Abstract
Studies in mice have shown that CD70 on dendritic cells (DCs) is sufficient to convert T-cell tolerance into immunity and hence induce anti-tumour immune responses. Therefore, it is important to investigate (i) optimal stimuli to induce CD70 on human monocyte-derived DCs (MoDCs), which are widely used for tumour immunotherapy, and (ii) the role of CD70 in functional differentiation of naive CD4+ and CD8+ T cells stimulated with MoDCs. We show that interferon-α (IFN-α) is a key cytokine to differentiate monocytes into DCs with the capacity to express CD70 upon maturation. CD70 expression on IFN-α-induced MoDCs was elicited by different categories of maturation-inducing factors (Toll-like receptor ligands, CD40 ligand and pro-inflammatory mediators), among which prostaglandin E2 was most effective. Naive T cells stimulated with MoDCs also expressed CD70. Stimulation with MoDCs promoted naive CD4+ T cells to acquire the ability to produce T helper type 1 and 2 cytokines in a CD70-dependent manner. In contrast, the CD70–CD27 interaction diminished the production of an immunoregulatory cytokine IL-10. The CD27 signal did not play a dominant role in the induction of effector molecules in naive CD8+ T cells during the stimulation with MoDCs. This study adds a novel function to the versatile cytokines, type I IFNs, that is, the induction of CD70 on MoDCs. CD70 promotes naive CD4+ T cells to acquire immunostimulatory activity through the DC–T-cell and T-cell–T-cell interactions during the stimulation with MoDCs. Hence, the CD70–CD27 interaction may play an important role in inducing effective immune responses in DC-based immunotherapy.
Keywords: CD70, CD27, costimulation, cytokines, dendritic cells, T cells
Introduction
Dendritic cells (DCs) play a pivotal role in determining the magnitude and quality of naive T-cell responses. In humans, monocyte-derived DCs (MoDCs) have been widely used for investigating basic functions of DCs and as a tool for tumour immunotherapy. It is therefore important to elucidate how MoDCs control naive T-cell differentiation so as to extend the knowledge about DC biology and its clinical application.
A variety of costimulatory molecules expressed on DCs greatly influence the consequences of naive T-cell stimulation. Among such costimulatory molecules, tumour necrosis factor (TNF) family members play a critical role in augmenting the responses of T cells that express receptors belonging to the TNF receptor family.1 Recent studies have revealed that, among these ligand–receptor pairs, the CD70–CD27 interaction plays an important role in enhancing T-cell responses2 by promoting T-cell survival3,4 and by enhancing effector functions.5–8 CD27 is constitutively expressed on naive T cells, in contrast to other members of the TNF receptor family.9 This suggests that the CD70–CD27 interaction plays a role in the activation of naive T cells during primary immune responses. Furthermore, recent studies have shown that sole expression of CD70 on DCs is sufficient to convert CD8+ T-cell tolerance into immunity10 and so to induce anti-tumour immune responses11 in mice. Therefore, it is crucial to elucidate optimal stimulation to induce CD70 on human MoDCs and the role of CD70 during activation of naive T cells with MoDCs, given the importance of CD70 in the induction of robust immune responses in mice and the wide use of MoDCs for tumour immunotherapy in humans.
Different cytokines have been used in combination with granulocyte–macrophage colony-stimulating factor (GM-CSF) to induce immature DCs from monocytes. Whereas interleukin-4 (IL-4) is most commonly used, interferon-α (IFN-α),12 TNF-α13,14 and IL-1515 have been shown to induce MoDCs. Although monocytes are most often cultured with GM-CSF and IL-4 for 5–7 days, it has been reported that 2-day culture with GM-CSF and IL-4 is sufficient to induce equivalently competent DCs.16,17 Monocytes are also cultured with GM-CSF and IFN-α for only 3 days to develop into potent DCs.12 There are three categories of maturation-inducing factors for DCs: (i) Toll-like receptor (TLR) ligands, (ii) CD40 ligand (CD40L) and (iii) soluble pro-inflammatory mediators [e.g. cytokines and prostaglandins (PGs)]. Moreover, although RPMI-1640 containing fetal calf serum (FCS) is most often used to culture MoDCs for in vitro studies, a serum-free medium is desirable for clinical use from the viewpoints of safety and lot-to-lot consistency. There are four parameters of culture conditions to induce mature MoDCs: (i) basal cytokines (IL-4, IFN-α, TNF-α and IL-15), (ii) culture periods to induce immature MoDCs, (iii) maturation-inducing factors and (iv) culture media.
Recent studies investigated part of these parameters to find out the conditions to induce CD70 on MoDCs. For example, it has been shown that MoDCs induced with IL-418 or TNF-α14 and matured with lipopolysaccharide (LPS) as well as MoDCs induced with IL-4 and matured with PGE2-containing stimuli19 express CD70. However, these studies are not comprehensive, and some of the conditions14,18 are not compatible with clinical application because of the use of calf serum. Furthermore, there has been no study that examined functional consequences of the CD70–CD27 interaction during stimulation of naive T cells with MoDCs.
Here we comprehensively examined (i) clinically applicable stimulation that induces MoDCs to express CD70 most efficiently and (ii) the effects of the CD70–CD27 interaction on functional differentiation of naive CD4+ and CD8+ T cells during the stimulation with MoDCs. This is the first study showing that (i) IFN-α is a basal cytokine to induce CD70 most efficiently on MoDCs and that (ii) the CD70–CD27 interaction promotes naive CD4+ T cells to produce a broad range of immunostimulatory molecules during the stimulation with MoDCs. This study indicates an importance of the CD70–CD27 interaction in DC-based immunotherapy.
Materials and methods
Media and reagents
RPMI-1640 (Sigma-Aldrich, St Louis, MO) supplemented with 10% heat-inactivated FCS (ThermoTrace, Victoria, Australia), 2 mm l-glutamine, penicillin G, streptomycin (Gibco BRL, Carlsbad, CA), and 10 mm HEPES (Nacalai tesque, Kyoto, Japan) (referred to as R10) and CellGro DC Medium (CellGenix Technologie Transfer GmbH, Freiburg, Germany) were used as culture media. Mouse anti-human CD70 monoclonal antibody (mAb) 2F11 (immunoglobulin G1)20 was used to block the interaction between CD70 and CD27.
