 1). Age and clinical status were not associated with viability of cervical cytobrush cells in this study (data not shown). None of the women in the study were menstruating at the time of cytobrush collection although we did not control for menstrual cycle stage. We have previously shown that ex vivo cervical cytobrush mononuclear cell yields are significantly associated with genital tract inflammation.22 Although we have not measured inflammatory cytokine concentrations in genital secretions in this study, genital inflammatory mediators are likely to have a substantial impact on ex vivo cervical mononuclear cell yield.
Cervical T cells were polyclonally expanded for either 28 days (18/22) or 42 days (4/22) to increase the yield of cells from the cervix for the identification of peptide responses to Gag by IFN-γ ELISPOT. Of the 22 cervical T-cell lines initiated, 16/22 (72·7%) expanded. We obtained a median of 1·3 × 106 cells (range 0·5 × 106 to 2·9 × 106 cells) at day 28, which represented a 13-fold increase in T-cell yield compared with ex vivo cell yields (Table 1 and Fig. 1a). Six of 22 lines (27·3%) were lost to contamination during the first few days of culture. Of these, three of the six contaminated samples were collected from women with visible cervical discharge.
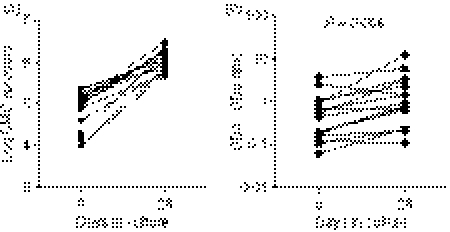
Figure 1.
Impact of ex vivo yield on expansion of cervical cytobrush-derived cervical mononuclear cells (CMC). (a) Comparison between day 0 and day 28 CMC yields in donors whose cervical CD3 cells expanded. (b) Comparison of CD4 : CD8 ratio ex vivo (day 0) and day 28 in expanders. CMC were cultured in vitro with anti-CD3 monoclonal antibody in the presence of recombinant human interleukin-2 (rhIL-2). The CMC counts and viability were performed by Trypan blue counting. CD4 : CD8 ratios on cervical cell lines were analysed before and after polyclonal expansion using allophycocyanin conjugated CD3 and fluorescein isothiocyanate-conjugated CD8 and CD4 staining on a FACSCalibur flow cytometer and analysis was performed using flowjo software. Wilcoxon Rank Test was applied to compare yield and CD4 : CD8 ratios at day 0 and day 28. P-values < 0·05 were considered significant. Each data point represents an individual donor.
The CD8 T cells were found to be the dominant T-cell subset detected ex vivo (CD4 : CD8 of 0·5); however, 28 days of in vitro expansion resulted in the ratio equalizing such that we obtained a CD4 : CD8 ratio of 1 (Fig. 1b; P = 0·006, Wilcoxon Rank Test). Of the cervical lymphocytes isolated ex vivo, 90·9% (± 7·5; mean ± SD) were viable (range 70–100%). We found a significant positive correlation between the absolute number of cells obtained following expansion and both the number of cervical cells isolated ex vivo (P = 0·01; R = 0·62) and the ex vivo viability of cells (P = 0·0002; R = 0·71). Conversely, we found a significant negative correlation between fold expansion by 28 days and ex vivo cell number (P = 0·004; R = −0·68), indicating that ex vivo cervical cells with the lowest yield exhibited the highest relative rate of expansion.
Gag-specific responses by cervical T cells
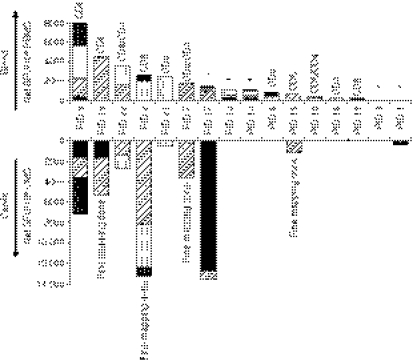
The HIV-1 Gag-specific responses were mapped by IFN-γ ELISPOT for 16 of 22 cervical T-cell lines (72·7%) from chronically HIV-infected women. These were compared with similarly identified responses identified in PBMCs ex vivo of these women (Fig. 2). Nine of the 16 (56·2%) cervical lines from the chronically HIV-infected women showed clear evidence of HIV-specific T-cell responses to Gag with a cumulative frequency of IFN-γ-producing cells ranging from 350 to 13 553 SFU/106 cells. We found no correlation between cervical ex vivo yield, viability, expansion (day 28) yield, or plasma viral load and the magnitudes of HIV Gag-specific cervical T-cell responses (data not shown). Eight of the nine cervical lines that exhibited Gag specificity were derived from women with clearly detectable Gag-specific blood responses (>600 SFU/106 cells; Fig. 2). There was only one participant who had a detectable cervical response to Gag but no matching blood response.
Figure 2.
