Abstract
Kynurenine aminotransferase (KAT) catalyzes the formation of kynurenic acid (KYNA), the natural antagonist of ionotropic glutamate receptors. This study tests potential substrates and assesses the effects of amino acids and keto acids on the activity of mosquito KAT. Various keto acids, when simultaneously present in the same reaction mixture, display a combined effect on KAT catalyzed KYNA production. Moreover, methionine and glutamine show inhibitory effects on KAT activity, while cysteine functions as either an antagonist or an inhibitor depending on the concentration. Therefore, the overall level of keto acids and cysteine might modulate the KYNA synthesis. Results from this study will be useful in the study of KAT regulation in other animals.
Keywords: Kynurenic acid, Kynurenine aminotransferase, Mosquito, Aedes, Cysteine, Keto acid, Oxoacid
1. Introduction
In mammals, kynurenic acid (KYNA) functions as a broad-spectrum antagonist of ionotropic excitatory amino acid receptors, which protects the central nervous system (CNS) from being overstimulated by excitatory cytotoxins, and its inhibitory actions underlie its neuroprotective and anticonvulsant effects [1–4]. Fluctuations in endogenous brain KYNA levels greatly influence neuronal excitation and vulnerability to excitotoxic attack [5–8]. It is apparent that KYNA is critical for maintaining normal physiological functions of the CNS in mammals. Indeed, it has been proposed that altered KYNA metabolism might play a role in seizure phenomena and neurodegenerative processes associated with excessive activation of glutamate receptors [9].
In the CNS, KYNA synthesis occurs primarily in glial cells, due to the conversion of its immediate bioprecursor, kynurenine, by kynurenine aminotransferases (KATs) [10]. At least two distinct KATs (KAT I and KAT II) were described in human and rodent brains [11,12]. KAT I is located in forebrain astrocytes [13], and also in a small percentage of neurons, such as in rat medulla and the spinal cord [14]. The cDNA clone encoding for a soluble protein with KAT I activity has been isolated from a rat brain library [15], suggesting that KAT I plays a role in the brain KYNA production. Moreover, mammalian KAT I is identical to glutamine transaminase K and is also a cysteine S-conjugate β-lyase [16].
KYNA production is likely affected by a number of factors, including the concentration of KAT and the concentration of KAT substrates, products and potential inhibitors. To achieve a comprehensive understanding of the mechanisms maintaining a physiological level of endogenous KYNA in living organisms, it is essential to critically evaluate the factors affecting KAT activity. Our laboratory has been focusing on tryptophan metabolism in mosquitoes and mainly on the kynurenine pathway. Based on the studies of the involved enzymes, tryptophan dioxygenase (Li, et al., unpublished data), kynurenine monooxygenase [17], Aedes aegypti KAT (AeKAT) [18], and 3-hydoxykynurenine transaminase [19,20], mosquitoes have a similar kynurenine pathway in the production of KYNA as that in mammals. By studying AeKAT, a homolog of the mammalian KAT I, we demonstrated its transcriptional up-regulation in the mosquito adult brain, suggesting that it might play a role in CNS functions [18]. Although KAT I has been implicated in KYNA production in the mammalian brain [13–15], it is generally agreed that quantitatively the role of KAT I is minor [2], and some authors, e.g. [16], have questioned whether KAT I contributes at all to KYNA production in the mammalian brain. However, our work has provided strong evidence that the mosquito equivalent to KAT I might indeed contribute to KYNA production in the insect’s brain. It is anticipated that through extensive characterization of the enzyme, we may better understand the physiological functions and molecular regulation of this group of enzymes. After screening the effects of various amino acids and keto acids on AeKAT catalyzed kynurenine transamination, we found that cysteine significantly enhanced kynurenine to KYNA pathway within a certain range of concentrations, but inhibited the pathway when its concentration was above a certain level. In addition, AeKAT was active to most keto acids, but due to substrate inhibition, their concentrations could have a major impact on AeKAT activity. Results from this study provide some basis for suggesting the potential regulatory roles keto acids and cysteine might play in KYNA biosynthesis. These data should be highly useful in comparative studies between AeKAT and its mammalian homologs and for a better understanding of the regulatory mechanisms of KYNA production in the CNS.