Generation and phenotypic analysis of MoDCs
Peripheral blood mononuclear cells (PBMCs) were obtained from buffy coats of healthy donors, obtained with written informed consent under the approval by the Institutional Review Board at Graduate School of Medicine, Kyoto University. Monocytes were purified from PBMCs by positive selection using anti-CD14-cojugated microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and cultured for 3 or 7 days in the presence of 50 ng/ml GM-CSF (a gift from Schering-Plough Research Institute, Kenilworth, NJ) together with either 1000 U/ml IFN-α (Intron A, Schering-Plough), 40 ng/ml IL-4, 10 ng/ml TNF-α or 200 ng/ml IL-15 (PeproTech, London, UK) to induce immature MoDCs (referred to as IFN-DCs, IL-4-DCs, TNF-DCs and IL-15-DCs, respectively). On days 3 and 7, half of the medium was exchanged for a fresh medium supplemented with the cytokines used during the first 3 days, and maturation-inducing stimuli were added as follows: 12·5 μg/ml polyinosinic-polycytidylic acid (poly I:C) (Pharmacia Biotech, Uppsala, Sweden), 100 ng/ml LPS (from Escherichia coli O111:B4, Sigma-Aldrich), human CD40L-transfected L cells21 (irradiated with 5500 rads) at one L cell for five DCs, or a mixture of 10 ng/ml TNF-α, 10 ng/ml IL-1β, 1000 U/ml IL-6 (PeproTech), and 1 μg/ml PGE2 (MP Biomedicals, Solon, OH) (referred to as a PGE2 cocktail). Dendritic cells were stained with fluorescein isothiocyanate (FITC)-labelled anti-CD86 mAb (BD Biosciences, San Jose, CA, USA), anti-CD70 mAb (clone BU69; Ancell Corporation, Bayport, MN), anti-CD70 mAb (clone Ki-24; BD Biosciences), phycoerythrin (PE) -labelled anti-OX40 ligand (OX40L) mAb (clone ik-1; BD Biosciences), or anti-4-1BB ligand (4-1BBL) mAb (clone C65-485; BD Biosciences) after blocking Fcγ receptors with human immunoglobulin G. To show reactivity of the anti-4-1BBL mAb, we stained L cells, human CD32-transfected L cells, human CD32/CD40L-transfected L cells, and human CD32/4-1BBL-double-transfected L cells (gifts from Dr Yong-Jun Liu, M.D. Anderson Cancer Center, Houston, TX)22 with PE-labelled anti-CD32 mAb, anti-CD40L mAb (Beckman Coulter Immunotech, Marseille, France) or anti-4-1BBL mAb. Dead cells were excluded by staining with propidium iodide. Cells were analysed by FACSCalibur™ flow cytometer (BD Biosciences), and data were analysed with CellQuest™ software (BD Biosciences).
Isolation of naive CD4+ and CD8+ T cells
The PBMCs were stained with FITC-labelled anti-CD3, PE-labelled anti-CD45RA and PC5-labelled anti-CD4 mAbs (Beckman Coulter Immunotech), and CD3+ CD4+ CD45RA+ cells were isolated using a FACSAria™ cell sorter as naive CD4+ T cells. The PBMCs were stained with FITC-labelled anti-CCR7 (R&D Systems, Minneapolis, MN), PE-labelled anti-CD45RA and PC5-labelled anti-CD8 mAbs (Beckman Coulter Immunotech), and CD8+ CD45RA+ CCR7+ cells were isolated using FACSAria™ cell sorter as naive CD8+ T cells. Reanalysis of the sorted cells confirmed purity > 98%.
T-cell culture with DCs, analysis of CD70 expression on T cells, and analysis of cytokine production by enzyme-linked immunosorbent assay
The IFN-DCs or IL-4-DCs were induced by culturing monocytes in CellGro DC Medium for 3 days and were stimulated either with TNF-α, IL-1β and IL-6 or with the PGE2 cocktail for 3 days. CD4+ CD45RA+ T cells or CD8+ CD45RA+ CCR7+ T cells (1 × 105 cells) were cocultured for 8 days with 1 × 104 allogeneic MoDCs in R10 in 200 μl/well in 96-well round-bottom culture plates in the presence of 10 μg/ml anti-CD70 mAb 2F11, 1 μg/ml human cytotoxic T-lymphocyte antigen 4 (CTLA-4)/Fc chimeric protein (R&D Systems), or 10 μg/ml isotype-matched control mAb. On day 1, 15 U/ml IL-2 (teceleukin, Shionogi & Co., Ltd., Osaka, Japan) and 5 ng/ml IL-7 (PeproTech) were added to the culture of CD8+ T cells. The stimulated T cells were stained with FITC-labelled anti-CD70 mAb (BD Biosciences), and were analysed for CD70 expression by FACSCalibur™. The T cells were also restimulated at 1 × 106 cells/ml with plate-bound 10 μg/ml anti-CD3 (OKT3) and 1 μg/ml soluble anti-CD28 mAbs (BD Biosciences) for 24 h. The supernatants were harvested and analysed for cytokine by enzyme-linked immunosorbent assay (ELISA). The following reagents were used for ELISA: human IFNg mAb clone 2G1, human IFNg mAb biotin-labelled, and horseradish peroxidase-conjugated streptavidin (Endogen, Rockford, IL), OptEIA™ human TNF, IL-4 and IL-10 ELISA set (BD Biosciences), human IL-5CytoSets™ kit (Biosource International, Camarillo, CA), and human IL-13 ELISA kit (Biosource International).
Analysis of intracellular granzyme B by flow cytometry
CD8+ CD45RA+ CCR7+ T cells stimulated with DCs for 8 days were harvested, fixed, permeabilized, and stained with FITC-labelled anti-granzyme B mAb (BD Biosciences) or an isotype-matched control mAb. Cells were analysed using FACSCalibur™.
Results
IFN-α is the most potent basal cytokine for the induction of CD70 on MoDCs
We cultured monocytes in different conditions to search optimal stimulation to induce the expression of CD70 on MoDCs. We used (i) IL-4, IFN-α, TNF-α or IL-15 in combination with GM-CSF as basal cytokines to induce monocytes to differentiate into immature DCs, (ii) different types of maturation-inducing factors: TLR ligands [poly I:C (TLR3 ligand) and LPS (TLR4 ligand)], CD40L or the PGE2 cocktail, and (iii) RPMI-1640 containing 10% FCS (referred to as R10) or a serum-free medium, CellGro DC Medium, as culture medium. CellGro DC Medium is optimized for the generation of DCs from monocytes and has been shown to induce potent MoDCs.23 Furthermore, we cultured monocytes for 3 days (a short period) or 7 days (a standard period) to induce immature DCs. We examined the expression of CD70 with anti-CD70 mAb clone BU69, and two other TNF family members, OX40L and 4-1BBL, for comparison. We examined the expression of CD86 as a marker of maturation.
