Abstract
An increased proportion of CD4+ CD25+ T cells has been reported in Wegener’s granulomatosis (WG) and may represent an accumulation of regulatory T cells (Treg). CD25 is also expressed on recently activated effector T cells. We have determined the relative proportion of these subsets in a large patient cohort. The fraction of Treg in peripheral blood mononuclear cells from patients and healthy controls was determined by assessment of Foxp3 expression on CD4+ CD25+ T cells. The functional activity of Treg was determined by their ability to suppress proliferation and cytokine production in response to proteinase-3. Although WG patients demonstrated an increased fraction of CD4+ CD25+ T cells, the percentage of Foxp3-positive cells was decreased. In addition, the percentage of Treg was inversely related to the rate of disease relapse. CD4+ CD25hi T cells were able to suppress T-cell proliferation to proteinase-3 in healthy controls and anti-neutrophil cytoplasm antibody (ANCA)- negative patients (at time of sampling) but not in ANCA-positive patients. In patients with active disease, an increased proportion of CD4+ Foxp3+ cells was associated with a more rapid disease remission. Patients with WG demonstrate abnormalities in the number and function of Treg and this is most pronounced in those with most active disease. This information is of value in understanding the pathogenesis and potential treatment of this disease.
Keywords: autoimmune diseases, autoimmunity, systemic vasculitis, T cells, Wegener’s granulomatosis
Introduction
Wegener’s granulomatosis (WG) is an autoimmune disease characterized by a necrotizing granulomatous, small-vessel vasculitis. Anti-neutrophil cytoplasm antibodies (ANCA) are associated with WG,1 are pathogenic,2 and are high-affinity class-switched antibodies that will require T-cell help for production.3 T-cell activation markers are raised in active disease4,5 and remission6 with T-cell accumulation in affected tissues. Intrarenal T cells correlate with renal impairment7 and anti-T-cell therapies may be effective.8,9 Recent studies have demonstrated increased CD25 expression on CD4+ T cells and a possible correlation between CD25 on CD45RO– T cells and severity of vasculitis disease.10,11 Although this T-cell population was interpreted as a naive population, it may also represent a memory subset that has undergone CD45RA reversion, a population that is known to be expanded in WG.12
Regulatory CD4+ CD25+ T cells (Treg) are critical for preventing development of autoimmune disease in mice.13 In humans, CD25 is expressed on effector cells and Treg appear to be confined to the CD25hi subset.14,15 Foxp3 has been identified as a specific marker for Treg.16–18
The role of Treg in human disease has been difficult to define because of the difficulties in distinguishing between effector and Treg using CD25 alone. In healthy control (HC) subjects CD4+ CD25+ T cells appear to play a role in controlling the T-cell response to several autoantigens.19,20 In autoimmune diseases, fewer circulating CD4+ CD25+ Treg are reported compared with controls21 but this may be accompanied by increased CD4+ CD25+ cells at the site of disease.22 Few studies have characterized Treg by Foxp3 expression and they do not address antigen-specific populations.
Using Foxp3 to identify Treg, there is no difference in numbers of Treg in systemic lupus erythematosus or rheumatoid arthritis patients23 compared with HC. However CD4+ CD25hi cells from patients with active rheumatoid disease had less Foxp3 messenger RNA than HC and patients post-treatment with infliximab.24 Measuring Foxp3 expression in this way would not distinguish between less Foxp3 expression in individual Treg, or whether there were more effector T cells than Treg cells within the cell population.
The aim of this study was two-fold; first to address the role of CD4+ CD25hi Treg in WG by investigating the frequency and phenotype of CD25+ and Foxp3+ T cells within the CD4+ population in a patient cohort. This would help to more accurately define the increase in T-cell CD25 expression10 and to identify whether specific patient sub-groups have differing CD4+ T-cell phenotypes. Second, to investigate Treg control of auto-antigen-specific T cells in WG. As immunoglobulin G-ANCA production is thought to require T-cell help we determined the ability of CD25hi T cells to suppress T-cell proliferation to the proteinase-3 (PR3) autoantigen.
Patients and methods
Patient characteristics
Patients were recruited at the Wellcome Trust Clinical Research Facility, Birmingham or New Cross Hospital, Wolverhampton, UK. Local Research Ethics Committee approval and informed consent were obtained. Blood samples from 33 HC volunteers (median age 52 years; range 30–82) were used. The HC donors were identified from laboratory staff and a local cohort of healthy elderly research volunteers.
Three separate cohorts of patients (Patient groups, PG) took part in this study. Patients whose blood was used for investigating the expression of Foxp3 and CD25 and investigating the relationship between the expression of these markers and disease relapses (PG1) (n= 55) were identified in the outpatient department (Table 1). Patients whose blood was used for serial Foxp3 analysis (PG2) (n= 21) were identified at the time of presentation with new disease or disease relapse (Table 1). Blood from a subgroup of patients in PG1 was used for proliferation experiments (n= 15) or cytokine analyses (n= 14) (PG3, total n= 16) (Table 2).
