Abstract
The current study investigated the neural correlates that underlie the processing of ambiguous words and the potential effects of semantic competition on that processing. Participants performed speeded lexical decisions on semantically related and unrelated prime-target pairs presented in the auditory modality. The primes were either ambiguous words (e.g., ball) or unambiguous words (e.g., athlete), and targets were either semantically related to the dominant (i.e., most frequent) meaning of the ambiguous prime word (e.g., soccer) or to the subordinate (i.e., less frequent) meaning (e.g., dance). Results showed increased activation in the bilateral inferior frontal gyrus (IFG) for ambiguous related compared to unambiguous related stimulus pairs, demonstrating that prefrontal areas are activated even in an implicit task where participants are not required to explicitly analyze the semantic content of the stimuli and to make an overt selection of a particular meaning based on this analysis. Additionally, increased activation was found in the left IFG and the left cingulate gyrus for subordinate meaning compared to dominant meaning conditions, suggesting that additional resources are recruited in order to resolve increased competition demands in accessing the subordinate meaning of an ambiguous word.
Keywords: fMRI, homophones, inferior frontal gyrus, prefrontal cortex, semantic ambiguity, semantic competition
Introduction
Semantic ambiguity is an inherent feature of words in many languages, including English. While listening to speech, we constantly encounter ambiguous words, such as the word “bank”; in this case, the same word form has several different meanings, including “a shore of a river” and “a financial institution”. Auditory comprehension requires that the listener be able to process this ambiguity and to select the appropriate meaning. And yet, as listeners, we are able to do so quickly as the linguistic message is unfolding without apparent difficulty and often without being aware that the word is even ambiguous. The goal of this research is to investigate the processing of ambiguous words in the presence of words that were semantically related to one or the other meaning of the ambiguous word in order to examine the effects of semantic competition that arise due to the resolution of ambiguity, and to identify the neural correlates that underlie these processes.
Psycholinguistic evidence suggests that during early stages of processing, all meanings of ambiguous words are initially activated, regardless of the frequency of these meanings or the biasing context in which the ambiguous words are presented (Onifer & Swinney, 1981; Swinney, 1979). The results of these experiments also demonstrate that after all meanings of ambiguous words are activated, a single interpretation is ultimately selected based on meaning frequency and context. Thus, at early stages of processing there is competition between the multiple lexical-semantic representations of ambiguous words and this competition is resolved later in the processing stream.
In recent years, Thompson-Schill and colleagues have proposed that the left inferior frontal gyrus (IFG) is involved in the selection of information among competing alternatives from semantic memory (Kan & Thompson-Schill, 2004; Thompson-Schill et al., 1997, 1999, 2002). In their landmark study, Thompson-Schill and colleagues (Thompson-Schill et al., 1997) investigated the role of the left IFG in selection and semantic retrieval by varying selection demands among semantically competing alternatives in unambiguous words across three different tasks including generation, classification, and comparison. Their results showed left IFG activation for the comparison of high and low selection conditions in all three tasks, with a large region of overlap among the three tasks. There was greater activation in the high selection conditions compared to the low selection conditions suggesting that the left IFG was involved in selection among competing alternatives. All three tasks used in this experiment required the subjects to make an overt selection from an array of alternatives. Such a process requires the subjects to consider competing alternatives and consciously choose one of them. Given these findings, accessing the meanings of ambiguous words should invoke left IFG activation. What is less clear is whether such activation will emerge for ambiguous words even when overt selection is not required. One of the aims of the current study is to investigate this issue.
Several neuroimaging experiments have investigated the neural systems underlying the processing of ambiguous words (Chan et al., 2004; Copland et al., 2003, 2007; Mason & Just, 2007; Rodd et al., 2005; Zempleni et al., 2007), and while some studies have shown increased activation in the left IFG during processing of ambiguous words (Mason & Just, 2007; Rodd et al., 2005; Zempleni et al., 2007), others have not (Chan et al., 2004; Copland et al., 2003), leading to some uncertainty as to the role of the left IFG in the processing of ambiguity.
In one of the first neuroimaging studies to focus on the processing of ambiguous words, Chan and colleagues (Chan et al., 2004) used a word generation task with single word stimuli to investigate ambiguous word processing in an experiment that also included unambiguous words as a control condition. Participants were visually presented with ambiguous and unambiguous words and they were required to silently generate words that were semantically related to the stimuli. Results showed that ambiguous words elicited increased activation in bilateral MFG, superior frontal gyri (SFG) and in the anterior cingulate, whereas unambiguous words elicited increased activation in bilateral IFG, middle temporal gyrus (MTG), and superior temporal gyrus (STG). Thus, the presentation of single ambiguous words failed to show increased activation in the IFG despite the fact that participants were required to ‘select’ and silently produce a word related to one of the meanings of the ambiguous word. The failure to show IFG activation could have been due to several factors. First, it is not clear whether the meanings of the ambiguous words differed in their frequencies or were weighted toward a single highly frequent dominant meaning. In the latter case, selection demands would not have been high reducing the likelihood of IFG activation. Second, because participants silently generated the words, it is not clear what their response choices were. If they consistently selected the highly frequent dominant meaning, competition demands would again have been minimal.
A crucial role of context in invoking left IFG activation in the processing of ambiguity is supported by the results of another study of ambiguous words that employed sentence stimuli (Rodd et al., 2005). Participants in this study heard sentences containing several ambiguous words and matched sentences containing only unambiguous words. In the first experiment, the participants decided whether a target word that followed each sentence was semantically related to the meaning of the sentence, and in the second experiment, the participants simply attended to the sentences. The results showed increased activation in bilateral IFG and left posterior inferior temporal cortex for ambiguous sentences compared to unambiguous sentences for both tasks. These findings suggest that the IFG is recruited for the processing of ambiguous words in the presence of a sentence context. They also suggest that IFG activation is recruited even in the absence of an overt decision or selection of a response by the participant.
The results of Rodd and colleagues (Rodd et al., 2005) were replicated in two recent studies (Mason & Just, 2007; Zempleni et al., 2007). In the study by Zempleni and colleagues (Zempleni et al., 2007), participants silently read sentences containing ambiguous and unambiguous words. The results revealed increased activation in bilateral IFG and bilateral MTG during processing of ambiguous sentence stimuli. In the study by Mason and Just (Mason & Just, 2007), participants silently read sentence stimuli and answered yes/no comprehension questions that followed each sentence. The results of this study also showed increased activation in the left IFG during processing of ambiguous stimuli compared to unambiguous stimuli. This combined evidence supports the view that ambiguous word processing in sentence contexts recruits the IFG.
Taken together, existing fMRI evidence suggests that the presence or absence of a disambiguating context plays a role in whether the IFG is recruited in ambiguous word processing. While the IFG may not be necessary for processing of single ambiguous words (Chan et al., 2004), it is recruited in the processing of sentences containing ambiguous words (Mason & Just, 2007; Rodd et al., 2005; Zempleni et al., 2007). Nonetheless, sentence comprehension is a complex process requiring not only access to individual word meanings, but also the integration of the meanings of words into the syntactic frame of the sentence. It has been shown that integration of meaning into sentence context recruits the left IFG (Baumgaertner et al., 2002; Hagoort et al., 2004). For example, reading sentences with unexpected endings (e.g., “The pilot flies the kite”) elicits increased activation in the left IFG compared to reading sentences with highly expected endings (e.g., “The pilot flies the plane”) (Baumgaertner et al., 2002). Thus, it is uncertain whether the increased activation in the left IFG reported in the studies with ambiguous sentence stimuli (Mason & Just, 2007; Rodd et al., 2005; Zempleni et al., 2007) is due to the competition among alternative meanings of ambiguous words or whether it is the result of increased resources required for integrating ambiguous word meanings into the larger sentence context. If increased activation in the IFG is due to the competition inherent in accessing the meaning of ambiguous words and not due to increased demands on integrating meanings into the sentence context, then increased IFG activation should emerge for ambiguous words presented in the absence of sentence context, but in the presence of disambiguating cues such as single words semantically related to one of the meanings of the ambiguous words.