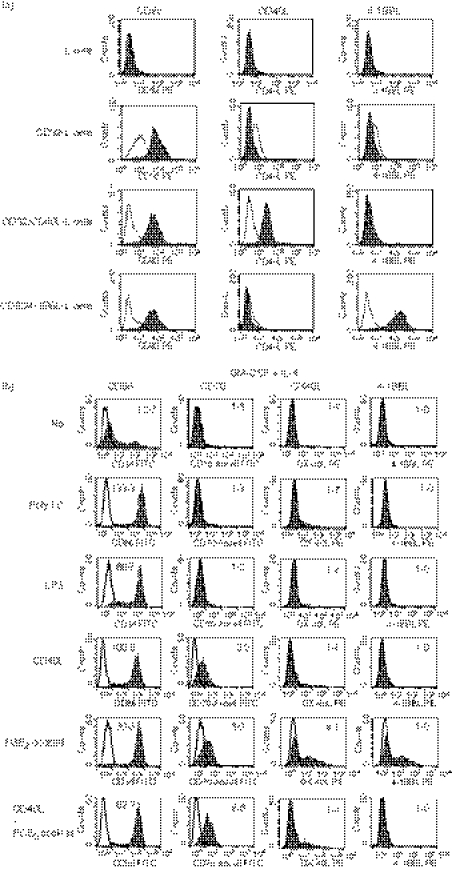
First, we cultured monocytes with IL-4 or IFN-α together with GM-CSF in R10 for 3 days, and then cultured immature DCs without or with maturation-inducing factors (poly I:C, LPS, CD40L or the PGE2 cocktail). Because the expression levels of CD70 and CD86 gradually increased until day 3 after stimulation of IFN-DCs with the PGE2 cocktail and did not further increase on day 4 (data not shown), we stimulated immature MoDCs with maturation-inducing factors for 3 days. The anti-4-1BBL mAb we used stained CD32/4-1BBL-double-transfected L cells but not parental L cells, CD32-single-transfected L cells or CD32/CD40L-double-transfected L cells (Fig. 1a), indicating that the anti-4-1BBL mAb specifically binds to 4-1BBL.
Figure 1.
Expression of CD70, OX40 ligand (OX40L), 4-1BBL and CD86 on monocyte-derived dendritic cells (MoDCs) cultured in different conditions. (a) Parental L cells and those transfected with CD32 (CD32-L), CD32 and CD40L (CD32/CD40L-L) or CD32 and 4-1BBL (CD32/4-1BBL-L) were stained with phycoerythrin (PE) -labelled anti-CD32, anti-CD40L or anti-4-1BBL monoclonal antibody (mAb). Open histograms represent cells stained with isotype-matched control mAbs. (b, c) Monocytes were cultured for 3 days in R10 in the presence of interleukin-4 (IL-4) (b) or interferon-α (IFN-α) (c) together with granulocyte–macrophage colony-stimulating factor (GM-CSF) to induce immature IL-4-DCs or IFN-DCs, respectively. Thereafter, they were cultured for 3 days in the absence or presence of maturation-inducing stimuli [poly I:C, lipopolysaccharide (LPS), CD40L-L cells, or the prostaglandin E2 (PGE2) cocktail]. The cells were stained with fluorescein isothiocyanate (FITC) -labelled anti-CD86, anti-CD70 (clone BU69), PE-labelled anti-OX40L, or anti-4-1BBL mAb. Open histograms represent cells stained with isotype-matched control mAbs. The numbers shown with each histogram represent [mean fluorescence intensity (MFI) of each costimulatory molecule]/(MFI of isotype-matched control]. Data are representative of three experiments.
When IL-4 was used, immature DCs expressed a low level of CD86 and did not express detectable levels of CD70, OX40L or 4-1BBL (Fig. 1b). All the maturation-inducing stimuli up-regulated the expression of CD86. CD40L or the PGE2 cocktail, but not the two TLR ligands (poly I:C and LPS), induced moderate levels of CD70. The combination of CD40L and the PGE2 cocktail did not exhibit an additive effect on the induction of CD70. Among these maturation-inducing stimuli, the PGE2 cocktail induced a moderate level of OX40L on about half of the DCs. The expression of 4-1BBL was not detectable on any of the DCs.
Similarly, when IFN-α was used, immature DCs expressed a low level of CD86 and did not express detectable levels of CD70, OX40L or 4-1BBL (Fig. 1c). All the maturation-inducing stimuli up-regulated the expression of CD86. In contrast to the effects on IL-4-DCs, poly I:C and LPS induced moderate levels of CD70 on IFN-DCs, indicating that IFN-α but not IL-4 is capable of inducing CD70 in combination with the TLR ligands. CD40L and to a greater extent the PGE2 cocktail induced higher levels of CD70 than poly I:C and LPS. The combination of CD40L and the PGE2 cocktail did not exhibit an additive effect on the induction of CD70. All the expression of CD70 was confirmed with another clone of anti-CD70 mAb (clone Ki-24) (data not shown). Among these maturation-inducing stimuli, poly I:C and the PGE2 cocktail slightly induced OX40L, whereas LPS or CD40L did not induce OX40L. The expression of 4-1BBL was not detectable on any of the DCs.
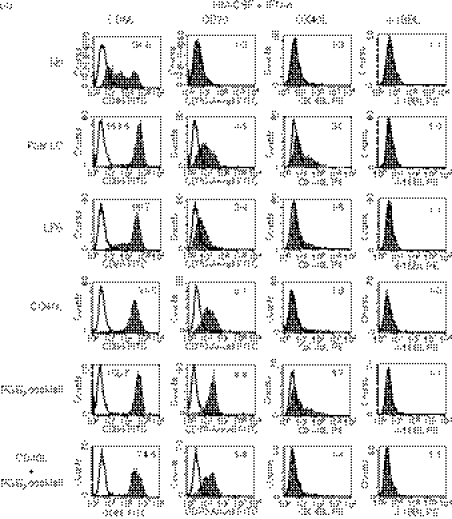
It is possible that a standard period (5–7 days) culture for the induction of immature DCs increases the expression of CD70 compared with a short period (3 days) of culture. Hence, we cultured monocytes with IL-4 or IFN-α together with GM-CSF for 3 or 7 days, and then cultured immature DCs without or with maturation-inducing factors (LPS, CD40L or the PGE2 cocktail) for another 3 days. The expression levels of CD70 and CD86 on IL-4-DCs (Fig. 2a) and IFN-DCs (Fig. 2b) did not increase but rather decreased in the 7-day culture compared with the 3-day culture. The short period (3 days) of culture to induce immature DCs was therefore sufficient to induce maximal levels of CD70 expression after maturation.
Figure 2.
