Abstract
Evidence has accumulated to suggest that individuals with schizophrenia are at increased risk for violent offending. Furthermore, converging evidence suggests that abnormalities in the fronto-limbic system, including the prefrontal cortex, hippocampus, and the parahippocampal gyrus, may contribute towards both neuropsychological disturbances in schizophrenia and violent behavior. Since the behavioral and clinical consequences of disturbed fronto-limbic circuitry appear to differ in schizophrenia and violence, it may be argued that patients with schizophrenia who exhibit violent behavior would demonstrate different structural abnormalities compared to their non-violent counterparts. However, the neurobiological basis underlying homicide offenders with schizophrenia remains unclear and little is known regarding the cross-cultural applicability of the findings. Using a 2 × 2 factorial design on a total Chinese sample of 92 males and females, we found reduced gray matter volume in the hippocampus and parahippocampal gyrus in murderers with schizophrenia, in the parahippocampal gyrus in murderers without schizophrenia, and in the prefrontal cortex in non-violent schizophrenia compared to normal controls. Results provide initial evidence demonstrating cross-cultural generalizability of prior fronto-limbic findings on violent schizophrenia. Future studies examining subtle morphological changes in frontal and limbic structures in association with clinical and behavioral characteristics may help further clarify the neurobiological basis of violent behavior.
Keywords: MRI, homicide offenders, gray matter, schizophrenia, prefrontal cortex, limbic structures
1. Introduction
Evidence has accumulated to suggest that individuals with schizophrenia are at increased risk for committing violent offenses and disproportionately over-represented amongst homicide offenders compared to the general population (Hodgins, 2008; Naudts and Hodgins, 2006). Behaviors characterizing schizophrenia can be marked by a lack of impulse control, poor planning and executing, and aggressive tendencies, thus it is of crucial importance to understand the risk factors for violent behavior in patients with schizophrenia. Furthermore, violent individuals with schizophrenia have been found to be distinguishable from non-violent schizophrenia and normal controls in their performance on neuropsychological tasks and numbers of neurological soft signs (Naudts and Hodgins, 2006; Schug and Raine, 2009), which suggests that neuropathological predispositions contribute towards violent behavior in schizophrenia.
Although many different brain systems have been implicated in schizophrenia, converging evidence suggests that abnormalities in the fronto-limbic system, including the prefrontal cortex, hippocampus, and the parahippocampal gyrus, may contribute towards neuropsychological disturbances in the disorder (Antonova et al., 2004; Harrison et al, 2004). Specifically, prefrontal deficits may lead to executive dysfunction and poor decision-making, whereas hippocampal/ parahippocampal deficits have been linked to memory impairments and affective dysregulation. The frontal-limbic circuit, in particular its role in emotion regulation, has also been implicated in the neuropathology of violence (Schug et al., 2009; Davidson et al., 2000). Therefore, it may be argued that patients with schizophrenia who exhibit violent behavior would demonstrate structural abnormalities that differ from their non-violent counterparts. Despite the supporting evidence provided by several structural brain imaging studies examining violent schizophrenia (Barkataki et al., 2006; Narayan et al., 2007; Puri et al., 2008; Kumari et al., 2009; see Naudts and Hodgins, 2006 for review), the neurobiological basis underlying homicide offenders with schizophrenia remains unclear and little is known regarding the cross-cultural applicability of these findings.
In this study, we employed a 2 × 2 factorial design on structural magnetic resonance imaging data collected on murderers with schizophrenia, murderers without schizophrenia, non-violent patients with schizophrenia, and normal controls in Nanjing, China. This design allowed the examination of separate effects of diagnosis and homicide on regional gray matter volumes in the frontal-limbic circuit, as well as the interaction between the two. It was hypothesized that murderers with schizophrenia would show structural deficits that differ from those observed in murderers without schizophrenia and non-violent patients with schizophrenia compared to normal controls.
