Abstract
The selective delivery of therapeutic radionuclides is a promising approach for treating cancer. Antibody-targeted radionuclides are of particular interest, with two products approved for the treatment of certain forms of non-Hodgkin lymphoma. However, for many other cancers, radioimmunotherapy (RAIT) has been ineffective, being limited by prolonged exposure to the highly radiosensitive bone marrow. An alternative approach, known as pretargeting, separates radionuclide from the antibody, allowing the radiation to be delivered on a small molecule that can quickly and efficiently migrate into the tumor, and then rapidly clear from the body with minimal retention in tissues. Several pretargeting methods have been developed that differ in the way they selectively capture the radionuclide. This review focuses on the development of a novel form of bispecific monoclonal antibody (bsMAb) pretargeting that uses a unique radiolabeled hapten-peptide system that can be modified to bind a number of therapeutic and imaging radionuclides. Together with a specialized recombinant humanized bsMAb prepared with by a technique known as the Dock-and-Lock (DNL) method, this pretargeting procedure has been examined in a number of different animal models, showing a high level of sensitivity and specificity for localizing tumors, and improved efficacy with less hematologic toxicity associated with directly radiolabeled IgG. The bsMAb is a tri-Fab structure, having 2 binding arms for the tumor antigen and one capable of binding a hapten-peptide. Preclinical studies were preformed to support the clinical use of a bsMAb (TF2) and a hapten-peptide bearing a single DOTA moiety (IMP-288). A Phase 0 trial found an 131I-TF2 that targets carcinoembryonic antigen (CEA) was stable in vivo, quickly clears from the blood, and localizes known tumors. The first-in-patient pretargeting experience with the 111In-IMP-288 also observed rapid clearance and low tissue (kidney) retention, as well as localization of tumors, providing initial promising evidence for developing these materials for radioimmunotherapy.
Keywords: bispecific antibody, carcinoembryonic antigen, pretargeting, radioimmunotherapy
The delivery of radionuclides by antibodies for cancer therapy started in the early 1950’s that culminated in the first radioimmunotherapy (RAIT) studies in patients reported in 1966 by McCardle et al., who used 131I-labeled polyclonal rabbit anti-fibrinogen antibody for the treatment of various cancers.1–4 It was not until the late 1970’s, when clinical investigations of a radiolabeled polyclonal antibody to the human oncofetal tumor-associated antigen, carcinoembryonic antigen (CEA), that interest in radiolabeled antibody targeting reemerged.5 Goldenberg et al. reported the first therapy studies in a human colonic cancer-hamster model,6 but clinical studies also had just begun, first as part of a multimodal approach, and later with RAIT alone.7–10 Although many preclinical and clinical studies focused on a variety of solid tumors,11 the most promising results were found clinically in hematopoietic malignancies.12–16 Today, the only approved RAIT agents are directly radiolabeled 90Y- and 131I-anti-CD20 antibodies used in follicular and transformed non-Hodgkin lymphoma (NHL).17–19
It is not surprising that RAIT’s first clinical success occurred in lymphoma, given the radiation sensitivity of hematologic malignancies that could achieve complete responses with ~700 cGy, but responses have even been observed even when tumor uptake is not visualized.20–22 Indeed, it is important to appreciate that the lymphoma treatments combine therapeutically effective anti-CD20 IgG with the radiolabeled antibody, and are therefore a combination therapy. Appreciable efficacy in solid tumors has been much more difficult, perhaps because in these indications the antibody alone is not itself effective, and therefore RAIT’s efficacy is based almost entirely on its ability to deliver an effective radiation dose uniformly to the metastatic disease. This is compounded by the problem that Phase I/II RAIT trials are commonly performed in refractory or relapsed advanced metastatic disease, a setting where animal studies predicted that RAIT would be less likely to succeed.23,24 Clinical trials have shifted largely to localized treatment, while other efforts have focused on initiating treatment in minimal or microscopic disease, as well as combinations with chemotherapy.25–28
While there may be several issues impeding efficacy, such as uniform penetration within the tumor, dosimetry data have consistently found that RAIT delivers <2000 ccGy to tumors at the maximum tolerated dose.11 RAIT is limited by hematologic toxicity as a result of the slow clearance of the antibody from the blood that exposes the red marrow to low, but continuous radiation. A few trials have escalated RAIT in solid tumors with the aid of hematologic support, but despite the additional risk, there still was no indication of improved responses.29–31 There have been several attempts to reduce the red marrow exposure. Enzymatically-cleaved antibodies lacking the Fc portion of the IgG, such as F(ab')2, or engineered antibodies lacking an Fc or even just critical portions of the Fc responsible for maintaining the IgG in the blood all clear more quickly.32–36 These antibody fragments/constructs, like an IgG, are cleared from the blood primarily by the liver, while antibody fragments ≤60 kD clear even faster and are removed by renal filtration. IgG can also be removed from the blood in a more timed-control manner by using an anti-antibody to form complexes that are then cleared by the body’s reticuloendothelial cells in the liver and spleen.37,38 Others have used extracorporeal methods to filter the body’s blood, selectively removing the radiolabeled antibody without diverting the cleared product to other tissues.39
Although all these methods have lowered the red marrow exposure, none has allowed for adequate tumor accretion to occur. For example, while antibody fragments clear more quickly from the blood so that higher doses can be given, unfortunately tumor uptake also decreases. There have been reports in animal models of improved efficacy using radiolabeled antibody fragments,40,41 but there have been relatively few clinical therapy trials using radiolabeled antibody fragments or engineered constructs. One problem facing the use of such agents is that directly radiolabeled fragments often provide favorable dosimetry only when radioiodinated. 131I is certainly a useful therapeutic, and it is readily available at a reasonable cost, but its beta-emission penetration is more ideally suited for the treatment of small lesions (perhaps <1.0 cm depending on the antibody’s distribution within the tumor), being well matched for treatments involving minimal or microscopic disease. However, its high-energy gamma emission detracts from its wider acceptance.
Today, most procedures for radiolabeling IgG result in products with good plasma stability, but when taken into cells, most radiometals become trapped or are only very slowly released from the cells. In contrast, the radioiodine that is linked to tyrosine by traditional methods is released back into the blood, where it is accreted by the thyroid or eliminated primarily in the urine. Antibody forms >60 kD usually are catabolized in the liver, so a substantial fraction of the radiometals are retained there, whereas radiometals attached to smaller fragments are retained in the kidneys. Thus, while the more rapidly clearing fragments reduce the risk for hematologic toxicity, accumulation in the kidneys increases the risk of renal toxicity. However, the kidneys can tolerate at least 2000 cGy, based on external beam data,42 and clinical trials with 90Y-DOTATOC (a somatostatin-binding peptide) have indicated patients can tolerate up to 2700 cGy.43,44 Therefore, with faster clearing agents, higher radioactivity levels can be administered, providing an opportunity to compensate for the lower tumor uptake.
Unfortunately, renal uptake with directly radiolabeled fragments is several-fold higher than that found in tumors,32,34 and thus radiometal-labeled fragments only deliver a fraction of the total dose tolerated to the kidneys. Behr et al.45 first reported that cationic amino acids, such as lysine, could reduce renal uptake several-fold, and interest in using radiolabeled peptides for therapy have lead to investigations of a number of other procedures that can reduce either radiometal uptake in the kidneys or temper the radiation effects, further expanding the therapeutic window for these agents.46–50 However, most of these procedures only provide modest improvement,51–53 which has been important for radiolabeled peptides, but has not been sufficient to compensate for the more highly elevated concentrations deposited in the kidneys with a directly radiometal-labeled fragment, and therefore the primary therapeutic choice for directly radiolabeled, systemically delivered, small antibody fragments remains radioiodine. As mentioned above, procedures that clear radiolabeled antibodies directly from the blood with second antibodies would deposit high concentrations of radiometal-labeled antibodies in the liver and spleen, but removal by extracorporeal perfusion avoids this complication. However, extracorporeal removal still requires the radiolabeled product to remain in the blood for 12–48 h, which still leads to substantial decreases in blood counts, though faster recoveries have been reported.39 Thus, the effort involved in using this procedure may outweigh what might be only a modest improvement in the therapeutic window.
Why Pretargeting?
It is ironic that it is the stability of radionuclide-antibody conjugates that causes the radionuclide to stay in the blood for so long and for radiometals to be retained by the liver and kidneys. Directly radiolabeled IgG prepared with linkages that are more labile when specifically catabolized in the liver to reduce hepatic uptake have been examined,54 but this approach does not impact hematologic toxicity associated with directly antibodies catabolized in the liver, and methods to reduce renal retention have not been able to compensate for the high uptake that occurs with a directly radiolabeled fragments. Therefore, in order to take advantage of the selective targeting afforded by an antibody, an alternative approach for delivering the radionuclide with an antibody is needed.
