Abstract
Wound healing involves a number of physiologic mechanisms including coagulation, inflammation, formation of granulation tissue, and tissue remodeling. Coagulation with robust thrombin generation leading to fibrin formation is necessary for wound healing. It is less clear if there is a requirement for ongoing coagulation to support tissue remodeling. We have studied wound healing in mice with defects in both the initiation (low tissue factor) and propagation (hemophilia B) phases. In hemophilia B mice, dermal wound healing is delayed; this delay is associated with bleeding into the granulation tissue. Mice can be treated with replacement therapy (factor IX) or bypassing agents (factor VIIa) to restore thrombin generation. If treated just prior to wound placement, mice will have normal hemostasis in the first day of wound healing. As the therapeutic agents clear, the mice will revert to hemophilic state. If the primary role of coagulation in wound healing is to provide a stable platelet/fibrin plug that is loaded with thrombin, then treating hemophilic animals just prior to wound placement should restore normal wound healing. The results from this study did not support that hypothesis. Instead the results show that restoring thrombin generation only at the time of wound placement did not improve the delayed wound healing. In preliminary studies on low tissue factor mice, there also appears to be a delay in wound healing with evidence of bleeding into the granulation tissue. The current data suggests that ongoing coagulation function needs to be maintained to support a normal wound healing process.
Keywords: wound healing, hemophilia, factor IX, factor VIIa, tissue factor
Phases of wound healing
The term wound healing has been used in a variety of animal models of incisional and excisional injuries to skin, muscle, and corneas; the term has also been used to describe experiments carried out in tissue culture models. In that setting it refers to the migration of cells to cover a denuded portion of a culture well. In this paper we use the term to mean reepithelialization of an excisional skin wound. This model does not attempt to be a model of joint injury; for models of hemophilic joint injury see [1,2]. Rather, this skin injury model has allowed us to examine the role of coagulation in some aspects of wound healing.
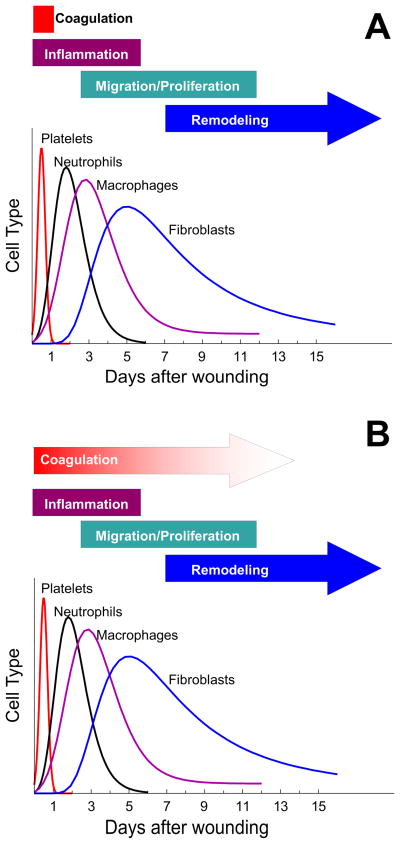
Wound healing is a process that involves a number of physiologic mechanisms (figure 1A) including coagulation, inflammation, formation of granulation tissue, and tissue remodeling [3,4]. Coagulation leading to thrombin generation is an immediate response to the initial injury. Thrombin generation results in aggregation of a platelet and fibrin mass that fills and stabilizes the wound preventing further blood loss. There is also a very rapid response by neutrophils and macrophages migrating along the fibrin matrix to prevent infection of the wound and clean up invading organisms and tissue debris. Subsequently fibroblasts crawl along the fibrin matrix and begin to form connective tissue, and endothelial sprouts begin revacularization of the site, transitioning the wound into granulation tissue. During all of this, the epithelial cells migrate and proliferate across the surface of the wound, displacing the scab, until the wound is closed (reepithelialized).
Figure 1.
Classical view of cells involved in different stages of wound healing. A. This figure is derived from figure 1 of Witte and Barbul [3]. The various stages of healing are shown associated with days in which that stage occurs. The relative involvement of various cell types is plotted against the days in which those cell types appear. B. TVisually presents the hypothesis that coagulation plays a significant role in the wound healing process for longer than just the period of initial hemostasis required to form a fibrin/platelet plug.
