Abstract
Nuclear factor of activated T cells (NFAT) is a transcription factor that translocates from cytosol to nucleus following dephosphorylation by the Ca2+/calmodulin dependent protein phosphatase calcineurin (CN). In nervous tissue, aberrant CN signaling is increasingly linked to a variety of pathologic features associated with Alzheimer’s disease (AD), including synaptic dysfunction, glial activation, and neuronal death. Consistent with this linkage, our recent work on postmortem human hippocampal tissue discovered increased nuclear accumulation of select NFAT isoforms at different stages of AD. Some of these changes occurred at the early stages of the disease process and/or paralleled diminishing cognitive status. In addition, inhibition of astrocytic NFAT activity in primary cultures of neurons and glia dampened glutamate levels and alleviated neuronal death in response to pathogenic amyloid-β peptides. In this article, we discuss our recent findings and expand upon the possible isoform specific contributions of NFATs to the progression of AD. We also consider the possible benefits of using NFAT inhibitors to treat AD and other neurodegenerative disorders, as well.
Keywords: NFATs, Alzheimer’s disease, Calcineurin, Neurodegenerative disorders
Introduction
Alzheimer’s disease (AD) is an aging-dependent, progressive neurodegenerative disease, characterized by β-amyloid (Aβ) plaques, neurofibrillary tangles, gliosis, oxidative stress, neuronal degeneration, and dementia. More than two decades ago, it was proposed that the dysregulation of cellular Ca2+ across the lifespan leads to impaired cognition and increased susceptibility to AD and other neurodegenerative conditions (1–4). Numerous studies on human AD tissue, and especially on multiple animal models of AD, have largely confirmed the importance of Ca2+ dysregulation during the disease process (5, 6). Recently, our group and several other labs have compiled strong evidence to suggest that the Ca2+-dependent protein phosphatase calcineurin (CN) provides an important link among the multiple pathologic hallmarks that emerge during AD. This article will discuss some of the recent findings concerning CN signaling and AD, with particular emphasis on our new research on the CN-dependent transcription factor, nuclear factor of activated T cells (NFAT) (7).
Calcineurin
CN signaling properties and regulation have been described in several excellent reviews, e.g. (8), and therefore will not be described in detail here. Briefly, the CN holoenzyme consists of an ~60 kDa catalytic subunit (CN A) and an ~19 kDa Ca2+ binding regulatory subunit (CN B). Multiple isoforms of each subunit (e.g. CN Aα, CN Aβ and CN Bα, and CN Bβ) have been identified and are expressed in brain. CN is strongly regulated by cooperative interactions between Ca2+, CN B, and Ca2+/calmodulin. Maximal catalytic activity is achieved when Ca2+/ calmodulin is bound to the CN A subunit. Several unique regulatory, expressional, and functional properties of CN make it a molecule of interest in AD and other neurodegenerative disorders. First, while most enzyme classes (e.g. protein kinases and proteases) consist of multiple Ca2+ sensitive family members, CN is the only Ca2+-activated protein phosphatase in mammalian cells. Moreover, of the many Ca2+ dependent enzymes, CN is among the most sensitive to fluctuating intracellular Ca2+ levels and is therefore especially vulnerable to the Ca2+ dysregulation that arises with AD. Second, CN is one of the most abundantly expressed proteins in the brain, with particularly high levels found in structures (e.g. the hippocampus) showing the earliest and most extensive signs of AD pathology (9). Third, elevated CN signaling is strongly associated with numerous deleterious processes linked to AD including, synaptic dysfunction and spine loss (10, 11), neuritic atrophy and neuronal death (12), astrocyte activation (13), β-amyloid (Aβ) production (14), and impaired cognition (15, 16). Fourth, pathogenic molecules in AD brain, especially oligomeric Aβ peptides, robustly activate CN activity in neurons (17, 18) and astrocytes (7). Moreover, numerous deleterious effects of oligomeric Aβ including synaptic depression (19), NMDA receptor endocytosis and synaptic spine retraction (20, 21), dendritic atrophy (22), neuronal apoptosis (18), and glutamate dyshomeostasis are blocked or ameliorated by inhibition of CN signaling pathways. Finally, several recent studies have shown that neural CN activity/expression is increased in mouse models of AD, as well as postmortem AD tissue (7, 13, 23, 24).