A short-term culture is sufficient to induce maximal levels of CD70 expression on monocyte-derived dendritic cells (MoDCs). Monocytes were cultured for 3 or 7 days in R10 in the presence of interleukin-4 (IL-4) (a) or interferon-α (IFN-α) (b) together with granulocyte–macrophage colony-stimulating factor (GM-CSF) to induce immature IL-4-DCs or IFN-DCs, respectively. Thereafter, the DCs were cultured for 3 days in the absence or presence of maturation-inducing stimuli [lipopolysaccharide (LPS), CD40L-L cells, or the prostaglandin E2 (PGE2) cocktail]. The cells were stained with fluorescein isothiocyanate (FITC) -labelled anti-CD70 or anti-CD86 monoclonal antibody (mAb). Open histograms represent cells stained with isotype-matched control mAbs. The numbers shown with each histogram represent [mean fluorescence intensity (MFI) of each costimulatory molecule]/(MFI of isotype-matched control). Data are representative of three experiments.
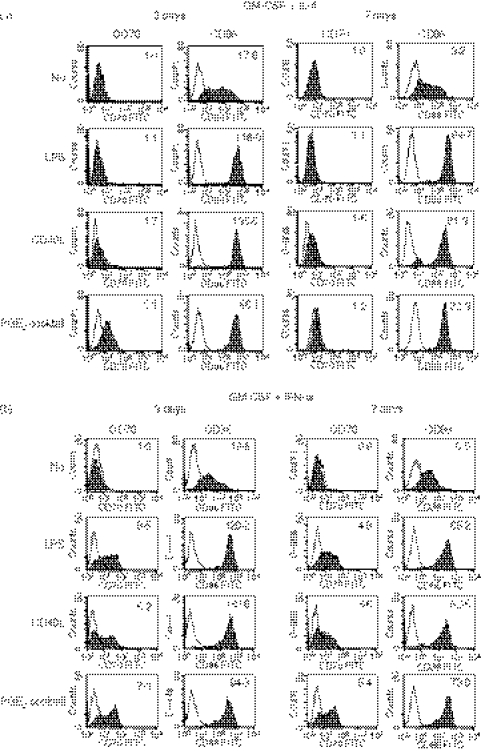
We also analysed the expression of CD70 on MoDCs induced by TNF-α or IL-15 together with GM-CSF. We cultured monocytes with these cytokines for 3 or 7 days, and then cultured immature DCs without or with maturation-inducing factors (LPS or the PGE2 cocktail) for another 3 days. Many cells strongly adhered to the plastic bottom when we used TNF-α or IL-15 without maturation and to a lesser extent after maturation, whereas most of the IL-4-DCs and IFN-DCs were non-adherent (data not shown). Hence, IL-4 and IFN-α are more suitable than TNF-α and IL-15 to harvest as many DCs as possible. We analysed the expression of CD70 and CD86 on immature and mature TNF-DCs and IL-15-DCs. Although both of the DCs expressed CD70 after stimulation with LPS or the PGE2 cocktail (Fig. 3), the levels were lower than those of CD70 on IFN-DCs (Fig. 2b). Consequently, IFN-α is most efficient to obtain MoDCs that express the maximal level of CD70 with minimal loss of cells by adherence.
Figure 3.
Tumour necrosis factor-α (TNF-α) or interleukin-15 (IL-15) is not efficient to induce the expression of CD70 on monocyte-derived dendritic cells (MoDCs). Monocytes were cultured for 7 days in R10 in the presence of TNF-α or IL-15 together with granulocyte–macrophage colony-stimulating factor (GM-CSF) to induce immature TNF-DCs or IL-15-DCs, respectively. Thereafter, they were cultured for 3 days in the absence or presence of maturation-inducing stimuli (LPS or the PGE2 cocktail). The cells were stained with fluorescein isothiocyanate (FITC) -labelled anti-CD70 or anti-CD86 monoclonal antibody (mAb). Open histograms represent cells stained with isotype-matched control mAbs. The numbers shown with each histogram represent [mean fluorescence intensity (MFI) of each costimulatory molecule]/(MFI of isotype-matched control). Data are representative of two experiments. Because there were many dead cells when TNF-DCs and IL-15-DCs were stimulated with CD40L-transfected L cells, these data are not shown. When monocytes were cultured for 3 days to induce immature DCs, similar data were obtained.
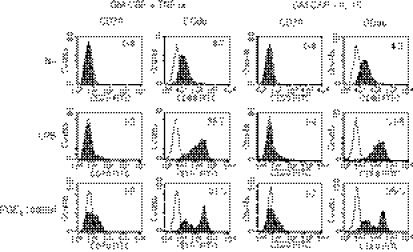
It is desirable to culture MoDCs in a serum-free medium for clinical use so we compared R10 with CellGro DC Medium for the induction of CD70 (Fig. 4). Higher levels of CD70 were induced in CellGro DC Medium than in R10 when immature IL-4-DCs and IFN-DCs were stimulated with CD40L or the PGE2 cocktail.
Figure 4.
Serum-free CellGro DC Medium is efficient to induce the expression of CD70 on monocyte-derived dendritic cells (MoDCs). Monocytes were cultured for 3 days in R10 or CellGro DC Medium (CG) in the presence of interleukin-4 (IL-4) or interferon-α (IFN-α) together with granulocyte–macrophage colony-stimulating factor (GM-CSF) to induce immature DCs. Thereafter, they were stimulated with CD40L-L cells or the prostaglandin E2 (PGE2) cocktail for 3 days in the same medium used for the first 3 days. The cells were stained with fluorescein isothiocyanate (FITC) -labelled anti-CD70 or anti-CD86 monoclonal antibody. Data are shown by [mean fluorescence intensity (MFI) of CD70]/(MFI of isotype-matched control). Data are representative of three experiments.
Collectively, these data indicate that IFN-α induces CD70 on MoDCs more efficiently than IL-4, TNF-α or IL-15 in combination with different categories of maturation-inducing stimuli within the short period of culture in both FCS-containing and serum-free media.
PGE2 is the most important factor within the cytokine cocktail for the induction of CD70 and CD86
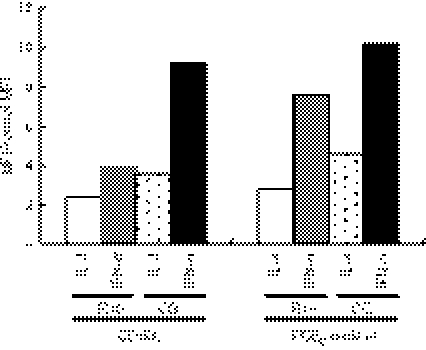
Prostaglandin E2 has been used as an important factor to induce maturation of MoDCs24 and their migratory capacity.25,26 For this reason, we next examined whether PGE2 is also important for inducing the expression of CD70 on IFN-DCs when the mixture of TNF-α, IL-1β, IL-6 and PGE2 is used. We stimulated immature IFN-DCs with TNF-α, IL-1β, IL-6 or PGE2 in various combinations. As shown in Fig. 5(a), TNF-α alone did not induce CD70. Prostaglandin E2 alone slightly induced CD70. The combination of TNF-α and PGE2 strongly induced CD70. The combination of all four factors (TNF-α, IL-1β, IL-6 and PGE2) further increased the expression level. Removal of TNF-α, and to a lesser extent IL-1β, reduced the expression of CD70. In contrast, removal of IL-6 did not reduce it. Importantly, removal of PGE2 greatly reduced the expression of CD70 down to the level of immature DCs. The induction of CD86 exhibited a similar pattern to that of CD70 (Fig. 5b). However, the combination of TNF-α and PGE2 was sufficient to induce a maximal level of CD86, and neither IL-1β nor IL-6 contributed to the up-regulation of CD86. Removal of PGE2 reduced the expression of CD86 more than removal of TNF-α, indicating that PGE2 contributes to the up-regulation of CD86 more than TNF-α does.