2. Methods
2.1. Participants
The structural magnetic resonance imaging (sMRI) data of 22 murderers with schizophrenia, 18 murderers without schizophrenia, 19 non-violent patients with schizophrenia, and 33 normal controls collected at Nanjing Brain Hospital in Nanjing, China were examined. Murderers were detainees accused of homicide who were undergoing forensic psychiatric evaluation, whereas non-violent schizophrenia patients were hospital inpatients. Normal controls were community members, cleared for any history of mental illness. For all participants, diagnostic interviews were conducted by two independent psychiatrists, who had no knowledge of the group membership of the subjects, at Nanjing Brain Hospital to assess the lifetime presence of Axis I and Axis II psychopathology using the Chinese Classification of Mental Disorder Version 3 (Chinese Society of Psychiatry, 2001) and the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychological Association, 1994). A schizophrenia diagnosis was confirmed by consensus as determined by both CCMD-3 and DSM-IV. All participants were free of lifetime and current substance abuse/ dependence. Information concerning the history of hospitalization for head injuries and the socioeconomic status of the subject (determined based on Hollingshead Four-Factor Index of Social Status; Hollingshead, 1975) were also collected during diagnostic interviews (Raine et al., 2000) and listed in Table 1. Additionally, full scale IQ was measured using the Wechsler Adults Intelligence Scale: Revised in China (WAIS-RC; Gong, 1992) by prorating four subtests – two from the Verbal Scale (Similarities, Arithmetic), and two from the Performance Scale (Picture Completion, Digit Symbol Coding). Raw subtest scores were converted to scaled scores based upon the areas in which participants reported being raised (i.e. city or country). Written informed consent was obtained from all subjects and procedures approved by the IRB at the University of Southern California.
Table 1.
Demographic measures, cognitive measures, physical measures and current medication use of the groups.
| Schizophrenia Murderers (n = 22) |
Murderers (n = 18) |
Non-violent Schizophrenia (n = 19) |
Normal Controls (n = 32) |
Statistics | |||||
|---|---|---|---|---|---|---|---|---|---|
| Demographic Measures | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age | 34.68 | 13.0 | 31.39 | 12.89 | 33.11 | 10.09 | 32.03 | 9.89 | F (3,88) = 0.35, p = 0.79 |
| Socioeconomic status | 61.7 | 17.55 | 57.92 | 21.33 | 49.56 | 18.93 | 55.59 | 20.44 | F (3,88) = 1.25, p = 0.30 |
| Gender (Male/Female) | 3 / 19 | 2 / 16 | 3 / 16 | 4 / 28 | χ2 (3,88) = 0.20, p = 0.98 | ||||
| Cognitive Measures | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Full scale IQ | 84.9 | 14.38 | 80.1 | 14.9 | 86.6 | 17.7 | 101.5 | 15.1 | F (3,88) = 9.09, p < 0.001 |
| (IQ range) | (78.2 – 91.6) | (72.2 – 88.0) | (78.1 – 95.2) | (96.1 – 107.0) | |||||
| Education (Years) | 7.32 | 4.22 | 9.00 | 5.45 | 11.63 | 2.97 | 8.83 | 4.26 | F (3,88) = 3.51, p = 0.019 |
| Physical Measures | |||||||||
| Taken to hospital for head injury (Yes/ no) |
3 / 19 | 3 / 15 | 0 / 19 | 5/ 27 | χ2 (3,88) = 3.84, p = 0.28 | ||||