Such an alternative was proposed in 1985 by Reardan et al., who developed antibodies to chelates,55 but it was how they envisioned using these antibodies that led to a radically new form of antibody-based targeting, known as pretargeting. These investigators postulated that a bispecific monoclonal antibody (bsMAb) could be prepared with one arm binding selectively to a tumor, while the other arm would be derived from an anti-chelate antibody. Chelated radiometals were known to clear efficiently and rapidly from the blood and tissues, so the investigators reasoned that by pretargeting an unlabeled bsMAb first to the tumor, its anti-chelate binding arm could capture a chelate-radiometal complex and retain it in the tumor, while the remaining product would clear, minimizing red marrow and tissue exposure. Indeed, given its small size, the chelate can traverse the blood vessels quickly, easily penetrating to localize within tumor where the bsMAb had been deposited. This concept eventually came to clinical fruition with the first studies performed in colorectal cancer patients who received a chemically-conjugated bsMAb composed of an anti-CEA (carcinoembryonic antigen) Fab’ x anti-(In)EDTA Fab’ (EOTUBE is a hydroxyethylthiourido-benzyl-EDTA).56 After allowing 4 days for the bsMAb to localize and clear from the body, 111In-EOTUBE was co-administered with different amounts of the bsMAb. The co-administration of EOTUBE with the bsMAb was performed because preclinical studies found that radiometal-chelates alone cleared exceptionally fast. By slowing EOTUBE’s clearance, they hoped to avoid the same problem found with antibody fragments that had lower tumor uptake than slower-clearing whole IgG. Since the EOTUBE-bsMAb complexes were held together monovalently, they would readily dissociate, providing a “slow-release” of EOTUBE, which would then be removed rapidly. While the pretargeting procedure was more complex than injecting a directly radiolabeled antibody, this method showed metastatic lesions in the liver with good contrast from surrounding normal liver, while 111In-labeled anti-CEA IgG being used at the time often showed tumors as “cold” lesions due to higher uptake in normal liver.57,58
This initial pretargeting system relied on the monovalent binding of the chelate to the anti-chelate antibody, but Le Doussal et al.59 rationalized that by joining two haptens together with a short peptide, uptake and retention of the radiolabeled hapten-peptide would be enhanced locally within the tumor. Their affinity enhancement system (AES) relied on the higher concentration of bsMAb within the tumor that would permit for greater interaction of a divalent hapten-peptide over a monovalent form, increasing its retention in a tumor, a concept that was confirmed later by others.60,61 While enhancing retention locally, the lower concentration of bsMAb in the blood would favor the less stable monovalent binding, allowing the divalent hapten-peptide to readily dissociate and clear rapidly, giving high tumor/blood ratios. A number of preclinical and clinical investigations using this new AES procedure followed, and in each, the radiolabeled hapten-peptide was given several days after the bsMAb was administered, leaving sufficient time for the bsMAb to localize in the tumor and clear from the blood.60 Pretargeting procedures that were later developed using the ultra-high affinity binding of streptavidin/avidin for biotin have relied on the administration of clearing agents after the primary targeting conjugate is given, since even at very low concentrations in the blood, the radiolabeled biotin will form an irreversible bond with the streptavidin conjugate and extend its clearance time.62
The separation of the radionuclide-targeting from the antibody-targeting step by using a small hapten-peptide effectively reduced retention in normal tissues, but for optimal visualization and certainly for therapy, tumor uptake also must be optimized. Based on prior experiences with various forms of directly radiolabeled antibodies that generally showed tumor uptake decreased as smaller, more rapidly clearing forms were used,32,34,35 the exceptionally fast clearance of the very small radiolabeled hapten-peptide (e.g., <1.5 kD) might be expected to result in even lower tumor uptake. Initial targeting studies in a melanoma model indicated that maximum tumor uptake of a pretargeted 111In-di-DTPA-TL (tyrosine-lysine) hapten-peptide was 4-fold lower than an 111In-F(ab')2 targeting the same antigen, but the pretargeting procedure had superior tumor/nontumor ratios.63,64 Gautherot et al. later reported, using an anti-CEA Fab’ x anti-DTPA(In) bsMAb in human colon cancer xenografts, maximum tumor uptake for the anti-CEA IgG and F(ab')2 to be ~33% and ~13% at 48 and 6 h, respectively, while for pretargeting, tumor uptake peaked within 1 h after the injection of the 131I-di-DTPA(In)-TL hapten-peptide with levels similar to that of the 131I-F(ab')2.65 Dosimetry derived from these biodistribution data suggested that, depending on how the pretargeting conditions were adjusted, pretargeting compared favorable to the directly radiolabeled IgG or F(ab')2 with respect to the dose delivered to the tumor, but with higher tumor/blood ratios. Later studies, using this same CEA-pretargeting system, found similar or improved efficacy, but with reduced toxicity in colorectal and medullary thyroid cancer xenograft models.66–68 Phase I/II trials with the murine F6 anti-CEA Fab’ × murine 734 anti-DTPA(In) bsMAb pretargeting 131I-di-DTPA examined patients with different types of CEA-producing tumors, and more recently, data with a humanized anti-CEA Fab’ x murine 734 anti-DTPA(In) bsMAb were reported.69 A review of their clinical experience with an 131I-labeled hapten peptide and anti-CEA bsMAbs in medullary thyroid cancer found promising results for a poor prognostic subgroup of patients who had a calcitonin-doubling time of <2 years.70 They had a significantly improved overall survival (OS) as compared with contemporaneous untreated MTC patients with comparable prognostic indicators (median OS, 110 vs. 61 months; P < .030), which was thought to be due to reduction in micrometastatic disease that is often found in the bone marrow.
Our pretargeting experience began with an anti-CEA IgG-streptavidin/90Y-biotin system that was similar to the approach of Axworthy et al., who had reported 90Y-biotin uptake in tumor at similar levels as with the 90Y-IgG, but with better tumor/nontumor ratios and enhanced efficacy.71 Dosimetry suggested that the therapeutic window in our system was not improved over directly radiolabeled IgG,72 and the concern that any approach using streptavidin or avidin would be limited by immunogenicity shifted our interest to a bsMAb-pretargeting approach. Karacay et al. reported initial results, using a chemically-conjugated, humanized anti-CEA x murine 734 anti-DTPA(In) bsMAb, finding favorable dosimetry for therapy with a pretargeted 188Re-labeled di-DTPA hapten-peptide.73 Encouraged by these findings, further investigations with various forms of bsMAbs prepared by chemical conjugation methods lead to the discovery that bsMAbs with divalent binding to a tumor that also cleared rapidly from the blood would provide the best tumor uptake and tumor/nontumor ratios.74 Advances in molecular engineering, particularly with antibodies, made preparing bsMAb chemically obsolete, so a program to define a suitable bsMAb for clinical use was initiated by our group.
A Universal Hapten-Binding System
Before initiating studies to prepare engineered bsMAbs, we first needed to examine the anti-hapten/hapten binding component that would be used for pretargeting. The initial studies by LeDoussal et al. used an anti-DNP (dinitrophenyl) as the anti-hapten antibody,59 but many of the later bsMAb-pretargeting studies used antibodies against a chelate, DTPA (diethylenetriaminepentaacetic acid). In this situation, DTPA served as the hapten, binding to the anti-DTPA portion of the bsMAb, but it also was the effector, carrying the radionuclide. Anti-chelate antibodies usually have an optimal affinity to the chelate it was developed against, and often, immunizations are performed with the chelate loaded with a particular metal. For example, the 734 anti-DTPA(In) antibody was derived by immunizing mice with cyclic anhydride DTPA loaded with InCl3.63 It had optimal affinity for In-DTPA, with affinity reduced 100-fold when indium was replaced with other metals, but coupling one of DTPA’s carboxylic groups to the alpha-amino of TL did not affect binding affinity, allowing for the formation of a di-DTPA-TL hapten-peptide for optimal binding with the 734 antibody. Although the DTPA could be radiolabeled with 111In for imaging and the tyrosine moiety in this hapten-peptide radioiodinated for pretargeted therapy,65–68 the 734/di-DTPA-TL hapten could not be used with 90Y or 177Lu, because (a) cyclic DTPA was not a strong chelator for either of these metals, and (b) the affinity of 734 was markedly reduced when DTPA was loaded with these metals. A new hapten-peptide bearing 2 DTPAs and a thiosemicarbonylglyoxl-cysteinyl group was used to capture 99mTc and 188Re, since when loaded with indium, it was able to bind to the 734 antibody.73 Consideration was given to developing a peptide that included 2 (In)DTPA molecules for binding 734 and a DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) moiety for more stable binding of 90Y and 177Lu, but concern over the need to load DTPA with indium to ensure high-affinity binding to 734, and then to also selectively load DOTA with 90Y for improved stability, lead to an evaluation of another hapten-binding system. Janevik-Ivanovska et al.75 had reported earlier on a pretargeting method using an anti-hapten antibody that bound selectively with high affinity to a derivative of histamine, histamine-succinyl-glycine (HSG), that was first prepared in an effort to make an immunoassay to measure histamine.76 They prepared di-HSG-peptides that were radioiodinated, but later, our group prepared di-HSG derivatives containing a single DOTA or a single cysteinyl moiety that was joined to a peptide core composed of 4 D-amino acids to reduce susceptibility to enzymatic degradation in vivo. Feasibility studies were performed using 111In, 90Y, 177Lu, and 99mTc-labeled peptides pretargeted with 2 chemically-conjugated bsMAbs, each using the 679 anti-HSG anti-hapten antibody.77 Later, we and others expanded this method to include 68Ga, 124I, and 18F for positron-emission tomography (PET) imaging, and a variety of beta- and alpha-emitters for therapy,78–82 illustrating the broad flexibility of a single binding system based on the anti-HSG anti-hapten antibody for accommodating a number of radionuclides for imaging and therapeutic applications.83–91 An attractive feature of the pretargeted peptides is that the amino acid composition of the peptide core can be modified to alter the properties of the hapten-peptide to ensure minimal retention in tissues. For example, in our first report, 2 different 99mTc-di-HSG-peptides were made, but one had higher hepatic uptake than the other, based on its peptide composition.77 Changes to the amino acid composition of the initial DOTA-di-HSG peptide that was used, IMP-241,77 lead to a new DOTA-di-HSG peptide, IMP-288,81 which reduced renal retention nearly 30%, and has allowed for further escalation of 90Y-IMP-288 activity in animal testing.91
Engineering Bispecific Antibodies: The Dock-and-Lock (DNL) Method
Perhaps no single molecule has undergone so many changes since the advent of molecular engineering as IgG.92 Molecular engineering is almost essential to the reliable production of bsMAbs. Using the platform of the humanized MN-14 anti-CEA IgG for tumor targeting and the anti-HSG antibody for binding di-HSG haptens, we have assessed a number of different types of constructs, starting with a diabody format that produced a 50-kD bsMAb with monovalent binding to CEA and HSG, to a novel ~80-kDa heterodimer of two complementary polypeptides, each having three variable domains connected by short peptide linkers; showing divalent binding to CEA and monovalent binding to HSG.93,94 Consistent with our earlier experience using chemically-conjugated bsMAbs, the trivalent form with divalent binding to CEA was preferred over the diabody form.94 Using the trivalent hBS14 bsMAb, animal studies using a human colonic cancer xenograft revealed the high targeting sensitivity with this pretargeting procedure that was a significant improvement over a 99mTc-anti-CEA Fab’ fragment,82 better vi sualization of tumors using an 124I-di-HSG-peptide as compared to an 124I-anti-CEA Fab’, and the absence of any normal tissue uptake provided less ambiguous localization compared to 18F-FDG (fluorodeoxyglucose).81 In addition, improved therapeutic responses with a 90Y-di-HSG-peptide were observed.85 However, since these products could not be produced in adequate yields in mammalian cell culture, a new strategy capable of preparing a trivalent bsMAb was required.