2. Materials and methods
2.1. Recombinant KAT expression and purification
AeKAT (NCBI accession number AF395204) was expressed in an insect cell line/baculovirus expression system and subsequently purified through different chromatography methods [18]. The purity of the enzyme was assessed by SDS–PAGE analysis. Protein concentration was determined by a Bio-Rad protein assay kit using bovine serum albumin as a standard. Purified recombinant AeKAT was used for biochemical assays.
2.2. Biochemical characterization
All chemicals were purchased from Sigma unless otherwise specified. The stock solution for amino acids (L-form) and keto acids was prepared in 100 mM Tris, adjusted to pH 8.5 before being used for enzyme activity assay. KAT activity assay was based on the described method [18]. Briefly, a reaction mixture of 50 μl containing 15 mM kynurenine, 0.16–20 mM different keto acids, 40 μM pyridoxal 5′-phosphate (PLP), and 2 μg purified recombinant AeKAT was prepared using a 100 mM Tris buffer, pH 8.5. The mixture was incubated for 10 min at 45 °C and the reaction was stopped by adding an equal volume of 0.8 M formic acid. Supernatant was obtained by centrifugation of the reaction mixture at 15 000 × g at 4 °C for 10 min and analyzed by HPLC-UV at 330 nm for KYNA.
To determine the effect of cysteine on AeKAT activity, a varying concentration of cysteine from 0.02 to 40 mM was added to the reaction mixture containing 3 mM kynurenine and 2 mM ketobutyrate, and the enzyme activity was assayed in the same manner. To determine possible transamination activity and kinetics of AeKAT towards other amino acids, the same reaction condition was used except with the reaction mixture containing 0.4–100 mM of a different amino acid, 10 mM ketobutyrate or pyruvate. The product was quantified based on the detection of o-phthaldialdehyde thiol (OPT)–amino acid product conjugate by HPLC with electrochemical detection after their corresponding reaction mixtures were derivatized by OPT reagent [20,21].
All assays were performed at least in triplicate. The results for the effects of keto acids and amino acids were analyzed by a student t test. The kinetic parameters of the recombinant enzyme towards different amino acids or keto acids were calculated by fitting the experimental data to the Michaelis–Menten equation using the Enzyme Kinetics Module (SPSS Science). Protein was assayed by a Bio-Rad protein assay kit using bovine serum albumin as a standard.
3. Results
3.1. Substrate study of AeKAT
AeKAT was tested for aminotransferase activity towards 20 protein amino acids, aminoadipate, aminobutyrate, kynurenine and 3-hydroxykynurenine using ketobutyrate or pyruvate as an amino acceptor. The enzyme displayed fairly high activity towards a number of amino acids (Table 1). Based on the parameter of kcat/Km, it is clear that AeKAT is very efficient in catalyzing the transamination of cysteine, tyrosine, glutamine, methionine, histidine, phenylalanine and kynurenine (Table 1), which is consistent with the previous studies showing that glutamine transaminase K has activity towards glutamine, methionine, aromatic amino acids, and cysteine, but poor affinity towards alanine [22–24].
Table 1.
Kinetic parameters of AeKAT towards different amino acids
| Amino acids | Km (mM) | kcat (min−1) | kcat/Km (min−1 mM−1) |
|---|---|---|---|
| Cysteine | 1.3 ± 0.6 | 206 ± 19 | 159 |
| Tyrosine | 0.9 ± 0.2 | 139 ± 10 | 155 |
| Glutamine | 3.8 ± 0.8 | 561 ± 34 | 148 |
| Methionine | 1.4 ± 0.4 | 163 ± 10 | 117 |
| Histidine | 1.5 ± 0.3 | 168 ± 10 | 112 |
| Phenylalanine | 3.5 ± 0.3 | 283 ± 10 | 81 |
| Kynurenine | 4.3 ± 0.5 | 172 ± 10 | 40 |
| Asparagine | 6.1 ± 0.8 | 230 ± 10 | 38 |
| Tryptophan | 12.9 ± 4.2 | 283 ± 38 | 22 |
| Leucine | 34.5 ± 7.5 | 758 ± 62 | 22 |
| Serine | 32.7 ± 7.9 | 345 ± 43 | 11 |
| Alanine | 246 ± 164 | 1540 ± 782 | 6 |
| Amino-butyrate | 92.7 ± 15.9 | 317 ± 34 | 3 |
The activities were measured as described in Section 2.2. The reaction mixture contains 0.4–100 mM of a different amino acid, 10 mM ketobutyrate or pyruvate (only for amino-butyrate). The kinetic parameters of the enzyme to amino acids were calculated by fitting the experimental data to the Michaelis–Menten equation using the Enzyme Kinetics Module (SPSS Science).
