Abstract
Minocycline is neuroprotective in clinical and experimental stroke studies, due in part to its ability to inhibit poly (ADP-ribose) polymerase. Previous preclinical data have shown that interference with poly (ADP-ribose) polymerase signaling leads to sex-specific neuroprotection, reducing stroke injury only in males. In this study, we show that minocycline is ineffective at reducing ischemic damage in females after middle cerebral artery occlusion, likely due to effects on poly (ADP-ribose) polymerase signaling. Clinical trials must consider possible sex differences in the response to neuroprotective agents, if we hope to translate promising therapies to stroke patients of both sexes.
Keywords: middle cerebral artery occlusion (MCAO), minocycline, poly(ADP-ribose) polymerase (PARP), sex differences
Introduction
Minocycline is a tetracycline derivative shown to be neuroprotective in experimental models of stroke and other neurodegenerative disorders (Lampl et al, 2007; Yrjanheikki et al, 1999). A recent open-label trial of minocycline for acute stroke found that patients treated with minocycline had improved outcomes compared with placebo-treated patients (Lampl et al, 2007). Minocycline may influence neuronal survival by acting as a poly (ADP-ribose) polymerase (PARP) inhibitor as its PARP-inhibiting potency approaches that of PJ34, one of most potent PARP inhibitors available (Alano et al, 2006; Jagtap et al, 2002). The PARP activation promotes cell death through bioenergetic failure (Alano et al, 2004). Male PARP-1 (the isoform that accounts for 90% of total brain PARP activity (Kauppinen and Swanson, 2005)) knockout mice were found to be resistant to focal cerebral ischemia, as are primary neuronal sex-mixed cell cultures derived from PARP-1 null mice (Eliasson et al, 1997; McCullough et al, 2005). However, data have suggested that the PARP’s importance in ischemic neuronal death is dependent on the sex of the animal, because loss of PARP-1 protects males but not females (Hagberg et al, 2004; McCullough et al, 2005). In fact, interference with PARP signaling led to an unexpected exacerbation in ischemic injury in female mice, an effect that was independent of estrogen (McCullough et al, 2005). As previous experimental studies have not evaluated possible sex-dependent effects of minocycline, which mediates part of its neuroprotective actions through inhibition of PARP signaling, we evaluated the neuroprotective efficacy of this agent in male and female mice after focal cerebral ischemia.
Materials and methods
Animals
Animals were wild-type (wt) C57/B6 male and female mice (Charles River) as well as PARP-1 null male mice (20 to 25 g). For female mice, ovariectomy was performed to remove gonadal estrogen 7 days before middle cerebral artery occlusion (MCAO) surgery. Estrogen levels and uterine weights were assessed to confirm loss of estrogenic effects. We chose to evaluate ovariectomized female mice to reflect a more relevant translational model, because the majority of women are postmenopausal at the time of their stroke, as well as to reduce experimental variability associated with varying estrogen levels during estrous. The mice were anesthetized with 4% isoflurane mixed with room air and oxygen for induction and then maintained with 1% to 2% isoflurane/room air and oxygen mixture for all surgical procedures. The mice were given two doses of minocycline (45 mg/kg) or vehicle (sterile water) every 12 h, the first dose 30 mins after the onset of MCAO.
Focal Cerebral Ischemia Model
We used a MCAO model to evaluate our hypothesis. The MCAO was performed as described previously (McCullough et al, 2005). Reperfusion was achieved by withdrawal of the occludder 90 mins after the occlusion.
Estrogen Levels of Ovariectomzed Mice
Blood samples from all female mice and four randomly selected wt male mice were collected at the time of killing. The samples were spun at 6,000 r.p.m. for 10 mins at 4 degrees. A measure of 25 µL of supernatant (serum) was used to measure estrogen by enzyme-linked immunosorbent assay (IBL, Germany).
Behavioral Assessments
Neurologic deficits were scored at 24 or 72 h after stroke. The score system was as follows (0) no deficit; (1) forelimb weakness and torso turning to the ipsilateral side when held by the tail; (2) circling to affected side; (3) unable to bear weight on the affected side; and (4) no spontaneous locomotor activity (McCullough et al, 2005).