| Whole brain volume (cm3) | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| 1235.14 | 109.12 | 1255.42 | 110.6 | 1274.55 | 113.9 | 1264.39 | 106.16 | F (3,88) = 0.49, p = 0.69 | |
| Medications | |||||||||
| Currently on anti- psychotic medications (Yes/ no) |
3/ 19 | 0/ 18 | 19/ 0 | 0/ 32 | χ2 (3,88) = 76.87, p < 0.001 | ||||
2.2. Imaging Procedures
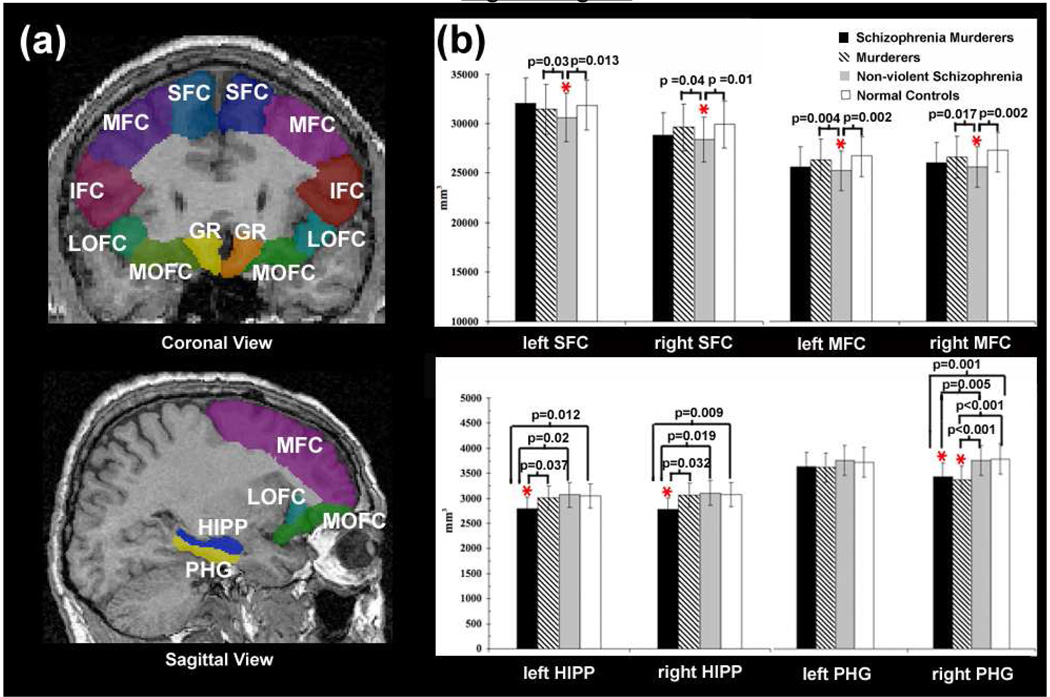
For all participants, sMRI data was collected on a 1.5T GE Signa scanner using a single-shot gradient echo MPRAGE sequence (TR = 25 ms, TE = 6ms, field of view = 24 cm, matrix = 256 × 256, flip angle = 45°, thickness = 1.2 mm, 124 continuous sagittal slices without gap). Before the segmentation, several pre-processing steps were applied to the data including correction for magnetic field inhomogeneity artifacts and head tilt, alignment and transformation of images into a common stereotaxic space without scaling, and automated tissue classification using a partial volume classifier method (Yang et al., 2009). A previously validated automated segmentation program (Tu et al., 2008) was then employed to delineate the prefrontal and limbic regions of interest (ROIs). In brief, a hybrid model using a general learning theory, auto-context, was applied to the images. By combining short-range and long-range appearance features and shape information as well as high level spatial configuration of different anatomical structures, 8 ROIs were segmented based on the reference image of a probabilistic atlas (http://www.loni.ucla.edu/Atlases/LPBA40) as follows: superior frontal gyrus (SFG), middle frontal gyrus (MFG), inferior frontal gyrus (IFG), middle orbitofrontal gyrus (mOFG), lateral orbitofrontal gyrus (lOFG), gyrus rectus (GR), parahippocampal gyrus (PHG), and hippocampus (HIPP) (Figure 1). The mOFG here consisted of the inferior aspect of the middle frontal gyrus. Its anterior boundary is approximately where the MFG curves above the eye sockets and the posterior boundary is where the MFG meets the temporal pole and where the H-shaped orbital sulcus ends. Laterally, the mOFG is bound by the transverse orbital sulcus and medially by the medial segment of the H-shaped orbital sulcus. The lOFG here consisted of the inferior aspect of the IFG. Its superior boundary is the lateral orbital sulcus and its posterior boundary is where the IFG meets temporal pole. Its medial boundary is the lateral segment of the H-shaped orbital sulcus. The parcellated ROIs were then combined with tissue-classified brain volumes (Yang et al., 2007) to estimate left and right ROI gray matter volumes for each individual.
Figure 1.
(a) Illustrations of the segmentation of the frontal cortex of one subject on the coronal (top) and sagittal view (bottom). SFC: superior frontal cortex, MFC: middle frontal cortex, IFC: inferior frontal cortex, lOFC: lateral orbitofrontal cortex, mOFC: medial orbitofrontal cortex, GR: gyrus rectus, HIPP: hippocampus, PHG: parahippocampal gyrus. (b) Gray matter volumes of the left and right SFC and MFC (top) and HIPP and PHG (bottom) in schizophrenia murderers, murderers, non-violent schizophrenia patients, and normal controls. P values indicate significant group comparisons while controlling for whole brain volume. The vertical lines represent the standard error bars.