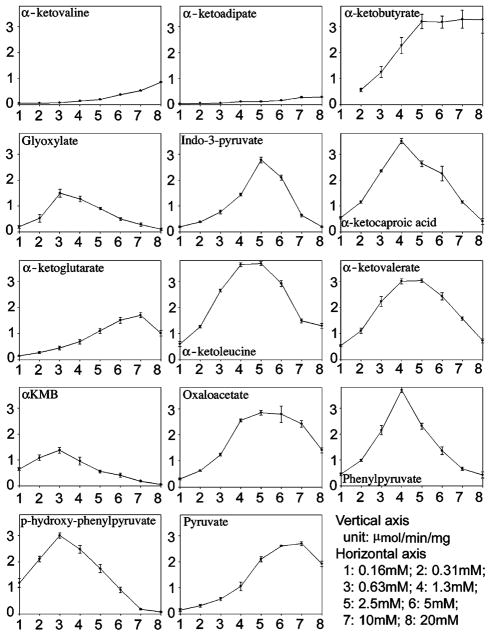
Fourteen keto acids were tested for their potential as amino group acceptors for AeKAT with 15 mM kynurenine as the amino group donor. Among them, twelve keto acids displayed high activity and two (ketoadipate and ketovaline) showed low activity (Fig. 1). Unlike amino group donors, however, all keto acids, with exception of ketobutyrate, showed substrate inhibition at relatively high concentration, especially p-hydroxy-phenylpyruvate, glyoxylate, and α-keto-methylthiobutyric acid (αKMB) with substrate inhibition starting at 1.3 mM (Fig. 1). Pyruvate and ketoglutarate were the most commonly used amino group acceptors; however, they were apparently not efficient amino group acceptors for AeKAT. Similar results have also been observed in previous studies of glutamine transaminase K: i.e., it generally had wide α-keto acid specificity, high activity with αKMB and glyoxylate, poor affinity towards pyruvate and strong substrate inhibition by αKMB and phenylpyruvate [22,23,25].
Fig. 1.
Cosubstrate specificity of AeKAT. AeKAT was incubated in the presence of 15 mM kynurenine and 0.16–20 mM different keto acids. The activity was quantitated by the amount of KYNA produced in the reaction mixture. The units are labeled at the right corner.
3.2. Effects of amino acids on AeKAT catalyzed kynurenine transamination
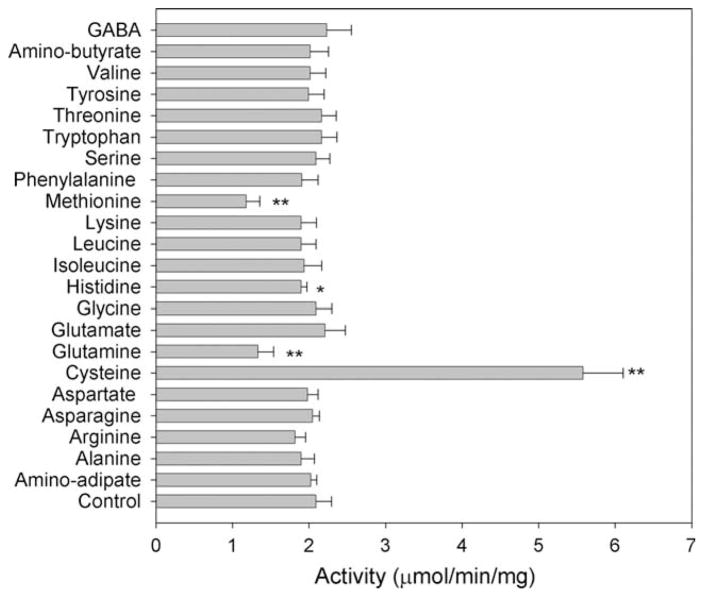
Based on the Km of AeKAT to the amino acids, cysteine, tyrosine, glutamine, methionine, histidine and phenylalanine have either similar or better affinity to AeKAT than kynurenine; accordingly, the presence of any of these amino acids in the kynurenine/AeKAT/keto acid mixture should lead to competitive inhibition of AeKAT activity towards kynurenine. When 2 mM of a different amino acid was incorporated into the kynurenine/AeKAT/ketobutyrate reaction mixture with kynurenine concentration at 3 mM, the rate of KYNA production was significantly decreased in the presence of glutamine, methionine and histidine. Surprisingly, however, no significant inhibition was observed in the presence of tyrosine and phenylalanine (Fig. 2). KYNA production was not seriously affected in the presence of other competing amino acids. These findings require further study.