Figure 5.
Tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), plus prostaglandin E2 (PGE2) induce full expression of CD70. Immature interferon-treated dendritic cells (IFN-DCs) were cultured for 3 days in the absence or presence of the indicated combinations of components of the PGE2 cocktail in R10. Expression levels of CD70 (a) and CD86 (b) are shown by [mean fluorescence intensity (MFI) of each costimulatory molecule]/(MFI of isotype-matched control). Data are representative of three experiments.
Taken together, among the four factors in the cocktail, PGE2 is most important for the induction of both CD70 and CD86 on IFN-DCs. Whereas the combination of TNF-α, IL-1β and PGE2 was necessary for the full induction of CD70, IL-1β was dispensable for that of CD86. Interleukin-1β therefore has unique activity to enhance the expression of CD70 among the combination of the four factors.
Naive CD4+ and CD8+ T cells acquire the expression of CD70 after stimulation with MoDCs
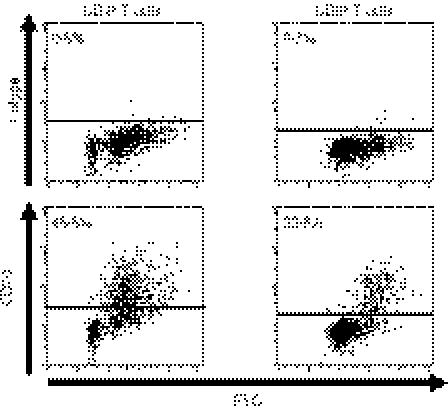
It has been shown that CD70 is expressed on activated T cells27,28 so we examined whether naive CD4+ and CD8+ T cells gain the expression of CD70 after stimulation with DCs. We stimulated CD4+ CD45RA+ or CD8+ CD45RA+ CCR7+ naive T cells with allogeneic IFN-DCs stimulated with the PGE2 cocktail, and examined the expression of CD70 on T cells. Most of the activated CD4+ and CD8+ T cells, as shown by increased forward scatter, expressed CD70 (Fig. 6). Hence, naive CD4+ and CD8+ T cells gain the expression of CD70 after stimulation with DCs, indicating that DCs as well as activated T cells can be sources of CD70 during the stimulation of T cells with DCs.
Figure 6.
Naive CD4+ and CD8+ T cells gain the expression of CD70 after stimulation with dendritic cells (DCs). Purified CD4+ CD45RA+ T cells or CD8+ CD45RA+ CCR7+ T cells were cocultured for 8 days with interferon-DCs stimulated with the prostaglandin E2 cocktail. The T cells were harvested and stained with fluorescein isothiocyanate-labelled anti-CD70 monoclonal antibody, and were analysed by flow cytometry. The percentages of CD70+ cells are indicated on the plot. Data are representative of three experiments.
The CD70–CD27 interaction enhances the production of a broad spectrum of cytokines whereas suppresses the production of IL-10 by naive CD4+ T cells during the stimulation with MoDCs
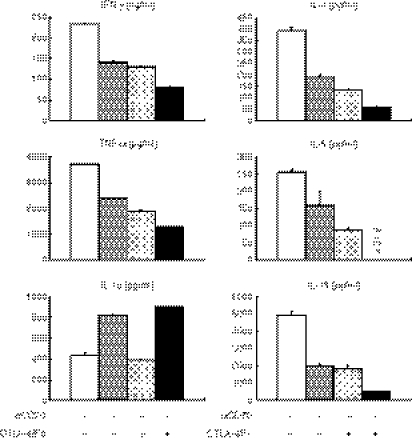
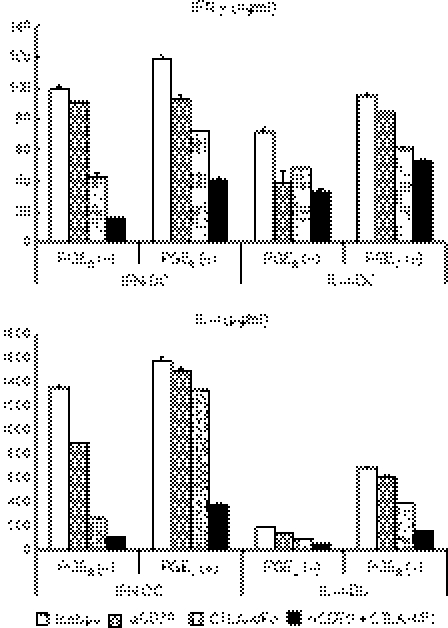
Next we examined whether the CD70–CD27 interaction contributes (i) to an increase in the number of naive CD4+ T cells and (ii) to the acquisition of cytokine-producing capacity by naive CD4+ T cells during the stimulation with IFN-DCs stimulated with the PGE2 cocktail, which express a high level of CD70. We cocultured naive CD4+ CD45RA+ T cells with allogeneic MoDCs in the presence or absence of anti-CD70 mAb and/or a CTLA-4/Fc chimeric protein. Neither anti-CD70 mAb nor CTLA-4/Fc diminished the increase in the number of T cells stimulated with DCs (data not shown), possibly because other costimulatory molecules and/or high-affinity interactions between allogeneic MHC class II molecules and T-cell receptors compensated for the blockade of the CD70–CD27 and CD80/CD86–CD28 interactions. In contrast, anti-CD70 mAb diminished the production of a T helper type 1 (Th1) cytokine (IFN-γ), Th2 cytokines (IL-4, IL-5, IL-13), and TNF-α by the CD4+ T cells (Fig. 7). Interestingly, however, the addition of anti-CD70 mAb increased, rather than diminished, the production of IL-10. The CTLA-4/Fc similarly diminished the production of IFN-γ, IL-4, IL-5, IL-13 and TNF-α by the CD4+ T cells, whereas the level of IL-10 did not change. The addition of both anti-CD70 mAb and CTLA-4/Fc exhibited additive inhibitory effects on the cytokine production. These data indicate that the CD70–CD27 interaction promotes naive CD4+ T cells to acquire the capacity to produce a broad spectrum of immunostimulatory cytokines including Th1 and Th2 types, whereas the interaction suppresses the production of an immunoregulatory cytokine IL-10 during the stimulation with MoDCs.