Histologic Assessments
Mice were euthanized at 24 or 72 h for infarct volume analysis after the onset of MCAO. Then the brains were removed and cut into 5-mm slices and stained with 1.5% 2,3,5-tripenyltetrazolium chloride for 30 mins at 37 degrees, then fixed with 4% paraformaldehyde in water. The digitalized and the infarct volumes (expressed as percentage of the contralateral hemisphere) were analyzed by computer program (Scan Pro 5) (McCullough et al, 2005).
Western Blots
The brains were removed 6 h after MCAO, and the ischemic hemispheres were separated and lysed for Western blot. Western blots were performed as described previously (McCullough et al, 2005). The brains were homogenized using lysis buffer (20 mmol/L Tris–HCL, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 50 mmol/L NaF, 1% Triton X-100, 1 mmol/L PMSF, 0.1 mmol/L Benzamidine, 50 µg/mL Soybean Trypsin inhibitor, 50 µg/mL leupeptin) and protein was loaded on a 4% to 15% gradient sodium dodecyl sulfate–polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. The membrane was probed with anti-PAR antibody (1:1000 dilution; Trevigen, Gaithersburg, MD, USA). Actin (1:5000; Sigma St Louis, MO, USA) was used as the loading control. All blots were incubated overnight in primary antibody at 4°C in Tris-buffered saline buffer containing 4% bovine serum albumin and 0.1% Triton X-100. Secondary antibodies (goat anti-rabbit IgG1:5000 for PAR; Chemicon, Billerica, MA, USA) were diluted and the ECL (pico) detection kit (Amersham Biosciences, Piscataway, NJ, USA) was used for signal detection. The PAR levels were expressed as the ratio to the control actin band with densitometry analysis (Adobe Photoshop 7.0).
Statistics
Data were expressed as mean ± s.e.m. Statistics was performed by Student T-test (for infarct volume), one-way analysis of variance with Tukey post hoc test for multiple comparisons (for PAR levels) or by Mann–Whitney U test (the neurologic deficit scores). Investigators performing behavioral and infarct size analysis were blinded to treatment group. P value < 0.05 was considered to be statistically significant.
Results
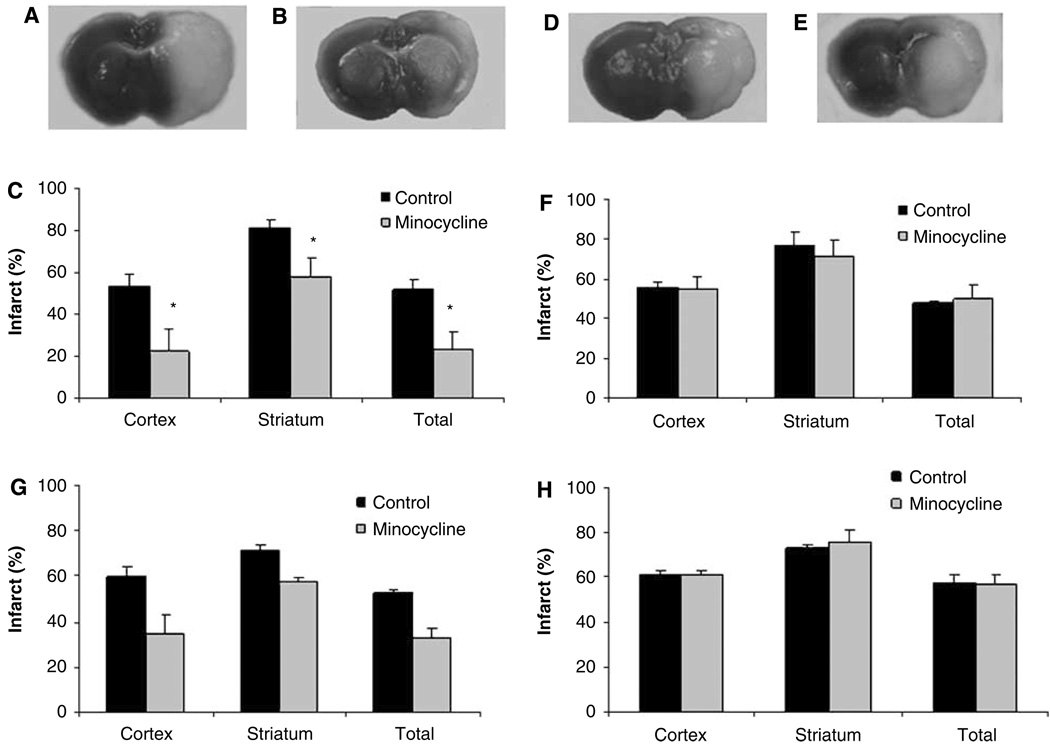