Most ambiguous words have a dominant (i.e., more frequent) meaning and a subordinate (i.e., less frequent) meaning. For example, for the ambiguous word “bank”, the meaning “a financial institution” is dominant compared to the meaning “a shore of a river”, because it is more frequently used in the former interpretation. It has been shown that the subordinate meaning requires more time to achieve its activation threshold compared to the dominant meaning, and that differences in activation have an effect on ambiguous word processing. Behavioral evidence is consistent with this hypothesis and shows that meaning frequency has an effect on processing of ambiguous words. In particular, it takes longer to access the subordinate meaning (Martin et al., 1999), there is a greater magnitude of priming for the dominant than for the subordinate meaning (Burgess & Simpson, 1988; Simpson & Burgess, 1985), and while the presence of a subordinate-biased context is unable to override access to the dominant meaning, the presence of a dominant-biased context is able to override access to the subordinate meaning (Tabossi et al., 1987; Tabossi, 1988; Tabossi & Zardon, 1993).
Results of the above-mentioned studies suggest that when the meanings of ambiguous words compete, the extent of competition among the meanings varies as a function of meaning frequency or dominance. Because subordinate meanings are less frequent, it takes increased processing resources for the subordinate meaning to reach its activation threshold and hence to be accessed. Additionally, it has been proposed that the subordinate meaning may actually never override the dominant meaning (Duffy et al., 1988; Rayner et al., 1994). In this case, when an ambiguous word is presented in a subordinate-biased sentence context, the subordinate meaning ultimately is activated to the same extent as the dominant meaning, resulting in increased competition between these two meanings, and thus requiring greater processing resources to access the subordinate meaning. According to either interpretation, there should be increased activation in the left IFG for the subordinate meaning of ambiguous words compared to the dominant meaning.
Nonetheless, existing fMRI evidence looking at semantic priming of ambiguous words in the visual modality failed to show such an effect (Copland et al., 2003). In particular, although Copland and colleagues (Copland et al., 2003) showed differential activation patterns for dominant related word pairs versus matched unrelated word pairs and subordinate related word pairs versus matched unrelated word pairs with decreased MTG activation for the dominant related comparison and decreased IFG activation for the subordinate related comparison, there was no difference in the neural activation patterns comparing dominant and subordinate pairs directly. As the authors indicate, the failure to show this effect could have been due to a number of factors relating to power, namely, number of subjects, number of events, and/or magnetic field strength.
It is the goal of the current study to investigate the neural correlates that underlie the processing of ambiguous words in the presence of disambiguating single word cues and the potential effects of semantic competition on that processing. The question is whether increased competition inherent in ambiguous words recruits the left IFG even in the absence of overt selection of word meaning. To this end, subjects are asked to perform an auditory lexical decision (LD) task on semantically related and unrelated pairs of stimuli. The resolution of ambiguity to one of the meanings of an ambiguous word should require greater processing resources than resolving the meaning of an unambiguous word. Thus, the processing of a word target semantically related to one of the meanings of a preceding ambiguous prime stimulus will be compared to the processing of the same target preceded by a semantically related unambiguous prime stimulus. The effect of meaning dominance is investigated by comparing the processing of target words that are related to either the dominant or the subordinate meanings of the ambiguous words (e.g., ball – soccer, ball – dance).
The behavioral results should show semantic priming for related stimuli compared to unrelated stimuli, replicating previous studies (Howard et al., 1981; Meyer & Schvaneveldt, 1971; Moss et al., 1995). However, differences should emerge in reaction-time latencies for a target word when it is preceded by an ambiguous word semantically related to one of the meanings of the target word compared to when it is preceded by a semantically related unambiguous word. Similar to the results from psycholinguistic investigations of lexical ambiguity, it is expected that the conditions containing ambiguous words followed by subordinate targets will show a smaller semantic priming effect than the condition with ambiguous words followed by dominant targets (Burgess & Simpson, 1988; Simpson & Burgess, 1985).
The expectations for the fMRI data are that there will be increased left IFG activation associated with conditions of increased competition. These findings should emerge even in an implicit task such as LD where participants are not required to explicitly analyze the semantic content of the stimuli and to make a selection based on this analysis but rather are required to make a decision based on another attribute of the stimulus, namely its lexicality. Thus, it is hypothesized that there will be increased left IFG activation for semantically related word pairs that contain ambiguous primes compared to those that contain unambiguous primes. Additionally, there should be increased left IFG activation for semantically related word pairs which are related to the subordinate meaning of the ambiguous word compared to the dominant meaning, because of the greater processing requirements for accessing the less frequent interpretation. Finally, overall semantic priming effects should replicate previous fMRI findings (Copland et al., 2003; Kotz et al., 2002; Mummery et al., 1999; Rissman et al., 2003; Rossell et al., 2001, 2003). In particular, there should be activation in the left STG and left frontal areas including the IFG and MFG, with more activation associated with semantically unrelated conditions compared to related conditions.
Methods
Participants
Sixteen right-handed native speakers of English (9 women) participated in this experiment. Their age range was 18–21 years, and the mean age was 19.5 years. Handedness was confirmed by the Edinburgh Handedness Inventory (Oldfield, 1971). None of the participants had any history of psychiatric or neurological problems, and none had any ferromagnetic implants or devices in their body. The participants provided informed consent according to the guidelines approved by the Human Subjects Committees of Brown University and Memorial Hospital of Rhode Island. All participants were familiarized with the experimental task and the fMRI procedure prior to performing the experiment in the scanner. The participants received monetary compensation and copies of images of their brain for their involvement in the study.
Stimuli
Twenty-eight ambiguous words served as prime stimuli. The ambiguous words were selected from ambiguity norms with meaning frequency values taken from Twilley and colleagues (Twilley et al., 1994). The average frequency of the dominant meaning was 82%, and the range was 60–98%. Each of the ambiguous words occurred in two semantically related conditions. In one condition, the semantically related target corresponded to the dominant meaning of the ambiguous word (e.g., ball - soccer) and in the other condition, the target corresponded to the subordinate meaning of the ambiguous word (e.g., ball - dance). These two conditions will be referred to as Ambiguous Dominant Related and Ambiguous Subordinate Related, respectively. For each of these semantically related pairs a corresponding semantically unrelated unambiguous pair was created, matched for word frequency (Francis & Kucera, 1982). In this case, the same target as the one used in the related pair was preceded by an unambiguous semantically unrelated word (e.g., item - soccer; item - dance), which was matched in frequency to the ambiguous prime (Francis & Kucera, 1982). These two conditions will be referred to as Unrelated Dominant A and Unrelated Subordinate A, respectively, to reflect the relationship of the targets to the ambiguous words in the related conditions. The letter A indicates that these sets of stimuli were constructed to correspond to the ambiguous word conditions, although the stimuli themselves in these conditions were unambiguous.
Fifty-six unambiguous words also served as prime stimuli in two semantically related conditions. The targets for these conditions were the same as those used in the ambiguous stimulus pairs (e.g., athlete – soccer; music – dance). Half of these semantically related pairs were matched in association strength (Nelson et al., 1998) with the dominant ambiguous word pairs and the other half were matched with the subordinate ambiguous word pairs, as confirmed by nonsignificant t-tests (dominant: t(27) = .766, p = .45; subordinate: t(27) = .053, p = .96). These conditions will henceforth be referred to as Unambiguous Dominant Related and Unambiguous Subordinate Related to reflect the fact that they are matched in association strength with the two dominant and subordinate related conditions; nonetheless, the primes in these two conditions have only one meaning, and so there is technically no meaning dominance. Analogous to the ambiguous word conditions, two corresponding sets of semantically unrelated pairs were constructed using the same targets as in these semantically related conditions (e.g., rabbit – soccer, enter – dance). These conditions will be referred to as Unrelated Dominant U and Unrelated Subordinate U. Once again, the words “dominant” and “subordinate” in this case are used to indicate the relationship of the targets to the ambiguous words, and the letter U is used to show that these sets of stimuli were constructed to correspond to the unambiguous word conditions. In total, there were eight conditions, with 28 pairs of stimuli in each. A full list of all stimulus pairs is provided in the Appendix.
Appendix.