This was solved by examining other biological systems that naturally form self-annealing structures. One such system is the interaction between the regulatory subunit of the human type II A-kinase and the human A-kinase anchor protein.95,96 A 44 amino acid sequence derived from the regulatory subunit of human type II A-kinase, called the dimerization and docking domain (or DDD), spontaneously forms a very stable homodimer, which in turn provides a docking site for the second peptide, called the anchor domain (or AD), which consists of a 17-amino acid sequence derived from an interactive human A-kinase anchor protein. By fusing these sequences on biomolecules, a variety of multivalent, mono- or multi-specific constructs can be formed.97–103 Our initial studies showed that while these structures combine efficiently, the resulting structures are not held together strongly enough for in vivo targeting, and thus the DDD and AD sequences were modified to insert cysteine residues in strategic locations so that when docked, they would be further locked in place by the formation of disulfide bonds between inserted cysteines (these modified sequences are designated AD2 and DDD2).97
The first DNL constructs were trivalent bsMAbs prepared using a DDD2-Fab from a humanized anti-CEA antibody and humanized 679 anti-HSG Fab (Figure 1).97 Several linkage strategies were explored initially, but ultimately the TF2 trivalent bsMAb was selected for pretargeting use. Despite being similar in size as an IgG, the 157-kD TF2 cleared quickly when injected in mice, with ~99% of an 131I-labeled TF2 eliminated in ~16 h (Figure 2). Uptake in a human colonic cancer xenograft peaked at ~4% injected dose per gram (ID/g) at 6 h and then decreased to ~1% at 16 h, clearing more slowly thereafter. This behavior was very different from that seen previously with the 131I-hMN-14 IgG in this same model system,104 and therefore additional pharmacokinetic studies were performed in rabbits to determine if there were species-specific differences in clearance rates. Rabbits injected with 131I-TF2 had a much slower clearance than mice, but still, TF2’s clearance in rabbits was much faster than 131I-hMN14 IgG (131I-hMN-14 IgG: T½-alpha = 7.3 h, T½-beta = 133 h; 131I-TF2: T½-alpha = 14.0 h, T½-beta = 36.5 h) (Figure 3). Indeed, 131I-hMN14 IgG clearance in rabbits was essentially identical to that found in colorectal cancer patients who had been given 131I-hMN-14 IgG (T½-alpha = 12.0 ± 8.4 h, T½-beta = 126 ± 45 h; n = 8, and includes only patients with plasma CEA <100 ng/mL),105 suggesting that the rabbits were a better model for predicting the pharmacokinetic behavior of a humanized IgG. BIACore analysis of TF2 binding to an HSG-coupled chip that was then probed with an anti-CEA idiotype antibody to illustrate continued binding to the CEA-binding arms showed that TF2 remained intact in fresh plasma at 37° C over 7 days. Size-exclusion HPLC studies of the 131I-TF2 given to mice and rabbits showed no evidence that the bsMAb decomposed to the individual Fab components, but rather only the intact 157 kD structure was found in samples taken from mice through 24 h and rabbits through 48 h (not shown). Interestingly, subsequent studies performed with another tri-Fab bsMAb, TF4 (anti-CD20 × anti-HSG) radiolabeled with 131I or 111In concurred with these findings; namely, only the intact bsMAb is present in the blood, but the tissue distribution and blood concentrations were different,87 suggesting there are catabolic processes occurring in the tissues, but not in the blood.
Figure 1.
Schematic representation of the Dock-and-Lock modules and how the are assembled to form bispecific monoclonal antibodies.
Figure 2.
Biodistribution of 131I-TF2 tri-Fab anti-CEA bsMAb in nude mice bearing the human colonic tumor xenograft, GW-39.
Figure 3.
Comparison of the blood clearance kinetics of 131I-TF2 in mice and rabbits and to 131I-hMN-14 IgG in rabbits. TF2’s Fab CEA-binding arms are donated from hMN-14.
Pretargeting with DNL Constructs
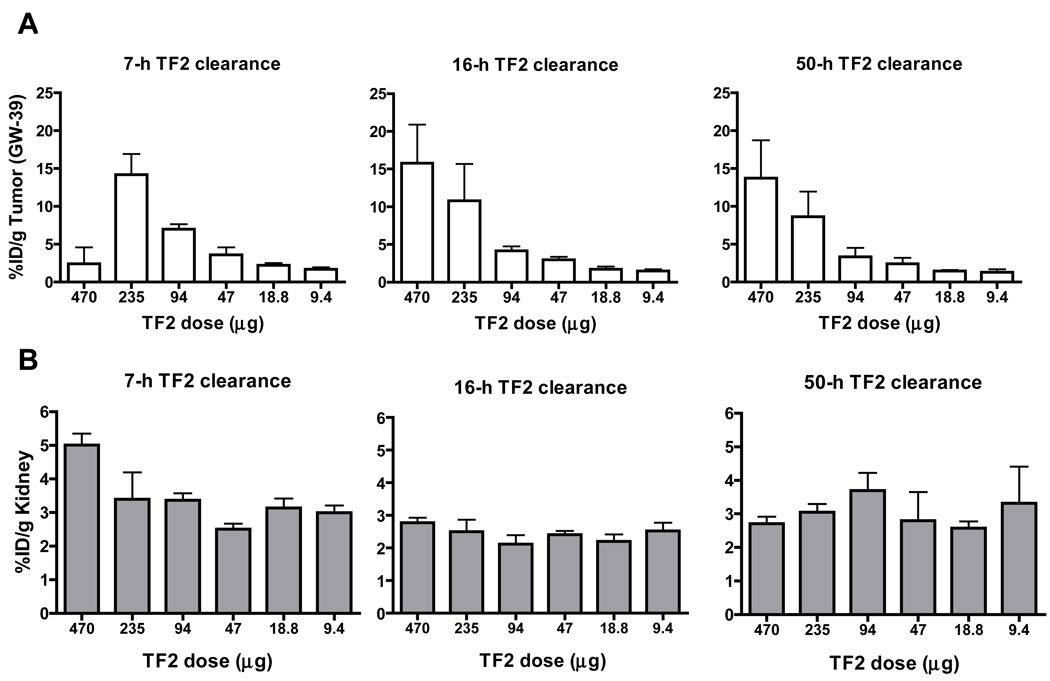
Optimization of the bsMAb pretargeting procedure requires an assessment of how much bsMAb and hapten-peptide should be given and the interval that should be given between the 2 injections. Ideally, the radiolabeled hapten-peptide should be prepared at its maximum specific activity to ensure the highest delivery of radioactivity to the tumor per mole hapten-peptide delivered, so in practice, its dose will be fixed based on the administered radioactivity dose. We had earlier observed in human xenograft-mouse models with other bsMAb that one could achieve rapid clearance of the radiolabeled hapten-peptide with good tumor targeting if the injection of the radiolabeled hapten-peptide was delayed until the bsMab’s concentration in the blood had decreased to ≤1.0% ID/g.106 Thus, to illustrate how these various parameters affect the targeting of the radiolabeled hapten-peptide, 3 sets of nude mice bearing a human colon cancer cell line (GW-39) were given a fixed amount of 111In-IMP-288 di-HSG-DOTA-peptide at 7, 16, or 50 h after receiving varying amounts of TF2. The average concentration of TF2 in the blood at 7 h was 3.3% ID/g, decreasing to ~0.3% ID/g by 16 h, and by 50 h, the amount was not measurable, suggesting that the best pretargeting interval would be ~16 h.
Figure 4 shows that irrespective of the interval used, tumor uptake of 111In-IMP-288 1 h after its injection increased as the TF2 dose was increased. At the 7-h interval, tumor uptake peaked at ~15% ID/g with 235 µg of TF2 (this represents 40-fold more moles of TF2 were given as compared to the moles of IMP-288, a.k.a. a bsMAb/peptide mole ratio of 40:1106). However, tumor uptake decreased significantly when 470 µg of TF2 was given, because more 111In-IMP-288 was bound to the residual bsMAb in the blood (e.g., 23.6% vs. 3.3% ID/g at the 470 µg vs. 235 µg TF2 dose, respectively), which also resulted in higher liver and spleen uptake (~18% and 31% ID/g vs. 3.9 and 8.1% ID/g at the 470 and 235 µg doses, respectively). Nevertheless, tumor uptake at the 270 µg TF2 dose in the 7-h interval group compared favorably to the uptake achieved when IMP-288 was given at later intervals, and tumor/blood and liver ratios at 1 h averaged 5.7 ± 2.9 and 4.8 ± 2.4, respectively.
Figure 4.
Evaluation of varying conditions to optimize pretargeting of 111In-IMP-288 di-HSG-DOTA-hapten-peptide. Nude mice bearing GW-39 human colonic tumor xenografts were injected iv with increasing amounts of TF2, and then at either 7, 16, or 50 h later, a fixed amount of 111In-IMP-288 was given (e.g., TF2/IMP-288 mole ratio at 470 µg of TF2 was ~80:1), and animals were then euthanized with determination of tumor (A) and tissue uptake. The bottom panel (B) shows uptake in the kidney in each of these groups of animals.
When the hapten-peptide injection was delayed until 16 h, maximum tumor uptake occurred between 470 µg (15.8 ± 5.1% ID/g) and 235 µg (10.3 ± 4.4% ID/g), with no significant differences at these 2 doses (P = 0.8). However, because more time was given for the TF2 to clear from the blood and tissues, tumor/blood ratios at these doses averaged ~70:1, a significant improvement over that found with the 7-h interval. Blood concentrations of 111In-IMP-288 were <0.15% ID/g for all groups given ≤235 µg of TF2 at the 16-h interval, which was similar to that found in animals given only 111In-IMP-288. At the 470 µg dose, 111In-IMP-288 concentrations were beginning to rise, but were not significantly higher than the 235 µg dose (0.24 ± 0.07% ID/g; P = 0.13). Thus, even at the highest TF2 dose given (470 µg), the molar concentration of TF2 in the blood was sufficiently low enough to avoid appreciable interaction with the hapten-peptide in a manner to affect with its clearance from the blood. At the 50-h interval, optimal uptake also occurred at the highest TF2 dose, but tumor/nontumor ratios were the same as those obtained at the 16-h interval. Thus, the 16-h interval was the earliest time at which the radiolabeled IMP-288 could be given where optimal tumor/nontumor ratios occurred and with maximum tumor uptake when ~40 to 80 more nmoles of bsMAb was injected compared to the nmoles of IMP-288 administered.