Minocycline significantly reduced infarct volume (expressed as percentage of the contralateral hemisphere, %) in wt male mice (cortex: drug 22.6 ± 10 versus vehicle 53.5 ± 5.9, P < 0.05; striatum: drug 57.9 ± 8.9 versus vehicle 81.0 ± 3.9, P < 0.05; total: drug 23.1 ± 8.6 versus vehicle 51.6 ± 5.1, P < 0.05) (Figure 1a–1c) at 24 h after stroke. However, no neuroprotection was seen in wt ovariectomized female mice (cortex: drug 54.9 ± 6.0 versus vehicle 56.1 ± 2.6; striatum: drug 71.3 ± 8.4 versus vehicle 77.1 ± 6.4; total: drug 49.3 ± 1.3 versus vehicle 47.3 ± 1.3) (Figure 1d–1f). This sex difference in neuroprotective efficacy was reflected in the neurologic deficits scores; treatment with minocycline reduced behavioral deficits in wt male mice but not in female mice (P < 0.05) (Supplementary Table 1).
Figure 1.
Minocycline is neuroprotective in male mice but not in ovariectomized female mice after stroke. (A, B, D, E) Representative images of tripenyltetrazolium chloride stained brain slice assessed at 24 h after stroke. (A) Male treated with vehicle, (B) male treated with minocycline, (D) female treated with vehicle, (E) female treated with minocycline; (C) (male mice) and (F) (female mice): infarct volume measured 24 h after stroke; (G) (male mice) and (H) (female mice): infarct volume measured 72 h after stroke. Infarct volume was expressed as percentage of the contralateral hemisphere (n = 6 in each group). *P < 0.05 (minocycline-treated group compared with vehicle-treated group, two-tailed Student t-test). Data were expressed as mean ± s.e.m.
We then extended the survival time to 72 h after MCAO in a separate cohort of animals. In wt males, infarct volume (expressed as percentage of the contralateral hemisphere) was again significantly reduced with minocycline treatment (total: drug 32.9 ± 4.7 versus vehicle 52.6 ± 1.3, P < 0.05) (Figure 1g). Similar to the 24-h endpoint, minocycline had no protective effect in ovxed wt females (total: drug 57.3 ± 3.6 versus vehicle 57.9 ± 2.9) (Figure 1h). The neurologic deficits scores were also consistent with the 24-h time point data; deficits at 72 h were reduced in minocycline-treated male mice, with no effect in drug-treated female mice (P < 0.05) (Supplementary Table 1). Estrogen levels were equivalent in male mice 6.5 ± 1.2 pg/mL (n = 4) and ovxed female mice 6.1 ± 1.0 pg/mL (n = 12).