Comparison of cervical and blood human immunodeficiency virus (HIV) Gag-specific interferon-γ (IFN-γ) responses in women with chronic HIV infection. Blood (top panel) and cervical (bottom panel) IFN-γ responses to HIV-1 subtype C Gag peptide pools 1–5. Net spot forming units (SFU)/106 was calculated by subtracting background IFN-γ frequency from Gag peptide pool-specific IFN-γ frequencies in 16 women with chronic HIV infection. Each stacked bar represents the cumulative IFN-γ frequency for all five Gag peptide pools and the subjects have been ordered from highest to lowest blood T-cell magnitude to Gag. The contribution of CD4+ and CD8+ T-cell subsets to overall IFN-γ responses to HIV Gag peptides was determined by intracellular cytokine staining and fluorescence-activated cell sorting. The T-cell subset contributing to Gag-specific responses per donor is shown above each stacked bar for blood responses. CD8 indicates CD8-only response, CD4 indicates CD4 responses only and CD8/CD4 indicates that responses in both subsets were detected. Asterisks indicate that responses were not evaluated in these individuals. ‘Fine mapping done’ denotes the four women for whom this analysis was carried out.
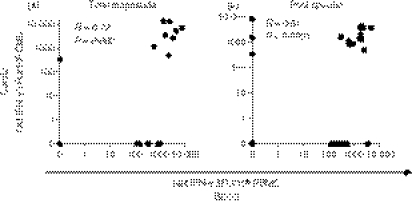
From the 16 women for which HIV Gag ELISPOT mapping was performed (Fig. 2), there was a significant correlation between the cervix and blood in both the total magnitude of IFN-γ responses to Gag (Table 2 and Fig. 3a; rho = 0·7, P = 0·002; Spearman Ranks test) and the individual Gag pool magnitudes of IFN-γ responses per individual (Table 2 and Fig. 3b; rho = 0·51, P < 0·0001; Spearman Ranks test). Despite good maintenance of specific Gag pool targeting between cervix and blood, four of the nine women (44·4%) with detectable HIV-specific responses at the cervix had unique pool specificities that were not detected in blood (PID 1, 2, 17 and 19; Fig. 2 and Table 2).
Table 2.
Comparison between breadth of response at cervix and in blood
| Cumulative magnitude (spot-forming units/106) |
Gag pools differently targeted |
||||
|---|---|---|---|---|---|
| Patient ID | Blood | Cervix | Gag pools shared | Blood | Cervix |
| 9 | 8040 | 7130 | 1,2,5 | 3,4 | – |
| 19 | 4605 | 5280 | 2 | – | 1 |
| 22 | 3565 | 2640 | 2,3 | – | – |
| 2 | 2610 | 13 240 | 3,5 | – | 2 |
| 21 | 2440 | 490 | 3 | – | – |
| 7 | 1740 | 3540 | 2 | – | – |
| 17 | 1480 | 13 553 | 2 | 3,5 | 1 |
| 13 | 1000 | 0 | – | 1,2,3 | – |
| 14 | 1100 | 0 | – | 1,2,3,4,5 | – |
| 6 | 850 | 0 | – | 3,5 | – |
| 15 | 600 | 1140 | – | 2 | – |
| 10 | 360 | 0 | – | 2,4 | – |
| 16 | 0 | 0 | – | 2 | – |
| 12 | 165 | 0 | – | 1 | – |
| 3 | 120 | 0 | – | – | – |
| 1 | 0 | 350 | – | – | 5 |
| Median | 1050 | 420 | |||
| Range | 0–8040 | 0–13 553 | |||
Figure 3.
Correlation between human immunodeficiency virus (HIV) Gag-specific interferon-γ (IFN-γ) response magnitudes detected at the cervix and in blood of HIV-infected subjects. (a) Correlation between cumulative net IFN-γ responses to Gag pools (sum of five pools) per chronically HIV-infected individual in each compartment (n = 16 women for whom matched enzyme-linked immunosorbent spot-forming cell assay was conducted on cervical lines and peripheral blood mononuclear cells). (b) Correlation between individual Gag pools (1–5) at the cervix and in blood in HIV-infected women. Each data point represents an individual’s IFN-γ response at the cervix and in blood. Spearman Rank Test was applied to test correlations and P-values < 0·05 were considered significant. Spearman R-value is shown on each plot.
Individual peptide mapping was performed on cervical lines derived from four of the nine women with detectable Gag pool responses who had sufficient cervical cell numbers to allow finer mapping (PID 2, 7, 15 and 19; Table 3). All four women had cervical T-cell responses that preferentially targeted peptides in p24 and three of the women targeted the same peptide within p24 containing the human leucocyte antigen (HLA) class I TL9 epitope (178GATPQDLNTMLNTVGGH194). Although the HLA for the participants in this study are not known, the peptides recognized by these four women had a number of previously characterized HLA class I and fewer HLA class II epitopes embedded within them (Table 3; predicted by the Los Alamos Database; http://www.hiv.lanl.gov/content/sequence/ELF/epitope_analyzer.html).
Table 3.