Table 1.
Characteristics of patients whose cells were used in flow cytometry analysis to establish regulatory T-cell frequencies
| Characteristics of patients (PG1) whose cells were used for CD25 and Foxp3 expression (n= 55) | Characteristics of patients (PG2) whose cells were used for serial Foxp3 expression (n= 21) | |
|---|---|---|
| Median age | 59·5 years (22–82) | 63 years (25–81) |
| Median creatinine | 113 μmol/l (65–947) | 211 μmol/l (78–657) |
| Active disease | 17 | 21 |
| Remission | 38 | 0 |
| ANCA+ at sampling | 35 | 17 |
| ANCA− at sampling | 20 | 4 |
| Sampled at new presentation of disease | 10 | 17 |
| Median time since diagnosis of disease to sampling | 142 weeks (0–1158) | 43 weeks (24–165) for four relapsed patients 0 weeks for patients with new diagnosis |
| Median follow-up | 335 weeks (20–1200) | |
| Death during follow-up | 6 | 0 |
ANCA, anti-neutrophil cytoplasm antibody.
Table 2.
Characteristics of patients (PG3) whose cells were used for proliferation and cytokine experiments
| Age | Gender | CRP | ANCA | Anti-PR3 (normal < 10) | Disease activity | Creatinine (μmol/l) | Treatment | Duration of disease (weeks) | |
| 1 | 77 | M | 12 | 1 in 25 | 18 | R | 244 | P M | 291 |
| 2 | 47 | F | 16 | Neg | < 2 | R | 140 | P R | 1157 |
| 3 | 59 | M | < 2 | Neg | < 2 | R | 125 | P A | 374 |
| 4 | 24 | M | 16 | Neg | 5 | R | ESRF | P R | 332 |
| 5 | 40 | F | < 1 | 1 in 25 | 18 | R | 90 | P | 142 |
| 6 | 64 | M | 17 | Neg | 10 | R | 110 | P R | 167 |
| 7 | 73 | M | 3 | Neg | < 2 | R | 233 | P A | 167 |
| 81 | 43 | M | < 3 | Neg | < 2 | R | 105 | P | 285 |
| 9 | 73 | M | 3 | Neg | < 2 | R | 150 | P A | 59 |
| 10 | 39 | M | 56 | 1 in 100 | 55 | A | 94 | P R | 858 |
| 11 | 47 | M | 1·4 | 1 in 100 | 26 | A | 289 | P C | 14 |
| 12 | 74 | F | < 3 | Neg | 27 | R | 92 | P A | 135 |
| 13 | 62 | F | < 1 | Neg | 3 | R | 98 | P A | 183 |
| 141 | 62 | F | 7 | 1 in 100 | > 100 | A | 108 | P A | 104 |
| 15 | 49 | M | < 2 | Neg | < 2 | R | 346 | P A | 431 |
| 162 | 60 | M | 1 in 25 | 17 | A | 130 | P R | 354 |
Proliferation only.
Cytokine only.
ANCA, anti-neutrophil cytoplasm antibody (by immunofluoresence); anti-PR3, antiproteinases 3 activity as measured by enzyme-linked immunosorbent assay; CRP, C-reactive protein. Disease activity: A, active disease; R, remission; ESRF, end-stage renal failure. Treatment: P, prednisolone; M, mycophenolate mofetil; A, azathioprine; R, rituximab.
Proliferation experiments were carried out in 13 HC and 15 patients who had been PR3-ANCA-positive (ANCA+) at some point during their disease. All patients had ANCA tested by indirect immunofluorescence and anti-PR3 enzyme-linked immunosorbent assay (ELISA). A finding of ANCA+ was defined as either cytoplasmic ANCA detection by immunofluoresence or anti-PR3 antibody titre of > 10 units by direct ELISA. All patients met the American College of Rheumatology criteria for diagnosis of WG.25
For serial analysis of Foxp3 expression, paired samples were collected from 21 patients with newly diagnosed (n= 17) or relapsed (n= 4) WG or microscopic polyangiitis and after 14 weeks of immunosuppressive treatment. Disease activity was defined using the Birmingham Vasculitis Activity Score (BVAS).26 Remission was defined as BVAS score of ≤ 1.
Treatment of patients was determined by severity, organ involvement and age.27 It is not ethically possible to standardize therapy within this type of study. Seven of those sampled at initial diagnosis were treatment free (n= 7); active disease was treated with cyclophosphamide and high-dose prednisolone, with plasma exchange if creatinine was > 500 μmol/l. Patients in remission were maintained on low-dose prednisolone with either azathioprine or mycophenolate mofetil. In addition, several patients received either infliximab (n= 7) or rituximab (n= 6) for remission induction.