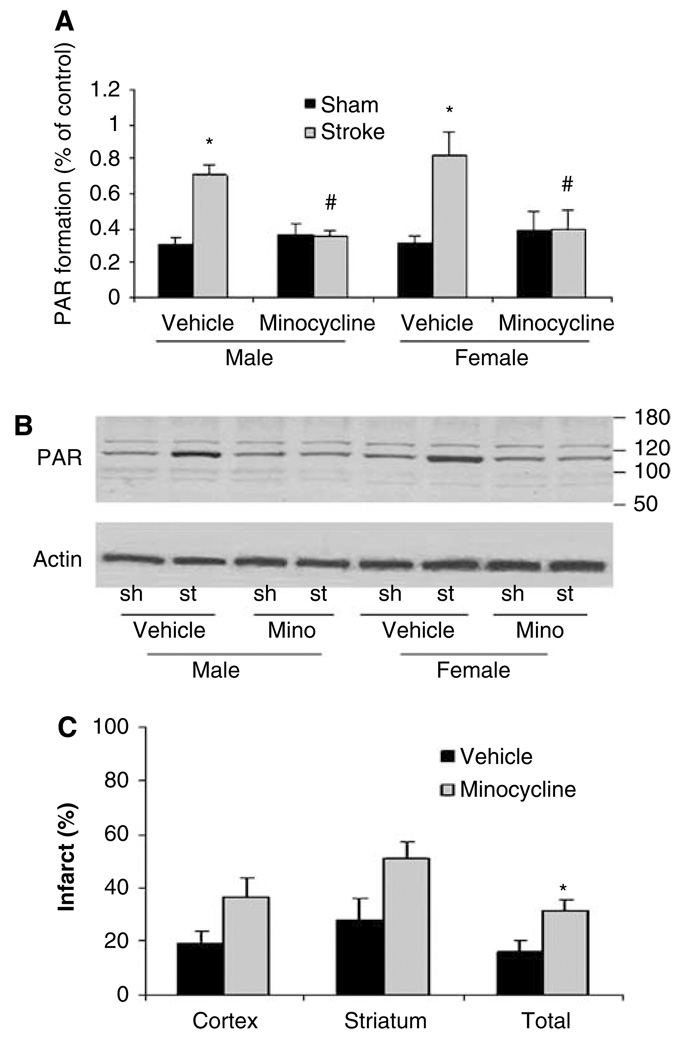
To directly examine the effect of minocycline on PARP activation in our model, we assessed PAR formation. The PAR levels were significantly elevated 6 h after stroke in both male and ovxed female mice (male: stroke 0.71 ± 0.06 versus sham 0.31 ± 0.03, P < 0.05; female: stroke 0.82 ± 0.13 versus sham 0.32 ± 0.034, P < 0.05). Minocycline treatment significantly reduced stroke-induced PAR formation when compared with the vehicle-treated stroke group in both sexes (male: drug 0.35 ± 0.04 versus vehicle 0.71 ± 0.06, P < 0.05 and female: drug 0.4 ± 0.1 versus 0.82 ± 0.13, P < 0.05) (Figure 2a and 2b). To further confirm PAPP inhibition is part of the mechanism by which minocycline protects male animals from stroke, we also examined the effect of minocycline in PARP-1 null male mice after MCAO. As expected, the neuroprotective effect of minocycline was lost in male PARP knockout mice, suggesting the protective effects of minocycline are mediated by PARP (Figure 2c).
Figure 2.
Minocycline treatment reduces stroke-induced PAR formation. (A and B) Minocycline reduced stroke-induced PAR accumulation in both wt male mice and ovariectomized wt female mice. (A) Quantification of PAR. *P < 0.05, vehicle-treated stroke group versus vehicle-treated sham. #P < 0.05, minocycline-treated stroke group versus vehicle-treated stroke group (one-way analysis of variance with Tukey post hoc for multiple comparison), n = 3 in each group. Sh, sham-operated group; st, stroke group; mino, minocycline-treated group. (B) Representative Western blots for PAR. The PAR is seen as a discrete, major band at 116 kDA. The PAR levels were expressed as the ratio to the control actin band with densitometry analysis. (C) Minocycline worsened stroke outcome in male PARP null mice after MCAO. Infarct volume was measured 24 h after stroke; infarct volume was expressed as percentage of the contralateral hemisphere (n = 7 per group). *P < 0.05 (minocycline-treated group versus vehicle-treated group, two-tailed Student t-test). Data were expressed as mean ± s.e.m.
Discussion
Consistent with previous reports from other investigators, our data show that minocycline is an effective neuroprotectant in males (Alano et al, 2006; Yrjanheikki et al, 1999), however, this drug is completely ineffective in reducing ischemic damage in females. This is the first report of ischemic sex differences in the response to a drug that is currently in clinical trials for stroke.