NFATs are CN dependent transcription factors
NFATs are the most thoroughly characterized CN substrate and are the subject of several excellent reviews, e.g. (25). These transcription factors tend to reside in the cytosol in a highly phosphorylated state when intracellular Ca2+ levels are low, but are bound tightly by activated CN and dephosphorylated when Ca2+ levels rise. CN-mediated dephosphorylation of NFATs reveals a nuclear import sequence which permits transport into the nucleus. Conversely, a nuclear export sequence is exposed and NFATs are shuttled back to the cytosol upon re-phosphorylation by a variety of protein kinases (e.g. glycogen synthase kinase 3β). Elevated CN activity is therefore generally required to maintain the nuclear localization of NFATs. Once in the nucleus, NFATs interact with specific DNA binding elements to regulate gene expression in conjunction with other transcription factors (e.g. AP1, MEFs, and NFκB). NFATs play an important role in immune/inflammatory signaling and are best known for coordinating T cell activation through the regulation of cytokine genes. Nonetheless, NFATs have been found in most other mammalian tissues, as well, where they regulate numerous and diverse cellular processes. In brain, CN-mediated effects on Ca2+ homeostasis, neuronal viability, Aβ production, and neuroinflammation have been shown to depend, in part, on NFAT activation (14, 26–30), suggesting a possible role for these transcription factors in AD. Based on these observations, we set out to determine whether AD and cognitive decline were associated with changes in NFAT signaling properties.
Isoform specific changes in NFAT signaling properties with AD
In our study (7), postmortem human hippocampal and cerebellar tissue was collected from non-demented control cases and confirmed AD cases. Subjects diagnosed with mild cognitive impairment (MCI) were also studied, as these patients appear to be at a transition between normal age-related cognitive decline and dementia associated with AD. Hippocampus and cerebellum were chosen because of their different vulnerabilities to AD pathology (hippocampus = high vulnerability; cerebellum = low vulnerability). Tissue from each subject was separated into cytosolic and nuclear fractions using differential centrifugation and protein levels were measured in each fraction. At least four CN-dependent NFAT isoforms have been identified (NFATs1-4), all of which are expressed in brain (31). In our study, we measured levels for NFATs 1–3 (due to the lack of a satisfactory antibody, NFAT4 was not investigated). Levels for multiple CN A isoforms and glycogen synthase kinase 3β (GSK3β) were also measured. The proportion of NFAT protein associated with the nuclear fraction (nucNFAT) was obtained by dividing nuclear protein levels by total (nuclear + cytosolic) protein levels. Table 1 summarizes changes in the nuclear localization of NFATs 1, 2, and 3, CN Aα and CN Aβ, and GSK3-β during MCI and AD, as reported in Abdul et al., 2009 (7).
Table 1.
Changes in nuclear protein levels for each NFAT isoform (nucNFATs) in patients with mild cognitive impairment (MCI) and Alzheimer’s disease (AD) and their relationships with other nuclear-localized proteins: CN Aα, CN Aβ, and GSK3-β, as reported in Abdul et al., 2009
| MCI | AD | Correlation with nucCNAα |
Correlation with nucCNAβ |
Correlation with nucGSK3β |
|
|---|---|---|---|---|---|
| nucNFAT1 | UP | Down Slightly |
Yes, Positive | Yes, Positive | No |
| nucNFAT2 | No Δ | No Δ | No | No | No |
| nucNFAT3 | Down Slightly |
Up | Yes, positive | No | No |
Because NFATs tend to accumulate in the nucleus when active, we predicted that AD and/or MCI would be associated with elevated nucNFAT levels in the hippocampus. Our results both supported and refuted this prediction. Of the three NFAT isoforms studied, only the NFAT2 isoform remained unchanged in the hippocampus across subject categories (see Table 1). Moreover, changes in NFATs1 and 3 occurred during different stages of disease progression. Hippocampal nucNFAT1 levels were increased during MCI, but then fell precipitously as pathology and cognitive status worsened. In contrast, hippocampal nucNFAT3 levels dipped slightly in MCI but were markedly increased with intermediate to severe AD. In contrast to the hippocampus, cerebellar nucNFAT levels were very similar across subject groups, suggesting that changes in NFAT signaling with AD may be relatively restricted to the most vulnerable structures.
To determine whether isoform specific changes in NFAT localization were attributable to different expression patterns across neuron and glial populations, we double-labeled postmortem hippocampal sections from each of the subject groups with anti-NFAT antibodies and an antibody to glial fibrillary acidic protein (GFAP), an intermediate filament highly enriched in astrocytes. Using confocal microscopy, we showed that NFATs1 and 3 were expressed in both neurons (dentate granule cells) and astrocytes in the hippocampus, regardless of clinical and pathological symptoms. However, relative to controls and AD cases, NFAT1 tended to show greater localization to astrocyte nuclei in the MCI group, whereas nuclear localization of NFAT3 in astrocytes was more readily apparent in the AD group. Within dentate granule neurons of all subject groups, NFATs1 and 3 each showed ‘donut-like’ expression patterns, consistent with cytosolic localization. These expression changes are generally consistent with our Western blot results and suggest that alterations in NFAT signaling during AD progression may occur to a greater extent in astrocytes. Caution is needed with this interpretation, however, as NFAT expression in CA pyramidal neurons were not examined closely.