Fig. 2.
Effect of other amino acids on enzyme activity. Assay conditions were similar to those described in Section 2.2, except that kynurenine and ketobutyrate concentrations were 3 and 2 mM, respectively. The concentration of the additional amino acid incorporated into the reaction mixture was 2 mM. The activity was quantitated by the amount of KYNA produced in the reaction mixture. The star labeled bars are significantly different from that of the control without additional amino acid. *P < 0:5, **P < 0:01.
3.3. Effects of keto acids on AeKAT activity
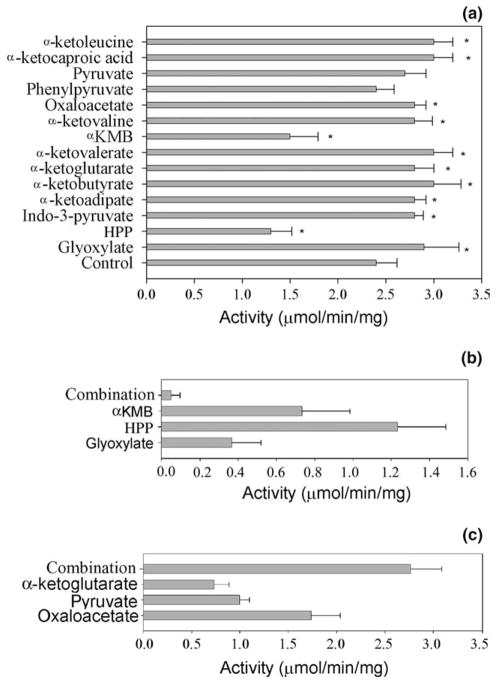
The ability of a number of biologically relevant keto acids to function as the amino group acceptors for AeKAT indicated that their presence might have some combined effect on KYNA production. To test this hypothesis, different keto acids were incorporated into the kynurenine/AeKAT/keto-butyrate mixture and the rate of KYNA production was compared to that of the control reaction mixture in the absence of the additional amino group acceptor(s). Results showed that pyruvate and phenylpyruvate had no significant effect on KYNA production, p-hydroxy-phenylpyruvate and αKMB decreased KYNA production, and the other tested keto acids at the applied concentration (2 mM) increased KYNA production (Fig. 3(a)). Oxaloacetate, ketoglutarate and pyruvate showed no substrate inhibition to AeKAT at physiological concentration (see Fig. 1). When these three keto acids each at 1 mM concentration were incorporated together into a kynurenine/AeKAT reaction mixture, the rate of KYNA production was higher than with each keto acid alone at 1 mM in the same reaction mixture (Fig. 3(b)), suggesting that the overall level and contents of keto acids may modulate KYNA biosynthesis. In contrast, glyoxylate, αKMB and p-hydroxy-phenylpyruvate showed substrate inhibition at 1.3 mM (see Fig. 1). When these three keto acids at 2 mM final concentration each were incorporated into the kynurenine/AeKAT reaction mixture, production of KYNA was much lower than with each keto acid alone at 2 mM in the same kynurenine/AeKAT reaction mixture (Fig. 3(c)), which again suggests that the overall level of keto acids modulates KYNA biosynthesis.