Epitope prediction from confirmed peptide specificities on cervical samples
| PID | HIV protein | Net SFU/million cells | Peptide | HLA I epitope | HLA I type | HLA II epitope | HLA II type |
|---|---|---|---|---|---|---|---|
| 19 | p24 | 880 | 24 | 170PMFTALSEGATPQDLNTM187 | 170PMFTALSEGATPQDLNTM187 | ||
| 170PMFTALSEGATPQDLNTM187 | B42, B*4403 | ||||||
| p24 | 4680 | 25 | 178GATPQDLNTMLNTVGGH194 | B*3910, B*4201, B*8101, B*0702, Cw*0802 | 178GATPQDLNTMLNTVGGH194 | ||
| 2 | p24 | 5600 | 22 | 155WVKVIEEKAFSPEVIPMF172 | B*1503 | 155WVKVIEEKAFSPEVIPMF172 | DRB1*0101 |
| 155WVKVIEEKAFSPEVIPMF172 | B*4006 | 155WVKVIEEKAFSPEVIPMF172 | DQ5 | ||||
| 155WVKVIEEKAFSPEVIPMF172 | B*4501 | ||||||
| 155WVKVIEEKAFSPEVIPMF172 | B*4501 | ||||||
| 155WVKVIEEKAFSPEVIPMF172 | Cw*0602 | ||||||
| p24 | 2040 | 25 | 155WVKVIEEKAFSPEVIPMF172 | B57, B63 | 178GATPQDLNTMLNTVGGH194 | ||
| p24 | 6120 | 33 | 155WVKVIEEKAFSPEVIPMF172 | B63, B57, B58 | 234SDIAGTTSTLQEQIAWM250 | ||
| p2p7p1p6 | 680 | 57 | 155WVKVIEEKAFSPEVIPMF172 | B*35 | |||
| 178GATPQDLNTMLNTVGGH194 | B*3910, B*4201, B*8101, B*0702, Cw*0802 | ||||||
| 234SDIAGTTSTLQEQIAWM250 | B*5701, B*5703, B*5801 | ||||||
| 414WKCGKEGHQMKDCTERQA431 | A11 | ||||||
| 15 | p24 | 3210 | 25 | 178GATPQDLNTMLNTVGGH194 | B*3910, B*4201, B*8101, B*0702, Cw*0802 | 178GATPQDLNTMLNTVGGH194 | |
| 7 | p24 | 3820 | 23 | 163AFSPEVIPMFTALSEGA179 | 163AFSPEVIPMFTALSEGA179 | ||
| 163AFSPEVIPMFTALSEGA179 | B63, B57, B58 | ||||||
| 163AFSPEVIPMFTALSEGA179 | A*2601, A*2603 |
HLA, human leucocyte antigen; HIV, human immunodeficiency virus; PID, patient identity; SFU, spot-forming units. Previously defined immunodominant epitope are represented in bold.
Although the low cervical cell numbers generated in this study did not allow us to evaluate whether the cervical Gag-specific responses detected (Fig. 2) were specific to the CD8+ or CD4+ T-cell subsets, we evaluated T-cell subset-specific IFN-γ responses to Gag in matched blood samples from 11/16 women by intracellular cytokine staining. The majority of these women had only CD8+ T-cell responses to Gag (7/11; PID 2, 6, 9, 12, 15, 19, 21), three women had Gag-specific responses in both CD4+ and CD8+ subsets (PID 7, 10, 22) and one woman had only a CD4+ response (PID 16). Of these 11 women, seven had detectable cervical responses to Gag. Five of these seven women had exclusively CD8+ T-cell responses to Gag in blood and two had both CD4+ and CD8+ T-cell responses.
Although it was not possible to confirm which T-cell subset was contributing to the cervical T-cell responses detected in this study (Fig. 2), the fact that the individual peptides mapped in four individuals (Table 3) contained both HLA class I and II epitopes and that flow cytometry on matched blood samples showed both CD8+ and CD4+ responses systemically to Gag implies that both T-cell subsets may be involved. The dominance of CD8+ responses in blood together with the number of HLA class I predicted epitopes suggests, however, that CD8+ T-cells are likely to be the dominant T-cell subset involved in the female genital tract as well.