Figure 7.
The CD70–CD27 interaction promotes naive CD4+ T cells to acquire the ability to produce immunostimulatory cytokines while prevents them from producing interleukin-10 (IL-10) during the stimulation with CD70+ monocyte-derived dendritic cell s(MoDCs). CD4+ CD45RA+ T cells were cocultured for 8 days with allogeneic interferon-DCs stimulated with the prostaglandin E2 cocktail in the presence of 10 μg/ml anti-CD70 monoclonal antibody (mAb), 5 μg/ml human CTLA-4/Fc chimeric protein, or 10 μg/ml isotype-matched control mAb. The stimulated T cells were harvested, and were restimulated at 1 × 106 cells/ml with plate-bound 10 μg/ml anti-CD3 and 1 μg/ml soluble anti-CD28 mAbs for 24 h. The supernatants were harvested and analysed for cytokines using enzyme-linked immunosorbent assay. Error bars indicate standard deviation of duplicate measurements. Data are representative of four experiments.
The CD70–CD27 interaction enhances the production of Th1 and Th2 cytokines by naive CD4+ T cells irrespective of the expression of CD70 on DCs before coculture
We examined whether the expression of CD70 on MoDCs before coculture with T cells correlates with the induction of IFN-γ and IL-4. We cocultured CD4+ CD45RA+ T cells with four types of allogeneic MoDCs that express different levels of CD70: (i) IFN-DCs stimulated with TNF-α, IL-1β and IL-6, (ii) IFN-DCs stimulated with the PGE2 cocktail, (iii) IL-4-DCs stimulated with TNF-α, IL-1β and IL-6, or (iv) IL-4-DCs stimulated with the PGE2 cocktail, in the presence or absence of anti-CD70 mAb and/or a CTLA-4/Fc chimeric protein. For all four DCs, anti-CD70 mAb or CTLA-4/Fc diminished the production of IFN-γ and IL-4 by the CD4+ T cells (Fig. 8). The addition of both anti-CD70 mAb and CTLA-4/Fc exhibited additive inhibitory effects on the cytokine production. We did not reproducibly obtain data showing correlation between the levels of CD70 expression on DCs before coculture and the levels of cytokine production by T cells. These data suggest that not only CD70 expressed on DCs at the beginning but also CD70 induced on DCs and T cells during coculture serve to trigger the signal through CD27.
Figure 8.
The CD70–CD27 signal enhances the production of T helper type 1 and 2 cytokines by naive CD4+ T cells irrespective of the expression of CD70 on dendritic cells (DCs) before coculture. CD4+ CD45RA+ T cells were cocultured for 8 days with the following allogeneic monocyte-derived (Mo) DCs: (i) interferon (IFN) -DCs stimulated with tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6, (ii) IFN-DCs stimulated with the prostaglandin E2 (PGE2) cocktail, (iii) IL-4-DCs stimulated with TNF-α, IL-1β and IL-6, or (iv) IL-4-DCs stimulated with the PGE2 cocktail, in the presence of 10 μg/ml anti-CD70 monoclonal antibody (mAb), 5 μg/ml human CTLA-4/Fc chimeric protein, or 10 μg/ml isotype-matched control mAb. The stimulated T cells were harvested, and were restimulated at 1 × 106 cells/ml with plate-bound 10 μg/ml anti-CD3 and 1 μg/ml soluble anti-CD28 mAbs for 24 h. The supernatants were harvested and analysed for cytokine by enzyme-linked immunosorbent assay. Error bars indicate standard deviation of duplicate measurements. Data are representative of two experiments.
The CD70–CD27 interaction does not play a dominant role in the induction of IFN-γ and granzyme B in naive CD8+ T cells during the stimulation with MoDCs
Finally, we cocultured naive CD8+ T cells with allogeneic IFN-DCs stimulated with the PGE2 cocktail in the presence or absence of anti-CD70 mAb and/or a CTLA-4/Fc chimeric protein. Neither anti-CD70 mAb nor CTLA-4/Fc diminished the increase in the number of naive CD8+ T cells stimulated with DCs (data not shown), possibly because exogenous IL-2 and IL-7, other costimulatory molecules, and/or high-affinity interaction between allogeneic MHC class I molecules and T-cell receptors compensated for the blockade of the CD70–CD27 and CD80/CD86–CD28 interactions. Neither anti-CD70 mAb nor CTLA-4/Fc, alone or in combination, reproducibly diminished the production of IFN-γ or the expression of granzyme B by naive CD8+ T cells (data not shown). We therefore concluded that the CD70–CD27 interaction does not play a dominant role in the induction of IFN-γ and granzyme B in naive CD8+ T cells during the stimulation with MoDCs.
Discussion
Unlike other members of the TNF receptor family, CD27 is already expressed on naive T cells, suggesting the importance of the CD70–CD27 interaction in T-cell priming. Recent studies using mice have shown that transgenic expression of CD70 on DCs is sufficient for naive CD8+ T cells to clonally expand and to acquire the capacity for IFN-γ production and cytotoxic activity after immunization with peptides.10 Furthermore, adoptive transfer of peptide-pulsed immature DCs constitutively expressing CD70 induce anti-tumour immune responses in mice,11 indicating the importance of CD70–CD27 interaction in DC-based tumour immunotherapy. Given these findings in mice and the wide use of MoDCs in tumour immunotherapy in humans, it is crucial to examine (i) what kinds of stimuli most strongly induce CD70 on human MoDCs and (ii) what kinds of functions human naive CD4+ and CD8+ T cells acquire through the CD70–CD27 interaction during the stimulation with MoDCs, so as to translate the findings in mice into clinical applications. We have shown that (i) IFN-α is the most important basal cytokine to induce the highest level of CD70 expression, (ii) the CD70–CD27 interaction induces naive CD4+ T cells to produce immunostimulatory cytokines during stimulation with MoDCs, and (iii) the CD70–CD27 interaction does not play a dominant role in promoting naive CD8+ T cells to develop into effector cells upon stimulation with MoDCs. This is the first study to reveal the importance of IFN-α in the induction of CD70 on MoDCs and to analyse the effects of the CD70–CD27 interaction during the stimulation of naive CD4+ and CD8+ T cells with MoDCs, hence providing important information for DC-based immunotherapy.