Cell staining and flow cytometry analysis
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation using Ficoll-Paque Plus (Amersham Biosciences, Little Chalfont, UK). Cells were suspended at 5 × 106/ml in phosphate-buffered saline and 100-μl samples were stained for 15 min with antibodies or isotype controls as detailed below. Permeabilization of cells and intracellular Foxp3 staining using allophycocyanin-conjugated PCH-101 (eBioscience, Hatfield, UK) was performed following manufacturer’s instructions. Fluorescence-activated cell sorting (FACS) analysis was performed using Becton Dickinson FACSCalibur (Becton and Dickinson (BD), Oxford, UK) and Dako Cyan (Dako, Ely, UK). Analysis was performed using cellquest version 3.3 and summit version 4.3 (Dako). Antibodies used were against CD4, CD25 (BD), conjugated with fluorescein isothiocyanate, phycoerythrin, peridinin chlorophyll protein (all BD) or Alexa Fluor 405 (Caltag, Buckingham, UK).
CD25hi depletion
Fifty microlitres of CD25 Dynabeads (Dynal Biotech, Oslo, Norway) per 1 × 107 cells were washed in 2 ml RPMI-1640/10% fetal calf serum (FCS) (both Sigma, Poole, UK) in a Dynal MPC (Dynal Biotech) following the manufacturer’s instructions. CD25− cells were resuspended in T-cell medium (TCM: RPMI/10%FCS/1% l-glutamine/1% penicillin–streptomycin).
To remove CD25hi cells from beads, 20 μl of DETACHABEAD (Dynal Biotech) was added for each 50 μl of beads used following the manufacturer’s instructions.
Proliferation and add back
The PBMC were prepared as described above and half the sample was depleted of CD25hi cells as described. Pre-depletion and post-depletion cells were resuspended in 500 μl RPMI-1640. Five micromoles carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE; Molecular Probes, Invitrogen, Paisley, UK) was added to each sample for 10 min at 37° then the samples were placed on ice for 5 min and washed three times in ice-cold RPMI/10% FCS before cell counting.
Cells in TCM at 1 × 106/ml were plated into U-bottomed 96-well plates (Falcon; BD Biosciences,) at 200 μl per well. Either antigen [heat-inactivated purified human PR3 at 5 μg/ml (Athens Research and Technology, Athens, GA) or purified protein derivative (PPD) at 10 μg/ml (Statens Serum Institut, Copenhagen, Denmark)] or medium alone was added. Plates were incubated at 37°, 5% CO2 for 11 days. At day 5, 100 μl supernatant was removed from each well and frozen at − 80° for future cytokine analysis. At day 11, cells were removed from the wells, washed and stained for surface expression of CD4 and proliferation was analysed by flow cytometry. Initial analysis of proliferation at day 5, 8 or 11 showed that at day 11 reasonable proliferation to control antigen, with minimal non-specific background proliferation, could be seen in both HC and patients. Cell proliferation was recorded by measuring progressive loss of membrane CFDA-SE.
In add-back experiments, PBMC were separated as described. Cells were then resuspended in 1·2 ml TCM. Two hundred microlitres was removed to use as non-depleted cells, the remainder underwent CD25hi depletion as described. CD25− cells were kept and CD25+ cells were removed from the beads as described. The non-depleted cells and CD25− fractions were stained with CFDA-SE. All cells were washed and re-suspended in TCM at 1 × 106/ml. One hundred microlitres of non-depleted and CD25− cells was plated into 96-well U-bottom plates. Fifty microlitres of CD25− cells was added to 50 μl of unstained CD25+ cells; 2 × 104 anti-CD3/anti-CD28 (Dynal Biotech) beads were added to each well. Cultures, in duplicate, were incubated at 37°, 5% CO2 for 4 days before proliferation was measured.
Cytokines
Interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) were measured in 50 μl culture supernatant using a custom cytokine reagent kit (Bio-Rad Laboratories, Hemel Hempstead, UK) following the manufacturer’s instructions on a Luminex 100 (Bio-Rad Laboratories) and analysed using bio-plex Manager Software (Bio-Rad).
Statistical analysis
Comparisons between groups were carried out using Mann–Whitney U-tests for non-paired data and Wilcoxon signed rank test for paired data using spss for Windows (version 14; SPSS, Chicago, IL) and GraphPad Prism (version 4; Graphpad software, La Jolla, CA). The multivariable analysis was performed using a linear regression. The relapse rate was calculated per 100 patient weeks for each patient.
Results
Preliminary studies confirmed previous reports28 that patients (n= 21) had significantly lower lymphocyte counts and CD4+ T-cell counts than healthy controls (n= 27) (data not shown) therefore the data presented refer to percentages of CD4+ cells rather than absolute numbers.
An increased fraction of CD4+ T cells are CD25hi in patients with Wegener’s granulomatosis but expression of Foxp3+ is reduced in this subset
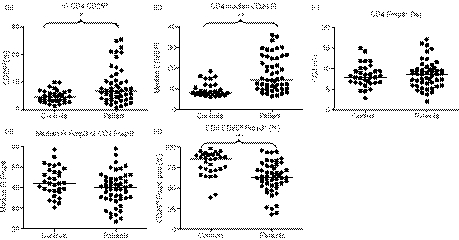
Compared with HC, PG1 showed a significant increase in the percentage of CD25hi CD4+ cells (Fig. 1a) and the median fluorescence intensity (MFI) of CD25 (Fig. 1b). There was no difference between PG1 and HC in the percentage of Foxp3+ CD4+ cells (Fig. 1c). There was a non-significant decrease in Foxp3 expression in the PG1 (Fig. 1d) with a decreased proportion of Foxp3+ cells in the CD4+ CD25hi fraction in the patient group (Fig. 1e).