2.3. Data Analyses
All statistical analyses were conducted using SPSS (SPSS Inc, Chicago, Il). Chi-square analyses were used to examine group differences in categorical variables for demographic and clinical measures whereas one-way analyses of variances (ANOVAs) were used to examine continuous variables. Variables that differed significantly between groups were then included in the analyses on regional brain volumes to examine the cumulative effect of these potential confounds. Differences in gray matter volumes were analyzed using Repeated Measure Analyses of Covariances (ANCOVAs) to examine the main and interaction effects of homicide (i.e., murderers/ non-murderers) by diagnosis (i.e. schizophrenia/ non-schizophrenia) by ROI (i.e., SFG/ MFG/ IFG/ lOFG, mOFG/ RG/ HIPP/ PHG) by hemisphere (i.e., left/ right). Specifically, left and right gray matter volumes of the 8 ROIs were entered as dependent variables with hemisphere as a within subjects factor, and the 2 group variables (i.e., homicide and diagnosis) were entered as between-subject factors, while controlling for whole brain volumes, as well as any potential demographic and clinical confounds. If significant main/ interaction effects were found, follow-up lower-order ANOVAs for individual group pair-wise comparison were conducted to examine differences between each group separately. The test of Wilks’ Lambda was used to obtain the probability (p) value, Fisher-Snedecor distribution (F) value, and observed power. Significance was established based on a two-tailed α level of .05 for all tests.
3. Results
Groups did not differ in age, gender, whole brain volume and head injury (all ps > .08), but differed significantly in Full Scale IQ, anti-psychotic medications and years of education, (all ps ≤ 0.001) (Table 1). Specifically, all non-violent patients with schizophrenia were on anti-psychotic medication at the time of the testing (risperidone: n = 6, clozapine: n = 4, other anti-schizophrenic medication: n = 9) whereas only 3 out of 22 murderers with schizophrenia were on anti-psychotic medication (chlorpromazine: n = 1, other anti-schizophrenic medication: n = 1, traditional Chinese medication: =1). n Findings also demonstrated that murderers with schizophrenia, murderers, and non-violent patients with schizophrenia showed lower Full Scale IQ compared to normal controls (all ps < 0.001), but the three groups did not differ from each other (all ps > 0.22). These variables were included as covariates in the statistical analyses to examine the cumulative effects of these potential confounds.
The Repeated Measure ANCOVAs showed a main effect of homicide by diagnosis by ROI by hemisphere while controlling for whole brain volume, IQ, education, and anti-psychotic medication (F (7, 67) = 2.22, p = 0.041, observed power = 0.80). In addition, significant interaction effects were observed for diagnosis by ROI (F (7, 67) = 2.17, p = 0.046; observed power = 0.79) and homicide by ROI by hemisphere (F (7, 67) = 2.30, p = 0.035, observed power = 0.81), while controlling for whole brain volume, current anti-psychotic medication, IQ and education. Follow-up ANOVAs showed murderers with schizophrenia exhibited significant gray matter volume reductions in the right and left hippocampus compared to murderers without schizophrenia, non-violent schizophrenia, and normal controls (all ps < 0.037, Figure 1 and Table 2). After additionally controlling for whole brain volume, IQ, anti-psychotic medication, and education, results remained significant for right hippocampus (all ps < 0.014) but not left (all ps > 0.07). In addition, both murderers with and without schizophrenia were found to show reduced gray matter volume in the right parahippocampus gyrus compared to non-violent schizophrenia and normal controls (all ps < 0.005, Figure 1 and Table 2), where results remained significant after controlling for whole brain volumes, anti-psychotic medications, IQ and education (all ps < 0.008). Non-violent schizophrenia patients were found to show significant gray matter reduction in the bilateral SFC and MFC compared to murderers without schizophrenia and normal controls (all ps < 0.04, Figure 1 and Table 2), however only findings for bilateral MFC remained significant after controlling for whole brain volumes, IQ, anti-psychotic medication, and education (all ps < 0.028). No significant group difference was observed for gray matter volumes of other ROIs (see Table 2).
Table 2.