Interferon-α induced CD70 in combination with any categories of maturation-inducing factors, among which the PGE2 cocktail was most efficient. Among the four factors in the PGE2 cocktail, PGE2 was indispensable, consistent with the recent report.19 Whereas TNF-α and PGE2 were sufficient for the maximal induction of CD86, the addition of IL-1β was necessary for the highest expression of CD70 but not of CD86, indicating that the optimal induction of CD70 is more demanding than that of CD86. This suggests that there is a positive correlation between the strength of inflammation and the repertoire of induction of costimulatory molecules on DCs. In other words, this might reflect correlation between the strength of innate and adaptive immunity through activation of DCs. Importantly, a serum-free medium, CellGro DC Medium, induced a higher level of CD70 than R10 after stimulation with CD40L or the PGE2 cocktail. This finding is therefore applicable to clinical use. In addition, it is convenient for clinical application that the short-term culture is sufficient to induce MoDCs to express the highest level of CD70.
We also compared the up-regulation of CD70 with that of other TNF family members, OX40L and 4-1BBL, which are also important for T-cell stimulation. As recently reported,19 IL-4-DCs stimulated with the PGE2 cocktail expressed OX40L, and this was also observed on IFN-DCs. Although the recent study showed increased expression of 4-1BBL messenger RNA in IL-4-DCs stimulated with CD40L and PGE2, the expression of 4-1BBL protein was not clearly assessed because of the lack of 4-1BBL-expressing positive control cells.19 Here, by using 4-1BBL-transfected cells as a control, we unequivocally showed that all the DCs in this study do not express a detectable level of 4-1BBL on their surfaces. The comprehensive comparison of CD70, OX40L and 4-1BBL expression on IL-4-DCs and IFN-DCs in this study demonstrates differential control of expression of these TNF family members on human MoDCs.
Consistent with the previous findings that activated T cells express CD70,27,28 naive CD4+ and CD8+ T cells gained the expression of CD70 after stimulation with MoDCs. This suggests that CD70 on mature DCs interacts with CD27 on naive T cells at the initial stage, and that thereafter CD70, which subsequently emerges on activated T cells, interacts with CD27 on primed T cells, providing a sustained signal throughout the activation process of naive T cells.
Next, we investigated the role of the CD70–CD27 interaction during the activation of naive CD4+ T cells with CD70+ IFN-DCs. Because CD27 and CD28 are the two major costimulatory receptors expressed on T cells from the naive stage, we compared the effects of these two costimulatory signals. The data indicate that the CD70–CD27 signal may enhance a broad spectrum of immune responses by augmenting the production of immunostimulatory cytokines including Th1 and Th2 types and at the same time by suppressing the production of an immunoregulatory cytokine IL-10 during the stimulation of naive CD4+ T cells by MoDCs. The suppression of IL-10 is reminiscent of the recent report that OX40 signalling inhibits the induction of IL-10-producing cells from naive CD4+ T cells.22 Both of these two important members of the TNF receptor family, CD27 and OX40, are likely to contribute to enhancing immune responses by transmitting a signal that inhibits the production of IL-10 by CD4+ T cells. The effect of MoDCs on naive CD4+ T cells shown in this study are consistent with our recent finding of the effect of myeloid DCs directly isolated from blood,29 and therefore it is a general phenomenon observed in human myeloid DCs directly obtained from blood and in those induced from monocytes.
We examined whether the expression of CD70 on MoDCs before coculture with T cells correlates with the induction of cytokines, using four types of MoDCs that express different levels of CD70: IFN-DCs and IL-4-DCs with or without PGE2 stimulation. For all four DCs, anti-CD70 mAb diminished the production of IFN-γ and IL-4 by the CD4+ T cells. Because CD70 is expressed on IFN-DCs and IL-4-DCs stimulated with CD40L (Fig. 1b, c), CD70 is likely to be induced on immature IFN-DCs and IL-4-DCs during the interaction with activated CD4+ T cells expressing CD40L, and such CD70 may interact with CD27 on T cells. In addition, CD70 is expressed on activated T cells (Fig. 6), and such CD70 is also likely to interact with CD27. Therefore, during priming of naive CD4+ T cells with MoDCs, CD70 induced on DCs by pro-inflammatory mediators (IFN-α, TNF-α, IL-1β, PGE2) and CD40L as well as CD70 induced on activated T cells may enhance functional development of T cells through DC–T-cell and T-cell–T-cell interactions, respectively.
In contrast to the findings of CD4+ T cells, neither anti-CD70 mAb nor CTLA-4/Fc diminished the production of IFN-γ and granzyme B by naive CD8+ T cells. The role of the CD70–CD27 interaction in effector differentiation of naive CD8+ T cells in mice is controversial. Studies using CD70 transgenic mice have shown that constitutive CD27 signalling promotes effector differentiation of naive CD8+ T cells.5,6,10 In contrast, studies using CD27-deficient mice or blocking anti-CD70 mAb have shown that the CD27 signal promotes survival but not effector differentiation of naive CD8+ T cells,3,4 or intriguingly even suppresses their effector differentiation.30 From these studies, together with our present data, it is likely that there are multiple signals that induce naive CD8+ T cells to acquire effector functions, and that the other signals compensate for the absence of the CD27 signal, whereas forced signalling through CD27 is sufficient for effector differentiation of naive CD8+ T cells. Our data together with studies in mice using blocking anti-CD70 mAb8,30 suggest that the CD27 signal may be more important for inducing functional differentiation of naive CD4+ T cells than that of naive CD8+ T cells in mice and humans.
In conclusion, this study adds a novel function to the versatile cytokines, type I IFNs, that is, the induction of CD70 on MoDCs. It is intriguing to consider the possibility that type I IFNs in infected tissues together with microbial components and/or the pro-inflammatory mediators induce monocytes to differentiate into CD70+ DCs, so enhancing protective immunity. From the perspective of tumour immunotherapy, this study shows that CD70 plays an important role in inducing naive CD4+ T cells to acquire robust immunostimulatory activity after administration of MoDCs through the DC–T-cell and T-cell–T-cell interactions. This finding has significant implications for DC-based immunotherapy.
Acknowledgments
We thank M. Hirai, K. Fukunaga and M. Utsumi for technical assistance, and Dr Yong-Jun Liu for providing us with L cells, CD32-transfected L cells, CD32/CD40L-transfected L cells, and CD32/4-1BBL-transfected L cells. This study was supported by research funding from Ministry of Education, Culture, Sports, Science, and Technology of Japan and Jishokai Health System, Inc.
Glossary
Abbreviations:
- 4-1BBL
4-1BB ligand
- CD40L
CD40 ligand
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- DC
dendritic cell
- ELISA
enzyme-linked immunosorbent assay
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- MFI
mean fluorescence intensity
- MoDC
monocyte-derived dendritic cell
- OX40L
OX40 ligand
- PBMC
peripheral blood mononuclear cell
- PE
phycoerythrin
- PG
prostaglandin
- poly I:C
polyinosinic-polycytidylic acid
- Th
T helper
- TLR
Toll-like receptor
- TNF
tumour necrosis factor
Disclosures
None.