Fig. 3.
Effect of the multiple keto acids on AeKAT-catalyzed KYNA production. (a) Rate of KYNA production in the AeKAT and kynurenine mixture in the presence of 3 mM of ketobutyrate and 2 mM of a different keto acids. The star labeled bars are significantly different from the bar for the control without adding any other keto acid. *P < 0:5, **P < 0:01. (b) Inhibition of KYNA production in the Ae-KAT/kynurenine mixture in the presence of three keto acids, αKMB, glyoxylate, and p-hydroxy-phenylpyruvate. They were used either simultaneously at 2 mM each (combination) or 2 mM any one of them alone. (c) Enhancement of KYNA production by AeKAT in the presence of three different keto acids, pyruvate, ketoglutarate and oxaloacetate. They were used either simultaneously at 1 mM each (combination) or 1 mM any one of them alone. HPP, p-hydroxy-phenylpyruvate.
3.4. Effects of cysteine on AeKAT
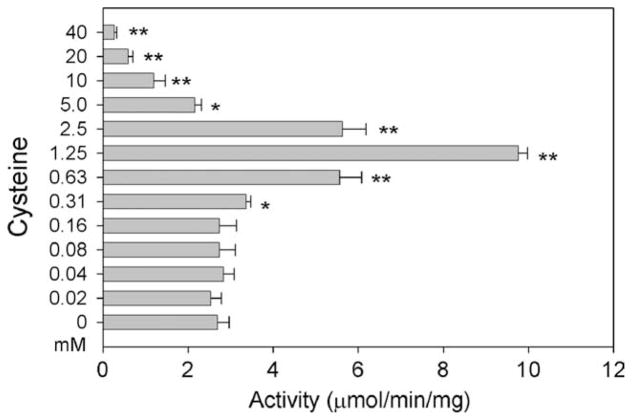
Cysteine is one of the favorable amino group donors for AeKAT, but the presence of 2 mM cysteine in the kynurenine/AeKAT reaction mixture resulted in more than a 2-fold increase in the rate of KYNA production (Fig. 2). When varying concentrations of cysteine from 0.02 to 40 mM were incorporated into kynurenine/AeKAT/ketobutyrate reaction mixtures with kynurenine and ketobutyrate at 3 and 2 mM, respectively, the rate of KYNA production was not substantially changed with cysteine concentration from 0.02 to 0.16 mM, was significantly increased with cysteine concentration from 0.31 to 2.5 mM, and was greatly decreased with cysteine concentration at and above 5 mM (Fig. 4).
Fig. 4.
Effect of cysteine on enzyme activity. Assay conditions were similar to those described in Section 2.2. The activity was calculated by the amount of KYNA produced in the reaction mixture. The bars labeled with stars are significantly different from the bar for the control without adding any cysteine. *P < 0:5, **P < 0:01.
4. Discussion
KYNA functions as a broad-spectrum antagonist against excitatory cytotoxins in the CNS [2,3] and is derived from kynurenine by transaminase-mediated reactions. Factors affecting KAT activity undoubtedly influence KYNA biosynthesis. It has been established that in humans, KAT I is suggested as one of at least two KATs responsible for KYNA production in the brain [12,26,27]. There have been a number of studies dealing with the biochemical characterization of mammalian and human KAT I [11,15,26–29]. However, most studies used partially purified enzymes from different species, so that the amount of the enzyme available and the purity of the protein for extensive characterization were often limiting factors. Under in vivo conditions, multiple amino group donors and acceptors are likely to co-exist with KAT in the local environment, so that KAT activity may be continuously fine-tuned by the levels of these components. Understanding the factors that modulate KAT activity could provide insight into strategies for maintaining physiological KYNA concentrations under disease conditions. Using a baculovirus/insect cell expression system, we were able to express and purify a large quantity (in mg level each time) of pure AeKAT, which enabled us to perform extensive biochemical characterization of the enzyme. Our data revealed some details in the biochemical behavior of AeKAT towards various amino acids and keto acids, which should be essential towards a comprehensive understanding of KAT in living organisms.
