Abstract
In the ANRS CO8 APROCO-COPILOTE cohort of patients treated with combination antiretroviral therapy since 1997–1999, the incidence density of bone fractures was 3.3 for 1,000 patient-years (95% CI: 2.0–4.6). Rate was 2.9-fold (95% CI: 1.3–6.5) higher among patients with excessive alcohol consumption and 3.6-fold (95% CI: 1.6–8.1) higher in those with Hepatitis C virus (HCV) co-infection. Specific monitoring of HCV/HIV-coinfected patients and active promotion of alcohol cessation should be recommended for the prevention of bone fractures.
Keywords: fractures, HIV, alcohol, protease inhibitor, hepatitis
Among emergent co-morbidities in the era of combination antiretroviral therapy (cART), bone complications such as osteonecrosis or osteoporosis are estimated to be more frequent in HIV infected treated patients than in the general population [1, 2], Many traditional determinants such as smoking, alcohol consumption, low body mass index, sedentary lifestyle, digestive, renal and endocrine disorders can be identified in this population, while the role of HIV infection and antiretroviral treatment remains controversial [1, 3–6]. As regards to the prevalence of usual risk factors in HIV-infected patients, incidence rate of bone fractures in those patients could increase over time [7]. However, there are little available data to quantify the risk in this population of patients. Our study estimated the incidence of bone fractures over a follow-up of 10 years in a national cohort of HIV1-infected patients on cART, described the type of fractures, and assessed potential determinants.
A total of 1,281 HIV1-infected adults were included into the ANRS CO8 APROCO-COPILOTE cohort when they were first prescribed a cART including a protease inhibitor, in 47 French centres between March 1997 and June 1999. Follow-up visits were performed one and four months after inclusion, then every four months. At each visit, a clinical examination and laboratory tests were performed; alcohol and tobacco consumption was assessed by a self-questionnaire administered to the patient. Severe and serious adverse events (SAE) were prospectively reported to the coordinating centre and reviewed by an Events Validation Committee [8].
All grade 3 or 4 fractures, i.e. leading to severe or complete limitation of activity and/or to patient’s hospitalization during follow-up, were included into the analysis. Incidence density was estimated by dividing the number of patients who underwent a first bone fracture by the period at risk for all included patients expressed in patient-years. Potential risk factors for fracture were assessed using a Poisson regression model. Between March 1997 and August 2007, a total of 27 fractures were recorded in 26 patients during a median follow-up of 7.1 years (interquartile range: 3.8–9.0). The incidence of a first bone fracture was 3.3 per 1,000 patient-years (95% confidence interval [CI]: 2.0 to 4.6) (Figure 1). In most cases (81%), an acute traumatism (eg, fall, mugging, skiing or other accident, blow) was reported as having caused a fracture. In 19% of patients, fractures occurred during acute alcoholization. Fourteen fractures were located in lower limbs: ankle in 4 patients (bi-malleolar in 3 patients; malleolar in 1 patient), femur in 7 patients (secondary to a femoral head osteonecrosis in one case), rotula, tibia, and foot in 1 patient, each; 5 fractures involved upper limbs: wrist, hand, elbow, clavicle and shoulder, in 1 patient each, fractured pelvis in 3 patients, vertebrae in 3 patients, and multiple fractured sites in 1 patient. Among the 26 patients who experienced bone fracture, osteoporosis was present in four of the five patients who had available osteodensitometry results. Two cases (located in foot and pelvis in 1 patient each) were considered by the physician in charge of the patient as possibly related to antiretroviral (d4T in one case and d4T/3TC/Nevirapine/Nelfinavir in the other case). Patients who experienced at least one fracture did not differ from others for age (median: 36.2 years overall), gender (77.2% male overall), AIDS (20.4% overall), baseline plasma HIV RNA (median: 4.5 log10 copies/mL overall), body mass index (median: 22.0 kg/m2 overall), birth place (83.1% France or Europe overall) and type of first protease inhibitor used (41% indinavir overall). Patients who experienced a bone fracture were more frequently excessive alcohol drinkers than patients who did not (44% with ≥ 5 glasses per day versus 19.5%), were more frequently co-infected with HCV (48% versus 24.5%) and had a lower baseline level of CD4 cell count (194 cells/mm3 versus 277 cells/mm3). In the multivariate analysis, the incidence rate of bone fracture was 2.9-fold (95% CI: 1.3–6.5) higher in patients with excessive alcohol consumption and 3.6-fold (95% CI: 1.6–8.1) higher in HCV co-infected patients than in others.
Figure 1.