The probability (p) values for pair-wise group comparisons conducted for gray matter volumes of each ROI, while controlling for whole brain volume.
| Schizophrenia Murderers vs Murderers |
Schizophrenia Murderers vs Non-violent Schizophrenia |
Schizophrenia Murderers vs Normal Controls |
Non-violent Schizophrenia vs Murderers |
Murderers vs Normal Controls |
Non-violent Schizophrenia vs Normal Controls |
Statistics | ||
|---|---|---|---|---|---|---|---|---|
| p | p | p | p | p | p | p (observed power) | ||
| SFG | Left | 0.14 | 0.43 | 0.12 | 0.03 | 0.90 | 0.01 | 0.054 (0.63) |
| Right | 0.33 | 0.22 | 0.42 | 0.04 | 0.88 | 0.01 | 0.071 (0.59) | |
| MFG | Left | 0.25 | 0.07 | 0.29 | 0.004 | 0.82 | 0.002 | 0.013 (0.80) |
| Right | 0.48 | 0.09 | 0.31 | 0.02 | 0.84 | 0.002 | 0.035 (0.69) | |
| IFG | Left | 0.74 | 0.29 | 0.58 | 0.18 | 0.87 | 0.09 | 0.37 (0.28) |
| Right | 0.69 | 0.33 | 0.35 | 0.19 | 0.65 | 0.05 | 0.26 (0.35) | |
| IOFG | Left | 0.53 | 0.46 | 0.10 | 0.92 | 0.37 | 0.43 | 0.53 (0.20) |
| Right | 0.64 | 0.53 | 0.24 | 0.87 | 0.54 | 0.66 | 0.32 (0.31) | |
| mOFG | Left | 0.19 | 0.51 | 0.88 | 0.52 | 0.20 | 0.56 | 0.42 (0.25) |
| Right | 0.70 | 0.18 | 0.72 | 0.35 | 0.45 | 0.07 | 0.70 (0.14) | |
| GR | Left | 0.39 | 0.42 | 0.45 | 0.96 | 0.83 | 0.87 | 0.80 (0.11) |
| Right | 0.20 | 0.24 | 0.44 | 0.91 | 0.52 | 0.60 | 0.55 (0.19) | |
| HIPP | Left | 0.04 | 0.02 | 0.012 | 0.81 | 0.68 | 0.89 | 0.033 (0.70) |
| Right | 0.03 | 0.02 | 0.009 | 0.78 | 0.66 | 0.90 | 0.018 (0.77) | |
| PHG | Left | 0.68 | 0.77 | 0.82 | 0.50 | 0.51 | 0.93 | 0.90 (0.08) |
| Right | 0.34 | 0.005 | 0.001 | < 0.001 | < 0.001 | 0.58 | < 0.001 (0.99) |
4. Discussion
Findings support the hypothesis that gray matter volume deficits in hippocampal and parahippocampal regions may predispose to violent behavior. In humans, the hippocampus and surrounding parahippocampal gyrus are critical components of a behavioral inhibition mechanism through which information processing for impulse control, emotion regulation, and moral reasoning is modulated (Gray and McNaughton, 2000). Furthermore, across species, the associated cortical structures such as the prefrontal cortex funnel information through the parahippocampal regions to the hippocampus (Eichenbaum and Lipton, 2008). This hierarchy of connectivity highlights the importance of the parahippocampal gyrus in the behavioral inhibition mechanism. Thus, deficits in the parahippocampal gyrus found in both murderer groups may contribute to poor impulse control and ultimately lead to violence.
Despite the shared neuropathology, murderers with schizophrenia were found to show additional volume reductions in the hippocampus, findings consistent with prior reports on violent schizophrenia (Barkataki et al., 2006; Kumari et al., 2009). Animal studies have demonstrated that lesions to the hippocampus may be linked to changes in social behavior including increased excitability and reduced response to social cues (Machado and Bachevalier, 2006). Therefore, volume reductions in the hippocampus may predispose individuals with schizophrenia to be less sensible to social and emotional signs, which contribute to the generation of conflicts and the inability to recognize signals for solution, leading to conflict escalation. This biological predisposition to violence, when further impaired by additional deficits in the parahippocampal gyrus that disrupts the input from cortical structures for behavioral control, may result in these schizophrenia patients resorting to violent offending during conflict situations.