Role of fibrin structure
Having an appropriate fibrin structure is necessary for a number of the subsequent healing steps [5]. Mice that lacked fibrinogen could form wounds that would reepithelialize, but the initial wound strength was reduced and long term cellular migration within wound fields was altered [6]. Mice which have a defect in fibrin stabilization (factor XIII knockout) had defects in wound healing with a delay in reepithelialization [7]. This is consistent with the observation that fibroblast proliferation as well as expression of growth factors and interleukins is altered by changes in fibrin structure [8]. Further evidence for the importance of fibrin structure comes from studies in mice with defects in fibrin removal (plasminogen deficiency). In these mice the reepithelialization was significantly delayed, with as many as a third of the mice having open wounds 6 weeks after wounding [9].
Among the determinants that influence fibrin structure is the rate and pattern of thrombin generation [10]. Since fibrin sequesters thrombin, it is easy to envision a model in which thrombin generated initially during the coagulation phase could have long term effects on wound healing. And it is not uncommon to find in the literature a discussion of the wound healing process similar to that shown in figure 1 where coagulation is described as being critical only for the initial formation of the platelet/fibrin plug.
Roles of thrombin
While thrombin generation is required for fibrin formation, thrombin and other coagulation proteins have functions beyond hemostasis (for reviews see [11,12]). Thrombin influences wound healing by acting as a chemotactic agent and a mitogenic agent for macrophage influx as well as promoting angiogenesis [13–15]. Thrombin can cleave the protease activated receptor 1 (PAR1) [16] and it seems reasonable that thrombin cleavage of PAR1 might play a role in wound healing. However, a study on PAR1 knockout mice showed no changes in time to closure of open wounds, tensile strength of healed incisional wounds, or wound histology [17]. A later, unpublished study did note some differences in healing of an excisional wound in PAR1 knockout mice including reductions in wound contraction and reduced leukocyte infiltration [18]. And hydrogel dressings impregnated with the PAR1 agonist peptide promoted more rapid closure of excisional wounds than dressing alone [19].
Wound healing model
To examine the role of coagulation and thrombin generation in both the initial and the overall process of wound healing, we initiated studies on hemophilia B mice [20]. These mice have a clear bleeding defect in a tail transection model as well as in the Whinna saphenous vein injury model [21]. To study wound healing, a single 3-mm biopsy punch wound was placed on the back of each animal [22]. The wound was through the skin down to, but not into, the muscle. In both wild type and hemophilic animals there was evidence of some fibrin deposition in the wound bed indicating that some level of thrombin had been generated. Immediately after wounding neither wild type nor hemophilic wounds consistently showed evidence of bleeding. However, as expected, the extent of fibrin deposition (as determined by immunohistochemical staining) was dramatically reduced in the hemophilic wounds. Of interest was the finding that fibrin staining extended well into the edematous and inflamed tissue surrounding the wound site, and was not confined to the injury site in wild type mice. However, in hemophilic mice fibrin was only found at the edges of the tissue defect adjacent to the remaining epithelium. This localization is likely because epithelial cells express high level of tissue factor and are able to support some thrombin generation in the absence of factor VIII or IX. Consistent with defective thrombin and fibrin formation, wounds placed on hemophilic animals typically showed signs of bleeding (Figure 2) by 24 hours.
Figure 2.
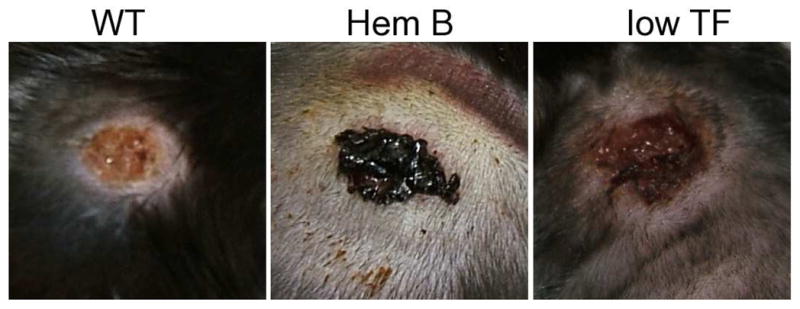
Day 3 wounds. Wounds in wild type (WT) animals did not generally show signs of bleeding in the wounds. Wounds placed on hemophilia B (Hem B) animals often showed clear evidence of bleeding into the wound bed. In preliminary studies, some, but not all, low tissue factor (low TF) mice showed evidence of some bleeding into the wound beds.