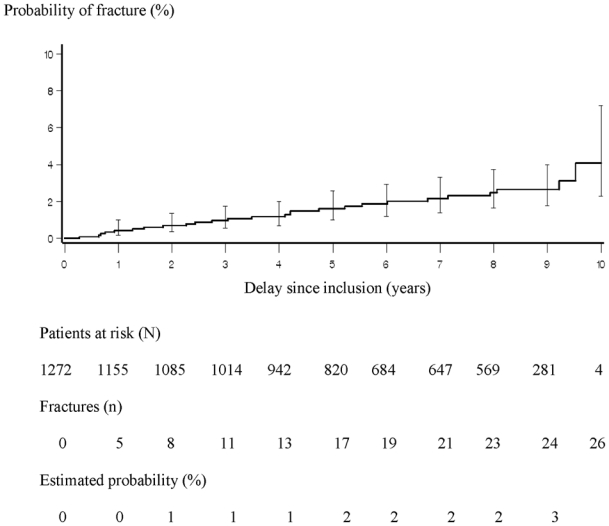
Cumulated probability of a first fracture estimated by the Kaplan-Meier method, ANRS CO8 APROCO-COPILOTE cohort (1997–2007).
Incidence rate of bone fractures estimated in our study population is in the same range as the one reported in the general population in Europe for the same age group, i.e 5 to 10 for 1,000 PY [9]. However, we acknowledge that our estimation might be slightly underestimated since only serious and severe adverse events were reported and monitored in our cohort and some fractures may have been treated on an outpatient basis.
Due to variable treatment exposure and frequent treatment modifications, we could not assess the role of a specific antiretroviral class or drug. In the context of a low number of fractures observed during this long follow-up, this study showed no association between specific markers of HIV disease (i.e HIV RNA, CD4 cell count, clinical stage or antiretroviral treatment) and the rate of bone fractures among our population. Alcohol consumption is a traditional determinant as it is known to increase both osteoporosis and risky behaviours and is, especially, higher in HCV/HIV-coinfected patients. Other studies have suggested that fractures and osteoporosis are more frequent in patients with chronic liver disease than in others, since patients with cirrhosis experience a decreased bone mass [10]. This decreased bone density might be due, in part, to vitamin D deficiency linked to cholestasis and/or hypogonadism.
Investigations in this field are to be carried on in a much larger number of patients and over extended follow-up periods. Although there is a lack of studies of specific drug treatment for prevention of bone fractures in HIV-infected patients, current HIV guidelines recommend the same preventive measures as in the general population, stressing the importance of non medicinal preventive measures. Meanwhile, specific information, monitoring of HCV/HIV-coinfected patients and active promotion of alcohol cessation programs in HIV-infected patients are to be recommended for the prevention of bone fractures.
Acknowledgments
We would like to thank Audrey Taïeb for advice regarding the statistical analyses, Catherine Barennes for her assistance and the patients for their participation.
F. Collin and G. Chene had full access to the data and take responsibility for the integrity, accuracy and analyses of the data. F. Collin contributed to the study concept and design, statistical analysis and interpretation of data, administrative, technical or material support, drafted and revised the manuscript. G. Chene and F. Raffi contributed to the study concept, design and supervision, interpretation of data, critical revision of the manuscript for important intellectual content, funding collection and administrative, technical or material support. X. Duval, V. Le Moing, L. Piroth, P. Massip, F. Raffi contributed to the acquisition of the data, critical revision of the manuscript for important intellectual content and administrative, technical or material support. F. Al Kaied and V. Villes contributed to the interpretation of the data, critical revision of the manuscript for important intellectual content and administrative, technical or material support.
Financial support: Collège des Universitaires de Maladies Infectieuses et Tropicales (CMIT ex APPIT), Sidaction Ensemble contre le Sida, and the following pharmaceutical companies: Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Glaxo- SmithKline, Gilead Sciences, Pfizer, Roche.
Appendix: ANRS CO8 APROCO-COPILOTE study group
Scientific Committee
Steering committee: investigator coordinators: C. Leport, F. Raffi, Methodology coordinators: G. Chêne, R. Salamon, Social sciences coordinators: J-P. Moatti, J. Pierret, B. Spire
Virology coordinators: F. Brun-Vézinet, H. Fleury, B. Masquelier, Pharmacology coordinators: G. Peytavin, R. Garraffo
Other members: D. Costagliola, P. Dellamonica, C. Katlama, L. Meyer, D. Salmon, A. Sobel.
Validation Committee for events: L. Cuzin, M. Dupon, X. Duval, V. Le Moing, B. Marchou, T. May, P. Morlat, C. Rabaud, A. Waldner-Combernoux
Project coordinator: F. Collin-Filleul
ANRS representatives: Nadine Job-Spira, Marcia Trumeau
Observers: C. Perronne.