Figure 1.
Flow cytometry analysis of markers of T-cell activation and regulation on peripheral blood mononuclear cells from patients with Wegener’s granulomatosis (PG1; n= 55) and age-matched healthy controls (HC, n= 33). Patients showed an increased percentage of CD4+ lymphocytes that were CD25hi (a) (*P= 0·0006) and an increase in CD25 median fluorescent intensity (MFI) (b) (**P< 0·0001). There was no difference between patients and HC in the percentage of Foxp3+ CD4+ cells (c). There was a non-significant decrease in Foxp3 expression in the patient group (d) with a decreased proportion of Foxp3+ cells in the CD4+ CD25hi fraction in the patient group (e) (***P< 0·0001) and therefore the increase in CD4+ CD25hi in the patient group is likely to be the result of an increase in the frequency of activated and not regulatory CD4+ T cells.
Decreased Foxp3 expression on CD4+ T cells from patients who are ANCA-antibody positive
In the PG1 group there was no difference between active disease and remission, or currently ANCA+ and ANCA− patients, in the percentage of CD25hi Foxp3+ CD4+ T cells (data not shown). There was less Foxp3 expression in the CD4+ Foxp3+ T cells of PG1 patients who were currently ANCA+ (MFI 36, range 24–86) compared with HC (MFI 44, range 30–60; P= 0·0037). There was a trend to increased Foxp3 expression in CD4+ Foxp3+ T cells in the PG1 who were currently ANCA− (MFI 41, range 28–60) compared with currently ANCA+ patients (P= 0·059).
Higher CD25 expression, or reduced proportion of Foxp3+ cells, within the CD4+ CD25hi T-cell population, are both associated with disease relapse
In a multivariate analysis of the PG1 group using age, serum creatinine concentration, ANCA status, disease activity and relapse rate, CD25 expression showed a positive correlation with relapse rate, whether measured as percentage of CD25hi CD4+ T cells (beta coefficient = 0·38, P= 0·011) or as median CD25 expression (beta coefficient = 0·33, P= 0·025). The percentage of Foxp3+ CD4+ CD25hi showed a negative correlation with disease relapse rate (beta coefficient = − 0·32, P= 0·034).
An increased proportion of Foxp3+ cells in the CD4+ T-cell population is associated with a more rapid time to clinical remission
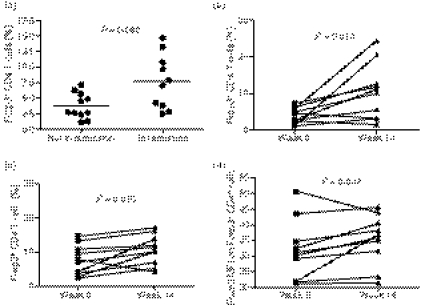
In the PG2 group the median time to remission was 14 weeks. At presentation, the percentage of Foxp3+ CD4+ T cells was higher in patients who went into remission by 14 weeks (n= 10) compared with those who still had active disease (n= 11) at week 14 (Fig. 2a). There was an increase in the percentage of Foxp3+ CD4+ T cells at week 14 compared with week 0 in both patients in remission as well as those in whom disease remained active (Fig. 2b,c). Only patients who were in remission had an increase of Foxp3 within CD4+ Foxp3+ cells at week 14 (Fig. 2d).
Figure 2.
Foxp3 expression on patients (PG2) with active disease and after 14 weeks of treatment. (a) The percentage of Foxp3+ CD4 T cells in patients with active disease (week 0) was higher in patients who subsequently entered remission by 14 weeks of treatment than those who had not entered remission by 14 weeks. The percentage of Foxp3+ CD4 T cells increased by week 14 of treatment compared with week 0 in both patients who had gone into remission by week 14 (b) and those who still had active disease at week 14 (c). Only patients who had gone into remission by week 14 showed an increase in the MFI for Foxp3 on Foxp3+ CD4 T cells by week 14 (d).
T-regulatory cells suppress proliferation to PR3 in healthy controls and ANCA– patients but suppression is lost in ANCA+ patients
Proliferation to PR3 and tuberculosis-derived recall antigen PPD was measured in both PG3 (n= 15) and HC (n= 13). (Clinical characteristics summarized in Table 2.) Proliferation was normalized to background proliferation in the absence of antigen (relative proliferation value of 1·0 indicates equal proliferation to medium alone).