To further examine how TF2’s concentration in the blood affected 111In-IMP-288 blood clearance in a larger animal, rabbits were given 0.445 mg/kg (2.83 nmoles/kg) TF2, and 0.283 nmoles/kg of 111In-IMP-288 was given either 5, 4, or 3 days later. These intervals were selected based on the slower TF2 clearance in rabbits as compared to mice. 111In-IMP-288 was given by intravenous push in a volume <0.5 mL. As seen in Figure 5A, in the absence of a prior TF2 injection, 99% of 111In-IMP-288 was cleared within 2–4 h (n =2). Additionally, ≤35% of the total injected product could be accounted for in the 5-min sample taken after the injection, suggesting that more than 60% of the product had already left the vascular volume. IMP-288 clearance increased as the interval was decreased, but still 99% cleared within 2–8 h at a 5-day interval (n = 4), 10–15 h with a 4-day interval (n =2), and 13–17 h with a 3-day interval (n = 2). As the interval was shortened, a higher fraction of the total injected dose could be accounted for, but it never exceeded 60%. When the molar concentration of TF2 in the serum, as determined by ELISA, on the day of IMP-288 injection was compared to an estimate of IMP-288’s molar concentration at the instant it was injected, one finds that when TF2’s molar concentration was ≥10-fold lower than IMP-288’s molar concentration, IMP-288 cleared as if no TF2 was present, and as long as TF2’s molar concentration was <2-fold that of IMP-288, 99% of IMP-288 would be cleared in <20 h. A similar relationship was found in mice, and therefore it is possible to predict the rate of IMP-288’s clearance with reasonably reliability based on the molar relationship of the 2 products in the blood.
Figure 5.
Blood and renal clearance of 111In-IMP-288 given to rabbits who received a fixed amount of TF2 (10:1 TF2/IMP-288 mole ratio) 3, 4, or 5 days later, or to rabbits who were only given 111In-IMP-288. (A) Blood clearance over the first 24 h showed more than 99% (dark line) had cleared before 18 h under the various conditions examined. Bars represent the range found in 2 to 4 rabbits that were studied. (B) Regions of interest analysis for the kidneys derived from whole-body images taken over 3 days, which provided an estimate of the percentage of the total injected activity that was given (biological values).
Since the kidney is the only tissue with appreciable uptake and retention, another important consideration in pretargeting is how renal uptake of the radiolabeled hapten-peptide might be affected over a range of pretargeting conditions. As shown in Figure 4B, renal uptake of 111In-IMP-288 1 h after injection usually averaged between ~2.5 to 3.0% ID/g. The only time renal uptake increased beyond these ranges was in the one set of animals given the highest TF2 dose in the 7-h interval group. As mentioned, this group had a substantial fraction of the IMP-288 complexed in the blood with TF2, but even under these conditions, renal uptake increased to only ~5% ID/g. In rabbits, whole-body scintigraphy starting within 15 min of the 111In-IMP-288 injection also showed renal uptake and clearance was unaffected at any of the intervals examined (Figure 5B), but the conditions evaluated in the rabbits were not designed to assess such extremes as those illustrated in the mice using a 7-h interval at the highest TF2 dose.
Thus, animal data indicated that TF2 was stable in blood (which was later confirmed in patients) and was rapidly cleared in rabbits and mice, but rabbits appeared to be a more reliably predict pharmacokinetic behavior. Mouse and rabbit studies indicated that IMP-288 cleared rapidly from the blood and tissues, with minimal uptake and retention in the kidneys. In a pretargeting setting, allowing TF2 to clear from the blood so its molar concentration is 10-fold lower than the concentration of IMP-288 when it was injected ensured the peptide would clear at a rate similar to that of the peptide alone. While higher TF2 concentrations slowed IMP-288’s clearance to some degree, it is still possible to have 99% clearance of IMP-288 in <20 h. Tumor-bearing mice indicated that the highest uptake of 111In-IMP-288 in the tumor required ~40–80-fold more moles of TF2 administered than moles of IMP-288; however, the precise TF2-dosing requirements would depend on the interval selected.
Additional preclinical testing focused on the safety of the di-HSG-DOTA-peptide, IMP-288. Immunohistology studies found no evidence of binding to human tissues, and plasma stability showed it was stable in vitro and in vivo. Although the HSG hapten was a derivative of histamine, in vitro binding studies on cells transfected to increase H1, H2, and H3 expression showed no evidence antagonistic or agonistic activity against histamine, and guinea pigs, which are highly sensitive to histamine, tolerated >2000 times the human equivalent dose of 100 nmoles IMP-288, providing further support that the HSG hapten is not pharmacologically active.
Initial Clinical Experience with 131I-TF2 in Colorectal Cancer
Since understanding the clearance kinetics of the bsMAb is critical to establishing appropriate pretargeting conditions, an IND to conduct a Phase 0 clinical study in patients with known or suspected colorectal cancer was undertaken at the Lombardi Cancer Center, Georgetown University, Washington, D.C. under the direction of Drs. Louis Weiner, Ruth He, and Giuseppe Esposito. Two patients were given ~2.0 mCi of 131I-TF2 containing ~1.2 mg of TF2. The products were infused safely over ~10–20 minutes, with blood sampling over 6–7 days and whole-body imaging over 3 days. Blood clearance data showed approximately 75 to 85% of the TF2 was cleared within the first 24 h, with 50% more removed each day thereafter (Figure 6). This clearance was similar to that found in the rabbits given 131I-TF2, supporting the view that bsMab’s pharmacokinetic behavior was best defined in rabbits. An ELISA for measuring TF2 in serum could detect TF2 only over the first 24 h. In each patient, there was reasonable concordance with concentrations estimated from the 131I-activity over the first few hours, but by 24 h, the ELISA predicted ~95% of the TF2 had cleared. Size-exclusion HPLC studies were only able to assess product integrity over the first 3 h, but in each patient, there was no evidence that the product had destabilized to the Fab fragments. In patient 001, whose plasma CEA was 53 ng/mL, two larger-sized peaks were seen, representing mono- and divalent binding with CEA. A large excess of 111In-IMP-288, a divalent HSG-peptide containing a single DOTA moiety, was added to the 24-h plasma from this patient. The chromatographic profile showed a peak corresponding to an elution time of the native TF2 and 3 additional larger peaks that likely represented TF2 × CEA complexes, and the other 2 could have been TF2 × TF2 complexes with and without CEA that were formed because the divalent HSG IMP-288 could crosslink 2 TF2 molecules; however, there were no peaks that would indicate the presence of the anti-HSG Fab (neither mono or divalent forms that could have been produced by interacting with di-HSG-peptide). This study confirmed (1) The TF2 in the blood was still capable of binding the di-HSG hapten-peptide, and (2) we again did not detect the presence of Fab-like structures, suggesting that TF2 was stable in plasma.
Figure 6.
Linear (top) and log (bottom) plots of the blood clearance of 131I-TF2 in 2 colorectal cancer patients.
Although the quality of the images were poor due to use of 131I and the small amount of activity administered, the studies found evidence that TF2 could localize the known lesions in each patient, and there was no evidence of non-specific uptake in any normal tissues. Figure 7 shows the targeting in patient 001 at 1 h, and at 1 and 3 days. At 1 h, 131I-TF2 was primarily seen in the heart, with less intensity in the liver. By 24 h, there was less activity in the heart, confirming the rapid blood clearance. Activity also was seen in the stomach, thyroid, sinuses, and urinary bladder, likely representing catabolic clearance of the radioiodinated product. Activity was also present in the transverse and descending colon, as well as focal uptake in the ascending colon. This patient had previous colonoscopy that revealed diverticulitis, but also cancer in the ascending colon, which was removed at surgery after the 131I-TF2 study and found to be ~ 5 cm in diameter. Images taken on day 3 showed no residual uptake in any tissue or the tumor, only thyroid activity. The second patient did not have evidence of nonspecific colonic uptake, and therefore the activity in the colon in patient 001 likely represented catabolic products, perhaps CEA-TF2 complexes, that had been processed in the liver that subsequently were transported through the bile duct, where they then were eliminated in the feces. The second patient had a single recurrent lesion in liver that was also targeted by 131I-TF2 (not shown). Although earlier clinical studies with 131I-hMN-14 IgG had shown no normal tissue binding,104,105 the findings in these 2 patients given 131I-TF2 indicate that the tri-Fab fragment, and hence the anti-HSG binding arm and associated peptide sequences used to tether there Fabs together, do not bind to normal tissues; a finding consistent with FDA-required immunohistology studies that found no evidence of tissue binding. One patient examined 14 weeks after treatment showed no evidence of an anti-TF2 antibody response, while the other did not return for these follow-up studies.
Figure 7.
Anterior whole-body images of a patient given 131I-TF2 anti-CEA × anti-HSG tri-Fab bsMAb. Initial images at 1 h showed the activity mainly in the heart (H) with some uptake in the liver (Lv). By 24 h, evidence of catabolic activity was observed with uptake in sinuses, and activity in stomach (S) and urinary bladder (UB). There was also activity in the transverse colon (TC) leading into the descending colon, but also there was distinct uptake in the cecum (arrow), where initial colonoscopy and later surgery had indicated a newly diagnosed 5.0 cm lesion. Heart activity was able appreciably less at 24 h, and by 72 h, the only activity remaining was in the thyroid, again likely representing catabolized radioiodine uptake.
Initial Clinical Experience with TF2 Pretargeting of 111In-IMP-288 in Colorectal Cancer
The pharmacokinetics determined from the 131I-TF2 patients suggested that if 10-fold more moles of TF2 were injected than IMP-288 that it would take ~5 days for the molar concentration in the blood to allow IMP-288 to clear without interference. Approval for clinical studies was obtained at the University of Radboud Medical Center, Nijmegen, The Netherlands, under the direction of Prof. Wim Oyen, Prof. Otto Boerman, and Dr. Rafke Schoffelen. The first patient had a history of metastatic colorectal cancer, and presented with several metastases in the lungs and a large liver lesion. He received 75 mg of TF2 (~35 mg/m2) by intravenous infusion over 2 h and then 5 days later received 100 µg 111In-IMP-288 by a 2-min IV push (185 MBq, ~69 nmoles; TF2:IMP-288 mole ratio ~7.5:1).