Isoform-specific contributions of NFATs to AD and dementia?
If multiple NFAT isoforms are expressed within the same cell populations, why would one isoform respond differently than the others during the progression of cognitive decline in AD? One possibility is that NFAT isoforms exhibit different sensitivities to CN, or to NFAT kinases. In fact, we found that nucNFAT 1 and 3 levels in human hippocampus were preferentially associated with different CN A isoforms (Table 1), but not with GSK-3β. NucNFAT1 levels were moderately, but positively, associated with the nuclear localization of both CN Aα and CN Aβ isoforms, whereas nucNFAT3 exhibited a much stronger association with CN Aα, and did not correlate with CN Aβ. Isoform differences in NFAT activation may also depend on the presence of other transcription factors and/or on the temporal and spatial properties of the Ca2+ signal, among other things. Regardless of the mechanism, our results demonstrate that NFATs 1 and 3 are excessively activated at different stages of cognitive decline and AD, and therefore may contribute to entirely different cellular processes.
Whether in peripheral tissues, or in brain, NFATs appear to play an important role in regulating immune/inflammatory signaling, which is an important component of most all neurodegenerative diseases, including AD. To determine the extent to which NFATs 1, 2, and 3 are associated with changes in inflammatory mediators in AD brain, we measured activated protein levels for several cytokines, including IL-1β, tumor necrosis factor α (TNFα), and granulocyte macrophage colony stimulating factor (GM-CSF) in the same postmortem samples used in the Abdul et al study (7), and compared these measures to nucNFAT levels (32). The results revealed an increase in the expression of all three cytokines with either MCI or AD. Moreover, each cytokine exhibited a significant positive correlation with nucNFAT1, as assessed within subjects. GM-CSF showed the strongest association (r = 0.51, p < 0.01), especially when evaluated across normal control cases, MCI cases, and so-called pre-clinical AD cases (i.e. individuals with prominent AD pathology but little to no clinical symptoms of cognitive decline). Thus, levels for several cytokine species increase as NFAT1 levels accumulate in the nucleus, consistent with a role for NFAT1 in neuroinflammation during the early stages of AD-related cognitive decline. Interestingly, none of the cytokines examined correlated significantly with NFAT3, suggesting that this isoform may have a limited role in neuroinflammation with AD.
In contrast to NFAT1, NFAT3 may have more of an impact on neurodegenerative processes, including cell death. In animal models of acute injury, NFAT3 translocates to nuclear compartments of dentate granule cells where it interacts with responsive elements within the promoter region of the pro-apoptotic Fas ligand (FasL) (30). Similar nuclear translocation of NFAT3 and FasL upregulation were observed in apoptotic cochlear neurons of mice following deafferentation (28). In both of these studies, blockade of CN/NFAT activity was neuroprotective. The link between NFAT3 and FasL is interesting as FasL protein levels are increased in the brain during end stage AD (33), similar to nucNFAT3. Moreover, recent work identified NFAT3 as a critical component of Aβ -mediated neuronal apoptosis (34). While these results suggest that elevated nucNFAT3 promotes or drives cell death cascades during AD, other reports have described neuroprotective properties of NFAT3. Benedito et al., (2005) found that NFAT3 mediated the pro-survival properties of serum in primary cerebellar neurons (35). Similarly, Vashishta et al., (2009) revealed anti-apoptotic effects of NFAT3 in cortical neuronal cultures following an NMDA insult (36). These results are generally consistent with earlier studies showing robust activation of NFAT3 in response to a variety of neuroprotective factors, including BDNF (37). Thus, as with other transcription factors, NFAT3 activation may promote, or prevent cell death. Additional work will be necessary to define the contextual variables that turn NFAT3 from killer to protector.