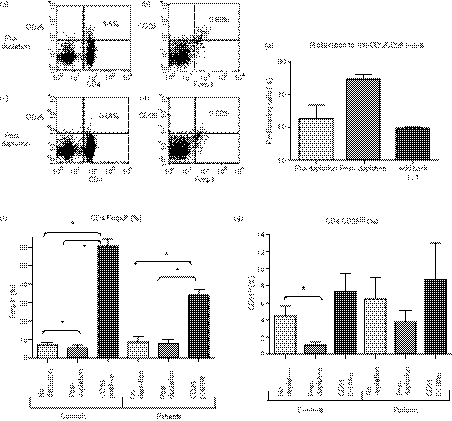
Anti-CD25-coated beads led to a good depletion of the CD4+ CD25hi subset (Fig. 3a–f).
Figure 3.
Magnetic bead depletion of CD25+ cells. Anti-CD25 coated magnetic beads were used to deplete CD25hi cells that were virtually all confined to the CD4+ subset (a, c). (b, d) are gated on CD4+ lymphocytes and demonstrate that in this healthy control CD4+ CD25hi cells are virtually all Foxp3+ (b, d; right upper quadrant). There are however some CD4+ Foxp3+ lymphocytes that express intermediate levels of CD25 (b, d; right lower quadrant). (f, g) In both healthy controls and patients, bead depletion led to a decrease in % of CD4+ cells that were CD25hi or Foxp3+. The removed cells were highly enriched in Foxp3 expressing Treg. Bead depletion led to an increase in proliferation to anti-CD3/anti-CD28 beads, measured at day 3 (e). Add-back of the depleted CD25hi cells led to suppression of this increased proliferation. (*P< 0·05).
Patients were divided into a group that were ANCA− and in remission (n= 9) and a group who were ANCA+ at the time of sampling (n= 6, active disease n= 3).
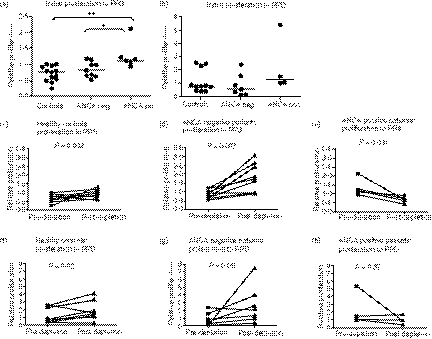
Before CD25 depletion there was more proliferation to PR3 in the ANCA+ patients compared with HC or ANCA− patients. There were no differences in proliferation to PPD. The HC showed less proliferation to PR3 than to medium alone (Fig. 4).
Figure 4.
The effect of CD25+ cell depletion on proliferative responses in T cells from PG3. Relative proliferation to the auto-antigen proteinase 3 (PR3) in the presence of CD25hi cells was significantly increased in the anti-neutrophil cytoplasm antibody positive (ANCA+) group compared with either the currently ANCA− group (*P= 0·036) or healthy controls (**P= 0·002) (a). There were no significant differences in proliferation to purified protein deriviative (PPD) between the three groups (b). Proliferation was measured at 11 days. Removal of CD25hi led to an increase in proliferation in response to PR3 in both healthy controls (c) and currently ANCA− patients (d) with this increase in proliferation being significantly greater in the ANCA− patients than controls (P= 0·001). The ANCA+ patients showed a significant decrease in proliferation in response to PR3 (e). Both healthy controls and currently ANCA− patients showed an increase in proliferation to PPD with removal of CD25hi cells but there was no difference in the degree of this increase between the two groups.
Healthy controls and ANCA− patients showed increased proliferation to PR3 following removal of CD25hi cells and this was greater in the currently ANCA− group than in HC. The ANCA+ patients showed decreased proliferation on removal of CD25hi cells. CD25 depletion resulted in increased proliferation to PPD in the HC and ANCA− groups (Fig. 4).
Proliferation to PR3 was confirmed to be antigen-specific by re-stimulating cells from the proliferation assay using autologous dendritic cells in an IFN-γ ELISpot (data not shown). Proliferation to PR3 was confined to CD3+ CD4+ cells and was not observed in the CD8+ or CD19+ cell fraction (data not shown).
Treg fail to suppress cytokine production in ANCA-positive patients
Cytokines were measured in the cell culture supernatants on day 5. There was significant variation between patients (all from PG3) in the absolute concentration of cytokines produced. Therefore, the results are expressed as the ratio of cytokine in the antigen-stimulated cell culture supernatant relative to that in the unstimulated cell culture supernatant. The PBMC from ANCA+ patients produced more TNF-α (median 12, range 4–44) in response to PR3 than ANCA− patients (median 5, range 1–29) (P= 0·047). T cells from the ANCA− patients produced less TNF-α in response to PR3 than the HC (median 8, range 4–12) (P= 0·047).
There were no differences between the groups in cytokine production to PPD.
Following CD25 depletion, there was increased production of IFN-γ (pre-depletion median 0·5, range 0–1·5; post-depletion median 1·3, range 0·2–2·4) (P= 0·039) and TNF-α (pre-depletion median 0·5, range 0·1–2·9; post-depletion median 0·9, range 0·7–4·9) (P= 0·039) in response to PR3 in the ANCA– group but not in HC.