Impact of expansion on HIV-specificity and T-cell Vβ repertoire in blood
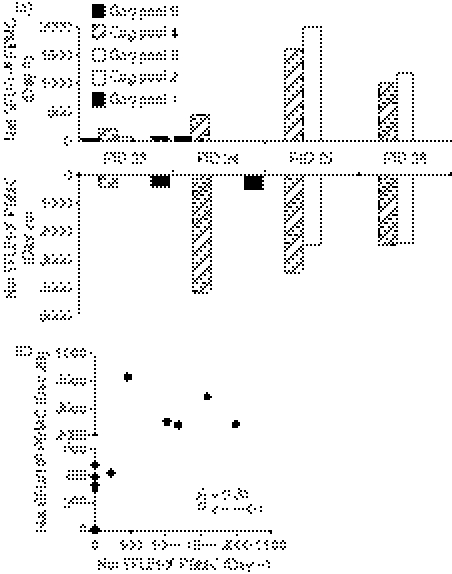
To evaluate the impact of polyclonal expansion with anti-CD3 mAb on skewing of Gag regions targeted by HIV-specific T cells, PBMCs from four HIV-infected individuals with well-characterized ex vivo Gag-specific responses were examined before and following expansion by IFN-γ ELISPOT (Fig. 4). The dominant ex vivo Gag regions targeted were maintained following in vitro expansion in all individuals. Compared with ex vivo responses (day 0), there was an overall increase in the cumulative frequency of IFN-γ-producing cells following in vitro expansion, ranging from twofold to 11-fold. The frequency of Gag pool responses at day 0 was, however, significantly correlated with the frequencies following expansion for 28 days (Fig. 4b; R = 0·81; P < 0·0001; Spearman Ranks Test) indicating that the specificity profile was largely maintained following expansion. In a single donor (PID 26), however, a unique specificity to pool 5 (485 SFU/106 cells) was observed after expansion that was not detectable ex vivo. This highlights the possibility that specificities of low frequency that are not apparent ex vivo may become detectable following expansion because of the observed overall increase in cumulative frequency. It is also important to note that only one of the four unique Gag specificities that we observed in cervical samples (PID 1) had a magnitude of < 500 SFU/106 cells whereas unique cervical specificities from the other three donors (PID 2, 17, 19) had magnitudes of > 1500 SFU/106 cells. This implies that the unique cervical specificities we recorded for at least three of the four cervical donors represent ‘real’ unique responses and are not the result of expansion of previously low-frequency events.
Figure 4.
Correlation between ex vivo and expanded human immunodeficiency virus (HIV) Gag-specific interferon-γ (IFN-γ) enzyme-linked immunosorbent spot-forming cell assay responses in blood from chronically HIV-infected subjects. (a) Peripheral blood mononuclear cells (PBMCs) from four HIV-infected women were assessed for HIV-specific IFN-γ production ex vivo (top panel) and following in vitro expansion with anti-CD3 monoclonal antibody for 28 days (bottom panel). (b) Correlation between IFN-γ responses detected in PBMCs ex vivo and after 28 days expansion. HIV-1 subtype C Gag overlapping peptides were divided into five pools and responses in these donors to each Gag pool were assessed. Net IFN-γ response to Gag was calculated by subtracting background IFN-γ production in each individual from Gag-specific responses. Each dot (b) represents an individual donor’s matched IFN-γ response to each of the five Gag pools in blood ex vivo and following expansion (day 28). Spearman Rank Test was used to test the correlation and a P-value <0·05 were considered significant.
We next evaluated changes in the TCR Vβ repertoire following 28-day expansion of PBMCs with anti-CD3 and rhIL-2 in these four individuals (Table 4). The profile of TCR Vβ frequencies on day 0 in three of the four study subjects correlated significantly with profiles following in vitro polyclonal expansion (patient 26: R = 0·97, P < 0·0001; patient 24: R = 0·69, P = 0·0002, patient : R = 0·84, P < 0·0001). In contrast, TCR Vβ frequencies for patient 23 following expansion did not correlate and this was probably as a result of the expansion of certain Vβ types. In this individual, Vβ4 expanded 9·9-fold, Vβ7.2 expanded 4·6-fold and Vβ13.1 contracted 7·6-fold over 28 days. We were unable to similarly compare fluctuations in cervical TCR Vβ specificities during the course of expansion because of limiting cervical cell numbers.
Table 4.
T-cell receptor (TCR) Vβ usage ex vivo and following 28 days of in vitro expansion with anti-CD3 and recombinant human interleukin-2
| PID 23 |
PID 24 |
PID 25 |