Positive feedback interactions between CN/NFAT signaling and Aβ
Aβ appears to be a primary factor for triggering aberrant CN/NFAT activity during the progression of AD. As discussed, Aβ robustly stimulates CN/NFAT activity in both neurons and astrocytes. Moreover, we observed a relatively strong positive correlation (r = 0.54, p < 0.01) between nucNFAT levels (particularly nucNFAT3) and soluble Aβ (1–42) in the Abdul et al., 2009 study (7). However, in addition to reacting to Aβ, CN/NFAT activity may also help drive Aβ production through the transcriptional regulation of the β-site APP-cleaving enzyme 1 (BACE-1). BACE1 is the rate-limiting protease involved in the conversion of APP to pathogenic Aβ peptides and is upregulated in AD brain (38). In both cell cultures and intact AD model mice, NFAT1 interacts with the BACE1 promoter and stimulates BACE1 protein levels (14, 39). Increased NFAT1 activation in the early stages of cognitive decline, as shown by our work (7) may therefore help potentiate amyloidosis through a BACE1 regulatory mechanism, leading to a precipitous loss of neuronal function and viability.
Working model for the role of CN/NFAT signaling in AD
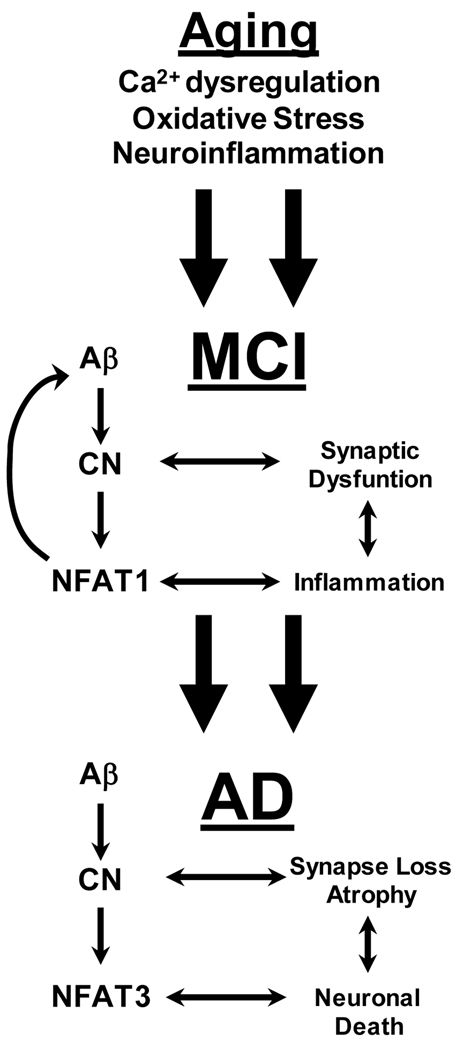
From these results, we constructed a working model for the role of CN/NFAT signaling in the progression of AD (Figure 1). This model suggests that aging related changes in Ca2+ homeostasis, glial activation, and oxidative stress lead to an increase in Aβ production, aberrantly high CN activity, or both. Aβ and CN engage in a positive feedback cycle that exacerbates the dysregulation of both molecules. In the early stages of cognitive decline, Aβ-CN interactions result in synaptic dysfunction/deterioration and glial activation, which also progress in a cyclical manner (i.e. synaptic deterioration ➔ glial activation ➔ synaptic deterioration). We propose that NFAT1 is especially critical at this stage as it stimulates neuroinflammatory responses and drives further production of Aβ through upregulation of BACE1. As these feedback interactions progress, critical mechanisms for maintaining neuronal viability are lost, such as astrocytic glutamate uptake (see below), leading to further Ca2+ dysregulation and oxidative stress in neurons. During this state, NFAT3 activation is selectively increased leading to further neurodegeneration, cell death, and ultimately, dementia.
Figure 1. Working model for the role of CN/NFAT signaling in AD.
During aging, gradual dysregulation of Ca2+, oxidative stress, and/or neuroinflammation lead to increased production of Aβ, increased activation of CN or both. In turn, aberrant CN activity leads to synaptic dysfunction and neuroinflammation, in part through increased stimulation of NFAT1. This chain of events leads to mild cognitive impairment (MCI). Note that cyclical interactions between neuroinflammatory processes (i.e. glial activation) and synaptic dysfunction) can maintain the CN/NFAT1 pathway in an activated state, which helps perpetuate deleterious neuronal and glial activities. NFAT1 also helps exacerbate amyloid toxicity through upregulation of BACE1 (see text). Eventually, these interactions progress to a degenerative state (i.e. Alzheimer’s disease) characterized by severe amyloidosis and severe Ca2+ dyshomeostasis leading to greater CN activity. CN triggers neuritic atrophy and cell death cascades through preferential activation of NFAT3.
Possible use of NFAT inhibitors for treating AD?