References
- 1.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 2.Borst J, Hendriks J, Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol. 2005;17:275–81. doi: 10.1016/j.coi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Hendriks J, Gravestein LA, Tesselaar K, van Lier RAW, Schumacher TNM, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 4.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–80. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arens R, Tesselaar K, Baars PA, et al. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFN-γ-mediated B cell depletion. Immunity. 2001;15:801. doi: 10.1016/s1074-7613(01)00236-9. [DOI] [PubMed] [Google Scholar]
- 6.Arens R, Schepers K, Nolte MA, van Oosterwijk MF, van Lier RAW, Schumacher TNM, van Oers MHJ. Tumor rejection induced by CD70-mediated quantitative and qualitative effects on effector CD8+ T cell formation. J Exp Med. 2004;199:1595–605. doi: 10.1084/jem.20031111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matter M, Odermatt B, Yagita H, Nuoffer J-M, Ochsenbein AF. Elimination of chronic viral infection by blocking CD27 signaling. J Exp Med. 2006;203:2145–55. doi: 10.1084/jem.20060651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soares H, Waechter H, Glaichenhaus N, et al. A subset of dendritic cells induces CD4+ T cells to produce IFN-γ by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med. 2007;204:1095–106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 10.Keller AM, Schildknecht A, Xiao Y, van den Broek M, Borst J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity. 2008;29:934. doi: 10.1016/j.immuni.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Keller AM, Xiao Y, Peperzak V, Naik SH, Borst J. Costimulatory ligand CD70 allows induction of CD8+ T-cell immunity by immature dendritic cells in a vaccination setting. Blood. 2009;113:5167–75. doi: 10.1182/blood-2008-03-148007. [DOI] [PubMed] [Google Scholar]
- 12.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–88. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chomarat P, Dantin C, Bennett L, Banchereau J, Palucka AK. TNF skews monocyte differentiation from macrophages to dendritic cells. J Immunol. 2003;171:2262–9. doi: 10.4049/jimmunol.171.5.2262. [DOI] [PubMed] [Google Scholar]
- 14.Iwamoto S, Iwai S, Tsujiyama K, et al. TNF-α drives human CD14+ monocytes to differentiate into CD70+ dendritic cells evoking Th1 and Th17 responses. J Immunol. 2007;179:1449–57. doi: 10.4049/jimmunol.179.3.1449. [DOI] [PubMed] [Google Scholar]
- 15.Mohamadzadeh M, Berard F, Essert G, et al. Interleukin 15 skews monocyte differentiation into dendritic cells with features of langerhans cells. J Exp Med. 2001;194:1013–20. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dauer M, Obermaier B, Herten J, et al. Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 2003;170:4069–76. doi: 10.4049/jimmunol.170.8.4069. [DOI] [PubMed] [Google Scholar]
- 17.Xu S, Koski GK, Faries M, et al. Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J Immunol. 2003;171:2251–61. doi: 10.4049/jimmunol.171.5.2251. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto S, Ishida M, Takahashi K, Takeda K, Miyazaki A. Lipopolysaccharide stimulation converts vigorously washed dendritic cells (DCs) to nonexhausted DCs expressing CD70 and evoking long-lasting type 1 T cell responses. J Leukoc Biol. 2005;78:383–92. doi: 10.1189/jlb.1104654. [DOI] [PubMed] [Google Scholar]
- 19.Krause P, Bruckner M, Uermosi C, Singer E, Groettrup M, Legler DF. Prostaglandin E2 enhances T-cell proliferation by inducing the costimulatory molecules OX40L, CD70, and 4-1BBL on dendritic cells. Blood. 2009;113:2451–60. doi: 10.1182/blood-2008-05-157123. [DOI] [PubMed] [Google Scholar]
- 20.Kobata T, Jacquot S, Kozlowski S, Agematsu K, Schlossman SF, Morimoto C. CD27–CD70 interactions regulate B-cell activation by T cells. Proc Natl Acad Sci USA. 1995;92:11249–53. doi: 10.1073/pnas.92.24.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadowaki N, Antonenko S, Ho S, Rissoan MC, Soumelis V, Porcelli SA, Lanier LL, Liu YJ. Distinct cytokine profiles of neonatal natural killer T cells after expansion with subsets of dendritic cells. J Exp Med. 2001;193:1221–6. doi: 10.1084/jem.193.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito T, Wang Y-H, Duramad O, Hanabuchi S, Perng OA, Gilliet M, Qin FX-F, Liu Y-J. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci USA. 2006;103:13138–43. doi: 10.1073/pnas.0603107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Napoletano C, Pinto D, Bellati F, et al. A comparative analysis of serum and serum-free media for generation of clinical grade DCs. J Immunother. 2007;30:567–76. doi: 10.1097/CJI.0b013e318046f396. [DOI] [PubMed] [Google Scholar]
- 24.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–42. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 25.Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–61. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 26.Luft T, Jefford M, Luetjens P, et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E2 regulates the migratory capacity of specific DC subsets. Blood. 2002;100:1362–72. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- 27.Hintzen RQ, Lens SM, Beckmann MP, Goodwin RG, Lynch D, van Lier RA. Characterization of the human CD27 ligand, a novel member of the TNF gene family. J Immunol. 1994;152:1762–73. [PubMed] [Google Scholar]
- 28.Agematsu K, Kobata T, Sugita K, Hirose T, Schlossman SF, Morimoto C. Direct cellular communications between CD45R0 and CD45RA T cell subsets via CD27/CD70. J Immunol. 1995;154:3627–35. [PubMed] [Google Scholar]
- 29.Hashimoto-Okada M, Kitawaki T, Kadowaki N, Iwata S, Morimoto C, Hori T, Uchiyama T. The CD70–CD27 interaction during the stimulation with dendritic cells promotes naive CD4+ T cells to develop into T cells producing a broad array of immunostimulatory cytokines in humans. Int Immunol. 2009;21:891–904. doi: 10.1093/intimm/dxp056. [DOI] [PubMed] [Google Scholar]
- 30.Carr JM, Carrasco MJ, Thaventhiran JED, Bambrough PJ, Kraman M, Edwards AD, Al-Shamkhani A, Fearon DT. CD27 mediates interleukin-2-independent clonal expansion of the CD8+ T cell without effector differentiation. Proc Natl Acad Sci USA. 2006;103:19454–9. doi: 10.1073/pnas.0609706104. [DOI] [PMC free article] [PubMed] [Google Scholar]