Wounds on hemophilic animals healed more slowly than wounds on wild type animals [22]. Some wild type animals had wounds that were reepithelialized by day 8 after wounding, with all wild type animals healed by day 10. By contrast no wounds on hemophilia B mice were healed until day 10; many were healed by day 12, and a few animals required up to 15 days for healing. This established that the coagulation defect in hemophilia is associated with delayed wound healing.
Role of thrombin beyond the initial hemostasis
Thrombin generation can be increased in hemophilia B mice by treatment with replacement therapy (factor IX) or treatment with bypassing agents (factor VIIa). Mice treated just prior to wound placement will have increased thrombin generation during the time the wound is placed; subsequently the animals will return to hemophilic levels. So, if the primary role of coagulation in wound healing is to provide a stable platelet/fibrin plug that is loaded with thrombin, then treating hemophilic animals just prior to wound placement should fully restore normal wound healing.
Studies on hemophilia B mice treated just prior to wounding did not support this hypothesis [23]. Pretreatment with either factor IX or factor VIIa at doses that completely corrected bleeding the in saphenous vein injury model did not shorten the time required for reepithelialization in hemophilic animals. Treatment did however have some effect on the histologic features of the wound. In untreated hemophilia B animals macrophage influx is delayed and decreased. Treated mice had a more robust early macrophage influx that was similar to wild type mice. This suggests that chemotactic signals generated during the initial coagulation phase are important for macrophage influx. However, in both treated and untreated hemophilia B mice, macrophage influx persisted much longer than in wild type animals. The macrophages stained for iron suggesting that there was bleeding into the granulation tissue. In wild type animals iron staining was seen early in the granulation tissue with the iron being carried off by the macrophages into the deep tissues and the draining lymph nodes. In both treated and untreated hemophilic animals, the iron staining persisted at a high level well past the point where the wounds were reepithelialized. This data points to ongoing bleeding into the granulation tissue and suggests that coagulation is necessary to limit bleeding into the granulation tissue during tissue remodeling.
Role of tissue factor
Tissue factor, in addition to being the primary initiator of coagulation, can play a role in a number of mechanisms that may be important in wound healing (for a review see [24]). In diabetic mice, tissue factor is not upregulated in excisional wounds to the same extent as in wild type mice [25]. When somatic gene transfer was used to increase tissue factor levels in the wounds of these diabetic mice: fibrin deposition in the wound bed increased; more tissue factor was seen in the migrating epidermal tips; and wound healing time was restored to normal [25]. These results suggest that both coagulant and non-coagulant functions of tissue factor might be important in the wound healing process.
In examining the granulation tissue of dermal wounds, one striking feature is the lack of staining for tissue factor [26]. Tissue factor staining can be observed in the surrounding dermal tissue and tissue factor is strongly expressed at the leading edge of the epithelial layer that is closing the wound. However, there is little or no staining for tissue factor in the granulation tissue, nor is it present around the newly formed angiogenic vessels. In addition, tissue factor that is normally present on the pericytes around blood vessels disappears in the area of inflammation surrounding the wound. Tissue factor expression does not completely reappear for several days (with delayed reappearance in hemophilic animals). This lack of tissue factor may account for the propensity of granulation tissue to bleed, and even in wild type animals some extravasated red blood cells can be observed in the granulation tissue. In hemophilic animals this bleeding tendency is exaggerated with red blood cells easily visible in the granulation tissue.