ELISA determinations found TF2 cleared as quickly over the first day in this patient as in the 2 previous patients who received just 1.2 mg of TF2 (Figure 8). When 111In-IMP-288 was given, it cleared exceptionally fast, with only ~20% of the total injected activity accounted for in the first blood sample taken at 4.5 min, indicating that nearly 80% of the product had already left the vascular volume before the first sample was drawn. The clearance was bi-exponential with an alpha- and beta-effective half-life of 20 and 149 min, respectively. The first image of the patient taken at ~10 min after the injection showed the activity had already dispersed throughout the body, with 18% of the activity already eliminated in the urinary bladder (Figure 8B). By 24 h, 95% of the activity had cleared the body, with the only remaining activity found in the kidneys and tumors. All sites of known disease were visualized, even an 18F-FDG positive lesion in the humerus (not shown); however, regions of interest analysis indicated tumor uptake was lower than the kidneys. Thus, like the data in tumor-bearing nude mice in Figure 4, these initial results indicate that an insufficient amount of TF2 was given to allow for optimal capture of IMP-288. However, these conditions provided important data to illustrate the pharmacokinetic behavior of 111In-IMP-288 without having any significant interaction with TF2 in the blood, illustrating IMP-288 is not retained by normal tissues. Dosimetry data, using standard-man criteria and OLINDA, predicted that with 90Y-IMP-288, the kidneys and red marrow would receive only 1.4 and 0.1 cGy per 37 MBq, respectively. The low red marrow dose is expected, but it is also important to predict that renal uptake of 111In-IMP-288 would be considerably lower than that found for 90Y-biotin in other clinical pretargeting experiences.107,108 Since preclinical data indicated renal uptake did not vary over a wide range of pretargeting conditions, renal doses may very well remain low in future testing. Other patient studies will include examinations of shorter intervals and higher TF2 doses that will likely lead to improved tumor uptake with modest increases in normal tissue uptake expected.
Figure 8.
Blood clearance of TF2 by ELISA (A) and whole-body distribution of 111In-IMP-288 (B) in a patient with extensive metastatic colorectal cancer. The patient was given 75 mg of TF2 followed 5 days later with 100 µg (~69 nmoles) of 111In-IMP-288 (5 mCi). TF2’s blood clearance, as determined by ELISA, in this patient (open squares) was similar to that of 2 earlier patients who had received ~1.2 mg of TF2 (open and closed triangles). The biodistribution of 111In-IMP-288 showed evidence of rapid dispersion through the body within 10 minutes. The heart (H), liver, lungs, and kidneys (K) can be discerned. Regions of interest indicated that 18% of the injected activity was already present in the urinary bladder (UB). By 24 h, most of the activity had been cleared from the blood and body, with only a small amount of activity in the urinary bladder. Known metastatic sites in the lungs and liver were disclosed (arrows), and there was residual activity in the kidneys.
Conclusions and Future Prospects
Preclinical studies have repeatedly shown that different pretargeting methods can improve therapeutic responses in solid tumors and hematologic malignancies as compared to a directly radiolabeled IgG or F(ab')2.65–68,71,83,85,87,91,109–112 This improvement is driven by the ability of the radiolabeled compounds to clear rapidly from the blood, minimizing red marrow exposure that limits the escalation of activity for directly radiolabeled antibodies. Despite the exceptionally fast clearance, it is also important that tumor uptake of the radiolabeled compound in a pretargeting setting can equal that of a directly radiolabeled F(ab')2 or even an IgG, yet their small size allows for rapid localization, reaching maximum accretion levels within minutes instead of days. Such rapid targeting increases the radiation dose-rate delivered to the tumor, which improves the biological effectiveness of this radiation delivery system.71,85
In animal testing, hematologic toxicity occurs with pretargeting, but it is significantly reduced, and instead renal toxicity becomes dose-limiting.85,91 Unlike radiometal-labeled antibody fragments that have tumor/kidney ratios much less than 1:1, tumor/kidney ratios have always been >2:1 in animal models. Thus, if the pretargeting system is optimized in patients as it has been in animal testing, and if a patient can tolerate at least 2000 cGy to the kidneys, pretargeting could conceivably deliver >5,000 cGy to the tumor, a level that would be expected to produce an objective response. Dosimetry from a pilot clinical trial using an anti-TAG-72-streptavidin fusion protein for pretargeting 90Y-biotin found about one-third of the patients studied would have had >5,000 cGy delivered to their tumors if they were administered 90Y-biotin to a level where 2,000 cGy would be delivered to the kidneys.108 Thus, there may be an advantage to have a pretargeting procedure adjusted to reduce the risk for hematologic toxicity. Interestingly, there has only been one clinical experience with a streptavidin-biotin pretargeting procedure that may have been limited by renal toxicity, but instead was first limited by gastrointestinal toxicity due to the selective binding of the targeting antibody to the intestine.113,114 Other clinical studies with a bsMAb-pretargeting procedure and another biotin-avidin pretargeting method found hematologic toxicity limiting for an 131I-hapten-peptide and 90Y-biotin, respectively.62,69 While such limitations can be caused by localization of disease in the bone marrow, as was found in medullary thyroid cancer,69 animal studies suggest that protocol adjustments that minimize hematologic toxicity may be required for optimal treatment. However, shifting the primary dose-limiting organ to the kidneys will place greater emphasis on ensuring adequate dose planning based on renal uptake, following the lead from similar experiences already reported with radiolabeled analogues for somatostatin receptor binding.43,115
Most radiolabeled agents used in a pretargeting setting have very low hepatic and renal uptake, a major advantage over most directly radiolabeled antibody forms and even lower than some peptides. The bsMAb pretargeting method described herein provides considerable flexibility in the design of the hapten-peptide that could be helpful in minimizing tissue uptake for a variety of different ligands that might be used to capture radionuclides or other imaging or therapeutic agents. The flexibility of the HSG-based hapten has allowed a number of different radionuclides of therapeutic and imaging interest to be examined. When properly adjusted, the hapten-peptide radiolabeled with a positron-emitter has a high level of sensitivity and specificity for localizing even very small tumor deposits, and the minimal tissue retention creates less ambiguous images as compared to 18F-FDG.81,88 The preparation of bsMAbs by the DNL method is highly versatile, and can be produced in good yields and with preferred bivalent binding to tumor antigens. The Fab fragments are humanized and the linkage sequences have been derived from human proteins, which will reduce immunogenicity as compared to other pretargeting agents using streptavidin. In addition to the preclinical studies described herein with TF2 that targets CEA, initial pretargeting with 2 other tri-Fab bsMAbs, one targeting CD20 and another targeting a mucin produced by pancreatic cancer, have reported enhanced imaging and therapy capabilities.87,89,90,91
Pretargeting has had its share of criticisms, being considered “too complex” by some, but while the technique requires more initial testing to derive optimal conditions, the final bsMAb pretargeting procedure will require just 2 separate injections, one of which could be administered by an oncologist and the other a few days later in a licensed nuclear medicine facility. Patients will be required to remain in the facility for a few hours, giving time for the activity to be flushed from the body, but otherwise side-effects, such as those associated with chemotherapy, should be minimal. Pretargeted therapy will require considerably higher amounts of radioactivity than is currently used with the approved anti-CD20 antibody therapies, and thus to meet the economic concerns of our health care system, the procedure must provide significant therapeutic advantages. Such improvements may require additional interventions, such as chemotherapy that has been found useful in some animal model testing,91,116,117 or even in combination with other effective therapies, such as consolidation with anti-CD20 antibody therapy that showed a higher cure rate in a human B-cell lymphoma xenograft model.90 Animal testing also has indicated that weekly fractionation of the pretargeting treatment instead of a single high-dose therapy improves responses.91
Thus, the Dock-and-Lock-derived bsMAb and HSG-hapten-peptide system provide a considerable number of opportunities for pretargeting. We are currently planning to engage in several therapy trials using 90Y and 177Lu-IMP-288 in several solid tumors (e.g., colorectal, lung, and pancreas), as well as in B-cell lymphomas. The technique can also be exploited for improved imaging with a variety of positron- or gamma-emitting radionuclides in conjunction with these therapeutic trials, or for certain indications that are currently not well served by 18F-FDG.
Acknowledgements