Discussion
Increased CD25 expression on CD4+ T cells has been seen in patients with WG.29,30 Both Treg and effector T cells express CD25 but the relative proportions of these cells had not been determined. This study investigated the phenotype of CD4+ CD25hi T cells from WG patients by measuring Foxp3 expression. We focused on differences in Treg and effector ratios rather than on absolute T-cell numbers because absolute numbers are consistently low in patients with WG.10
At present there is no ideal method for identifying human Treg. The transcription factor Foxp3 is widely accepted as necessary for the development of a Treg phenotype but as it is only present intracellularly it cannot be used to identify live Treg. Other markers, such as low expression of the interluekin-7 (IL-7) receptor (CD127), have been proposed as a specific way of identifying live Treg for sorting experiments. Previous investigations have shown that under in vitro conditions effector T cells activated by anti-CD3/CD28 antibodies will transiently express low levels of Foxp3 and lose CD127 expression.31 We investigated the relationship between low CD127 expression and Foxp3 expression and found a highly significant correlation between the two (data not shown) such that we have defined high CD25-expressing Foxp3+ cells as Treg in this study.
Patients with WG showed a raised percentage of CD25hi CD4+ T cells and increased CD25 expression compared with HC. This increase remained constant over time for each individual and was not associated with disease activity or ANCA expression. However, in the population, levels of CD4+ T-cell CD25 expression were positively associated with relapse rate.
This increase in CD4+ CD25hi cells was not accompanied by an increased percentage of Foxp3+ CD4+ T cells and therefore reflects an expansion of activated effector T cells. Patients therefore exhibit an imbalance of activated versus regulatory T cells, which may be important in the pathogenesis of this autoimmune condition.
The determinants of the marked T-cell activation seen in the patient group are unknown. This pattern is present before immunosuppression and remains in many patients throughout the disease. It may therefore reflect impairment in the control mechanisms for T-cell activation.
We have been able to demonstrate that although the percentage of CD25+ CD4+ T cells is not related to disease activity there is a relationship between the relative proportions of CD4+ Foxp3+ Treg and CD4+ CD25hi effector T cells and disease relapse rates. In addition, time to remission appears to be less in those with a higher proportion of CD4+ Foxp3+ Treg. These observations imply that the imbalance between effector and regulatory T cells is an important factor in disease control.
Patients that continue to express PR3-ANCA are at increased risk of relapse.32 Production of the immunoglobulin G-ANCA autoantibody is likely to require T-cell help and implies a breakdown in self-tolerance. The ability of Treg to suppress T-cell activation by PR3 was investigated in our patient cohort. The ANCA+ patients showed increased proliferation to PR3 compared with HC and those who had become ANCA−. Indeed, HC showed less proliferation to PR3 than to medium alone, implying that activation of antigen-specific Treg by PR3 can mediate bystander suppression of proliferation. To define the function of the Treg population, CD25hi cells were removed leading to increased proliferation in both the currently ANCA− patients and HC. This confirms the suppression of autoantigen-specific T cells by Treg in HC that has been shown by others.19,20 Removal of Treg in the ANCA− group led to greater production of IFN-γ and TNF-α in response to PR3 than in controls. With the proliferation data, this implies that Treg play an important role in controlling PR3-specific T cells during disease remission.
In contrast, ANCA+ patients showed higher proliferation and TNF-α production to PR3 than HC or ANCA− patients. This implies a loss of Treg control of PR3-specific T cells and is consistent with the presence of autoantibody in this group. CD25 depletion resulted in decreased proliferation in ANCA+ patients and probably reflects the removal of highly activated PR3-specific T cells.
There are several possible explanations for this loss of Treg function in the patient cohort. ANCA+ patients expressed reduced levels of Foxp3 in CD4+ T cells compared with HC, implying that a possible intrinsic defect in Treg function may contribute to disease progression. However, this phenotype was stable across the patient cohort and is unlikely to explain the relapsing nature of this condition. In rheumatoid arthritis, serum TNF-α may impair Treg function.24 Production of TNF-α in response to PR3 was raised in our ANCA+ cohort. Moreover, polyclonal stimulation results in increased production of IFN-γ and TNF-α by patients’ T cells, particularly in active disease.33,34 Other defects in regulatory components of the immune system have been shown in WG, such as polymorphisms associated with decreased cytotoxic T-lymphocyte antigen-4 function35,36 and IL-10,37 and decreased number and function of C1d-restricted natural killer T cells38 and may contribute to increased TNF-α leading to Treg suppression.
Our results differ from those of Abdulahad et al.39 who describe an increased percentage of CD4+ CD25hi Foxp3+ T cells in patients compared with HC and interpreted this as reflecting either an increase in Treg or the result of Foxp3 expression in recently activated effector cells. In our study we define Treg solely by a CD4+ Foxp3+ phenotype because the transcription factor has now emerged as a specific marker of Treg within the human immune system.