Test stimuli
| Type of Target |
Ambiguous Related |
Unrelated A | Unambiguous Related |
Unrelated U |
|---|---|---|---|---|
| Dominant | affair – love | slowly - love | hug - love | pump - love |
| Subordinate | affair – event | slowly - event | social - event | young - event |
| Dominant | ball - soccer | item - soccer | athlete - soccer | rabbit - soccer |
| Subordinate | ball - dance | item - dance | music - dance | enter - dance |
| Dominant | bowl – dish | blade - dish | tray - dish | realm – dish |
| Subordinate | bowl – alley | blade - alley | lane - alley | rice – alley |
| Dominant | bluff - fake | script - fake | genuine - fake | suitable - fake |
| Subordinate | bluff - cliff | script - cliff | ledge - cliff | pave - cliff |
| Dominant | cane – walk | confide - walk | jog - walk | sieve – walk |
| Subordinate | cane - sugar | confide - sugar | spice - sugar | wool – sugar |
| Dominant | fan - wind | host - wind | storm - wind | beer - wind |
| Subordinate | fan - cheer | host - cheer | yell - cheer | bunk – cheer |
| Dominant | grave – death | tongue - death | tombstone - death | puddle - death |
| Subordinate | grave – danger | tongue - danger | enemy - danger | recently - danger |
| Dominant | hamper - laundry | sever - laundry | detergent - laundry | vigorous - laundry |
| Subordinate | hamper – bother | sever - bother | tease - bother | luggage – bother |
| Dominant | kid - boy | concept - boy | guy - boy | text – boy |
| Subordinate | kid - goat | concept - goat | sheep - goat | red – goat |
| Dominant | mass - large | tooth - large | big - large | nation-large |
| Subordinate | mass - church | tooth - church | catholic - church | compare - church |
| Dominant | mold - fungus | depth - fungus | mushroom - fungus | kidnap - fungus |
| Subordinate | mold - clay | depth - clay | sculpture - clay | glory - clay |
| Dominant | net – tennis | sand - tennis | sport - tennis | slave - tennis |
| Subordinate | net – worth | sand - worth | appraise - worth | smolder - worth |
| Dominant | play – fun | member - fun | pleasure - fun | farmer – fun |
| Subordinate | play - actor | member - actor | singer - actor | illness - actor |
| Dominant | poach - eggs | hive - eggs | omelet - eggs | diaper - eggs |
| Subordinate | poach - kill | hive - kill | gun - kill | size - kill |
| Dominant | pool - water | brother - water | liquid - water | silence - water |
| Subordinate | pool - table | brother - table | dresser - table | cynic - table |
| Dominant | pot - kettle | fate - kettle | steam - kettle | false - kettle |
| Subordinate | pot - smoke | fate - smoke | fog - smoke | jaw - smoke |
| Dominant | present - birthday | effort - birthday | celebrate - birthday | discourage - birthday |
| Subordinate | present – future | effort - future | history - future | receive - future |
| Dominant | relish - ketchup | facet - ketchup | tomato - ketchup | single - ketchup |
| Subordinate | relish - enjoy | facet - enjoy | relax - enjoy | prevail - enjoy |
| Dominant | sage - herb | flaunt - herb | parsley - herb | mildew - herb |
| Subordinate | sage - wise | flaunt - wise | owl – wise | rodent – wise |
| Dominant | sign – stop | horse - stop | pause - stop | tube – stop |
| Subordinate | sign - name | horse - name | title - name | reflect - name |
| Dominant | stable – barn | nerve - barn | loft – barn | joy – barn |
| Subordinate | stable – secure | nerve - secure | protect – secure | liquor - secure |
| Dominant | staple - attach | juggle - attach | connect – attach | planet - attach |
| Subordinate | staple - food | juggle - food | barbecue - food | investor - food |
| Dominant | strain – pressure | path - pressure | tension - pressure | decade - pressure |
| Subordinate | strain – spaghetti | path - spaghetti | garlic - spaghetti | puncture - spaghetti |
| Dominant | strip – remove | lake - remove | detach – remove | acute - remove |
| Subordinate | strip – bacon | lake - bacon | ham – bacon | success - bacon |
| Dominant | stroke - caress | wipe - caress | soothe - caress | dusk - caress |
| Subordinate | stroke – heart | wipe - heart | surgery – heart | disclosure – heart |
| Dominant | tire - car | author - car | engine - car | lawyer - car |
| Subordinate | tire - weary | author - weary | weak - weary | dine - weary |
| Dominant | vessel - boat | sheriff - boat | motor - boat | suffer - boat |
| Subordinate | vessel - blood | sheriff - blood | wound - blood | queen - blood |
| Dominant | wake - morning | scream - morning | evening - morning | language - morning |
| Subordinate | wake - funeral | scream - funeral | burial - funeral | overlap - funeral |
Each ambiguous prime was repeated twice, once in the Ambiguous Dominant Related condition, and once in the Ambiguous Subordinate Related condition. Twenty-eight unambiguous primes were also repeated twice, once in condition Unrelated Dominant A and once in condition Unrelated Subordinate A, to correspond with the repetition in the ambiguous word conditions. Additionally, each target was repeated four times, with one half of the targets appearing in conditions Ambiguous Dominant Related, Unambiguous Dominant Related, Unrelated Dominant A, and Unrelated Dominant U, and the other half of the targets appearing in conditions Ambiguous Subordinate Related, Unambiguous Subordinate Related, Unrelated Subordinate A, and Unrelated Subordinate U.
All prime stimuli were matched for frequency (Francis & Kucera, 1982), as confirmed by a nonsignificant ANOVA [F(5, 23) = 1.41, p = .26]. Experimental targets were also matched for frequency across the conditions [t(27) = .77, p = .45]. Two hundred twenty-four filler trials with pronounceable nonword targets were also included. Similar to the word target conditions, there were 28 ambiguous primes, each repeated twice.
The current study was designed to explore the neural activation patterns recruited in resolving the meaning of an ambiguous word when that word is paired with a target related to one of its meanings. As a consequence, we did not investigate whether there would an overall effect of ambiguity (i.e. a main effect of ambiguity) in the context of a semantically unrelated word. For this reason, the design of this experiment did not include a condition in which a target stimulus was preceded by a semantically unrelated ambiguous word. While the overall effect of ambiguity on processing semantically unrelated as well as semantically related word pairs is of interest, this question is beyond the scope of the current investigation.
Task Design
The experiment was divided into 4 runs with 7 pairs of stimuli from each condition in each run, resulting in 112 trials per run. The stimuli were distributed in such a way that no words were repeated within a run. The trials were pseudo-randomized so that no more than three trials with the same response or from the same condition appeared consecutively.
The participants were asked to make a yes or no lexical decision indicating whether the second stimulus in each pair was a word of English or not by pressing one of two buttons with their right hand. They were asked to respond as quickly and accurately as possible. Button mapping for yes/no responses was counterbalanced across participants.
The Brown Lab Interactive Speech System (BLISS) software (Mertus, 2005) was used to set up and present the experimental stimuli. All stimuli were recorded digitally at a sampling rate of 22050 Hz by a male speaker of American English and edited to create the sound files used in the experiment.
Image Acquisition
All anatomical and functional image acquisition was performed using a 1.5T Symphony Magnetom MR System (Siemens Medical Systems, Erlangen, Germany). A standard whole-head coil was used. The experiment consisted of four 12-minute runs, with 112 pairs of stimuli in each run. The order of trials within each run was fixed, and the order of the runs was randomized across subjects, so that each subject received a unique order. The experimental task required the participants to listen to pairs of stimuli, with a 50 ms interstimulus interval (ISI) between the two stimuli in each pair. The intertrial interval (ITI) was jittered in increments equal to quarters of the TR, resulting in seven trial onset asynchrony (TOA) values: 3620 ms, 4525 ms, 5430 ms, 6335 ms, 7240 ms, 8145 ms, and 9050 ms. Jittering was done in accordance with a design optimization algorithm (Dale, 1999).
The stimuli were presented through MR-compatible headphones (Resonance Technologies, Inc.), and the participants responded via an MR-compatible response box. The participants’ responses and RTs from the onset of the second stimulus were recorded on a Dell laptop computer using the BLISS software (Mertus, 2005). All participants performed a 10-trial practice session during the acquisition of the anatomical data.
A high resolution 3D anatomical data set was acquired, using a rapid acquisition gradient-echo MPRAGE sequence (TR =1900 msec, TE = 4.15 msec, TI = 1100 msec, 1 mm isotropic voxels, 256 mm FOV). Functional data was acquired using a gradient-echo echo-planar pulse sequence in an interleaved fashion (45 axial slices, 3 mm isotropic voxels, TR = 3620 ms, TE = 38 ms, 192 mm FOV, 200 volumes per run).
fMRI Data Analysis
Processing and statistical analysis of the MR data was performed using AFNI (Cox, 1996; http://afni.nimh.nih.gov/afni/). A total of 800 echo-planar volumes were acquired, with 200 volumes per run. The first four volumes in each run were discarded to allow the scanner to reach equilibrium in order to avoid T1 saturation effects. Anatomical and functional data were co-registered using the position coordinates obtained from the scanner. The time-series corrections were performed in accordance to the interleaved slice-timing fMRI sequence. Motion correction was performed by realigning the subjects’ EPI images to the first volume in the experiment via a six-parameter rigid body transformation (Cox & Jesmanowicz, 1999). The MPRAGE anatomical dataset was normalized to the Talairach and Tournoux stereotaxic space (Talairach & Tournoux, 1988) using AFNI. The functional data was then Talairach-aligned using the normalized anatomical dataset, and finally spatially smoothed with a 6-mm full width half maximum Gaussian kernel.