Reduced prefrontal gray matter volumes found in non-violent schizophrenia are consistent with meta-analysis reviews on neuroimaging findings of schizophrenia patients (Naudts and Hodgins, 2006). Reduced gray matter volume in the DLPFC has been linked to neuropsychological deficits in patients with schizophrenia including poor planning and executing and impaired impulse control (Wright et al., 2000; Weinberger, 1988). Findings also suggest that, although structural deficits to the prefrontal cortex may interrupt the sending of inhibitory inputs to the limbic system (e.g. the hippocampus, parahippocampal gyrus) and may promote aggression, the structural integrity of the limbic system could serve as a protective factor against violent behavior in schizophrenia. In addition, non-violent patients with schizophrenia reported higher percentages of anti-psychotic medication use than their violent counterparts. Previous studies have linked reduced DLPFC gray matter volume with anti-psychotic medication (e.g., Taki et al., 2006), consistent with findings of this study. However, the lack of detailed information on the extent of lifetime exposure to anti-psychotic medication in the current sample prevented further investigating the potential contribution of anti-psychotic medication towards the findings. Furthermore, the possibility remains that subtle and/ or localized morphological alterations (e.g. cortical thickness) may be present in violent schizophrenia patients in the absence of grossvolumetric changes. Future studies examining the morphometric characteristics of the frontal-limbic system in murderers with schizophrenia are needed to confirm this speculation.
Several limitations should be considered while interpreting the findings. First, all non-violent patients with schizophrenia were on one or more antipsychotic medications at the time of the scanning, whereas only 3 of the 19 murderers with schizophrenia were on anti-psychotic medication. Therefore, we cannot fully rule out the confounding effect of anti-psychotic medication on the gray matter volumes, despite the inclusion of current anti-psychotic medication as a covariate in all analyses. Several studies have demonstrated that antipsychotic medication did not influence cortical thickness in both chronic and first-episode schizophrenia patients with little or no prior medication exposure (Kuperberg et al., 2003; Narr et al., 2005a, 2005b; Nesvåg et al., 2008), thus it is unlikely that gray matter volume differences were solely attributable to medication use. However, the information regarding past and current substance abuse was largely self-reported, thus it remains a possibility that substance abuse may contribute to findings of this study. Second, positive correlations have been found between reduced intelligence scores and reduced cerebral gray matter, particularly in the prefrontal regions (Reiss et al. 1996). In our sample, the lower IQ scores of homicidal and/ or schizophrenia individuals may be associated with the reduced gray matter volumes in the prefrontal-limbic circuit found in these individuals. However, it is worth mentioning that full scale IQ was included as a covariate in all analyses and most results remained the same. Also, since schizophrenia is characterized by a generalized cognitive impairment, correcting for IQ may remove variance overlapping with the disease effects of interest arguing against controlling for group differences in IQ. Third, due constraints of the auto-parsing algorithm employed in this study, the amygdala could not be precisely and reliably segmented without human intervention. Thus, this structure, although recognized as an important structure in the fronto-limbic system that has been found to be impaired in several violent and schizophrenia samples, was not examined in this study. Last, the orbitofrontal cortex was segmented into middle and lateral section based on a previously established atlas using the auto-parsing algorithm, thus we were unable to examine each of the neuroanatomically distinct sub-regions of the orbitofrontal cortex. Future studies using other automated or manual segmentation methods could help illuminate the potential contribution of these regions in violent schizophrenia.
Findings of this study demonstrated the presence of frontal-limbic neuroanatomical abnormalities in murderers with and without schizophrenia in China supporting the involvement of the hippocampus and parahippocampal gyrus in violent behavior specifically. Results provide initial evidence demonstrating cross-cultural generalizability of prior findings on violent individuals, particularly those with schizophrenia. Future studies examining subtle morphological changes in the frontal and limbic structures in association with clinical and behavioral characteristics may help further clarify the neurobiological basis of violent behavior.