Very little is known about the effects of immunosuppressive drugs on the number or function of Treg although there have been reports of increased CD25 expression on T cells in patients taking corticosteroids.40 In contrast, a decreased frequency and function of Treg has been reported following low-dose cyclophosphamide treatment.41 We could show no decrease in CD25 or Foxp3 expression by T cells in our cohort when this was stratified according to immunosuppressive therapy (data not shown).
Our findings demonstrate that within the CD4+ T-cell population there is an upset of the effector : regulatory balance particularly in those with multiple relapses. Moreover, we show that the proportion of Treg may be important in achieving disease remission. This implies that immune balance is important in control of this autoimmune disease, possibly irrespective of the tendency to reduced absolute numbers of CD4+ T cells. In addition, we have also demonstrated that Foxp3+ Treg may be important in the control of auto-antigen-specific effector T cells. These findings could have implications for disease management because the transfer of CD4+ CD25+ Treg has been shown to ameliorate disease in several animal models of autoimmune disease42 and methods have been developed to allow in vitro expansion of Treg isolated from patients with autoimmune disease.43 Adoptive transfer of autologous expanded Treg, alone or in combination with standard immunosuppressive strategies may therefore be a valuable approach in patients with treatment-resistant disease.
Acknowledgments
We thank Dr Mark Little for help with the preparation of figures. This work was supported by Medical Research Council Clinical Training Fellowship (C.J.D.) and The Healthcare Foundation project grant (M.D.M.).
Disclosures
None of the authors have competing interests to declare.
References
- 1.van der Woude FJ, Rasmussen N, Lobatto S, et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener’s granulomatosis. Lancet. 1985;1:425–9. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- 2.Xiao H, Heeringa P, Hu P, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–63. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellbye OJ, Mollnes TE, Steen LS. IgG subclass distribution and complement activation ability of autoantibodies to neutrophil cytoplasmic antigens (ANCA) Clin Immunol Immunopathol. 1994;70:32–9. doi: 10.1006/clin.1994.1007. [DOI] [PubMed] [Google Scholar]
- 4.Stegeman CA, Tervaert JW, Huitema MG, Kallenberg CG. Serum markers of T cell activation in relapses of Wegener’s granulomatosis. Clin Exp Immunol. 1993;91:415–20. doi: 10.1111/j.1365-2249.1993.tb05918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang G, Hansen H, Tatsis E, Csernok E, Lemke H, Gross WL. High plasma levels of the soluble form of CD30 activation molecule reflect disease activity in patients with Wegener’s granulomatosis. Am J Med. 1997;102:517–23. doi: 10.1016/s0002-9343(97)00049-1. [DOI] [PubMed] [Google Scholar]
- 6.Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Differential B- and T-cell activation in Wegener’s granulomatosis. J Allergy Clin Immunol. 1999;103:885–94. doi: 10.1016/s0091-6749(99)70434-3. [DOI] [PubMed] [Google Scholar]
- 7.Brouwer E, Cohen Tervaert J, Weening J, Kallenberg C. Immunohistopathology of renal biopsies in Wegener’s granulomatosis (WG): clues to its pathogenesis? Kidney Int. 2001;39:1051–6. [Google Scholar]
- 8.Mathieson PW, Cobbold SP, Hale G, Clark MR, Oliveira DB, Lockwood CM, Waldmann H. Monoclonal-antibody therapy in systemic vasculitis. N Engl J Med. 1990;323:250–4. doi: 10.1056/NEJM199007263230407. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt W, Hagen E, Neumann I, Nowack R, Flores-Suarez L, van der Woude F. European Vasculitis Study Group. Treatment of refractory Wegener’s granulomatosis with antithymocyte globulin (ATG): an open study in 15 patients. Kidney Int. 2004;65:1440–8. doi: 10.1111/j.1523-1755.2004.00534.x. [DOI] [PubMed] [Google Scholar]
- 10.Marinaki S, Neumann I, Kalsch AI, et al. Abnormalities of CD4 T cell subpopulations in ANCA-associated vasculitis. Clin Exp Immunol. 2005;140:181–91. doi: 10.1111/j.1365-2249.2005.02731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marinaki S, Kalsch AI, Grimminger P, et al. Persistent T-cell activation and clinical correlations in patients with ANCA-associated systemic vasculitis. Nephrol Dial Transplant. 2006;21:1825–32. doi: 10.1093/ndt/gfl097. [DOI] [PubMed] [Google Scholar]
- 12.Lamprecht P, Bruhl H, Erdmann A, et al. Differences in CCR5 expression on peripheral blood CD4+ CD28− T-cells and in granulomatous lesions between localized and generalized Wegener’s granulomatosis. Clin Immunol. 2003;108:1–7. doi: 10.1016/s1521-6616(03)00121-9. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 14.Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD, Isaacs JD, Lechler RI. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–44. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 15.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+ CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 16.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 17.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 18.Yagi H, Nomura T, Nakamura K, et al. Crucial role of FOXP3 in the development and function of human CD25+ CD4+ regulatory T cells. Int Immunol. 2004;16:1643–56. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 19.Taams LS, Vukmanovic-Stejic M, Smith J, et al. Antigen-specific T cell suppression by human CD4+ CD25+ regulatory T cells. Eur J Immunol. 2002;32:1621–30. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 20.Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol. 2004;172:5967–72. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]