Coordinator of clinical research group: V. Le Moing, C. Lewden
Investigating centres: Amiens (Pr JL. Schmit), Angers (Dr JM. Chennebault), Belfort (Dr JP. Faller), Besançon (Pr JL. Dupond, Dr JM. Estavoyer, Dr Drobachef), Bobigny (Pr O. Bouchaud), Bordeaux (Pr M. Dupon, Pr Longy-Boursier, Pr P. Morlat, Pr JM. Ragnaud), Bourg-en-Bresse (Dr P. Granier), Brest (Pr M. Garré), Caen (Pr R. Verdon), Compiègne (Dr D. Merrien), Corbeil Essonnes (Dr A. Devidas), Créteil (Pr A. Sobel), Dijon (Pr H. Portier), Garches (Pr C. Perronne), Lagny (Dr P. Lagarde), Libourne (Dr J. Ceccaldi), Lyon (Pr D. Peyramond), Meaux (Dr C. Allard), Montpellier (Pr J. Reynes), Nancy (Pr T. May), Nantes (Pr F. Raffi), Nice (Pr JG Fuzibet, Pr P. Dellamonica), Orléans (Dr P. Arsac), Paris (Pr E. Bouvet, Pr F. Bricaire, Pr P. Bergmann, Pr J. Cabane, Dr J. Monsonego, Pr P.M. Girard, Pr L. Guillevin, Pr S. Herson, Pr C. Leport, Pr MC. Meyohas, Pr J.M. Molina, Pr G. Pialoux, Pr D. Salmon), Poitiers (Pr B. Becq-Giraudon), Reims (Pr R. Jaussaud), Rennes (Pr C. Michelet), Saint-Etienne (Pr F. Lucht), Saint-Mandé (Pr T. Debord), Strasbourg (Pr JM Lang), Toulon (Dr JP. De Jaureguiberry), Toulouse (Pr B. Marchou), Tours (Pr JM. Besnier).
Monitoring and biostatistics: J. Biemar, S. Boucherit, AD Bouhnik, C. Brunet-François, M.P. Carrieri, F. Couturier, J.L. Ecobichon, V. Guiyedi, P. Kurkdji, S. Martiren, M. Préau, C. Protopopescu, C. Roy, J. Surzyn, A. Taieb, V. Villes, C. Wallet.
Sponsor: Agence Nationale de Recherches sur le Sida et les hépatites virales (ANRS, Action Coordonnée n°7).
Footnotes
Note: Data presented previously at the “9èmes Journées Nationales d’Infectiologie, Marseille - France – 4 au 6 juin 2008 » and «2ème Conférence Francophone d’Epidémiologie Clinique, Nancy – France – 22 et 23 mai 2008 »
Conflict of interest statement
Drs F. Collin, X. Duval, V. Le Moing, L. Piroth, F. Al Kaied, Patrice Massip, V. Villes, G. Chêne and F. Raffi have not commercial or other associations that might pose a conflict of interest.
References
- 1.Tebas P, Powderly WG, Claxton S, Marin D, Tantisiriwat W, Teitelbaum SL, et al. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. Aids. 2000;14(4):F63–7. doi: 10.1097/00002030-200003100-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Negredo E, Martinez E, Cinquegrana D, Estany C, Clotet B. Therapeutic management of bone demineralization in the HIV-infected population. Aids. 2007;21(6):657–63. doi: 10.1097/QAD.0b013e3280142191. [DOI] [PubMed] [Google Scholar]
- 3.Thomas J, Doherty SM. HIV infection—a risk factor for osteoporosis. J Acquir Immune Defic Syndr. 2003;33(3):281–91. doi: 10.1097/00126334-200307010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Bruera D, Luna N, David DO, Bergoglio LM, Zamudio J. Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. Aids. 2003;17(13):1917–23. doi: 10.1097/00002030-200309050-00010. [DOI] [PubMed] [Google Scholar]
- 5.Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Lo Y, Klein RS. Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. Aids. 2007;21(5):617–23. doi: 10.1097/QAD.0b013e3280148c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cazanave C, Dupon M, Lavignolle-Aurillac V, Barthe N, Lawson-Ayayi S, Mehsen N, et al. Reduced bone mineral density in HIV-infected patients: prevalence and associated factors. AIDS. 2008 Jan 30;22(3):395–402. doi: 10.1097/QAD.0b013e3282f423dd. [DOI] [PubMed] [Google Scholar]
- 7.Amorosa V, Tebas P. Bone disease and HIV infection. Clin Infect Dis. 2006;42(1):108–14. doi: 10.1086/498511. [DOI] [PubMed] [Google Scholar]
- 8.Duval X, Journot V, Leport C, Chêne G, Dupon M, Cuzin L, et al. Incidence of and risk factors for adverse drug reactions in a prospective cohort of HIV-infected adults initiating protease inhibitor-containing therapy. Clin Infect Dis. 2004;39(2):248–55. doi: 10.1086/422141. [DOI] [PubMed] [Google Scholar]
- 9.Van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29(6):517–22. doi: 10.1016/s8756-3282(01)00614-7. [DOI] [PubMed] [Google Scholar]
- 10.Collier J. Bone disorders in chronic liver disease. Hepatology. 2007;46(4):1271–8. doi: 10.1002/hep.21852. [DOI] [PubMed] [Google Scholar]