Acknowledgments
This study was supported by a grant to the first author from the National Institute of Mental Health (1F31MH079592) and a grant to the second author from the National Institute of Child Health and Development (I RO1 HD42259). Research grants from the National Center for Research Resources, the National Institutes of Health through the NIH Roadmap for Medical Research supported contributions of the UCLA co-authors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychological Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Barkataki I, Kumari V, Das M, Taylor P, Sharma T. Volumetric structural brain abnormalities in men with schizophrenia or antisocial personality disorder. Behavioral Brain Research. 2006;169:239–247. doi: 10.1016/j.bbr.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Chinese Society of Psychiatry. The Chinese Classification and Diagnostic Criteria of Mental Disorders Version 3 (CCMD-3) Jinan: Chinese Society of Psychiatry; 2001. [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation – a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Lipton PA. Towards a functional organization of the medial temporal lobe memory system: role of parahippocampal and medial entorhinal cortical areas. Hippocampus. 2008;18:1314–1324. doi: 10.1002/hipo.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullam RS, Dolan MC. Executive function and in-patient violence in forensic patients with schizophrenia. British Journal of Psychiatry. 2008;193:247–253. doi: 10.1192/bjp.bp.107.040345. [DOI] [PubMed] [Google Scholar]
- Gong YX. Manual for the Wechsler Adults Intelligence Scale: Revised in China. 2nd ed. Changsha, Hunan, China: Hunan Medical College; 1992. [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. 2nd ed. New York: Appleton-Century-Crofts; 2000. [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychophamacology (Bel) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Hodgins S. Violent behaviour among people with schizophrenia: a framework for investigations of causes, and effective treatment, and prevention. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363:2505–2518. doi: 10.1098/rstb.2008.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Barkataki I, Goswami S, Flora S, Das M, Taylor P. Dysfunctional, but not functional, impulsivity is associated with a history of seriously violent behavior and reduced orbitofrontal and hippocampal volumes in schizophrenia. Psychiatry Research. 173:39–44. doi: 10.1016/j.pscychresns.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Archive of General Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Narayan VM, Narr KL, Kumari V, Woods RP, Thompson PM, Toga AW, Sharma T. Regional cortical thinning in subjects with violent antisocial personality disorder or schizophrenia. Am J Psychiatry. 2007;164:1418–1427. doi: 10.1176/appi.ajp.2007.06101631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Toga AW, Szeszko P, Thompson PM, Woods RP, Robinson D, Sevy S, Wang Y, Schrock K, Bilder RM. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biological Psychiatry. 2005a;58:32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Szeszko PR, Robinson D, Sevy S, Gunduz-Bruce H, Wang YP, DeLuca H, Thompson PM. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cerebral Cortex. 2005b;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- Naudts K, Hodgins S. Neurobiological correlates of violent behavior among persons with schizophrenia. Schizophrenia Bulletin. 2006;32:562–572. doi: 10.1093/schbul/sbj036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvåg R, Lawyer G, Vernäs K, Fjell AM, Walhovd KB, Frigessi A, Jönsson EG, Agartz I. Regional thinning of the cerebral cortex in schizophrenia: effects of diagnosis, age, and antipsychotic medication. Schizophrenia Research. 2008;98:16–28. doi: 10.1016/j.schres.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Puri BK, Counsell SJ, Saeed N, Bustos MG, Treasaden IH, Bydder GM. Regional grey matter volumetric changes in forensic schizophrenia patients: an MRI study comparing the brains structure of patients who have seriously and violently offended with that of patients who have not. BMC Psychiatry. 2008;8 Suppl 1:S6. doi: 10.1186/1471-244X-8-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Schug RA, Raine A. Comparative meta-analyses of neuropsychological functioning in antisocial schizophrenic persons. Clinical Psychology Review. 2009;29:230–242. doi: 10.1016/j.cpr.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Goto R, Inoue K, Okada K, Ono S, Kawashima R, Fukuda H. Both global gray matter volume and regional gray matter volume negatively correlate with lifetime alcohol intake in non-alcohol-dependent Japanese men: a volumetric analysis and a voxel-based morphometry. Alcoholism, Clinical and Experimental Research. 2006;30:1045–1050. doi: 10.1111/j.1530-0277.2006.00118.x. [DOI] [PubMed] [Google Scholar]
- Tu Z, Narr KL, Dollar P, Dinov I, Thompson PM, Toga AW. Brain anatomical structure segmentation by hybrid discriminative/generative models. IEEE Transactions on Medical Imaging. 2008;27:495–508. doi: 10.1109/TMI.2007.908121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. Schizophrenia and the frontal lobe. Trends in Neuroscience. 1988;11:367–370. doi: 10.1016/0166-2236(88)90060-4. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. American Journal of Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr KL, Colletti P, Toga AW. Abnormal temporal and prefrontal cortical gray matter thinning in psychopaths. Molecular Psychiatry. 2009;14:561–562. doi: 10.1038/mp.2009.12. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr KL, Lencz T, LaCasse L, Colletti P, Toga AW. Localisation of increased prefrontal white matter in pathological liars. British Journal of Psychiatry. 2007;190:174–175. doi: 10.1192/bjp.bp.I06.025056. [DOI] [PMC free article] [PubMed] [Google Scholar]