We are indebted to our many colleagues who have contributed to this work, including Habibe Karacay, PhD at CMMI, Jean-François Chatal, Jacques Barbet, and Françoise Kraeber-Bodéré at INSERM, Nantes, France, to the clinical team of Aiwu Ruth He, MD, PhD, Giuseppe Esposito, MD, and Louis Weiner, MD at the Lombardi Cancer Center, Georgetown University, Washington, D.C., and Wim Oyen, MD, Otto Boerman, PhD, and Rafke Schoffelen, MD of Radboud University, Nijmegen, The Netherlands.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pressman D, Korngold L. The in vivo localization of anti-Wagner-osteogenic-sarcoma antibodies. Cancer. 1953;6:619–623. doi: 10.1002/1097-0142(195305)6:3<619::aid-cncr2820060319>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Barker PA, Flax MH, Lavia MF, et al. A study of the preparation, localization, and effects of antitumor antibodies labeled with I131. Cancer Res. 1956;16:761–773. [PubMed] [Google Scholar]
- 3.Bale WF, Spar IL. Studies directed toward the use of antibodies as carriers of radioactivity for therapy. Adv Biol Med Phys. 1957;5:285–356. doi: 10.1016/b978-1-4832-3111-2.50011-0. [DOI] [PubMed] [Google Scholar]
- 4.McCardle RJ, Harper PV, Spar IL, et al. Studies with iodine-131-labeled antibody to human fibrinogen for diagnosis and therapy of tumors. J Nucl Med. 1966;7:837–847. [PubMed] [Google Scholar]
- 5.Goldenberg DM, DeLand F, Kim E, et al. Use of radiolabeled antibodies to carcinoembryonic antigen for the detection and localization of diverse cancers by external photoscanning. N Engl J Med. 1978;298:1384–1386. doi: 10.1056/NEJM197806222982503. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg DM, Gaffar SA, Bennett SJ, et al. Experimental radioimmunotherapy of a xenografted human colonic tumor (GW-39) producing carcinoembryonic antigen. Cancer Res. 1981;41:4354–4360. [PubMed] [Google Scholar]
- 7.Ettinger DS, Dragon LH, Klein J, et al. Isotopic immunoglobulin in an integrated multimodal treatment program for a primary liver cancer: a case report. Cancer Treat Rep. 1979;63:131–134. [PubMed] [Google Scholar]
- 8.Order SE, Klein JL, Ettinger D, et al. Phase I–II study of radiolabeled antibody integrated in the treatment of primary hepatic malignancies. Int J Radiat Oncol Biol Phys. 1980;6:703–710. doi: 10.1016/0360-3016(80)90226-6. [DOI] [PubMed] [Google Scholar]
- 9.Order SE, Klein JL, Leichner PK. Antiferritin IgG antibody for isotopic cancer therapy. Oncology. 1981;38:154–160. doi: 10.1159/000225543. [DOI] [PubMed] [Google Scholar]
- 10.Carrasquillo JA, Krohn KA, Beaumier P, et al. Diagnosis of and therapy for solid tumors with radiolabeled antibodies and immune fragments. Cancer Treat Rep. 1984;68:317–328. [PubMed] [Google Scholar]
- 11.Sharkey RM, Goldenberg DM. Perspective on cancer therapy with radiolabeled monoclonal antibodies. J Nucl Med. 2005;46:115S–127S. [PubMed] [Google Scholar]
- 12.DeNardo SJ, DeNardo GL, O'Grady LF, et al. Treatment of a patient with B cell lymphoma by I-131 LYM-1 monoclonal antibodies. Int J Biol Markers. 1987;2:49–53. doi: 10.1177/172460088700200107. [DOI] [PubMed] [Google Scholar]
- 13.DeNardo SJ, DeNardo GL, O'Grady LF, et al. Treatment of B cell malignancies with 131I Lym-1 monoclonal antibodies. Int J Cancer Suppl. 1988;3:96–101. [PubMed] [Google Scholar]
- 14.Press OW, Eary JF, Badger CC, et al. Treatment of refractory non-Hodgkin's lymphoma with radiolabeled MB-1 (anti-CD37) antibody. J Clin Oncol. 1989;7:1027–1038. doi: 10.1200/JCO.1989.7.8.1027. [DOI] [PubMed] [Google Scholar]
- 15.Scheinberg DA, Lovett D, Divgi CR, et al. A phase I trial of monoclonal antibody M195 in acute myelogenous leukemia: specific bone marrow targeting and internalization of radionuclide. J Clin Oncol 1991. 1991;9:478–490. doi: 10.1200/JCO.1991.9.3.478. [DOI] [PubMed] [Google Scholar]
- 16.Kaminski MS, Fig LM, Zasadny KR, et al. Imaging, dosimetry, and radioimmunotherapy with iodine 131-labeled anti-CD37 antibody in B-cell lymphoma. J Clin Oncol. 1992;10:1696–1711. doi: 10.1200/JCO.1992.10.11.1696. [DOI] [PubMed] [Google Scholar]
- 17.Emmanouilides C. Radioimmunotherapy for non-Hodgkin lymphoma: historical perspective and current status. J Clin Exp Hematop. 2007;47:43–60. doi: 10.3960/jslrt.47.43. [DOI] [PubMed] [Google Scholar]
- 18.Schaefer-Cutillo J, Friedberg JW. Non-myeloablative radioimmunotherapy for non-Hodgkin's lymphoma. Semin Hematol. 2008;45:110–117. doi: 10.1053/j.seminhematol.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Buchegger F, Press OW, Delaloye AB, et al. Radiolabeled and native antibodies and the prospect of cure of follicular lymphoma. Oncologist. 2008;13:657–667. doi: 10.1634/theoncologist.2008-0020. [DOI] [PubMed] [Google Scholar]
- 20.Koral KF, Dewaraja Y, Clarke LA, et al. Tumor-absorbed-dose estimates versus response in tositumomab therapy of previously untreated patients with follicular non-Hodgkin's lymphoma: preliminary report. Cancer Biother Radiopharm. 2000;15:347–355. doi: 10.1089/cbr.2000.15.347. [DOI] [PubMed] [Google Scholar]
- 21.Sgouros G, Squeri S, Ballangrud AM, et al. Patient-specific, 3-dimensional dosimetry in non-Hodgkin's lymphoma patients treated with 131I-anti-B1 antibody: assessment of tumor dose-response. J Nucl Med. 2003;44:260–268. [PubMed] [Google Scholar]
- 22.Sharkey RM, Brenner A, Burton J, et al. Radioimmunotherapy of non-Hodgkin's lymphoma with 90Y-DOTA humanized anti-CD22 IgG (90Y-Epratuzumab): do tumor targeting and dosimetry predict therapeutic response? J Nucl Med. 2003;44:2000–2018. [PubMed] [Google Scholar]
- 23.Sharkey RM, Pykett MJ, Siegel JA, et al. Radioimmunotherapy of the GW-39 human colonic tumor xenograft with 131I-labeled murine monoclonal antibody to carcinoembryonic antigen. Cancer Res. 1987;47:5672–5677. [PubMed] [Google Scholar]
- 24.Boerman OC, Sharkey RM, Blumenthal RD, et al. The presence of a concomitant bulky tumor can decrease the uptake and therapeutic efficacy of radiolabeled antibodies in small tumors. Int J Cancer. 1992;51:470–475. doi: 10.1002/ijc.2910510322. [DOI] [PubMed] [Google Scholar]
- 25.Sharkey RM, Goldenberg DM. Use of antibodies and immunoconjugates for the therapy of more accessible cancers. Adv Drug Deliv Rev. 2008;60:1407–1420. doi: 10.1016/j.addr.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liersch T, Meller J, Bittrich M, et al. Update of carcinoembryonic antigen radioimmunotherapy with 131I-labetuzumab after salvage resection of colorectal liver metastases: comparison of outcome to a contemporaneous control group. Ann Surg Oncol. 2007;14:2577–2590. doi: 10.1245/s10434-006-9328-x. [DOI] [PubMed] [Google Scholar]
- 27.Shibata S, Raubitschek A, Leong L, et al. A phase I study of a combination of yttrium-90-labeled anti-carcinoembryonic antigen (CEA) antibody and gemcitabine in patients with CEA-producing advanced malignancies. Clin Cancer Res. 2009;15:2935–2941. doi: 10.1158/1078-0432.CCR-08-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pennington K, Guarino MJ, Serafini AN, et al. Multicenter study of radiosensitizing gemcitabine combined with fractionated radioimmunotherapy for repeated treatment cycles in advanced pancreatic cancer. J Clin Oncol. 2009;27:15s. (abstr 4620) [Google Scholar]
- 29.Tempero M, Leichner P, Baranowska-Kortylewicz J, et al. High-dose therapy with 90Yttrium-labeled monoclonal antibody CC49: a phase I trial. Clin Cancer Res. 2000;6:3095–3102. [PubMed] [Google Scholar]
- 30.Sharkey RM, Hajjar G, Yeldell D, et al. A phase I trial combining high-dose 90Y-labeled humanized anti-CEA monoclonal antibody with doxorubicin and peripheral blood stem cell rescue in advanced medullary thyroid cancer. J Nucl Med. 2005;46:620–633. [PubMed] [Google Scholar]
- 31.Richman CM, Denardo SJ, O'Donnell RT, et al. High-dose radioimmunotherapy combined with fixed, low-dose paclitaxel in metastatic prostate and breast cancer by using a MUC-1 monoclonal antibody, m170, linked to indium-111/yttrium-90 via a cathepsin cleavable linker with cyclosporine to prevent human anti-mouse antibody. Clin Cancer Res. 2005;11:5920–5927. doi: 10.1158/1078-0432.CCR-05-0211. [DOI] [PubMed] [Google Scholar]
- 32.Sharkey RM, Motta-Hennessy C, Pawlyk D, et al. Biodistribution and radiation dose estimates for yttrium- and iodine-labeled monoclonal antibody IgG and fragments in nude mice bearing human colonic tumor xenografts. Cancer Res. 1990;50:2330–2336. [PubMed] [Google Scholar]
- 33.Mueller BM, Reisfeld RA, Gillies SD. Serum half-life and tumor localization of a chimeric antibody deleted of the CH2 domain and directed against the disialoganglioside GD2. Proc Natl Acad Sci USA. 1990;87:5702–5705. doi: 10.1073/pnas.87.15.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colcher D, Goel A, Pavlinkova G, et al. Effects of genetic engineering on the pharmacokinetics of antibodies. Q J Nucl Med. 1999;43:132–139. [PubMed] [Google Scholar]
- 35.Batra SK, Jain M, Wittel UA, et al. Pharmacokinetics and biodistribution of genetically engineered antibodies. Curr Opin Biotechnol. 2002;13:603–608. doi: 10.1016/s0958-1669(02)00352-x. [DOI] [PubMed] [Google Scholar]
- 36.Kenanova V, Wu AM. Tailoring antibodies for radionuclide delivery. Expert Opin Drug Deliv. 2006;3:53–70. doi: 10.1517/17425247.3.1.53. [DOI] [PubMed] [Google Scholar]
- 37.Begent RHJ, Green AJ, Bagshawe KD, et al. Liposomally entrapped second antibody improves tumour imaging with radiolabeled (first) antitumour antibody. Lancet. 1982;320:739–742. doi: 10.1016/s0140-6736(82)90923-0. [DOI] [PubMed] [Google Scholar]
- 38.Sharkey RM, Primus FJ, Goldenberg DM. Second antibody clearance of radiolabeled antibody in cancer radioimmunodetection. Proc Natl Acad Sci USA. 1984;81:2843–2846. doi: 10.1073/pnas.81.9.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mårtensson L, Nilsson R, Ohlsson T, et al. Reduced myelotoxicity with sustained tumor concentration of radioimmunoconjugates in rats after extracorporeal depletion. J Nucl Med. 2007;48:269–276. [PubMed] [Google Scholar]