Statistical analyses
Deconvolution analysis was performed on the functional data using AFNI, so that an estimate of the hemodynamic response to each stimulus condition could be obtained. Time series files were created for each condition containing the time points at which stimuli in each condition were presented. The time series files were convolved with a gamma function to obtain the idealized hemodynamic response for each condition.
Statistical analyses were performed using multiple linear regression, using AFNI’s 3dDeconvolve program. The time-series vectors for each condition convolved with a stereotypic hemodynamic response function served as regressors. There were eleven regressors for condition types: eight word conditions with only correct trials included (Ambiguous Dominant Related, Ambiguous Subordinate Related, Unambiguous Dominant Related, Unambiguous Subordinate Related, Unrelated Dominant A, Unrelated Subordinate A, Unrelated Dominant U, Unrelated Subordinate U), nonwords, incorrect word trials, and incorrect nonword trials. The six realignment parameters obtained during motion correction were also included as regressors to account for any variance due to movement that was not removed by the earlier motion correction. Finally, linear and baseline trends were also estimated to correct for linear drift and mean signal fluctuation between runs. The condition coefficients for each subject were then converted to percent signal change by dividing the coefficient for each voxel by the mean estimated baseline for that voxel across the four runs.
The percent change values were then submitted to a two-way ANOVA, with experimental condition (excluding the incorrect trial conditions) as a fixed effect and subjects as a random effect. The following planned comparisons were performed: To examine the effect of ambiguity resolution towards dominant and subordinate meanings:
Ambiguous Related {Ambiguous Dominant Related + Ambiguous Subordinate Related} versus Unambiguous Related {Unambiguous Dominant Related + Unambiguous Subordinate Related}
Ambiguous Dominant Related versus Unambiguous Dominant Related
Ambiguous Subordinate Related versus Unambiguous Subordinate Related
To examine the effect of dominance:
Ambiguous Dominant Related versus Ambiguous Subordinate Related
To examine the effect of relatedness (priming):
Related vs. Unrelated across all conditions
Unambiguous Related {Unambiguous Dominant Related + Unambiguous Subordinate Related} versus Unambiguous Unrelated {Unrelated Dominant U + Unrelated Subordinate U}
Ambiguous Related {Ambiguous Dominant Related + Ambiguous Subordinate Related} versus Unrelated {Unrelated Dominant A + Unrelated Subordinate A}
Ambiguous Dominant Related versus Unrelated Dominant A
Ambiguous Subordinate Related versus Unrelated Subordinate A
Monte Carlo simulations were performed in order to determine the number of contiguous voxels to achieve a correct significance level of p < .05. These simulations model the likelihood that a cluster of a given size would occur in a randomly generated data set. To this end, 20,000 iterations of normally distributed random data were generated within a brain volume of the same dimensions used in this study. A voxel-level threshold of p < .05 and a cluster-level threshold of 122 contiguous voxels were ultimately adopted for a corrected significance level of p < .05. The maximum intensity points of the activated clusters were used to identify the location of activated anatomical regions, and an additional analysis was undertaken to investigate the proportion of voxels within a cluster that fell within different anatomical regions. The atlases used to locate anatomical structures were the Anatomy Toolbox atlases (Eickhoff et al., 2005; 2006; 2007) and the Duvernoy anatomical atlas (Duvernoy, 1991). The summary of significant activation that emerged in these analyses is provided in Table 3.
Table 3.
Clusters which exceed the minimum cluster threshold of p<0.05, corrected. For each cluster, Brodmann areas (BA), number of voxels in the activated cluster (# Vox), x,y,and z coordinates in Talairach and Tournoux, and mean difference in % signal change with standard error of the mean are listed. P statistics are calculated on the basis of Monte Carlo simulations of cluster size likelihood at a voxel-level correction of p<0.05 (see Methods).
| Region | BA | # Vox |
p statistic |
Coordinates [x, y, z] |
Difference in % signal change (SEM) |
|
|---|---|---|---|---|---|---|
| Ambiguous Related > Unambiguous Related | ||||||
| L inferior frontal gyrus | 45/44 | 253 | p<0.001 | [−52, 14, 23] | 0.089 (0.018) | |
| R inferior frontal gyrus | 45/44 | 167 | p<0.01 | [47, 14, 29] | 0.080 (0.017) | |
| Ambiguous Subordinate Related > Unambiguous Subordinate Related | ||||||
| L inferior frontal gyrus | 45 | 152 | p<0.025 | [−43, 41, 11] | 0.126 (0.035) | |
| L superior parietal lobule | 1 | 307 | p<0.001 | [−28, −61, 56] | 0.138 (0.033) | |
| L cingulate gyrus | 6 | 166 | p<0.01 | [−4, −1, 44] | 0.114 (0.031) | |
| L medial orbital gyrus | 11 | 420 | p<0.001 | [−1, 56, −4] | 0.169 (0.047) | |
| R superior parietal lobule | 7 | 127 | p<0.05 | [23, −73, 47] | 0.133 (0.038) | |
| R middle frontal gyrus | 45 | 139 | p<0.025 | [44, 20, 41] | 0.137 (0.035) | |
| Ambiguous Subordinate Related >Ambiguous Dominant Related | ||||||
| L temporal pole * | 38/45 | 152 | p<0.025 | [−46, 14, −7] | 0.106 (0.024) | |
| L posterior cingulate | 31 | 128 | p<0.05 | [−1, −43, 27] | 0.112 (0.024) | |
| Unrelated > Related | ||||||
| L lingual gyrus | 19 | 216 | p<0.005 | [−16, −61, −7] | 0.075 (0.020) | |
| R lingual gyrus | 18 | 213 | p<0.005 | [14, −73, −10] | 0.078 (0.021) | |
| R supplementary motor | 6 | 155 | p<0.025 | [14, 2, 65] | 0.057 (0.013) | |
| R middle frontal gyrus | 9 | 443 | p<0.001 | [29, 35, 35] | 0.060 (0.012) | |
| R postcentral gyrus | 3 | 183 | p<0.005 | [38, −31, 59] | 0.061 (0.015) | |
| Unambiguous Unrelated > Unambiguous Related | ||||||
| L superior temporal gyrus | 42 | 237 | p<0.001 | [−64, −31, 14] | 0.075 (0.012) | |
| L inferior frontal gyrus | 45 | 128 | p<0.05 | [−49, 17, 26] | 0.069 (0.009) | |
| L temporal pole * | 38/45/47 | 187 | p<0.005 | [−46, 14, −10] | 0.078 (0.012) | |
| L precuneus | 7 | 848 | p<0.001 | [−7, −70, 50] | 0.104 (0.024) | |
| R precentral gyrus | 6 | 2243 | p<0.001 | [23, −19, 65] | 0.084 (0.013) | |
| R angular gyrus | 19 | 140 | p<0.025 | [38, −67, 41] | 0.084 (0.022) | |
| R postcentral gyrus | 1/2 | 221 | P<0.001 | [62, −16, 17] | 0.083 (0.017) | |
Although the maximum intensity fell within the L temporal pole, only a small percentage of the activated voxels were in the temporal pole; the largest percentage of the activated voxels fell within the IFG (see text).