To further examine the role of tissue factor in wound healing, preliminary studies have begun with low tissue factor mice [27]. These mice express human tissue factor at roughly 1% of the levels of tissue factor seen in wild type mice. The mice develop normally and the majority of the mice do not have an obvious bleeding diathesis in the first 3 months. As with hemophilic animals, wounds placed on low tissue factor mice did not immediately bleed. However during healing some but not all animals showed signs of bleeding in the wound bed (figure 2). Wound healing was delayed by at least one day in the limited number of animals studied. Extravasated red blood cells could be observed in the granulation tissue suggesting ongoing bleeding in these animals.
Summary
Taken together, this data and published data suggests that coagulation function is required in the immediate response to wounding and that robust thrombin generation leading to fibrin formation is a necessary component for healthy wound healing. But this data also suggests that ongoing coagulation function needs to be maintained for an extended period to support a normal wound healing process (figure 1B). Whether this coagulation function is only required for hemostasis or whether modulation of signaling, possibly through through activation of PAR receptors including PAR-1, is also a key component is the subject of our ongoing investigations.
Acknowledgments
This work was supported by a research grant from Novo Nordisk, by NIH/NHLBI grant P01-HL06350, and by the US Department of Veterans Affairs.
Footnotes
Conflict of Interest
DMM and MH have received research support through their institutions from Novo Nordisk. Both have also received honorarium from Novo Nordisk for speaking.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sun J, Hakobyan N, Valentino LA, Feldman BL, Samulski RJ, Monahan PE. Intraarticular factor IX protein or gene replacement protects against development of hemophilic synovitis in the absence of circulating factor IX. Blood. 2008;112:4532–4541. doi: 10.1182/blood-2008-01-131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lafeber FPJG, Miossec P, Valentino LA. Physiopathology of haemophilic arthropathy. Haemophilia. 2008;14 (Suppl 4):3–9. doi: 10.1111/j.1365-2516.2008.01732.x. [DOI] [PubMed] [Google Scholar]
- 3.Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77:509–528. doi: 10.1016/s0039-6109(05)70566-1. [DOI] [PubMed] [Google Scholar]
- 4.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 5.Laurens N, Koolwijk P, de Maat MPM. Fibrin structure and wound healing. J Thromb Haemost. 2006;4:932–939. doi: 10.1111/j.1538-7836.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 6.Drew AF, Liu H, Davidson JM, Daugherty CC, Degen JL. Wound-healing defects in mice lacking fibrinogen. Blood. 2001;97:3691–3698. doi: 10.1182/blood.v97.12.3691. [DOI] [PubMed] [Google Scholar]
- 7.Inbal A, Lubetsky A, Krapp T, Castel D, Shaish A, Dickneitte G, Modis L, Muszbek L, Inbal A. Impaired wound healing in factor XIII deficient mice. Thromb Haemost. 2005;94:432–437. doi: 10.1160/TH05-04-0291. [DOI] [PubMed] [Google Scholar]
- 8.Cox S, Cole M, Tawil B. Behavior of human dermal fibroblasts in three-dimensional fibrin clots: dependence on fibrinogen and thrombin concentration. Tissue Eng. 2004;10:942–954. doi: 10.1089/1076327041348392. [DOI] [PubMed] [Google Scholar]
- 9.Romer J, Bugge TH, Pyke C, Lund LR, Flick MJ, Degen JL, Dano K. Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med. 1996;2:287–292. doi: 10.1038/nm0396-287. [DOI] [PubMed] [Google Scholar]
- 10.Wolberg AS. Thrombin generation and fibrin clot structure. Blood Rev. 2007;21:131–142. doi: 10.1016/j.blre.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Dugina TN, Kiseleva EV, Chistov IV, Umarova BA, Strukova SM. Receptors of the PAR family as a link between blood coagulation and inflammation. Biochemistry Mosc. 2002;67:65–74. doi: 10.1023/a:1013952114485. [DOI] [PubMed] [Google Scholar]