Ischemia leads to a dramatic increase in the activation of the DNA repair enzyme PARP (Andrabi et al, 2006), which uses NAD+ as a substrate to form polymers on ADP-ribose. Recently, it has been discovered that PAR polymers are the major trigger for stroke-induced mitochondrial release of apoptosis- inducing factor (Andrabi et al, 2006). Apoptosis-inducing factor then translocates to the nucleus and leads to caspase-independent cell death (Andrabi et al, 2006). However, the majority of data implicating the PARP/apoptosis-inducing factor pathway as a key cell death pathway have been derived from experiments that used only male mice. Emerging data from several laboratories have suggested that ischemic cell death pathways may differ in males and females (McCullough et al, 2005). In the female brain, ischemic cell death is triggered by activation of caspases (Lang and McCullough, 2008; Liu et al, 2009), rather than PARP activation. Minocycline dramatically reduced stroke-induced PAR formation in both males and females, showing that minocycline has equivalent ‘downstream’ effects in the male and female brain. However, the dramatic reduction in PAR by minocycline does not translate into neuroprotection in females. This suggests that the PARP-1 pathway, although activated in both sexes, does not mediate cell death in the female brain.
Interestingly, male PARP-1 null mice treated with minocycline had significantly larger infarcts than vehicle-treated knockout mice. This suggests that minocycline has effects beyond that of PARP inhibition. Both pharmacological and genetic approaches have limitations. Pharmacological agents often have unknown nonspecific effects on other pathways, and compensatory changes can occur in genetic models, especially when the gene is missing during early development. In any case, our data clearly show that minocycline is not protective in PARP-1 null male mice, suggesting that neuroprotective effect is mediated in part through PARP inhibition.
Minocycline is a pleiotropic agent with known antiinflammatory properties (Yrjanheikki et al, 1999). As inflammation in the brain may occur later after stroke (Rami et al, 2008), we extended our survival time to 72 h to ensure that there was no subsequent infarct growth that would ameliorate the sex differences seen after treatment. Minocycline continued to have a dramatic sex-dependent neuroprotective effect. It is well known that PARP inhibitors are antiinflammatory in stroke as well as in other models of inflammation (Koh et al, 2004). However, similar to our results, loss of PARP decreased the endothelial inflammatory response to LPS injection in males, but not in females (Mabley et al, 2005), suggesting that PARP-mediated sex differences are not limited to the CNS.
A recent open-label clinical trial suggested a potential benefit of minocycline treatment in ischemic stroke patients (Lampl et al, 2007). However, this initial trial was small (152 patients), with a low percentage of female patients (35%), and it was not powered or designed to evaluate sex differences. Larger trials will determine whether this sex difference has clinical relevance, but this requires an initial awareness of possible sex-dependent effects of treatment.
Do these sex differences matter? Several reports have shown sex differences in the response to pharmacological agents that are commonly used in stroke patients (Kassell et al, 1996; Ridker et al, 2005). For example, Tirilazad reduced mortality and enhanced functional recovery after subarachnoid hemorrhage in men, but not in women (Kassell et al, 1996). Aspirin reduces ischemic stroke risk more robustly in healthy women compared with men (Ridker et al, 2005), where much of the benefit of aspirin was from a reduction of cardiovascular events. Finally, sex differences in the response to acute stroke therapies (i.e., tissue plasminogen activator) are also emerging (Hill et al, 2006; Kent et al, 2005). The Stroke Therapy Academy Industry Roundtable recommends that neuroprotective studies be performed in both male and female rodents (Recommendations for standards regarding preclinical neuroprotective and restorative drug development, 1999), yet the majority of preclinical studies continue to examine only male animals. It is imperative that investigators are aware of the potential for erroneous conclusions when attempting to translate promising experimental findings into a clinical population at risk for stroke that includes women.
Supplementary Material
Acknowledgments
This work was supported by NIH R01 NS050505 and NS055215 (LDM).