Results
Behavioral Results
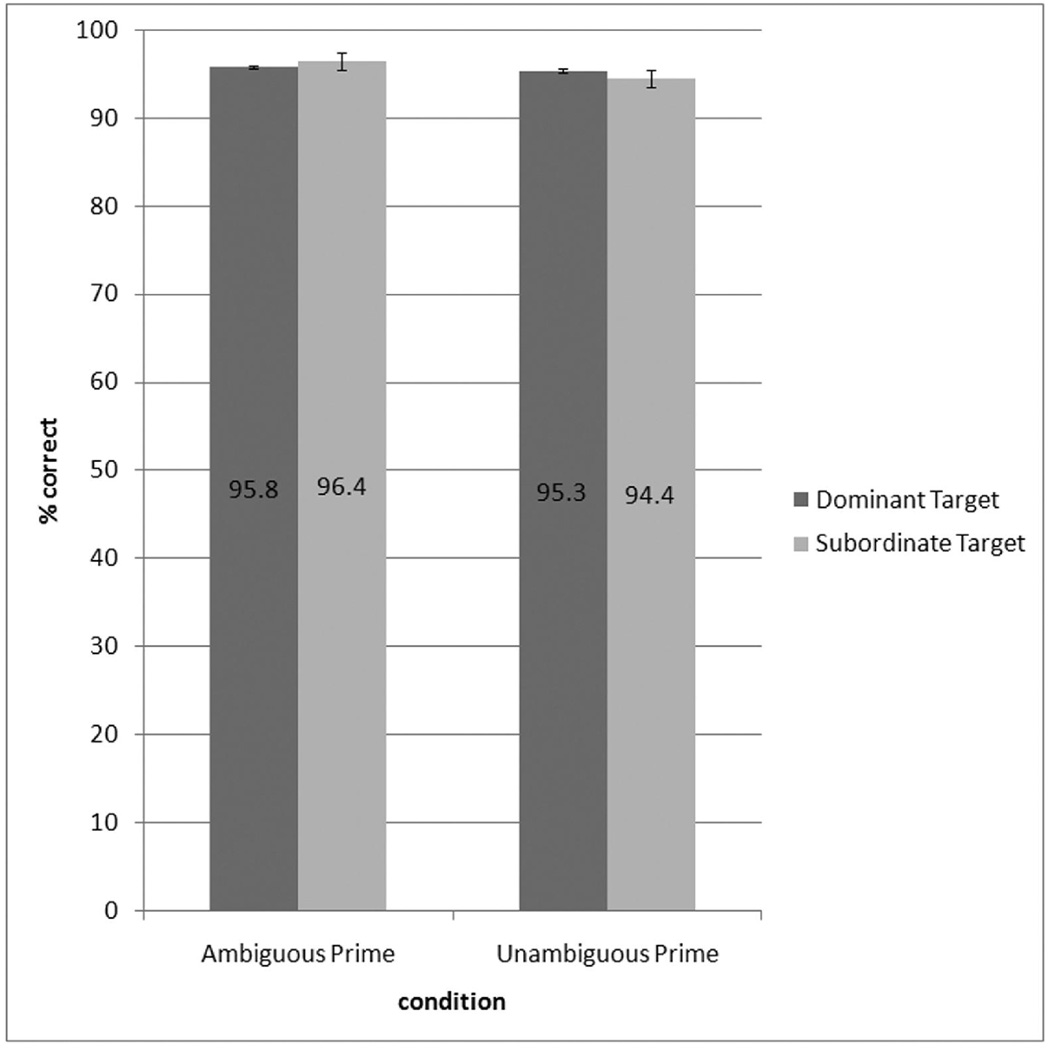
Mean RTs across subjects (n=16) for all experimental conditions are presented in Table 1, and mean RTs for the semantically related conditions are presented in Figure 1. All means were calculated after errors and outliers were removed. Outliers were defined as responses greater than 2000 ms or outside two standard deviations from the mean for a particular condition. Accuracy data for each condition are presented in Table 2, and accuracy data for all related conditions are presented in Figure 2. A 2 × 2 × 2 repeated measures analysis of variance (ANOVA) was performed on the RT and accuracy data for word conditions, with Ambiguity (ambiguous, unambiguous), Dominance (dominant, subordinate), and Relatedness (related, unrelated) as the three within-subjects factors. In the analysis of the RT data, Relatedness emerged as a significant main effect in both subject and item analyses [F1(1, 15) = 76.33, p < .0001; F2(1, 27) = 108.96, p < .0001]. RTs were significantly faster for related conditions compared to unrelated conditions in all cases: Ambiguous Dominant Related versus Unrelated Dominant A [t(15) = 3.44, p < .01], Ambiguous Subordinate Related versus Unrelated Subordinate A [t(15) = 5.31, p < .0001], Unambiguous Dominant Related versus Unrelated Dominant U [t(15) = 6.18, p < .0001], and Unambiguous Subordinate Related versus Unrelated Subordinate U [t(15) = 3.80, p < .01]. The interaction of Ambiguity X Relatedness approached significance in the subject analysis [F1(1, 15) = 4.30, p = .056], and reached significance in the item analysis [F2(1, 27) = 7.94, p < .01]. The interaction was due to a smaller priming effect for ambiguous than unambiguous words. No other main effects or interactions were found to be significant in the RT data. With respect to the accuracy data, there was a significant main effect of Relatedness [F1(1, 15) = 9.06, p < .01], due to subjects performing with higher accuracy on related trials than unrelated trials. No other main effects or interactions were significant for the accuracy data.
Table 1.
Mean RTs (ms) for the lexical decision task. Example stimuli are included for each condition. The dominant and subordinate targets are related to the two distinct meanings of the ambiguous prime and are repeated across the 4 prime conditions (see text).
| Prime Type | |||||
|---|---|---|---|---|---|
| Type of Target | Ambiguous Related (e.g.‘affair’) |
Unrelated A (e.g.‘slowly’) |
Unambiguous Related (e.g.‘hug”social’) |
Unrelated U (e.g.‘pump’/’young’) |
|
| Dominant (e.g.‘love’) | 811 | 865 | 778 | 889 | |
| Subordinate(e.g.‘event’) | 829 | 894 | 793 | 886 | |
Figure 1.
Mean RTs (ms) for the related conditions in the lexical decision task. Dominant and subordinate refer to the relationship of the target stimulus to one of the two meanings of the ambiguous prime stimulus (see Table 1, see text).
Table 2.
Mean accuracy (% correct) for the lexical decision task. Example stimuli are included for each condition. The dominant and subordinate targets are related to the two distinct meanings of the ambiguous prime and are repeated across the 4 prime conditions (see text).
| Prime Type | |||||
|---|---|---|---|---|---|
| Type of Target | Ambiguous Related (e.g.’affair’) |
Unrelated A (e.g.’slowly’) |
Unambiguous Related (e.g.’hug’’social’) |
Unrelated U (e.g.‘pump’/’young’) |
|
| Dominant (e.g.‘love’) | 96 | 93 | 95 | 92 | |
| Subordinate(e.g.‘event’) | 96 | 94 | 94 | 94 | |
Figure 2.
Mean accuracy (% correct) for the related conditions in the lexical decision task. Dominant and subordinate refer to the relationship of the target stimulus to one of the two meanings of the ambiguous prime stimulus (see Table 1, see text).
fMRI Results
A two-way repeated measures analysis of variance (ANOVA) was conducted on the functional data with two factors: Condition and Subjects. A number of planned contrasts were carried out as described above in the methods section.