- 40.Behr TM, Memtsoudis S, Sharkey RM, et al. Experimental studies on the role of antibody fragments in cancer radio-immunotherapy: Influence of radiation dose and dose rate on toxicity and anti-tumor efficacy. Int J Cancer. 1998;77:787–795. doi: 10.1002/(sici)1097-0215(19980831)77:5<787::aid-ijc19>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 41.Behr TM, Béhé M, Stabin MG, et al. High-linear energy transfer (LET) alpha versus low-LET beta emitters in radioimmunotherapy of solid tumors: therapeutic efficacy and dose-limiting toxicity of 213Bi- versus 90Y-labeled CO17-1A Fab' fragments in a human colonic cancer model. Cancer Res. 1999;59:2635–2643. [PubMed] [Google Scholar]
- 42.O'Donoghue J. Relevance of external beam dose-response relationships to kidney toxicity associated with radionuclide therapy. Cancer Biother Radiopharm. 2004;19:378–387. doi: 10.1089/1084978041425025. [DOI] [PubMed] [Google Scholar]
- 43.Barone R, Borson-Chazot F, Valkema R, et al. Patient-specific dosimetry in predicting renal toxicity with 90Y-DOTATOC: relevance of kidney volume and dose rate in finding a dose-effect relationship. J Nucl Med. 2005;46 Suppl:99S–106S. [PubMed] [Google Scholar]
- 44.van Essen M, Krenning EP, Kam BL, et al. Peptide-receptor radionuclide therapy for endocrine tumors. Nat Rev Endocrinol. 2009;5:382–393. doi: 10.1038/nrendo.2009.105. [DOI] [PubMed] [Google Scholar]
- 45.Behr TM, Sharkey RM, Sgouros G, et al. Overcoming the nephrotoxicity of radiometal-labeled immunoconjugates: improved cancer therapy administered to a nude mouse model in relation to the internal radiation dosimetry. Cancer. 1997;80:2591–2610. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2591::aid-cncr35>3.3.co;2-a. [DOI] [PubMed] [Google Scholar]
- 46.Lambert B, Cybulla M, Weiner SM, et al. Renal toxicity after radionuclide therapy. Radiat Res. 2004;161:607–611. doi: 10.1667/rr3105. [DOI] [PubMed] [Google Scholar]
- 47.van Eerd JE, Vegt E, Wetzels JF, et al. Gelatin-based plasma expander effectively reduces renal uptake of 111In-octreotide in mice and rats. J Nucl Med. 2006;47:528–533. [PubMed] [Google Scholar]
- 48.Vegt E, Wetzels JF, Russel FG, et al. Renal uptake of radiolabeled octreotide in human subjects is efficiently inhibited by succinylated gelatin. J Nucl Med. 2006;47:432–436. [PubMed] [Google Scholar]
- 49.Rolleman EJ, Forrer F, Bernard B, et al. Amifostine protects rat kidneys during peptide receptor radionuclide therapy with [177Lu-DOTA0,Tyr3]octreotate. Eur J Nucl Med Mol Imaging. 2007;34:763–771. doi: 10.1007/s00259-006-0291-3. [DOI] [PubMed] [Google Scholar]
- 50.Vegt E, Eek A, Oyen WJ, et al. Albumin-derived peptides efficiently reduce renal uptake of radiolabelled peptides. Eur J Nucl Med Mol Imaging. 2009 doi: 10.1007/s00259-009-1239-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valkema R, Pauwels SA, Kvols LK, et al. Long-term follow-up of renal function after peptide receptor radiation therapy with 90Y-DOTA0,Tyr3-octreotide and 177Lu-DOTA0, Tyr3-octreotate. J Nucl Med. 2005;46 Suppl 1:83S–91S. [PubMed] [Google Scholar]
- 52.Vegt E, Wetzels JF, Russel FG, et al. Renal uptake of radiolabeled octreotide in human subjects is efficiently inhibited by succinylated gelatin. J Nucl Med. 2006;47:432–436. [PubMed] [Google Scholar]
- 53.Cremonesi M, Ferrari M, Bodei L, et al. Dosimetry in peptide radionuclide receptor therapy: a review. J Nucl Med. 2006;47:1467–1475. [PubMed] [Google Scholar]
- 54.Peterson JJ, Meares CF. Enzymatic cleavage of peptide-linked radiolabels from immunoconjugates. Bioconjug Chem. 1999;10:553–557. doi: 10.1021/bc990010t. [DOI] [PubMed] [Google Scholar]
- 55.Reardan DT, Meares CF, Goodwin DA, et al. Antibodies against metal chelates. Nature. 1985;316:265–268. doi: 10.1038/316265a0. [DOI] [PubMed] [Google Scholar]
- 56.Stickney DR, Anderson LD, Slater JB, et al. Bifunctional antibody: a binary radiopharmaceutical delivery system for imaging colorectal carcinoma. Cancer Res. 1991;51:6650–6655. [PubMed] [Google Scholar]
- 57.Divgi CR, McDermott K, Johnson DK, et al. Detection of hepatic metastases from colorectal carcinoma using indium-111 (111In) labeled monoclonal antibody (mAb): MSKCC experience with mAb 111In-C110. Int J Rad Appl Instrum B. 1991;18:705–710. doi: 10.1016/0883-2897(91)90008-9. [DOI] [PubMed] [Google Scholar]
- 58.Collier BD, Abdel-Nabi H, Doerr RJ, et al. Immunoscintigraphy performed with In-111-labeled CYT-103 in the management of colorectal cancer: comparison with CT. Radiology. 1992;185:179–186. doi: 10.1148/radiology.185.1.1523304. [DOI] [PubMed] [Google Scholar]
- 59.Le Doussal JM, Martin M, Gautherot E, et al. In vitro and in vivo targeting of radiolabeled monovalent and divalent haptens with dual specificity monoclonal antibody conjugates: enhanced divalent hapten affinity for cell-bound antibody conjugate. J Nucl Med. 1989;30:1358–1366. [PubMed] [Google Scholar]
- 60.Goodwin DA, Meares CF, McTigue M, et al. Pretargeted immunoscintigraphy: effect of hapten valency on murine tumor uptake. J Nucl Med. 1992;33:2006–2013. [PubMed] [Google Scholar]
- 61.Boerman OC, Kranenborg MH, Oosterwijk E, et al. Pretargeting of renal cell carcinoma: improved tumor targeting with a bivalent chelate. Cancer Res. 1999;59:4400–4405. [PubMed] [Google Scholar]
- 62.Goldenberg DM, Sharkey RM, Paganelli G, et al. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J Clin Oncol. 2006;24:823–834. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 63.Le Doussal JM, Gruaz-Guyon A, Martin M, et al. Targeting of indium 111-labeled bivalent hapten to human melanoma mediated by bispecific monoclonal antibody conjugates: imaging of tumors hosted in nude mice. Cancer Res. 1990;50:3445–3452. [PubMed] [Google Scholar]
- 64.Le Doussal JM, Barbet J, Delaage M. Bispecific-antibody-mediated targeting of radiolabeled bivalent haptens: theoretical, experimental and clinical results. Int J Cancer. 1992;7 Suppl:58–62. [PubMed] [Google Scholar]
- 65.Gautherot E, Bouhou J, Le Doussal JM, et al. Therapy for colon carcinoma xenografts with bispecific antibody-targeted, iodine-131-labeled bivalent hapten. Cancer. 1997;80(12 Suppl):2618–2623. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2618::aid-cncr37>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 66.Gautherot E, Le Doussal JM, Bouhou J, et al. Delivery of therapeutic doses of radioiodine using bispecific antibody-targeted bivalent haptens. J Nucl Med. 1998;39:1937–1943. [PubMed] [Google Scholar]
- 67.Kraeber-Bodéré F, Faivre-Chauvet A, Saï-Maurel C, et al. Toxicity and efficacy of radioimmunotherapy in carcinoembryonic antigen-producing medullary thyroid cancer xenograft: comparison of iodine 131-labeled F(ab')2 and pretargeted bivalent hapten and evaluation of repeated injections. Clin Cancer Res. 1999;5:3183s–3189s. [PubMed] [Google Scholar]
- 68.Gautherot E, Rouvier E, Daniel L, et al. Pretargeted radioimmunotherapy of human colorectal xenografts with bispecific antibody and 131I-labeled bivalent hapten. J Nucl Med. 2000;41:480–487. [PubMed] [Google Scholar]
- 69.Kraeber-Bodéré F, Rousseau C, Bodet-Milin C, et al. Targeting, toxicity, and efficacy of 2-step, pretargeted radioimmunotherapy using a chimeric bispecific antibody and 131I-labeled bivalent hapten in a phase I optimization clinical trial. J Nucl Med. 2006;47:247–255. [PubMed] [Google Scholar]
- 70.Chatal JF, Campion L, Kraeber-Bodéré F, et al. Survival improvement in patients with medullary thyroid carcinoma who undergo pretargeted anti-carcinoembryonic-antigen radioimmunotherapy: a collaborative study with the French Endocrine Tumor Group. J Clin Oncol. 2006;24:1705–1711. doi: 10.1200/JCO.2005.04.4917. [DOI] [PubMed] [Google Scholar]
- 71.Axworthy DB, Reno JM, Hylarides MD, et al. Cure of human carcinoma xenografts by a single dose of pretargeted yttrium-90 with negligible toxicity. Proc Natl Acad Sci USA. 2000;97:1802–1807. doi: 10.1073/pnas.97.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharkey RM, Karacay H, Griffiths GL, et al. Development of a streptavidin-anti-carcinoembryonic antigen antibody, radiolabeled biotin pretargeting method for radioimmunotherapy of colorectal cancer. Studies in a human colon cancer xenograft model. Bioconjug Chem. 1997;8:595–604. doi: 10.1021/bc970101v. [DOI] [PubMed] [Google Scholar]
- 73.Karacay H, McBride WJ, Griffiths GL, et al. Experimental pretargeting studies of cancer with a humanized anti-CEA x murine anti-[In-DTPA] bispecific antibody construct and a 99mTc-/188Re-labeled peptide. Bioconjug Chem. 2000;11:842–854. doi: 10.1021/bc0000379. [DOI] [PubMed] [Google Scholar]
- 74.Karacay H, Sharkey RM, McBride WJ, et al. Pretargeting for cancer radioimmunotherapy with bispecific antibodies: role of the bispecific antibody's valency for the tumor target antigen. Bioconjug Chem. 2002;13:1054–1070. doi: 10.1021/bc0200172. [DOI] [PubMed] [Google Scholar]
- 75.Janevik-Ivanovska E, Gautherot E, Hillairet de Boisferon M, et al. Bivalent hapten-bearing peptides designed for iodine-131 pretargeted radioimmunotherapy. Bioconjug Chem. 1997;8:526–533. doi: 10.1021/bc970083h. [DOI] [PubMed] [Google Scholar]
- 76.Morel A, Darmon M, Delaage M. Recognition of imidazole and histamine derivatives by monoclonal antibodies. Mol Immunol. 1990;27:995–1000. doi: 10.1016/0161-5890(90)90122-g. [DOI] [PubMed] [Google Scholar]
- 77.Sharkey RM, McBride WJ, Karacay H, et al. A universal pretargeting system for cancer detection and therapy using bispecific antibody. Cancer Res. 2003;63:354–363. [PubMed] [Google Scholar]