Mammalian KAT I is identical to glutamine transaminase K and a cysteine S-conjugate β-lyase that is found mainly in kidney and liver [16], but in arthropods KAT activity or mRNA is mainly present in the adult head [18,30,31], which suggests that the enzyme primarily functions in CNS. It has been shown that the concentration of kynurenine in the brain is in μM range [32,33], which leads to a question about the efficiency of KAT in mediating KYNA biosynthesis in the brain. Many aspects of brain amino acid metabolism are sharply compartmented, with a particular metabolic pathway or transport process being restricted to either astrocytes or neurons [34]. In recent years, there have been a number of studies providing strong evidence of intracellular compartmentation of metabolism in both astrocytes and neurons in the brain (see reviews: [35–37] and references therein). Kynurenine may be present at a relatively high concentration locally at some sites in the brain, so that the mean kynurenine concentration in the brain might hardly reflect the true kynurenine concentration in specific sites where the kynurenine to KYNA pathway operates. Similar considerations apply to many neuroactive agents in the CNS [38]. An unexpected phenomenon in the study of different amino acids on KYNA production is the effect of cysteine on enhancing AeKAT-catalyzed KYNA production. Cysteine itself was a favorable amino group donor for AeKAT. Depending upon cysteine concentrations, its addition to the reaction mixture should, in principle, decrease the rate of KYNA production. The inability to substantially change the rate of KYNA production in the presence of low concentrations (0.02–0.16 mM) of cysteine and strong inhibition of KYNA production in the presence of high concentrations (5–40 mM) of cysteine can be logically explained by the status of AeKAT saturation by cysteine. Cysteine may also form a covalent ring structure with PLP cofactor, or form hemithioketals with α-keto acids that are stable enough to be crystallized [24], which might also contribute to the inhibition of KAT activity. However, the apparent increase in the rate of KYNA production in the presence of 0.31–2.5 mM cysteine was unexpected. The effects of cysteine on AeKAT in vitro suggest that further study is needed to elucidate the in vivo role of cysteine in regulation of the KYNA production in mosquitoes.
Some keto acids were found to regulate KYNA production in the rat brain [39,40], but the mechanism was not fully understood. Our data clearly showed that most keto acids could serve as amino group acceptors for AeKAT and that a combination of different keto acids could have either a positive or negative impact on AeKAT mediated kynurenine transamination. Unlike amino group donors, most of the tested keto acids showed substrate inhibition to AeKAT at and above a certain concentration; therefore, the applied keto acid concentration in the reaction mixture could greatly affect the rate of KYNA production. This is practically important, because αKMB, glyoxylate and p-hydroxy-phenylpyruvate could easily be concluded to be poor amino group acceptors for the enzyme if 5–10 mM concentrations of these keto acids (commonly used substrate concentrations for transaminase assay) were used for the initial screening. Among the keto acids tested, ketobutyrate was found to be the only one showing no substrate inhibition at the tested concentrations; therefore, it was often used as the primary amino group acceptor in different experiments. It is clear that the presence of more than one amino group acceptor at their optimal concentrations enhances AeKAT mediated KYNA production. For example, pyruvate, oxaloacetate and ketoglutarate showed no apparent substrate inhibition to AeKAT up to 10 mM in the reaction mixture. When a combination of these three keto acids at 1 mM each was added into the AeKAT/kynurenine mixture, the rate of KYNA production was much greater than any one of them alone, indicating that different keto acids are able to jointly function as the amino group acceptor for AeKAT. In contrast, some keto acids displayed substrate inhibition at relatively low concentrations, and a combination of these amino acids aggravated the inhibitory effect to AeKAT. For example, glyoxylate, αKMB and p-hydroxy-phenylpyruvate displayed substrate inhibition at 1.3 mM. When all three keto acids were present in the enzyme reaction mixture, the rate of KYNA production was significantly lower than any single keto acid alone at the same reaction condition. This result points out that the amino group acceptor is also a rate-limiting factor during KAT mediated transamination reactions.