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
References
- Alano CC, Kauppinen TM, Valls AV, Swanson RA. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc Natl Acad Sci USA. 2006;103:9685–9690. doi: 10.1073/pnas.0600554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano CC, Ying W, Swanson RA. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J Biol Chem. 2004;279:18895–18902. doi: 10.1074/jbc.M313329200. [DOI] [PubMed] [Google Scholar]
- Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW, Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, Hurn PD, Poirier GG, Dawson VL, Dawson TM. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci USA. 2006;103:18308–18313. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Wilson MA, Matsushita H, Zhu C, Lange M, Gustavsson M, Poitras MF, Dawson TM, Dawson VL, Northington F, Johnston MV. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem. 2004;90:1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- Hill MD, Kent DM, Hinchey J, Rowley H, Buchan AM, Wechsler LR, Higashida RT, Fischbein NJ, Dillon WP, Gent M, Firszt CM, Schulz GA, Furlan AJ. Sex-based differences in the effect of intra-arterial treatment of stroke: analysis of the PROACT-2 study. Stroke. 2006;37:2322–2325. doi: 10.1161/01.STR.0000237060.21472.47. [DOI] [PubMed] [Google Scholar]
- Jagtap P, Soriano FG, Virag L, Liaudet L, Mabley J, Szabo E, Hasko G, Marton A, Lorigados CB, Gallyas F, Jr, Sumegi B, Hoyt DG, Baloglu E, VanDuzer J, Salzman AL, Southan GJ, Szabo C. Novel phenanthridinone inhibitors of poly (adenosine 5′-diphosphate-ribose) synthetase: potent cytoprotective and antishock agents. Crit Care Med. 2002;30:1071–1082. doi: 10.1097/00003246-200205000-00019. [DOI] [PubMed] [Google Scholar]
- Kassell NF, Haley EC, Jr, Apperson-Hansen C, Alves WM. Randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in Europe, Australia, and New Zealand. J Neurosurg. 1996;84:221–228. doi: 10.3171/jns.1996.84.2.0221. [DOI] [PubMed] [Google Scholar]
- Kauppinen TM, Swanson RA. Poly(ADP-ribose) polymerase-1 promotes microglial activation, proliferation, and matrix metalloproteinase-9-mediated neuron death. J Immunol. 2005;174:2288–2296. doi: 10.4049/jimmunol.174.4.2288. [DOI] [PubMed] [Google Scholar]
- Kent DM, Price LL, Ringleb P, Hill MD, Selker HP. Sex-based differences in response to recombinant tissue plasminogen activator in acute ischemic stroke: a pooled analysis of randomized clinical trials. Stroke. 2005;36:62–65. doi: 10.1161/01.STR.0000150515.15576.29. [DOI] [PubMed] [Google Scholar]
- Koh SH, Park Y, Song CW, Kim JG, Kim K, Kim J, Kim MH, Lee SR, Kim DW, Yu HJ, Chang DI, Hwang SJ, Kim SH. The effect of PARP inhibitor on ischaemic cell death, its related inflammation and survival signals. Eur J Neurosci. 2004;20:1461–1472. doi: 10.1111/j.1460-9568.2004.03632.x. [DOI] [PubMed] [Google Scholar]
- Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med. 2008;6:33. doi: 10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Li Z, Li J, Siegel C, Yuan M, McCullough L. Sex differences in caspase activation after stroke. Stroke. 2009 doi: 10.1161/STROKEAHA.108.538686. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabley JG, Horvath EM, Murthy KG, Zsengeller Z, Vaslin A, Benko R, Kollai M, Szabo C. Gender differences in the endotoxin-induced inflammatory and vascular responses: potential role of poly(ADP-ribose) polymerase activation. J Pharmacol Exp Ther. 2005;315:812–820. doi: 10.1124/jpet.105.090480. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- Rami A, Bechmann I, Stehle JH. Exploiting endogenous anti-apoptotic proteins for novel therapeutic strategies in cerebral ischemia. Prog Neurobiol. 2008;85:273–296. doi: 10.1016/j.pneurobio.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable (STAIR) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.