Ambiguity Effects
Ambiguous Related versus Unambiguous Related
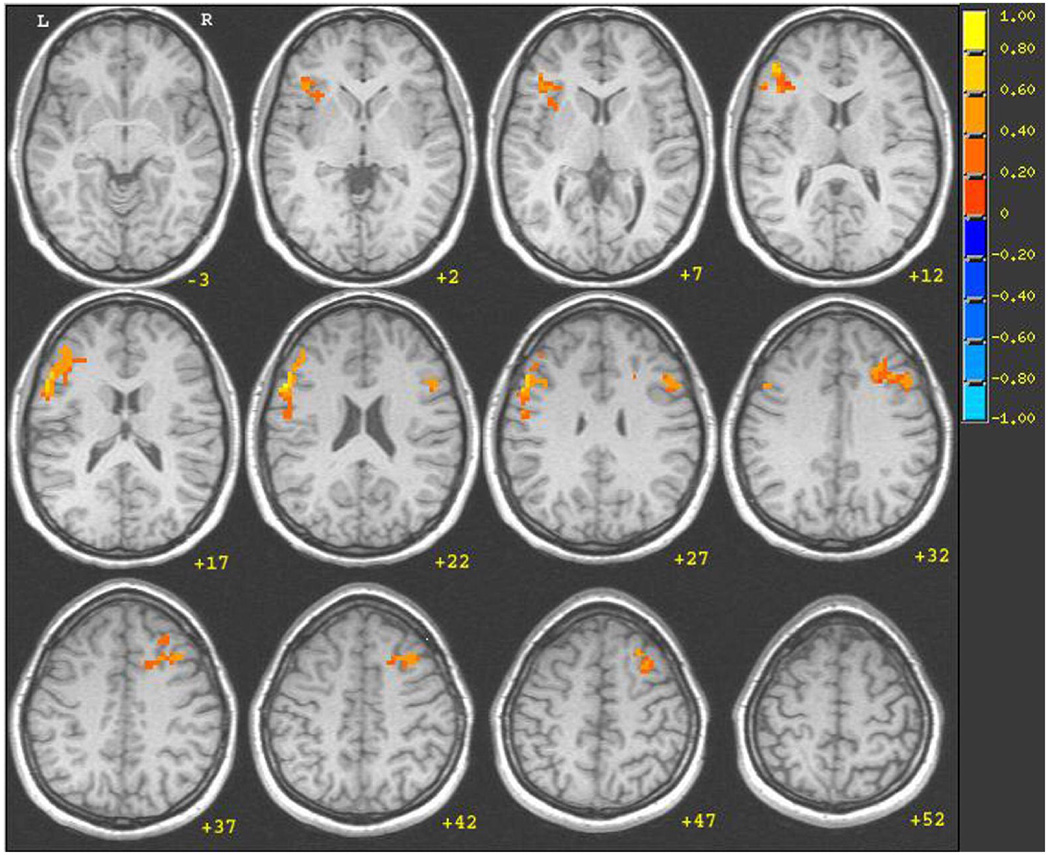
Activation was greater for the Ambiguous Related conditions {Ambiguous Dominant Related + Ambiguous Subordinate Related} than for the Unambiguous Related conditions {Unambiguous Dominant Related + Unambiguous Subordinate Related}. The significant clusters emerged in bilateral frontal areas (see Figure 3) including the left (253 voxels) and right (167 voxels) inferior frontal gyrus (IFG).
Figure 3.
Group functional map (N=16) for the Ambiguous Related > Unambiguous Related contrast. Significantly activated clusters shown: left IFG and right MFG (p < .05). Figure 4.
Ambiguous Dominant Related versus Unambiguous Dominant Related
No significantly activated clusters emerged in this comparison.
Ambiguous Subordinate Related versus Unambiguous Subordinate Related
The contrast between Ambiguous Subordinate Related and Unambiguous Subordinate Related trials showed more activation for ambiguous words than unambiguous words. Frontal activation in the left hemisphere (LH) was centered in the left IFG, the left cingulate gyrus, and the left medial orbital gyrus. There was also a cluster in the right hemisphere (RH) centered in the right MFG. Parietal activation occurred in the bilateral superior parietal lobules (SPL), with one cluster (307 voxels) in the LH, and another cluster (127 voxels) in the RH.
Dominance Effects
Ambiguous Dominant Related versus Ambiguous Subordinate Related
The comparison of Ambiguous Dominant Related and Ambiguous Subordinate Related trials revealed more activation for the subordinate trials than the dominant trials. Two significant clusters emerged in the frontal areas of the LH. Although one of the clusters (152 voxels) had its point of maximum intensity in the temporal pole, only 3% of the cluster was located in the temporal pole; while the largest part of the cluster (56%) was in the left IFG. The second cluster (128 voxels) was located in the left posterior cingulate gyrus (see Figure 4).
Figure 4.
Group functional map (N=16) for the Ambiguous Dominant Related < Ambiguous Subordinate Related contrast. Significantly activated clusters shown: left IFG and left cingulate gyrus (p < .05).
Relatedness (Priming) Effects
Unambiguous Related versus Unambiguous Unrelated {Unrelated Dominant U + Unrelated Subordinate U}
The contrast between Unambiguous Related conditions {Unambiguous Dominant Related + Unambiguous Subordinate Related} and corresponding unrelated conditions {Unrelated Dominant U + Unrelated Subordinate U} showed more activation for unrelated words. In the LH, two significant clusters emerged. One cluster was located in the left STG (237 voxels). Although the maximum intensity of the second cluster (187 voxels) was located in the left temporal pole, it constituted only 7% of this cluster; 72% of this cluster was located in the left IFG including BA45 and BA47. A third significantly activated LH cluster was located in the left IFG (128 voxels). In the RH, three significantly activated clusters emerged. One cluster (2243 voxels) was located in the right precentral gyrus, another cluster (221 voxels) was found in the right postcentral gyrus, and the third cluster (140 voxels) emerged in the right angular gyrus.
Related versus Unrelated
The contrast between Related conditions {Ambiguous Dominant Related + Ambiguous Subordinate Related + Unambiguous Dominant Related + Unambiguous Subordinate Related} and all Unrelated conditions {Unrelated Dominant A + Unrelated Subordinate A + Unrelated Dominant U + Unrelated Subordinate U} showed more activation for unrelated words than for related words. There were two significant clusters in the frontal areas of the RH, one cluster (443 voxels) in the MFG and another cluster (155 voxels) in the supplementary motor area (SMA). Parietal activation centered in one significant cluster (183 voxels) in the right postcentral gyrus, and occipital activation emerged in two significant clusters in the bilateral lingual gyrus (216 voxels in the LH and 213 voxels in the RH).
Discussion
The results of this experiment support the hypothesis that the bilateral IFG is involved in lexical-semantic processing under conditions of increased competition, showing increased activation for ambiguous words in the presence of a single disambiguating related word compared to unambiguous words. This study also demonstrates that left IFG activation is not dependent on overt selection, but is recruited in implicit tasks using stimuli that have competition as an intrinsic property.
Behavioral Results
As expected, behavioral findings revealed a semantic priming effect in all conditions with shorter RT latencies and greater accuracy for related pairs compared to unrelated pairs. This semantic priming effect occurred presumably because related primes partially activate the same lexical-semantic network as the target stimuli (cf. Rissman et al., 2003). These data also demonstrate that the subjects’ increased speed on related conditions did not compromise their accuracy. A significant Ambiguity X Relatedness interaction approached significance in the subject analysis and was significant in the item analysis. This interaction was due to increased RT latencies in the semantically related conditions for the ambiguous stimuli compared to the unambiguous stimuli. This result suggests that the processing demands resulting from competition of meanings inherent in ambiguous words were deleterious to the facilitation effects found for semantically related target stimuli.
fMRI Results
Ambiguity Effects
The contrast of neural activation patterns for the Ambiguous Related conditions compared to the Unambiguous Related conditions revealed increased bilateral prefrontal activation in the IFG associated with the processing of ambiguous words and their related targets. Increased activation in the left IFG for ambiguous stimuli expands on the hypothesis that the left IFG is recruited under conditions of increased competition (Thompson-Schill et al., 1997). These findings suggest that bilateral prefrontal areas are involved in the processing of competing meaning alternatives when subjects hear ambiguous words. Since the experimental task did not explicitly require the subjects to overtly select a particular meaning of the ambiguous word, these results show that increased processing resources recruit the bilateral prefrontal areas under conditions of competition, even in the absence of overt selection demands imposed by the task.
These results are consistent with some of the existing neuroimaging evidence that suggests that prefrontal areas are recruited in the processing of ambiguous words that occur in sentences (Mason & Just, 2007; Rodd et al., 2005; Zempleni et al., 2007). All three of these studies showed increased activation in the IFG for ambiguous words compared to unambiguous words in a sentence context. The results of the current study show that the activation of the IFG for ambiguous words is not contingent on sentence processing demands, and can be found in a minimal context such as paired priming. Existing neuroimaging evidence suggests that when ambiguous words are presented in isolation, in the absence of a disambiguating context, the IFG is not recruited (Chan et al., 2004). The results of the current study demonstrate that a single related word constitutes a sufficient cue for ambiguous word meanings to compete and hence to increase processing demands that lead to increased activation of the IFG. These results also suggest that the increased IFG activation in the processing of ambiguous words in sentence contexts is due at least in part to semantic competition demands of ambiguous words, and not due to increased demands imposed by integration of ambiguous word meanings into sentence context (Mason & Just, 2007; Rodd et al., 2005; Zempleni et al., 2007).