According to this model, blockade of CN/NFAT activity at each stage should help disrupt these positive feedback cycles and slow neurodegeneration and cognitive decline. In fact, CN inhibitors (such as tacrolimus) have been shown to improve cognition (23, 40), prevent degeneration of neuronal processes (22), and extend the lifespan (41) of transgenic mice expressing amyloid or tau pathology. However, the use of CN inhibitors for treating AD in humans may be limited because of the numerous serious, and possibly deadly, adverse effects associated with these drugs (42), which may be even more pronounced in elderly subjects (43). In the last 10 years, new immunosuppressive agents, such as the peptide, VIVIT, have been developed as possible alternatives to CN inhibitors. VIVIT mimics a CN docking site expressed in all CN-dependent NFATs (44), and possibly in a few other CN substrates, as well. When present, VIVIT prevents CN from binding to and activating NFATs, without suppressing CN activity. In animal models, VIVIT exerts powerful immunosuppressant/anti-inflammatory properties, and appears to be less toxic than CN inhibitors (45). Recently, we observed neuroprotective effects of VIVIT in primary cultures of neurons and astrocytes (7, 29). Specifically, delivery of VIVIT to primary astrocytes prevented the downregulation of critical astrocytic glutamate transporter levels during chronic exposure to IL-1β or Aβ Effects of VIVIT were especially robust for the type II excitatory amino acid transporter (EAAT2), which contains multiple NFAT binding sites in its promoter region. Selective delivery of VIVIT to astrocytes in a mixed (neurons/glia) primary culture model limited IL- and Aβ-dependent elevations in extracellular glutamate and ameliorated neurotoxicity.
Based on these findings, we initiated pilot studies to investigate the effects of VIVIT in a mouse model of AD. We used Alzet miniosmotic pumps to unilaterally deliver VIVIT peptide or a scrambled control peptide, IVEET, to the cerebral ventricles of 14-month-old APP/PS1 mice (VIVIT n = 3, IVEET n = 2) over a period of 14 days (~60 µg/day). Peptides were synthesized by Synbiosci (Livermore, CA) and contained a polyarginine tag at the N terminus (i.e. 11R-VIVIT, 11R-IVEET) to facilitate transport across cell membranes, as previously described (45). Mice were homozygous double knock-in mutants (APPNLh/NLh X PS-1P264L/P264L) that express humanized Aβ and the P264L familial Alzheimer’s disease presenilin-1 mutation. As described elsewhere (46, 47), these mice show extensive amyloid pathology and/or gliosis well before 14 months-of-age. At the end of the treatment period, mice were transcardially perfused and brains fixed, and cut into 60 µm sections for immunohistochemical labeling of astrocytes, using anti-GFAP primary antibody (Cell Signaling) and HRP-coupled secondary antibody. All mice were treated in accordance with institutional animal usage guidelines.
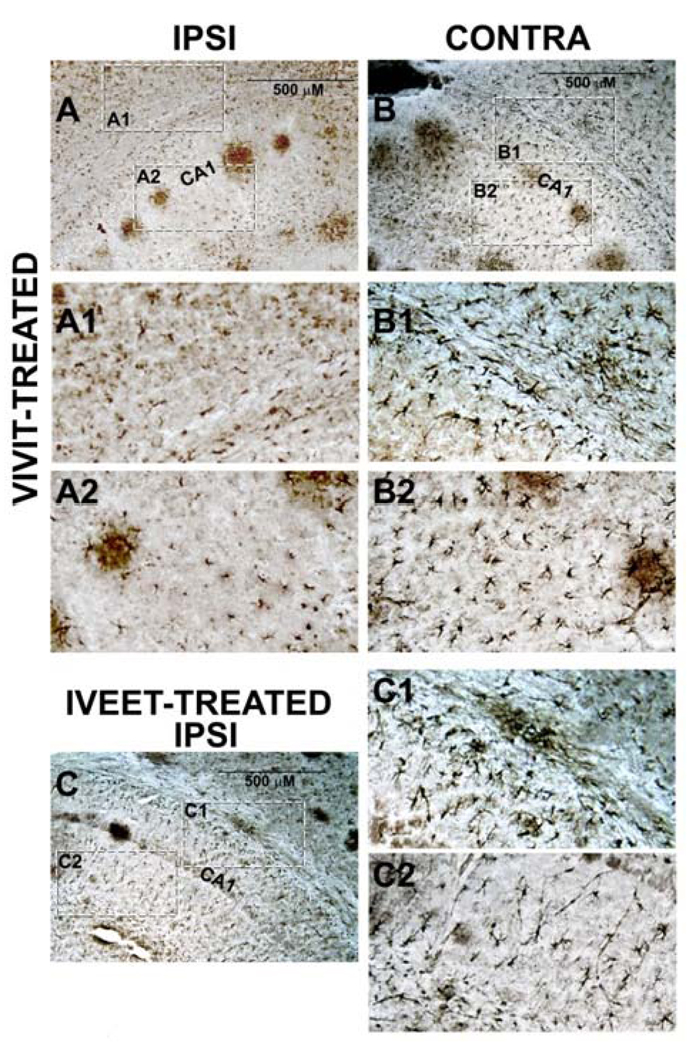
Figure 2 shows representative photomicrographs taken of the ipsilateral and contralateral hippocampus of a mouse treated with VIVIT peptide. Note that images in Figure 2A and 2B are from the same coronal section. Relative to the contralateral hemisphere, the ipsilateral VIVIT-perfused hemisphere shows far fewer and less ramified GFAP positive cells (compare Figs 2A1 and 2A2 with Figs 2B1 and 2B2). Similar inter-hemispheric differences were observed in the remaining two VIVIT-treated mice. Note also that the ipsilateral hippocampus of VIVIT-treated mice shows fewer GFAP positive cells relative to the ipsilateral hemisphere of IVEET-treated mice (compare Figs 1A1 and A2 with Figs 2C1 and 2C2). Though preliminary, these data suggest that VIVIT treatment blunts or limits astrocyte activation in APP/PS1 mice and may therefore be useful in treating synaptic dysfunction, amyloid pathology, and cognitive decline in this, and other animal models of AD.