- 12.Landis RC. Protease activated receptors: clinical relevance to hemostasis and inflammation. Hematol Oncol Clin North Am. 2007;21:103–113. doi: 10.1016/j.hoc.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Bar-Shavit R, Kahn A, Fenton JW, Wilner GD. Chemotactic response of monocytes to thrombin. J Cell Biol. 1983;96:282–285. doi: 10.1083/jcb.96.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawes KE, Gray AJ, Laurent GJ. Thrombin stimulates fibroblast chemotaxis and replication. Eur J Cell Biol. 1993;61:126–130. [PubMed] [Google Scholar]
- 15.Norfleet AM, Bergmann JS, Carney DH. Thrombin peptide, TP508, stimulates angiogenic responses in animal models of dermal wound healing, in chick chorioallantoic membranes, and in cultured human aortic and microvascular endothelial cells. Gen Pharmacol. 2000;35:249–254. doi: 10.1016/s0306-3623(01)00118-5. [DOI] [PubMed] [Google Scholar]
- 16.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 17.Connolly AJ, Suh DY, Hunt TK, Coughlin SR. Mice lacking the thrombin receptor, PAR1, have normal skin wound healing. Am J Pathol. 1997;151:1199–1204. [PMC free article] [PubMed] [Google Scholar]
- 18.Major CD, Santulli RJ, Derian CK, Andrade-Gordon P. Extracellular mediators in atherosclerosis and thrombosis: lessons from thrombin receptor knockout mice. Arterioscler Thromb Vasc Biol. 2003;23:931–939. doi: 10.1161/01.ATV.0000070100.47907.26. [DOI] [PubMed] [Google Scholar]
- 19.Strukova SM, Dugina TN, Chistov IV, Lange M, Markvicheva EA, Kuptsova S, Zubov VP, Glusa E. Immobilized thrombin receptor agonist peptide accelerates wound healing in mice. Clin Appl Thromb Hemost. 2001;7:325–329. doi: 10.1177/107602960100700414. [DOI] [PubMed] [Google Scholar]
- 20.Lin HF, Maeda N, Smithies O, Straight DL, Stafford DW. A coagulation factor IX-deficient mouse model for human hemophilia B. Blood. 1997;90:3962–3966. [PubMed] [Google Scholar]
- 21.Whinna HC, Monroe DM. A novel murine hemostasis model: studies on recombinant factor VIIa and NN1731. J Thromb Haemost. 2009;7:PP-MO-392. [Google Scholar]
- 22.Hoffman M, Harger A, Lenkowski A, Hedner U, Roberts HR, Monroe DM. Cutaneous wound healing is impaired in hemophilia B. Blood. 2006;108:3053–3060. doi: 10.1182/blood-2006-05-020495. [DOI] [PubMed] [Google Scholar]
- 23.McDonald A, Hoffman M, Hedner U, Roberts HR, Monroe DM. Restoring hemostatic thrombin generation at the time of cutaneous wounding does not normalize healing in hemophilia B. J Thromb Haemost. 2007;5:1577–1583. doi: 10.1111/j.1538-7836.2007.02647.x. [DOI] [PubMed] [Google Scholar]
- 24.Monroe DM, Key NS. The tissue factor-factor VIIa complex: procoagulant activity, regulation, and multitasking. J Thromb Haemost. 2007;5:1097–105. doi: 10.1111/j.1538-7836.2007.02435.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Kasper M, Heck T, Nakagawa K, Humpert PM, Bai L, Wu G, Zhang Y, Luther T, Andrassy M, Schiekofer S, Hamann A, Morcos M, Chen B, Stern DM, Nawroth PP, Bierhaus A. Tissue factor as a link between wounding and tissue repair. Diabetes. 2005;54:2143–2154. doi: 10.2337/diabetes.54.7.2143. [DOI] [PubMed] [Google Scholar]
- 26.McDonald AG, Yang K, Roberts HR, Monroe DM, Hoffman M. Perivascular tissue factor is down-regulated following cutaneous wounding: implications for bleeding in hemophilia. Blood. 2008;111:2046–2048. doi: 10.1182/blood-2007-05-092916. [DOI] [PubMed] [Google Scholar]
- 27.Parry GC, Erlich JH, Carmeliet P, Luther T, Mackman N. Low levels of tissue factor are compatible with development and hemostasis in mice. J Clin Invest. 1998;101:560–569. doi: 10.1172/JCI814. [DOI] [PMC free article] [PubMed] [Google Scholar]