As hypothesized, the findings for the contrast of the Ambiguous Subordinate Related condition with the Unambiguous Subordinate Related condition revealed areas of activation that were similar to the activation seen in the comparison of the Ambiguous Related condition with the Unambiguous Related condition, including a cluster in the left IFG and the right MFG. However, there were several other areas which showed significant activation, including the left medial orbital gyrus, left cingulate gyrus, and bilateral SPL, that were not found in the comparison of Ambiguous Related and Unambiguous Related conditions. The left cingulate has been shown to play a major role in cognitive control (Kan & Thompson-Schill, 2004; Miller, 2000; Miller & Cohen, 2001; Novick et al., 2005), so its involvement may be necessary for the inhibition of the dominant meaning or the increase in activation of the subordinate meaning when the subordinate meaning of an ambiguous word is primed. The role of the left medial orbital gyrus in language processing has not been directly shown in the literature, so further research is needed to shed more light on its potential involvement. However, it is part of the orbitofrontal complex, which has been implicated in decision-making (Bechara et al., 2000; Bolla et al., 2003; Manes et al., 2002), so its recruitment may be due to increased difficulty of making a relatedness decision about a subordinate meaning of an ambiguous word, compared to its dominant meaning.
The role of the bilateral SPL in ambiguous word processing is less certain. In previous fMRI studies, superior parietal areas have been implicated in maintaining representations in working memory (Wolpert et al., 1998). Thus, it is possible that the SPL is recruited in order to maintain meaning alternatives of ambiguous words in memory during competition among meaning alternatives. Inferior parietal areas have previously been shown to play a role in lexical retrieval (Badre et al., 2005), and although the LD task does not require explicit lexical retrieval, parietal areas may have a role in maintaining representations of possible meanings in case retrieval is required.
Although the comparison of the Ambiguous Dominant Related condition to the Unambiguous Dominant Related condition was expected to elicit similar results to the comparison of the Ambiguous Related to Unambiguous Related conditions, no significantly activated clusters were found. This may be due to the fact that the dominant meaning has a higher level of activation than the subordinate meaning (Duffy et al., 1988). As a result, recruitment of the IFG would not be required for the dominant condition, because competition demands are minimal.
The significantly greater activation that emerged in the IFG for semantically related ambiguous words compared to unambiguous words, and, as well, for subordinate meanings of ambiguous words, occurred largely in BA45. These findings are consistent with a number of studies in the literature suggesting different functional roles for cortical areas within the IFG (Badre & Wagner, 2007; Dapretto & Bookheimer, 1999; Fiez, 1997; Miller & Cohen, 2001; Snyder et al., 2007). What is less clear is whether the distinctions relate to different functional domains of language, e.g. semantics, phonology, and syntax (Buckner et al., 1995; Dapretto & Bookheimer, 1999; Fiez, 1997) or more domain general cognitive operations, e.g. activation of representations, selection among competing alternatives (Badre and Wagner, 2007; Snyder et al., 2007; cf. also Vuust et al., 2006). Because the current experiment investigated lexical-semantic processing in relation to competition, we are unable to distinguish between these two possibilities. What is the case, however, is that the extent of competition between lexical-semantic representations modulates activation in the IFG, particularly in BA45.
Dominance Effects
Comparison of the Ambiguous Subordinate Related condition to the Ambiguous Dominant Related condition showed increased activation in the left IFG and the left posterior cingulate gyrus. These findings are consistent with the view that there are greater processing demands for subordinate compared to dominant meanings of ambiguous words. It has been proposed that the subordinate meaning of an ambiguous word may require more time to reach its threshold of activation compared to the dominant meaning (Martin et al., 1999). As a consequence, even when the subordinate meaning is primed, greater processing resources are required to ultimately access the subordinate meaning instead of the dominant meaning. By comparison, when the dominant meaning is primed, competition between the dominant and subordinate meaning is minimal, because the dominant meaning achieves its threshold of activation faster than the subordinate meaning, so the processing demands are lower.
As discussed above, previous evidence shows that both the left IFG and the left cingulate are major players in cognitive control (Kan & Thompson-Schill, 2004; Miller, 2000; Miller & Cohen, 2001; Novick et al., 2005). Activation of these areas is observed when automatic processing has to be overridden, and cognitive conflict is detected and resolved. Thus, the left IFG and the left cingulate gyrus may be involved in resolution of cognitive conflict and competition when the subordinate meaning of an ambiguous word is primed.
Relatedness (Priming) Effects
A number of significant clusters emerged as a function of semantic relatedness. Priming effects, as shown by decreased activation for the related stimulus pairs in the comparison between the Unambiguous Related conditions and corresponding unrelated conditions {Unrelated Dominant U + Unrelated Subordinate U} revealed a network of increased activation. Areas showing significant activation were the left STG, left IFG, left precuneus, right precentral gyrus, right angular gyrus, and right postcentral gyrus. The activation of the left STG and left IFG is consistent with existing evidence of the neural correlates of semantic priming (Copland et al., 2003; Kotz et al., 2002; Mummery et al., 1999; Rissman et al., 2003; Rossell et al., 2001, 2003). The activation in the angular gyrus was also expected, as it has been implicated in the literature in lexical-semantic processing (Binder et al., 1997; Demonet et al., 1992; Newman et al., 2001). The activation in the right precentral gyrus was somewhat unexpected, although left precentral gyrus activation has been found before in semantic priming studies. It is possible that higher demands for motor planning of the response in the unrelated condition may account for this activation (Rissman et al., 2003). The role of the left precuneus is under debate in the literature, but previous evidence suggests that it plays a role in lexical-semantic processing, including the processing of associatively related words (Kotz et al., 2002; Koustaal et al., 2001). Thus, its increased activation in the unrelated condition may be due to the higher lexical-semantic processing demands in that condition. There is little evidence in the fMRI literature that would account for the postcentral gyrus activation, though it has been shown to play a role in language perception in multiple modalities (Kang et al., 2006).
Conclusion
The findings of this experiment demonstrate that competition between the multiple meanings of ambiguous words recruits bilateral prefrontal areas, including the bilateral IFG. Moreover, prefrontal activation is not contingent upon overt selection among competing alternatives, as observed in earlier studies (Thompson-Schill et al., 1997, 1998, 1999), but is found under conditions of implicit competition for ambiguous words in the presence of disambiguating single related words. Competition is present in the processing of ambiguous words because all meanings of ambiguous words are initially activated. The results of the current study not only show increased left IFG activation under conditions of competition for semantically related ambiguous stimuli compared to unambiguous stimuli, but they also show that this competition is modulated by the frequency of the ambiguous word meanings. In conditions where the less frequent, subordinate meaning, is primed, there is increased left IFG activation, presumably because additional resources are required in order for the activation level of the subordinate meaning to reach threshold, such that it is accessed instead of the more frequent, dominant meaning.
Acknowledgments
This research was supported in part by NIH Grant RO1 DC006220 to Sheila E. Blumstein, as well as a Brown University MRI Research Facility Undergraduate Student Fellowship and an Undergraduate Teaching and Research Assistantship to Natalia Bilenko. Many thanks to Brendan Britton for his help in the analysis of the fMRI data.