Figure 2. The NFAT inhibitor, VIVIT, reduces astrocyte activation in AD mice.
A and B, photomicrographs of a GFAP-labeled coronal section from a 14-month-old APP/PS1 mouse treated intraventricularly for two weeks with 11R-VIVIT. Dashed boxes indicate regions of higher magnification shown in A1 and A2 (ipsilateral, VIVIT-treated hemisphere—Ipsi) and B1 and B2 (contralateral hemisphere—contra). C, GFAP-labeled coronal section (Ipsilateral hemisphere) from a 14-month-old APP/PS1 mouse treated for two weeks with 11R-IVEET control peptide. Dashed boxes indicate regions of higher magnification shown in C1 and C2. IVEET-treated mice and the contralateral hemisphere of VIVIT-treated mice appear to express more numerous and more ramified astrocytes relative to the ipsilateral hemisphere of VIVIT-treated mice. CA1 = cornu ammonis 1 pyramidal cell layer.
Of course, it is possible that inhibition of CN/NFAT activity and blockade of astrocyte activation in intact animals could have detrimental rather than beneficial effects. Indeed, several studies have shown that glial activation helps clear amyloid peptides from the interstitial space (48, 49). In addition, Fernandez et al. (2007) found that transgenic mice overexpressing an activated form of CN in astrocytes were less vulnerable to neurotoxicity following an acute trauma (26). Anti-inflammatory effects of VIVIT, as shown in Figure 2, may therefore worsen amyloid pathology and hasten cognitive decline in animal models. On the other hand, chronic neuroinflammation observed in AD (or amyloidogenic animals) and inflammation arising from acute neural injury may have fundamentally different effects on neuronal viability. Ongoing work in our lab is therefore testing the effects of VIVIT in multiple animal models of injury and amyloidosis.
Conclusions and future directions
Mounting evidence from our laboratory and from several other groups suggest that aberrant CN/NFAT signaling significantly contributes to the pathologic and clinical features of AD. Interestingly, different CN/NFAT signaling pathways appear to be activated at different stages of AD: NFAT1 is selectively activated early in cognitive decline, while NFAT3 is recruited later in dementia. The precise isoform-specific functions of NFATs in nervous tissue, and their respective roles in AD, will require extensive investigation. Finally, CN inhibitors show promising anti-inflammatory, neuroprotective, and/or cognitive enhancing properties in a variety of experimental models of AD. Whether the beneficial properties of these drugs offset their numerous adverse effects remains to be seen. In addition to CN inhibitors, reagents with greater selectivity to NFAT activation (e.g. VIVIT) also hold promise for treating neurodegeneration. Studies are presently underway in our lab to test the neuroprotective and nootropic properties of VIVIT in multiple experimental models of injury and disease.
Acknowledgements
Work supported by NIH grants AG024190, AG027297, AG028383, AG010836 and a gift from the Kleberg Foundation.
Footnotes
Conflicts of Interest
No potential conflicts of interest to disclose.