PID 26 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCR Usage | Day 0 | Day 28 | Ratio Day 28 : 0 | Day 0 | Day 28 | Ratio Day 28 : 0 | Day 0 | Day 28 | Ratio Day 28 : 0 | Day 0 | Day 28 | Ratio Day 28 : 0 |
| Vβ1 | 4·27 | 2·2 | 0·52 | 5·84 | 5·61 | 0·96 | 3·65 | 5·94 | 1·63 | 6·93 | 6·56 | 0·95 |
| Vβ2 | 3·02 | 0·78 | 0·26 | 6·69 | 5·55 | 0·83 | 3·56 | 6·59 | 1·85 | 3·38 | 5·21 | 1·54 |
| Vβ3 | 5·52 | 6·34 | 1·15 | 4·17 | 5·14 | 1·23 | 1·64 | 2·27 | 1·38 | 4·48 | 5·73 | 1·28 |
| Vβ4 | 0·64 | 6·24 | 9·751 | 1·51 | 3·41 | 2·26 | 1·26 | 1·72 | 1·37 | 0·85 | 0·66 | 0·78 |
| Vβ5.1 | 3·22 | 3·52 | 1·09 | 3·85 | 4·26 | 1·11 | 3·12 | 6·19 | 1·98 | 4·5 | 5·71 | 1·27 |
| Vβ5.2 | 1·44 | 1·43 | 0·99 | 0·79 | 0·91 | 1·15 | 1·3 | 1·59 | 1·22 | 1·8 | 1·56 | 0·87 |
| Vβ5.3 | 2·19 | 0·96 | 0·44 | 1·45 | 1·44 | 0·99 | 1·43 | 1·32 | 0·92 | 0·92 | 1·09 | 1·18 |
| Vβ7.1 | 3·42 | 3·23 | 0·94 | 2·23 | 2·41 | 1·08 | 2·17 | 3·93 | 1·81 | 1·73 | 2·56 | 1·48 |
| Vβ7.2 | 0·97 | 4·47 | 4·61 | 1·36 | 1 | 0·74 | 0·66 | 1·56 | 2·36 | 0·34 | 0·85 | 2·50 |
| Vβ8 | 3·86 | 4·54 | 1·18 | 3·96 | 4·43 | 1·12 | 4·24 | 4·67 | 1·10 | 4·6 | 4·99 | 1·08 |
| Vβ9 | 3·4 | 1·69 | 0·50 | 2·18 | 2·58 | 1·18 | 3·56 | 2·37 | 0·67 | 5·09 | 2·52 | 0·50 |
| Vβ11 | 0·95 | 1·54 | 1·62 | 1·06 | 1·33 | 1·25 | 1·07 | 1·32 | 1·23 | 0·4 | 1·22 | 3·05 |
| Vβ12 | 2·17 | 0·82 | 0·38 | 1·47 | 1·4 | 0·95 | 2·17 | 3·02 | 1·39 | 1·53 | 3·57 | 2·33 |
| Vβ13.1 | 6·15 | 0·81 | 0·13 | 2·96 | 3·69 | 1·25 | 3·2 | 4·82 | 1·51 | 4·27 | 4·98 | 1·17 |
| Vβ13.2 | 1·3 | 1·41 | 1·08 | 2·62 | 2·61 | 1·00 | 1·24 | 1·59 | 1·28 | 0·97 | 1·66 | 1·71 |
| Vβ13.6 | 1·97 | 3·03 | 1·54 | 2·31 | 2·43 | 1·05 | 1·74 | 3·19 | 1·83 | 0·89 | 2·3 | 2·58 |
| Vβ14 | 2·72 | 2·73 | 1·00 | 10·55 | 11·1 | 1·05 | 6·27 | 4·24 | 0·68 | 3·15 | 3·52 | 1·12 |
| Vβ16 | 2·36 | 2·39 | 1·01 | 0·93 | 0·95 | 1·02 | 1·59 | 3·18 | 2·00 | 1·27 | 2·4 | 1·89 |
| Vβ17 | 2·54 | 4·06 | 1·60 | 3·57 | 3·8 | 1·06 | 2·47 | 6·23 | 2·52 | 5·96 | 4·39 | 0·74 |
| Vβ18 | 0·64 | 0·55 | 0·86 | 0·36 | 0·46 | 1·28 | 0·56 | 0·62 | 1·11 | 0·81 | 0·96 | 1·19 |
| Vβ20 | 3·03 | 3·37 | 1·11 | 2 | 2·41 | 1·21 | 2·61 | 3·48 | 1·33 | 2·38 | 3·19 | 1·34 |
| Vβ21.3 | 5·42 | 5·48 | 1·01 | 0·55 | 0·87 | 1·58 | 2·64 | 4·42 | 1·67 | 1·99 | 4·17 | 2·10 |
| Vβ22 | 2·55 | 5·16 | 2·02 | 3·87 | 3·94 | 1·02 | 2·43 | 4·68 | 1·93 | 3·54 | 3·94 | 1·11 |
| Vβ23 | 2 | 4·59 | 2·30 | 0·64 | 0·56 | 0·88 | 0·82 | 1·18 | 1·44 | 0·94 | 1·25 | 1·33 |
| Mean | 1·55 | 1·14 | 1·51 | 1·46 | ||||||||
| Pearson R-value | 0·18202 | 0·9736 | 0·6896 | 0·8408 | ||||||||
| Pearson P-value | 0·3946 | < 0·0001 | 0·0002 | < 0·0001 | ||||||||
Changes in any Vβ subset from day 28 to day 0 that were > 2 are denoted in bold.
The correlation between Vβ TCR usage at day 0 and day 28 was tested using a Pearson test and R-values and P-values are shown.
Discussion
Immunological events at the cervix are likely to influence susceptibility to heterosexually-transmitted HIV infection. There is an urgent need for the development of validated, non-invasive methodologies for investigating HIV-specific mucosal immune responses associated with HIV pathogenesis and for vaccine assessment specifically in the female genital tract.10,17 Although cervical cytobrushing is relatively non-invasive, low cervical cell yields associated with this approach have significantly impacted on thorough evaluation of HIV-specific T-cell responses.10,13,17 Although in vitro expansion of cervical cytobrush-derived T cells would circumvent the problem of low yield, there have been few studies investigating the ability of cervical cytobrush-derived T cells to expand in vitro either polyclonally or in response to HIV-1 antigens. We show here that cervical T cells can be isolated by cytobrushing and polyclonally expanded in the majority of women studied. Further, we show that HIV Gag-specific cervical T-cell responses can be detected in HIV-infected women and correlate with responses detected in blood.