References
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:6907–6918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Baumgaertner A, Weiller C, Buchel C. Event-related fMRI reveals cortical sites involved in contextual sentence integration. Neuroimage. 2002;16:736–745. doi: 10.1006/nimg.2002.1134. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decisión making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Rao SM, Hammeke TA, Yetkin YZ, Jesmanowicz A, Bandettini PA, Wong EC, Estkowski LD, Goldstein MD, Haughton VM, Hyde JS. Functional magnetic resonance imaging of human auditory cortex. Annals of Neurology. 1994;35:662–672. doi: 10.1002/ana.410350606. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderbunk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Petersen SE. Dissociation of human prefrontal cortical areas across different speech production tasks and gender groups. Journal of Neurophysiology. 1995;74:2163–2173. doi: 10.1152/jn.1995.74.5.2163. [DOI] [PubMed] [Google Scholar]
- Burgess C, Simpson GB. Cerebral hemispheric mechanisms in the retrieval of ambiguous word meanings. Brain and Language. 1988;33:86–103. doi: 10.1016/0093-934x(88)90056-9. [DOI] [PubMed] [Google Scholar]
- Chan AHD, Liu HL, Yip V, Fox PT, Gao JH, Tan LH. Neural systems for word meaning modulated by semantic ambiguity. Neuroimage. 2004;22:1128–1133. doi: 10.1016/j.neuroimage.2004.02.034. [DOI] [PubMed] [Google Scholar]
- Copland DA, de Zubicaray GI, McMahon K, Eastburn M. Neural correlates of semantic priming for ambiguous words: An event-related fMRI study. Brain Research. 2007;1131:163–172. doi: 10.1016/j.brainres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Copland DA, de Zubicaray GI, McMahon K, Wilson SJ, Eastburn M, Chenery HJ. Brain activity during automatic semantic priming revealed by event-related functional magnetic resonance imaging. Neuroimage. 2003;20:302–310. doi: 10.1016/s1053-8119(03)00279-9. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Bookherimer SY. Form and content: Dissociating suntax and semantics in sentence comprehension. Neuron. 1999;24:427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- Duffy SA, Morris RK, Rayner K. Lexical ambiguity and fixation times in reading. Journal of Memory and Language. 1988;27:429–446. [Google Scholar]
- Duvernoy HM. The human brain: structure, three-dimensional sectional anatomy, and MRI. Wien, NY: Springer-Verlag; 1991. [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32:570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amuants K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;26:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Fiez J. Phonology, semantics, and the role of the left inferior prefrontal cortex. Human Brain Mapping. 1997;5:79–83. [PubMed] [Google Scholar]
- Francis W, Kucera H. Frequency Analysis of English Usage. Boston, MA: Houghton Mifflin; 1982. [Google Scholar]
- Gabrieli JDE, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P, Hald L, Bastiaansen M, Petersson KM. Integration of word meaning and world knowledge in language comprehension. Science. 2004;304:438–441. doi: 10.1126/science.1095455. [DOI] [PubMed] [Google Scholar]
- Howard DV, McAndrews MP, Lasaga MI. Semantic priming of lexical decisions in young and old adults. Journal of Gerontology. 1981;36:707–714. doi: 10.1093/geronj/36.6.707. [DOI] [PubMed] [Google Scholar]
- Kan IP, Thompson-Schill SL. Selection from perceptual and conceptual representations. Cognitive, Affective and Behavioral Neuroscience. 2004;4:466–482. doi: 10.3758/cabn.4.4.466. [DOI] [PubMed] [Google Scholar]
- Kang E, Lee DS, Kang H, Hwang CH, Oh SH, Kim CS, Chung JK, Lee MC. The neural correlates of cross-modal interaction in speech perception during a semantic decision task on sentences: A PET study. Neuroimage. 2006;32:423–431. doi: 10.1016/j.neuroimage.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Cappa SF, von Cramon DY, Friederici AD. Modulation of the lexical-semantic network by auditory semantic priming: An event-related functional MRI study. Neuromage. 2002;17:1761–1772. doi: 10.1006/nimg.2002.1316. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL. Perceptual specificity in visual object priming: Functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39:184–199. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Martin C, Vu H, Kellas G, Metcalf K. Strength of discourse context as a determinant of the subordinate bias effect. Quarterly Journal of Experimental Psychology. 1999;52A:813–839. doi: 10.1080/713755861. [DOI] [PubMed] [Google Scholar]
- Mason RA, Just MA. Lexical ambiguity in sentence comprehension. Brain Research. 2007;1146:115–127. doi: 10.1016/j.brainres.2007.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNealy K, Mazziotta JC, Dapretto M. Cracking the language code: Neural mechanisms underlying speech parsing. Journal of Neuroscience. 2006;26:7629–7639. doi: 10.1523/JNEUROSCI.5501-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertus J. Brown Lab Interactive Speech System. Providence, RI: Brown University; 2005. [Google Scholar]
- Meyer DE, Schvaneveldt RW. Facilitation in recognizing pairs of words: Evidence of a dependence between retrieval operations. Journal of Experimental Psychology. 1971;90:227–234. doi: 10.1037/h0031564. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nature Reviews Neuroscience. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moss HE, Ostrin RK, Tyler LK, Marslen-Wilson WD. Accessing different types of lexical semantic information: Evidence from priming. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:863–883. [Google Scholar]
- Mummery CJ, Shallice T, Price CJ. Dual-process model in semantic priming: A functional imaging perspective. Neuroimage. 1999;9:516–525. doi: 10.1006/nimg.1999.0434. [DOI] [PubMed] [Google Scholar]
- Neely JH. Semantic priming effects in visual word recognition: A selective review of current findings and theories. In: Besner D, Humphreys G, editors. Basic Processes in Reading: Visual Word Recognition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1991. pp. 264–336. [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida word association, rhyme, and word fragment norms. 1998 doi: 10.3758/bf03195588. http://www.usf.edu/FreeAssociation/ [DOI] [PubMed]
- Newman AJ, Pancheva R, Ozawa K, Neville HJ, Ullman MT. An event-related fMRI study of syntactic and semantic violations. Journal of Psycholinguistic Research. 2001;30:339–364. doi: 10.1023/a:1010499119393. [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson-Schill SL. Cognitive control and parsing: Reexamining the role of Broca’s area in sentence comprehension. Cognitive, Affective and Behavioral Neuroscience. 2005;5:263–281. doi: 10.3758/cabn.5.3.263. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Onifer W, Swinney DA. Accessing lexical ambiguities during sentence comprehension: Effects of frequency of meaning and contextual bias. Memory and Cognition. 1981;9:225–236. [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Rayner K, Pacht JM, Duffy SA. Effects of prior encounter and global discourse bias on the processing of lexically ambiguous words: Evidence from eye fixations. Journal of Memory and Language. 1994;33:527–544. [Google Scholar]
- Rissman J, Eliassen JC, Blumstein SE. An event-related fMRI investigation of implicit semantic priming. Journal of Cognitive Neuroscience. 2003;15:1160–1175. doi: 10.1162/089892903322598120. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Davis MH, Johnsrude IS. The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cerebral Cortex. 2005;15:1261–1269. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. Journal of Cognitive Neuroscience. 2001;13:829–843. doi: 10.1162/08989290152541485. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Bullmore ET, Williams SCR, David AS. Brain activation during automatic and controlled processing of semantic relations: A priming experiment using lexical-decision. Neuropsychologia. 2001;39:1167–1176. doi: 10.1016/s0028-3932(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Price CJ, Nobre AC. The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia. 2003;41:550–564. doi: 10.1016/s0028-3932(02)00181-1. [DOI] [PubMed] [Google Scholar]
- Simpson GB, Burgess C. Activation and selection processes in the recognition of ambiguous words. Journal of Experimental Psychology: Human Perception and Performance. 1985;11:28–39. [Google Scholar]
- Snyder HR, Feigenson K, Thompson-Schill SL. Prefrontal cortical response to conflict during semantic and phonological tasks. Journal of Cognitive Neuroscience. 2007;19:761–775. doi: 10.1162/jocn.2007.19.5.761. [DOI] [PubMed] [Google Scholar]
- Swinney DA. Lexical access during sentence comprehension: (Re) consideration of context effects. Journal of Verbal Learning and Verbal Behavior. 1979;18:645–659. [Google Scholar]
- Tabossi P. Accessing lexical ambiguity in different types of sentential contexts. Journal of Memory and Language. 1988;27:324–340. [Google Scholar]
- Tabossi P, Colombo L, Job R. Accessing lexical ambiguity: Effects of context and dominance. Psychological Research. 1987;49:161–167. [Google Scholar]
- Tabossi P, Zardon F. Processing ambiguous words in context. Journal of Memory and Language. 1993;32:359–372. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Kan IP. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron. 1999;23:513–522. doi: 10.1016/s0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D’Esposito M, Kan IP, Knight RT, Swick D. Effects of frontal lobe damage on interference effects in working memory. Cognitive, Affective and Behavioral Neuroscience. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: A neuropsychological test of neuroimaging findings. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twilley LC, Dixon P, Taylor D, Clark K. University of Alberta norms of relative meaning frequency for 566 homographs. Memory and Cognition. 1994;22:111–126. doi: 10.3758/bf03202766. [DOI] [PubMed] [Google Scholar]
- Vuust P, Roepstorff A, Wallentin M, Mouridsen K, Østergaard L. It don’t mean a thing… Keeping the rhythm during polyrhythmic tension, activates language areas (BA47) Neuroimage. 2006;31:832–841. doi: 10.1016/j.neuroimage.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Goodbody SJ, Husain M. Maintaining internal representations: The role of the human superior parietal lobe. Nature Neuroscience. 1998;1:529–533. doi: 10.1038/2245. [DOI] [PubMed] [Google Scholar]
- Zempleni MZ, Renken R, Hoeks JCJ, Hoogduin JM, Stowe L. Semantic ambiguity processing in sentence context: Evidence from event-related fMRI. Neuroimage. 2007;34:1270–1279. doi: 10.1016/j.neuroimage.2006.09.048. [DOI] [PubMed] [Google Scholar]