References
- 1.Gibson GE, Peterson C. Calcium and the aging nervous system. Neurobiol Aging. 1987;8:329–343. doi: 10.1016/0197-4580(87)90072-8. [DOI] [PubMed] [Google Scholar]
- 2.Khachaturian ZS. Towards theories of brain aging. In: Kay DW, Burrows GW, editors. Handbook of Studies on Psychiatry and Old Age. Amsterdam: Elsevier; 1984. pp. 7–30. [Google Scholar]
- 3.Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- 4.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store. Aging Cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green KN, LaFerla FM. Linking calcium to Abeta and Alzheimer's disease. Neuron. 2008;59:190–194. doi: 10.1016/j.neuron.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 7.Abdul HM, Sama MA, Furman JL, et al. Cognitive decline in Alzheimer's disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci. 2009;29:12957–12969. doi: 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aramburu J, Rao A, Klee CB. Calcineurin: from structure to function. Curr Top Cell Regul. 2000;36:237–295. doi: 10.1016/s0070-2137(01)80011-x. [DOI] [PubMed] [Google Scholar]
- 9.Polli JW, Billingsley ML, Kincaid RL. Expression of the calmodulin-dependent protein phosphatase, calcineurin, in rat brain: developmental patterns and the role of nigrostriatal innervation. Brain Res Dev Brain Res. 1991;63:105–119. doi: 10.1016/0165-3806(91)90071-p. [DOI] [PubMed] [Google Scholar]
- 10.Winder DG, Mansuy IM, Osman M, Moallem TM, Kandel ER. Genetic and pharmacological evidence for a novel, intermediate phase of long-term potentiation suppressed by calcineurin. Cell. 1998;92:25–37. doi: 10.1016/s0092-8674(00)80896-x. [DOI] [PubMed] [Google Scholar]
- 11.Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang HG, Pathan N, Ethell IM, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 13.Norris CM, Kadish I, Blalock EM, et al. Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer's models. J Neurosci. 2005;25:4649–4658. doi: 10.1523/JNEUROSCI.0365-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho HJ, Jin SM, Youn HD, Huh K, Mook-Jung I. Disrupted intracellular calcium regulates BACE1 gene expression via nuclear factor of activated T cells 1 (NFAT 1) signaling. Aging Cell. 2008;7:137–147. doi: 10.1111/j.1474-9726.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- 15.Mansuy IM, Mayford M, Jacob B, Kandel ER, Bach ME. Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell. 1998;92:39–49. doi: 10.1016/s0092-8674(00)80897-1. [DOI] [PubMed] [Google Scholar]
- 16.Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A. Calcineurin links Ca2+ dysregulation with brain aging. J Neurosci. 2001;21:4066–4073. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reese LC, Zhang W, Dineley KT, Kayed R, Taglialatela G. Selective induction of calcineurin activity and signaling by oligomeric amyloid beta. Aging Cell. 2008;7:824–835. doi: 10.1111/j.1474-9726.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agostinho P, Lopes JP, Velez Z, Oliveira CR. Overactivation of calcineurin induced by amyloid-beta and prion proteins. Neurochem Int. 2008;52:1226–1233. doi: 10.1016/j.neuint.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snyder EM, Nong Y, Almeida CG, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 22.Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dineley KT, Hogan D, Zhang WR, Taglialatela G. Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol Learn Mem. 2007;88:217–224. doi: 10.1016/j.nlm.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F, Grundke-Iqbal I, Iqbal K, Oda Y, Tomizawa K, Gong CX. Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain. J Biol Chem. 2005;280:37755–37762. doi: 10.1074/jbc.M507475200. [DOI] [PubMed] [Google Scholar]
- 25.Im SH, Rao A. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol Cells. 2004;18:1–9. [PubMed] [Google Scholar]
- 26.Fernandez AM, Fernandez S, Carrero P, Garcia-Garcia M, Torres-Aleman I. Calcineurin in reactive astrocytes plays a key role in the interplay between proinflammatory and anti-inflammatory signals. J Neurosci. 2007;27:8745–8756. doi: 10.1523/JNEUROSCI.1002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graef IA, Mermelstein PG, Stankunas K, et al. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature. 1999;401:703–708. doi: 10.1038/44378. [DOI] [PubMed] [Google Scholar]