In summary, our data revealed that AeKAT is a multi-functional aminotransferase as has been shown for other mammalian KATs [16]. Most naturally occurring keto acids can serve as the amino group acceptor for AeKAT and the presence of multiple keto acids could either enhance or reduce the kynurenine to KYNA pathway, depending on their concentrations, and cysteine may function as an important endogenous AeKAT modulator in insects. The results, especially the enhancement effect of cysteine and inhibitory effects of some keto acids, should be critical for a comprehensive understanding of KATs, serve as a useful reference for comparative studies among KATs from different resources, and provide insight into novel therapeutic compounds to upregulate endogenous KYNA levels in the brain. The availability of an adequate amount of native AeKAT has led to the successful crystallization of AeKAT and its crystals diffract at a resolution of 2.0 Å. We anticipate achieving better understanding of the structure function relationship for AeKAT once its crystal structure is determined.
Acknowledgments
This study was supported by the National Institutes of Health Grant AI 44399. The authors thank all the reviewers for their valuable comments on the manuscript.
Abbreviations
- AeKAT
Aedes aegypti kynurenine aminotransferase
- KAT
kynurenine aminotransferase
- KYNA
kynurenic acid
- αKMB
α-keto-methylthiobutyric acid
- PLP
pyridoxal 5′-phosphate
References
- 1.Moroni F. Eur J Pharmacol. 1999;375:87–100. doi: 10.1016/s0014-2999(99)00196-x. [DOI] [PubMed] [Google Scholar]
- 2.Schwarcz R, Pellicciari R. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 3.Stone TW, Darlington LG. Nat Rev Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 4.Scharfman HE, Goodman JH, Schwarcz R. Amino Acids. 2000;19:283–297. doi: 10.1007/s007260070060. [DOI] [PubMed] [Google Scholar]
- 5.Erhardt S, Hajos M, Lindberg A, Engberg G. Synapse. 2000;37:104–108. doi: 10.1002/1098-2396(200008)37:2<104::AID-SYN4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 6.Carpenedo R, Chiarugi A, Russi P, Lombardi G, Carla V, Pellicciari R, Mattoli L, Moroni F. Neuroscience. 1994;61:237–243. doi: 10.1016/0306-4522(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 7.Cozzi A, Carpenedo R, Moroni F. J Cereb Blood Flow Metab. 1999;19:771–777. doi: 10.1097/00004647-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Wu HQ, Guidetti P, Goodman JH, Varasi M, Ceresoli-Borroni G, Speciale C, Scharfman HE, Schwarcz R. Neuroscience. 2000;97:243–251. doi: 10.1016/s0306-4522(00)00030-0. [DOI] [PubMed] [Google Scholar]
- 9.Schwarcz R, Ceresoli-Borroni G, Wu HQ, Rassoulpour A, Poeggeler B, Hodgkins PS, Guidetti P. Adv Exp Med Biol. 1999;467:113–123. doi: 10.1007/978-1-4615-4709-9_17. [DOI] [PubMed] [Google Scholar]
- 10.Stone TW. Trends Pharmacol Sci. 2000;21:149–154. doi: 10.1016/s0165-6147(00)01451-6. [DOI] [PubMed] [Google Scholar]
- 11.Guidetti P, Okuno E, Schwarcz R. J Neurosci Res. 1997;50:457–465. doi: 10.1002/(SICI)1097-4547(19971101)50:3<457::AID-JNR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Okuno E, Nakamura M, Schwarcz R. Brain Res. 1991;542:307–312. doi: 10.1016/0006-8993(91)91583-m. [DOI] [PubMed] [Google Scholar]
- 13.Du F, Schmidt W, Okuno E, Kido R, Kohler C, Schwarcz R. J Comp Neurol. 1992;321:477–487. doi: 10.1002/cne.903210313. [DOI] [PubMed] [Google Scholar]