With one exception, cervical responses to Gag were detected only in HIV-infected women with blood Gag-specific responses > 1000 SFU/106. Although we achieved a 13-fold expansion of cervical cell numbers, our inability to resolve genital tract responses to Gag in individuals with < 1000 SFU/106 cells in blood suggests that our approach may not be sensitive enough for the evaluation of vaccine efficacy. The reason for this is that vaccine induced T-cell responses to Gag are expected to be substantially lower than those found in HIV-infected individuals. Although the bottleneck imposed by non-invasive cytobrush sampling impinges on the sensitivity achievable in cellular assays such as those described in this study, it is likely that a combination of even moderately improved sampling and cellular expansion methodologies could substantially lower the threshold above which HIV-specific cellular responses could be detected.
Cervical cytobrush-derived T cells were polyclonally expanded in vitro from 73% of samples analysed. We achieved a median of 13-fold expansion of cervical mononuclear cells using anti-CD3 over 28 days. Ibarrondo et al.9 similarly expanded rectal biopsy-derived mucosal T cells from 12 HIV-infected individuals and reported a fourfold to 10-fold expansion of CD8+ T cells over 14 days with bi-specific CD4/3 antibodies. Whereas their method selectively expanded CD8+ T cells, anti-CD3 expansion used in the present study expanded both CD4 and CD8 T cells with an overall twofold enrichment of CD4+ cells following expansion. Although the overall relative expansion described here was higher than in previous studies,9 rectal biopsy mucosal sampling yielded 16-fold to 39-fold more cells than were obtained in our study from a single cytobrush. Although we have focused on a single expansion protocol with anti-CD3 mAb and rhIL-2, it will be important in future studies to compare cervical T-cell expansion kinetics using different expansion protocols, such as bi-specific antibodies,23 anti-CD3/28-coated beads (T-cell expander; Dynabeads; Invitrogen, Oslo, Norway); and addition of alternative or complementary cytokines such as IL-7 and IL-15.24,25
In this study, 27% of cytobrush-derived cervical cultures that were initiated became contaminated within the first 24 hr of culture. Of these cytobrush samples, half were from women with macroscopic evidence of vaginal discharge, cervical inflammation or yeast infection indicating that concomitant cervical infections impact on the sterility of the sample for culture. Although our culture medium for transport of these samples to the laboratory and for processing contains penicillin, streptomycin and amphotericin B, these contaminations could possibly be minimized by including higher concentrations of amphotericin B and antibacterial agents specific for genital tract bacterial infections.
From expanded cervical T-cell lines, we found that HIV Gag-specific cervical T-cell responses were detectable in ∼ 50% of women with chronic HIV infection. From matched blood samples, 88% of these women had systemic responses to Gag. The magnitude of these mucosal responses correlates significantly with the magnitude of Gag-specific responses measured in blood and was largely restricted to women with > 1000 Gag-responsive cells/106 cells. Similarly, Kaul et al.11 showed concordance between HIV-specific responses in blood and at the cervix ex vivo during chronic infection with 8/10 women studied having matching responses at the cervix and in blood. Kaul et al.11 selected participants based on high response frequencies to Gag in blood, which may have impacted on the strong compartmental overlap they described. In comparison, Shacklett et al.12 reported that only three of eight chronically HIV-infected women with detectable HIV-specific responses in blood had a corresponding response at the cervix. Shacklett et al.12 also focused their study on women with well-characterized high-frequency responses in blood. In contrast to these studies, Kaul et al.10 found in both HIV-infected and apparently HIV-resistant uninfected sex workers that ex vivo HIV-specific responses at the cervix were as prevalent as they were in blood, that responses in the two compartments largely correlated and that 95% of women with detectable HIV-specific cervical T-cell responses (40/42) had corresponding blood responses of < 1000 SFU/million cells.
Recently, our laboratory13 reported that ex vivo frequencies of HIV Gag-specific CD8 responses in blood and at the cervix did not correlate. Unlike Kaul et al.11 and Shacklett et al.,12 our previous study13 did not select women with high-magnitude responses in blood and this may have impacted on the lack of concordance observed between compartments ex vivo. Our previous study13 focused ex vivo functional assessment rather than in vitro expansion used in the present study. Ex vivo cytobrush-derived T-cell effector cell distribution is likely to differ substantially from phenotypes present after in vitro expansion, with the former being dominated by effector cells and the latter being dominated by expanded memory cells.26 Although we did not compare the maturational status of ex vivo or expanded cervical T cells, this may play a role in the success of in vitro expansion. Naïve and memory CD8+ T-cell subsets in humans have significantly different capacities to proliferate and differentiate in response to T-cell receptor stimulation or cytokines.27 There are a number of issues that may also impact on the extent of in vitro expansion of cytobrush-derived T cells including the stage of the menstrual cycle at which cytobrushes were collected, concomitant sexually transmitted infections, and vaginal hygiene practices.28–30
Although in vitro polyclonal expansion of T cells is a useful approach to increase the number of cells available from inaccessible mucosal sites, antigen bias and epitope skewing introduced by polyclonal expansion (potentially favouring the outgrowth of certain populations at the expense of others) cannot be entirely excluded. We show here that both CD4+ and CD8+ cervical T cells were expanded and that similarly expanding PBMCs did not significantly alter HIV Gag targeting nor TCR Vβ repertoire in most individuals. Some fluctuations were noted in TCR usage and the range of HIV Gag pools targeted in a minority of women, so we acknowledge that some bias may be introduced by expansion in a subset of individuals.