- 28.Luoma JI, Zirpel L. Deafferentation-induced activation of NFAT (nuclear factor of activated T-cells) in cochlear nucleus neurons during a developmental critical period: a role for NFATc4-dependent apoptosis in the CNS. J Neurosci. 2008;28:3159–3169. doi: 10.1523/JNEUROSCI.5227-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sama MA, Mathis DM, Furman JL, et al. Interleukin-1beta-dependent signaling between astrocytes and neurons depends critically on astrocytic calcineurin/NFAT activity. J Biol Chem. 2008;283:21953–21964. doi: 10.1074/jbc.M800148200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shioda N, Han F, Moriguchi S, Fukunaga K. Constitutively active calcineurin mediates delayed neuronal death through Fas-ligand expression via activation of NFAT and FKHR transcriptional activities in mouse brain ischemia. J Neurochem. 2007;102:1506–1517. doi: 10.1111/j.1471-4159.2007.04600.x. [DOI] [PubMed] [Google Scholar]
- 31.Vihma H, Pruunsild P, Timmusk T. Alternative splicing and expression of human and mouse NFAT genes. Genomics. 2008;92:279–291. doi: 10.1016/j.ygeno.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furman JL, Abdul HM, Xiong S, et al. Hippocampal cytokine expression and calcineurin/NFAT signaling associated with pre-clinical Alzheimer's disease and mild cognitive impairment. Soc Neurosci Abs. 2009;39:727–724. [Google Scholar]
- 33.Su JH, Anderson AJ, Cribbs DH, et al. Fas and Fas ligand are associated with neuritic degeneration in the AD brain and participate in beta-amyloid-induced neuronal death. Neurobiol Dis. 2003;12:182–193. doi: 10.1016/s0969-9961(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 34.Wu H-Y, Hudry E, Hashimoto T, Grosskreutz C, Hyman B. Dysregulation of calcinerin/NFAT links abeta to neurodegeneration in Alzheimer's disease. Soc Neurosci Abs. 2009;39:725–729. [Google Scholar]
- 35.Benedito AB, Lehtinen M, Massol R, et al. The transcription factor NFAT3 mediates neuronal survival. J Biol Chem. 2005;280:2818–2825. doi: 10.1074/jbc.M408741200. [DOI] [PubMed] [Google Scholar]
- 36.Vashishta A, Habas A, Pruunsild P, Zheng JJ, Timmusk T, Hetman M. Nuclear Factor of Activated T-Cells Isoform c4 (NFATc4/NFAT3) as a Mediator of Antiapoptotic Transcription in NMDA Receptor-Stimulated Cortical Neurons. J Neurosci. 2009;29:15331–15340. doi: 10.1523/JNEUROSCI.4873-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groth RD, Mermelstein PG. Brain-derived neurotrophic factor activation of NFAT (nuclear factor of activated T-cells)-dependent transcription: a role for the transcription factor NFATc4 in neurotrophin-mediated gene expression. J Neurosci. 2003;23:8125–8134. doi: 10.1523/JNEUROSCI.23-22-08125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 39.Cho HJ, Son SM, Jin SM, et al. RAGE regulates BACE1 and Abeta generation via NFAT1 activation in Alzheimer's disease animal model. FASEB J. 2009;23:2639–2649. doi: 10.1096/fj.08-126383. [DOI] [PubMed] [Google Scholar]
- 40.Taglialatela G, Hogan D, Zhang WR, Dineley KT. Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav Brain Res. 2009;8:95–99. doi: 10.1016/j.bbr.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshiyama Y, Higuchi M, Zhang B, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Krensky A. Immunosuppressants, Tolerogens, and immunostimulants. In: Brunton L, Lazo J, Parker K, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11 edition. McGraw Hill: New York; 2006. pp. 1405–1432. [Google Scholar]
- 43.Aliabadi AZ, Zuckermann AO, Grimm M. Immunosuppressive therapy in older cardiac transplant patients. Drugs Aging. 2007;24:913–932. doi: 10.2165/00002512-200724110-00004. [DOI] [PubMed] [Google Scholar]
- 44.Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 45.Noguchi H, Matsushita M, Okitsu T, et al. A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat Med. 2004;10:305–309. doi: 10.1038/nm994. [DOI] [PubMed] [Google Scholar]
- 46.Siman R, Reaume AG, Savage MJ, et al. Presenilin-1 P264L knock-in mutation: differential effects on abeta production, amyloid deposition, and neuronal vulnerability. J Neurosci. 2000;20:8717–8726. doi: 10.1523/JNEUROSCI.20-23-08717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy MP, Beckett TL, Ding Q, et al. Abeta solubility and deposition during AD progression and in APPxPS-1 knock-in mice. Neurobiol Dis. 2007;27:301–311. doi: 10.1016/j.nbd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 48.DiCarlo G, Wilcock D, Henderson D, Gordon M, Morgan D. Intrahippocampal LPS injections reduce Abeta load in APP+PS1 transgenic mice. Neurobiol Aging. 2001;22:1007–1012. doi: 10.1016/s0197-4580(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 49.Shaftel SS, Griffin WS, O'Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]