- 14.Kapoor R, Okuno E, Kido R, Kapoor V. Neuroreport. 1997;8:3619–3623. doi: 10.1097/00001756-199711100-00039. [DOI] [PubMed] [Google Scholar]
- 15.Alberati-Giani D, Malherbe P, Kohler C, Lang G, Kiefer V, Lahm HW, Cesura AM. J Neurochem. 1995;64:1448–1455. doi: 10.1046/j.1471-4159.1995.64041448.x. [DOI] [PubMed] [Google Scholar]
- 16.Cooper AJ. Neurochem Int. 2004;44:557–577. doi: 10.1016/j.neuint.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Han Q, Calvo E, Marinotti O, Fang J, Rizzi M, James AA, Li J. Insect Mol Biol. 2003;12:483–490. doi: 10.1046/j.1365-2583.2003.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang J, Han Q, Li J. Insect Biochem Mol Biol. 2002;32:943–950. doi: 10.1016/s0965-1748(02)00032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han Q, Fang J, Li J. J Biol Chem. 2002;277:15781–15787. doi: 10.1074/jbc.M201202200. [DOI] [PubMed] [Google Scholar]
- 20.Han Q, Li J. FEBS Lett. 2002;527:199–204. doi: 10.1016/s0014-5793(02)03229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han Q, Fang J, Li J. Biochem J. 2001;360:617–623. doi: 10.1042/0264-6021:3600617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper AJ, Meister A. J Biol Chem. 1974;249:2554–2561. [PubMed] [Google Scholar]
- 23.Cooper AJ, Meister A. Comp Biochem Physiol B. 1981;69B:137–145. [Google Scholar]
- 24.Cooper AJ, Haber MT, Meister A. J Biol Chem. 1982;257:816–826. [PubMed] [Google Scholar]
- 25.Cooper JL, Meister A. Biochemistry. 1972;11:661–671. doi: 10.1021/bi00755a001. [DOI] [PubMed] [Google Scholar]
- 26.Baran H, Okuno E, Kido R, Schwarcz R. J Neurochem. 1994;62:730–738. doi: 10.1046/j.1471-4159.1994.62020730.x. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt W, Guidetti P, Okuno E, Schwarcz R. Neuroscience. 1993;55:177–184. doi: 10.1016/0306-4522(93)90464-q. [DOI] [PubMed] [Google Scholar]
- 28.Perry SJ, Schofield MA, MacFarlane M, Lock EA, King LJ, Gibson GG, Goldfarb PS. Mol Pharmacol. 1993;43:660–665. [PubMed] [Google Scholar]
- 29.Mosca M, Cozzi L, Breton J, Speciale C, Okuno E, Schwarcz R, Benatti L. FEBS Lett. 1994;353:21–24. doi: 10.1016/0014-5793(94)01003-x. [DOI] [PubMed] [Google Scholar]
- 30.Meunpol O, Hall MR, Kapoor V. Comp Biochem Physiol B. 1998;120:139–143. [Google Scholar]
- 31.Real MD, Ferre J. Insect Biochem. 1991;21:647–652. [Google Scholar]
- 32.Chiarugi A, Carpenedo R, Moroni F. J Neurochem. 1996;67:692–698. doi: 10.1046/j.1471-4159.1996.67020692.x. [DOI] [PubMed] [Google Scholar]
- 33.Ceresoli-Borroni G, Schwarcz R. Amino Acids. 2000;19:311–323. doi: 10.1007/s007260070062. [DOI] [PubMed] [Google Scholar]
- 34.Yudkoff M. Glia. 1997;21:92–98. doi: 10.1002/(sici)1098-1136(199709)21:1<92::aid-glia10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 35.McKenna MC, Stevenson JH, Huang X, Hopkins IB. Neurochem Int. 2000;37:229–241. doi: 10.1016/s0197-0186(00)00042-5. [DOI] [PubMed] [Google Scholar]
- 36.Hertz L. Neurochem Int. 2004;45:285–296. doi: 10.1016/j.neuint.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Sonnewald U, Schousboe A, Qu H, Waagepetersen HS. Neurochem Int. 2004;45:305–310. doi: 10.1016/j.neuint.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Stone TW. Pharmacol Rev. 1993;45:309–379. [PubMed] [Google Scholar]
- 39.Hodgkins PS, Wu HQ, Zielke HR, Schwarcz R. J Neurochem. 1999;72:643–651. doi: 10.1046/j.1471-4159.1999.0720643.x. [DOI] [PubMed] [Google Scholar]
- 40.Hodgkins PS, Schwarcz R. Eur J Neurosci. 1998;10:1986–1994. doi: 10.1046/j.1460-9568.1998.00208.x. [DOI] [PubMed] [Google Scholar]