In summary, this study evaluates the feasibility and efficacy of polyclonal in vitro expansion of cervical cytobrush-derived T cells from women with HIV infection. The low cell yields that can be recovered non-invasively from the female genital tract necessitate focused efforts to optimize effective expansion methodologies. We do not fully understand what would constitute protective immunity against HIV, and immunity at the genital mucosa is likely to play an important role in preventing HIV acquisition, so the incorporation of mucosal sampling at the female genital tract and understanding HIV-specific events at this site should be a high priority during HIV vaccine trials. Our finding that cervical cytobrushing and anti-CD3-mediated polyclonal expansion only enable the detection of Gag-specific T-cell responses in the genital tracts of women with correspondingly high systemic Gag-specific responses sets a benchmark against which to measure future efforts in this field and highlights the need for more efficient expansion methodologies.
Acknowledgments
We thank the women from the Nyanga Day Hospital who kindly participated in the study, Mrs Janine Jones for collecting all the specimens, and Dr Darren Martin for reviewing the manuscript and for his constructive comments. We gratefully acknowledge the provision of recombinant human IL-2 and HIV subtype C Gag peptides by the NIH AIDS Research and Reference Reagent Program. This study was supported by grants from the Doris Duke Charitable Foundation HIV Pathogenesis Program, Wellcome Trust and South African AIDS Vaccine Initiative (SAAVI). A.B. received funding from the Doris Duke Charitable Foundation and Poliomyelitis Research Foundation. A.B., W.B. and J.P. received training in the USA as part of the Columbia University-Southern African Fogarty AITRP Program. J.P. is a Wellcome Trust Intermediate Fellow in Infectious Diseases.
Disclosures
None.
References
- 1.UNAIDS: Report on the Global AIDS epidemic . 2006. http://www.unaids.org/en/HIV_data/2006GlobalReport/default.asp [accessed on 26 May 2007] [Google Scholar]
- 2.Prakash M, Patterson S, Kapembwa MS. Evaluation of the cervical cytobrush sampling technique for the preparation of CD45+ mononuclear cells from the human cervix. J Immunol Methods. 2001;258:37–46. doi: 10.1016/s0022-1759(01)00464-1. [DOI] [PubMed] [Google Scholar]
- 3.Addo MM, Yu XG, Rathod A, et al. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1) -specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J Virol. 2003;77:2081–92. doi: 10.1128/JVI.77.3.2081-2092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, Koup RA, Picker LJ. Analysis of total human immunodeficiency virus (HIV)-specific CD4± and CD8± T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75:11983–91. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. Magnitude of functional CD8± T-cell responses to the gag protein of HIV-1 correlates inversely with viral load in plasma. J Virol. 2002;76:2298–305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufmann DE, Bailey PM, Sidney J, et al. Comprehensive analysis of human immunodeficiency virus type 1-specific CD4 responses reveals marked immunodominance of gag and nef and the presence of broadly recognized peptides. J Virol. 2004;78:4463–77. doi: 10.1128/JVI.78.9.4463-4477.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramduth D, Chetty P, Mngquandaniso NC, et al. Differential immunogenicity of HIV-1 clade C proteins in eliciting CD8± and CD4± cell responses. J Infect Dis. 2005;192:1588–96. doi: 10.1086/496894. [DOI] [PubMed] [Google Scholar]
- 8.Musey L, Ding Y, Cao J, Lee J, Galloway C, Yuen A, Jerome KR, McElrath MJ. Ontogeny and specificities of mucosal and blood human immunodeficiency virus type 1-specific CD8(+) cytotoxic T lymphocytes. J Virol. 2003;77:291–300. doi: 10.1128/JVI.77.1.291-300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibarrondo FJ, Anton PA, Fuerst M, et al. Parallel human immunodeficiency virus type 1-specific CD8+ T-lymphocyte responses in blood and mucosa during chronic infection. J Virol. 2005;79:4289–97. doi: 10.1128/JVI.79.7.4289-4297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaul R, Plummer FA, Kimani J, et al. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J Immunol. 2000;164:1602–11. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- 11.Kaul R, Thottingal P, Kimani J, Kiama P, Waigwa CW, Bwayo JJ, Plummer FA, Rowland-Jones SL. Quantitative ex vivo analysis of functional virus-specific CD8 T lymphocytes in the blood and genital tract of HIV-infected women. AIDS. 2003;17:1139–44. doi: 10.1097/00002030-200305230-00004. [DOI] [PubMed] [Google Scholar]