- 78.Griffiths GL, Chang CH, McBride WJ, et al. Reagents and methods for PET using bispecific antibody pretargeting and 68Ga-radiolabeled bivalent hapten-peptide-chelate conjugates. J Nucl Med. 2004;45:30–39. [PubMed] [Google Scholar]
- 79.Gruaz-Guyon A, Janevik-Ivanovska E, Raguin O, et al. Radiolabeled bivalent haptens for tumor immunodetection and radioimmunotherapy. Q J Nucl Med. 2001;45:201–206. [PubMed] [Google Scholar]
- 80.Morandeau L, Benoist E, Loussouarn A, et al. Synthesis of new bivalent peptides for applications in the Affinity Enhancement System. Bioconjug Chem. 2005;16:184–193. doi: 10.1021/bc0497721. [DOI] [PubMed] [Google Scholar]
- 81.McBride WJ, Zanzonico P, Sharkey RM, et al. Bispecific antibody pretargeting PET (immunoPET) with an 124I-labeled hapten-peptide. J Nucl Med. 2006;47:1678–1688. [PubMed] [Google Scholar]
- 82.McBride WJ, Sharkey RM, Karacay H, et al. A novel method of 18F radiolabeling for PET. J Nucl Med. 2009;50:991–998. doi: 10.2967/jnumed.108.060418. [DOI] [PubMed] [Google Scholar]
- 83.Sharkey RM, Karacay H, Chang CH, et al. Improved therapy of non-Hodgkin's lymphoma xenografts using radionuclides pretargeted with a new anti-CD20 bispecific antibody. Leukemia. 2005;19:1064–1069. doi: 10.1038/sj.leu.2403751. [DOI] [PubMed] [Google Scholar]
- 84.Sharkey RM, Cardillo TM, Rossi EA, et al. Signal amplification in molecular imaging by pretargeting a multivalent, bispecific antibody. Nat Med. 2005;11:1250–1255. doi: 10.1038/nm1322. [DOI] [PubMed] [Google Scholar]
- 85.Karacay H, Brard PY, Sharkey RM, et al. Therapeutic advantage of pretargeted radioimmunotherapy using a recombinant bispecific antibody in a human colon cancer xenograft. Clin Cancer Res. 2005;11:7879–7885. doi: 10.1158/1078-0432.CCR-05-1246. [DOI] [PubMed] [Google Scholar]
- 86.Sharkey RM, Karacay H, McBride WJ, et al. Bispecific antibody pretargeting of radionuclides for immuno single-photon emission computed tomography and immuno positron emission tomography molecular imaging: an update. Clin Cancer Res. 2007;13:5577s–5585s. doi: 10.1158/1078-0432.CCR-07-1087. [DOI] [PubMed] [Google Scholar]
- 87.Sharkey RM, Karacay H, Litwin S, et al. Improved therapeutic results by pretargeted radioimmunotherapy of non-Hodgkin's lymphoma with a new recombinant, trivalent, anti-CD20, bispecific antibody. Cancer Res. 2008;68:5282–5290. doi: 10.1158/0008-5472.CAN-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sharkey RM, Karacay H, Vallabhajosula S, et al. Metastatic human colonic carcinoma: molecular imaging with pretargeted SPECT and PET in a mouse model. Radiology. 2008;246:497–507. doi: 10.1148/radiol.2462070229. [DOI] [PubMed] [Google Scholar]
- 89.Gold DV, Goldenberg DM, Karacay H, et al. A novel bispecific, trivalent antibody construct for targeting pancreatic carcinoma. Cancer Res. 2008;68:4819–4826. doi: 10.1158/0008-5472.CAN-08-0232. [DOI] [PubMed] [Google Scholar]
- 90.Sharkey RM, Karacay H, Johnson CR, et al. Pretargeted versus directly targeted radioimmunotherapy combined with anti-CD20 antibody consolidation therapy of non-Hodgkin lymphoma. J Nucl Med. 2009;50:444–453. doi: 10.2967/jnumed.108.058602. [DOI] [PubMed] [Google Scholar]
- 91.Karacay H, Sharkey RM, Gold DV, et al. Pretargeted radioimmunotherapy of pancreatic cancer xenografts: TF10/90Y IMP-288 alone and combined with gemcitabine. J Nucl Med. doi: 10.2967/jnumed.109.067686. in press. [DOI] [PubMed] [Google Scholar]
- 92.Sharkey RM, Goldenberg DM. Novel radioimmunopharmaceuticals for cancer imaging and therapy. Curr Opin Investig Drugs. 2008;9:1302–1316. [PubMed] [Google Scholar]
- 93.Rossi EA, Sharkey RM, McBride W, et al. Development of new multivalent-bispecific agents for pretargeting tumor localization and therapy. Clin Cancer Res. 2003;9:3886S–3896S. [PubMed] [Google Scholar]
- 94.Rossi EA, Chang CH, Losman MJ, et al. Pretargeting of carcinoembryonic antigen-expressing cancers with a trivalent bispecific fusion protein produced in myeloma cells. Clin Cancer Res. 2005;11:7122s–7129s. doi: 10.1158/1078-0432.CCR-1004-0020. [DOI] [PubMed] [Google Scholar]
- 95.Wong W, Scott JD. AKAP signalling complexes : focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 96.Baillie GS, Scott JD, Houslay MD. Compartmentalisation of phosphodiesterases and protein kinase A: opposites attract. FEBS Lett. 2005;579:3264–3270. doi: 10.1016/j.febslet.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 97.Rossi EA, Goldenberg DM, Cardillo TM, et al. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci USA. 2006;103:6841–6846. doi: 10.1073/pnas.0600982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang CH, Rossi EA, Goldenberg DM. The dock and lock method: a novel platform technology for building multivalent, multifunctional structures of defined composition with retained bioactivity. Clin Cancer Res. 2007;13:5586s–5591s. doi: 10.1158/1078-0432.CCR-07-1217. [DOI] [PubMed] [Google Scholar]
- 99.Rossi EA, Goldenberg DM, Cardillo TM, et al. Novel designs of multivalent anti-CD20 humanized antibodies as improved lymphoma therapeutics. Cancer Res. 2008;68:8384–8392. doi: 10.1158/0008-5472.CAN-08-2033. [DOI] [PubMed] [Google Scholar]
- 100.Goldenberg DM, Rossi EA, Sharkey RM, et al. Multifunctional antibodies by the Dock-and-Lock method for improved cancer imaging and therapy by pretargeting. J Nucl Med. 2008;49:158–163. doi: 10.2967/jnumed.107.046185. [DOI] [PubMed] [Google Scholar]
- 101.Rossi EA, Goldenberg DM, Cardillo TM, et al. Hexavalent bispecific antibodies represent a new class of anticancer therapeutics: 1. Properties of anti-CD20/CD22 antibodies in lymphoma. Blood. 2009;113:6161–6171. doi: 10.1182/blood-2008-10-187138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rossi EA, Goldenberg DM, Cardillo TM, et al. CD20-targeted tetrameric interferon- {alpha}, a novel and potent immunocytokine for the therapy of B-cell lymphomas. Blood. 2009 Aug 26; doi: 10.1182/blood-2009-06-228890. [Epub ahead of print] PubMed PMID:19710501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang CH, Rossi EA, Cardillo TM, et al. A new method to produce monoPEGylated dimeric cytokines shown with human interferon-alpha2b. Bioconjug Chem. 2009 Sep 8; doi: 10.1021/bc9001773. [Epub ahead of print] PubMed PMID: 19736932. [DOI] [PubMed] [Google Scholar]
- 104.Sharkey RM, Juweid M, Shevitz J, et al. Evaluation of a complementarity-determining region-grafted (humanized) anti-carcinoembryonic antigen monoclonal antibody in preclinical and clinical studies. Cancer Res. 1995;55:5935s–5945s. [PubMed] [Google Scholar]
- 105.Hajjar G, Sharkey RM, Burton J, et al. Phase I radioimmunotherapy trial with iodine-131-labeled humanized MN-14 anti-carcinoembryonic antigen monoclonal antibody in patients with metastatic gastrointestinal and colorectal cancer. Clin Colorectal Cancer. 2002;2:31–42. doi: 10.3816/CCC.2002.n.009. [DOI] [PubMed] [Google Scholar]
- 106.Sharkey RM, Karacay H, Richel H, et al. Optimizing bispecific antibody pretargeting for use in radioimmunotherapy. Clin Cancer Res. 2003;9:3897S–3913S. [PubMed] [Google Scholar]
- 107.Breitz HB, Fisher DR, Goris ML, et al. Radiation absorbed dose estimation for 90Y-DOTA-biotin with pretargeted NR-LU-10/streptavidin. Cancer Biother Radiopham. 1999;14:381–395. doi: 10.1089/cbr.1999.14.381. [DOI] [PubMed] [Google Scholar]
- 108.Shen S, Foreo A, LoBuglio AF, et al. Patient-specific dosimetry of pretargeted radioimmunotherapy using CC49 fusion protein in patients with gastrointestinal malignancies. J Nucl Med. 2005;46:642–651. [PubMed] [Google Scholar]
- 109.Press OW, Corcoran M, Subbiah K, et al. A comparative evaluation of conventional and pretargeted radioimmunotherapy of CD20-expressing lymphoma xenografts. Blood. 2001;98:2535–2543. doi: 10.1182/blood.v98.8.2535. [DOI] [PubMed] [Google Scholar]
- 110.Subbiah K, Hamlin DK, Pagel JM, et al. Comparison of immunoscintigraphy, efficacy, and toxicity of conventional and pretargeted radioimmunotherapy in CD20-expressing human lymphoma xenografts. J Nucl Med. 2003;44:437–445. [PubMed] [Google Scholar]
- 111.Pagel JM, Matthews DC, Kenoyer A, et al. Pretargeted radioimmunotherapy using anti-CD45 monoclonal antibodies to deliver radiation to murine hematolymphoid tissues and human myeloid leukemia. Cancer Res. 2009;69:185–192. doi: 10.1158/0008-5472.CAN-08-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gopal AK, Pagel JM, Fromm JR, et al. 131I anti-CD45 radioimmunotherapy effectively targets and treats T-cell non-Hodgkin lymphoma. Blood. 2009;113:5905–5910. doi: 10.1182/blood-2009-02-205476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Breitz HB, Weiden PL, Beaumier PL, et al. Clinical optimization of pretargeted radioimmunotherapy with antibody-streptavidin conjugate and 90Y-DOTA-biotin. J Nucl Med. 2000;41:131–140. [PubMed] [Google Scholar]
- 114.Knox SJ, Goris ML, Tempero M, et al. Phase II trial of yttrium-90-DOTA-biotin pretargeted by NR-LU-10 antibody/streptavidin in patients with metastatic colon cancer. Clin Cancer Res. 2000;6:406–414. [PubMed] [Google Scholar]
- 115.Wessels BW, Konijnenberg MW, Dale RG, et al. MIRD pamphlet No. 20: the effect of model assumptions on kidney dosimetry and response: Implications for radionuclide therapy. J Nucl Med. 2008;49:1884–1899. doi: 10.2967/jnumed.108.053173. [DOI] [PubMed] [Google Scholar]
- 116.Kraeber-Bodéré F, Saï-Maurel C, Campion L, et al. Enhanced antitumor activity of combined pretargeted radioimmunotherapy and paclitaxel in medullary thyroid cancer xenograft. Mol Cancer Ther. 2002;1:267–274. [PubMed] [Google Scholar]
- 117.Graves SS, Dearstyne E, Lin Y, et al. Combination therapy with pretarget CC49 radioimmunotherapy and gemcitabine prolongs tumor doubling time in a murine xenograft model of colon cancer more effectively than either monotherapy. Clin Cancer Res. 2003;9:3712–3721. [PubMed] [Google Scholar]