- 21.Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117:289–300. doi: 10.1111/j.1365-2567.2005.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 23.Alvarado-Sanchez B, Hernandez-Castro B, Portales-Perez D, Baranda L, Layseca-Espinosa E, Abud-Mendoza C, Cubillas-Tejeda AC, Gonzalez-Amaro R. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2006;27:110–8. doi: 10.1016/j.jaut.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+ CD25hi T-regulatory cells. Blood. 2006;108:253–61. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leavitt R, Fauci A, Bloch D, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum. 1990;33:1101–7. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 26.Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, Savage C, Adu D. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. Q J Med. 1994;87:671–8. [PubMed] [Google Scholar]
- 27.Mukhtyar C, Flossmann O, Hellmich B, et al. Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League Against Rheumatism systemic vasculitis task force. Ann Rheum Dis. 2008;67:1004–10. [Google Scholar]
- 28.Ikeda M, Tsuru S, Watanabe Y, Kitahara S, Inouye T. Reduced CD4 : CD8 T cell ratios in patients with Wegener’s granulomatosis. J Clin Lab Immunol. 1992;38:103–9. [PubMed] [Google Scholar]
- 29.Giscombe R, Nityanand S, Lewin N, Grunewald J, Lefvert A. Expanded T cell populations in patients with Wegener’s granulomatosis: characteristics and correlates with disease activity. J Clin Immunol. 1998;18:404–13. doi: 10.1023/a:1023230722874. [DOI] [PubMed] [Google Scholar]
- 30.Schlesier M, Kaspar T, Gutfleisch J, Wolff-Vorbeck G, Peter H. Activated CD4+ and CD8+ T-cell subsets in Wegener’s granulomatosis. Rheumatol Int. 1995;14:213–9. doi: 10.1007/BF00262300. [DOI] [PubMed] [Google Scholar]
- 31.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–54. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 32.Sanders JS, Huitma MG, Kallenberg CG, Stegeman CA. Prediction of relapses in PR3-ANCA-associated vasculitis by assessing responses of ANCA titres to treatment. Rheumatology (Oxford) 2006;45:724–9. doi: 10.1093/rheumatology/kei272. [DOI] [PubMed] [Google Scholar]
- 33.Ludviksson BR. Active Wegener’s granulomatosis is associated with HLA-DR+CD4+ T-cells exhibiting an unbalanced Th1-type T-cell cytokine pattern: reversal with IL-10. J Immunol. 1998;160:3602–9. [PubMed] [Google Scholar]
- 34.Csernok E, Trabandt A, Muller A, Wang G, Moosig F, Paulsen J, Schnabel A, Gross W. Cytokine profiles in Wegener’s granulomatosis: predominance of type 1 (Th1) in the granulomatous inflammation. Arthritis Rheum. 1999;42:742–50. doi: 10.1002/1529-0131(199904)42:4<742::AID-ANR18>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 35.Yihua Zhou DH, Paris PL, Sauter CS, Prock KA, Hoffman GS. An analysis of CTLA-4 and proinflammatory cytokine genes in Wegener’s granulomatosis. Arthritis Rheum. 2004;50:2645–50. doi: 10.1002/art.20385. [DOI] [PubMed] [Google Scholar]
- 36.Giscombe R, Wang X, Huang D, Lefvert AK. Coding sequence 1 and promoter single nucleotide polymorphisms in the CTLA-4 gene in Wegener’s granulomatosis. J Rheumatol. 2002;29:950–3. [PubMed] [Google Scholar]
- 37.Zhou Y, Giscombe R, Huang D, Lefvert AK. Novel genetic association of Wegener’s granulomatosis with the interleukin 10 gene. J Rheumatol. 2002;29:317–20. [PubMed] [Google Scholar]
- 38.Takagi D, Iwabuchi K, Iwabuchi C, et al. Immunoregulatory defects of V alpha 24V+ beta 11+ 11+ NKT cells in development of Wegener’s granulomatosis and relapsing polychondritis. Clin Exp Immunol. 2004;136:591–600. doi: 10.1111/j.1365-2249.2004.02471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdulahad WH, Stegeman CA, van der Geld YM, Doornbos-van der Meer B, Limburg PC, Kallenberg CG. Functional defect of circulating regulatory CD4+ T cells in patients with Wegener’s granulomatosis in remission. Arthritis Rheum. 2007;56:2080–91. doi: 10.1002/art.22692. [DOI] [PubMed] [Google Scholar]
- 40.Suarez A, Lopez P, Gomez J, Gutierrez C. Enrichment of CD4+ CD25high T cell population in patients with systemic lupus erythematosus treated with glucocorticoids. Ann Rheum Dis. 2006;65:1512–7. doi: 10.1136/ard.2005.049924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–8. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 42.Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, de Vries RR, Toes RE. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–21. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 43.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+ CD25+ FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–47. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]