- 12.Shacklett BL, Cu-Uvin S, Beadle TJ, et al. Quantification of HIV-1-specific T-cell responses at the mucosal cervicovaginal surface. AIDS. 2000;14:1911–5. doi: 10.1097/00002030-200009080-00005. [DOI] [PubMed] [Google Scholar]
- 13.Gumbi PP, Nkwanyana NN, Bere A, et al. Impact of mucosal inflammation on cervical HIV-1-specific CD8 T cell responses in the female genital tract during chronic HIV infection. J Virol. 2008;82:8529–36. doi: 10.1128/JVI.00183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheth PM, Danesh A, Shahabi K, et al. HIV-specific CD8+ lymphocytes in semen are not associated with reduced HIV shedding. J Immunol. 2005;175:4789–96. doi: 10.4049/jimmunol.175.7.4789. [DOI] [PubMed] [Google Scholar]
- 15.Anton PA, Elliott J, Poles MA, et al. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. 2000;14:1761–5. doi: 10.1097/00002030-200008180-00011. [DOI] [PubMed] [Google Scholar]
- 16.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–17. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shacklett BL, Yang O, Hausner MA, et al. Optimization of methods to assess human mucosal T-cell responses to HIV infection. J Immunol Methods. 2003;279:17–31. doi: 10.1016/s0022-1759(03)00255-2. [DOI] [PubMed] [Google Scholar]
- 18.Critchfield JW, Lemongello D, Walker DH, Garcia JC, Asmuth DM, Pollard RB, Shacklett BL. Multifunctional human immunodeficiency virus (HIV) gag-specific CD8+ T-cell responses in rectal mucosa and peripheral blood mononuclear cells during chronic HIV type 1 infection. J Virol. 2007;81:5460–71. doi: 10.1128/JVI.02535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musey L, Hu Y, Eckert L, Christensen M, Karchmer T, McElrath MJ. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J Exp Med. 1997;185:293–303. doi: 10.1084/jem.185.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passmore JS, Milner M, Denny L, et al. Mucosal T cell responses to human papillomavirus (HPV) type 16 in women with HPV-associated cervical intraepithelial neoplasia (CIN) Immunology. 2006;119:507–14. doi: 10.1111/j.1365-2567.2006.02465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burns GF, Boyd AW, Beverley PC. Two monoclonal anti-human T lymphocyte antibodies have similar biologic effects and recognize the same cell surface antigen. J Immunol. 1982;129:1451–7. [PubMed] [Google Scholar]
- 22.Nkwanyana NN, Gumbi PP, Roberts L, et al. Impact of HIV-1 infection and inflammation on composition and yield of cervical mononuclear cells in the female genital tract. Immunology. 2009;128:e746–57. doi: 10.1111/j.1365-2567.2009.03077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones N, Agrawal D, Elrefaei M, Hanson A, Novitsky V, Wong JT, Cao H. Evaluation of antigen-specific responses using in vitro enriched T cells. J Immunol Methods. 2003;274:139–47. doi: 10.1016/s0022-1759(02)00510-0. [DOI] [PubMed] [Google Scholar]
- 24.Chen HW, Liao CH, Ying C, Chang CJ, Lin CM. Ex vivo expansion of dendritic-cell-activated antigen-specific CD4+ T cells with anti-CD3/CD28, interleukin-7, and interleukin-15: potential for adoptive T cell immunotherapy. Clin Immunol. 2006;119:21–31. doi: 10.1016/j.clim.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Kalamasz D, Long SA, Taniguchi R, Buckner JH, Berenson RJ, Bonyhadi M. Optimization of human T-cell expansion ex vivo using magnetic beads conjugated with anti-CD3 and anti-CD28 antibodies. J Immunother. 2004;27:405–18. doi: 10.1097/00002371-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Keating SM, Bejon P, Berthoud T, et al. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J Immunol. 2005;175:5675–80. doi: 10.4049/jimmunol.175.9.5675. [DOI] [PubMed] [Google Scholar]
- 27.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory and effector memory CD4+ T cells. Pathol Biol (Paris). 2003;51:64–6. doi: 10.1016/s0369-8114(03)00098-1. [DOI] [PubMed] [Google Scholar]
- 28.Cohn JA, Gagnon S, Spence MR, et al. The role of human papillomavirus deoxyribonucleic acid assay and repeated cervical cytologic examination in the detection of cervical intraepithelial neoplasia among human immunodeficiency virus-infected women. Cervical Disease Study Group Of The American Foundation For Aids Research Community Based Clinical Trials Network. Am J Obstet Gynecol. 2001;184:322–30. doi: 10.1067/mob.2001.109938. [DOI] [PubMed] [Google Scholar]
- 29.Iqbal SM, Ball TB, Kimani J, Kiama P, Thottingal P, Embree JE, Fowke KR, Plummer FA. Elevated T cell counts and RANTES expression in the genital mucosa of HIV-1-resistant Kenyan commercial sex workers. J Infect Dis. 2005;192:728–38. doi: 10.1086/432482. [DOI] [PubMed] [Google Scholar]
- 30.White HD, Crassi KM, Givan AL, Stern JE, Gonzalez JL, Memoli VA, Green WR, Wira CR. CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J Immunol. 1997;158:3017–27. [PubMed] [Google Scholar]