Abstract
Evidence from developmental and regeneration studies of the cochlea and other tissues give reason to hypothesize a role for non-neural cells in the growth and regeneration of cochlear spiral ganglion nerve fibers. We examined the spontaneous associations of regrowing neurites and non-neural cells in mixed cultures of dissociated newborn mouse spiral ganglia. After 7 days in vitro, non-neural cells formed a confluent layer in the culture well. Regrowing neurites grew atop this layer, forming non-uniform patterns that were similar to those formed by endogenously expressed laminin-1, entactin and integrin β4, but not fibronectin or tenascin. In cultures grown for 42 hours and maintained in three different growth media, all regrowing neurites were preferentially associated with spindle-shaped non-neural cells. The spindle shaped cells incorporated BrdU in culture and were immunoreactive for the proteins S100, laminin-1, laminin-2, Sox10, P75 and connexin29 but negative for fibronectin and GFAP. These cells existed in the culture within a much larger, general population of fibronectin positive cells. Immunolabeling of fixed cochleas from neonatal mice localized Sox10, P75 and connexin29, to peripheral nerve bundles. The observed expressions of protein markers and the bipolar, spindle shape of the neurite-associated cells indicates that they are derived in vitro from the original Schwann or satellite cells in the ganglion or spiral lamina. The spontaneous and preferential association of neurites in culture with mitotic Schwann cells highlights the potential contribution neurite-Schwann cell interactions may have in promoting the growth and regrowth of damaged spiral ganglion neurons in the cochlea.
Keywords: cochlea, regeneration, neurite, guidance, Schwann, in vitro
INTRODUCTION
In the mammalian peripheral nervous system, Schwann cells contribute to the mechanisms that underlie neuronal survival, growth and regeneration both in vivo and in vitro (Fallon, 1985; Kleitman et al., 1988a; Kleitman et al., 1988b; Seilheimer and Schachner, 1988; Meyer and Birchmeier, 1995; Chen et al., 2005; Morris et al., 2006). The neurite-promoting effects of Schwann cells on nerve fibers are mediated by the expression of trophic factors as well as by the expressions of cell surface and extracellular matrix proteins that are permissive for neurite growth (Kleitman et al., 1988a; Kleitman et al., 1988b; Seilheimer and Schachner, 1988; Letourneau et al., 1990; Schneider-Schaulies et al., 1990; Hansen et al., 2001; Wanner and Wood, 2002; Wanner et al., 2006b). The positive effects of Schwann cells on neurite growth are exerted mainly by immature, mitotic cells that have not yet begun to myelinate the nerve process (Domeniconi et al., 2002; Domeniconi and Filbin, 2005). After nerve damage, however, mature, myelinating Schwann cells are able to backtrack along their differentiation program to a more immature state that will again support neurite growth (Cheng and Zochodne, 2002).
In the cochlea, the role of non-neural cells during spiral ganglion neurite growth and their potential for aiding in neurite regeneration has not been closely examined. In adult cochleas, Schwann cells normally myelinate the peripheral nerve fibers of Type I spiral ganglion neurons along their length until they reach the habenula perforata, after which the fibers continue to grow unmyelinated into the epithelial region. In a mouse model in which Schwann cells are absent from the inner ear (erbB2 null mice), the developing cochlea displays nerve fiber projection deficits that are consistent with the idea that Schwann cells are necessary for the development of the correct, organized nerve fiber patterns in the organ (Morris et al., 2006).
In the present work, we have used cell culture of the dissociated newborn mouse spiral ganglion to examine the spontaneous associations of regrowing neurites with non-neural cells.
EXPERIMENTAL PROCEDURES
Animals
Newborn and postnatal day (P) 1 and P2 mice, CD-1 strain (Charles River Laboratories, Wilmington MA, USA) were used for preparation of cultures. Mice aged P3 and P9 were used for immunohistology. The care and use of the animals in this study were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and was approved by the Animal Care and Use Committee of Northwestern University. Every effort was made to minimize the suffering of research animals and to limit their numbers.
Reagents
Cell culture
Brain derived neurotrophic factor (BDNF) and neurotrophic factor 3 (NT3) (Promega, Madison, WI, USA; final 8 ng/ml); Fetal bovine serum – FBS (Sigma-Aldrich, St. Louis MO, USA; purchased and subsequently heat inactivated, final 10%); N2 Supplement (Invitrogen, Carlsbad, CA, USA); Leukemia Inhibitory Factor (LIF; Sigma-Aldrich, 50 ng/ml final); Bone morphogenetic protein 4 (BMP4; R&D Systems, Minneapolis, MN, USA; final 25–50 ng/ml); Bromodeoxyuridine (BrdU; BrdU labeling and detection kit II, Roche Applied Science; 1:1000); poly-D-lysine (Sigma-Aldrich P7280, 100µg/ml); laminin (Sigma-Aldrich, L2020, 10µg/ml); murine fibronectin (Invitrogen, 10µg/ml); human-tenascin C (Chemicon, now Millipore, Billerica MA; 10µg/ml);
Immunolabeling
mouse anti-βIII tubulin (TuJ1; 1:500–1:1000; Covance, Berkeley, CA); rabbit anti-βIII tubulin (Covance, Berkeley, CA; 1:5000); rabbit anti-mouse fibronectin (1:5000; Chemicon AB2033); rat anti-entactin (1:2500; Chemicon MAB 1946); rat anti-laminin (1:500, Chemicon MAB 1914); rat anti-mouse CD104 Integrin β4 chain (1:250; Pharmingen clone 346-11A); peroxidase labeled (Fab’)2 of either rat anti-mouse IgG (1:100), donkey anti-rabbit IgG (1:500), donkey anti-rat IgG (1:500), and alkaline phosphatase labeled (Fab’)2 of donkey anti-rabbit IgG, (1:1000) all from Jackson Immunoresearch (West Grove, PA); Vectastain DAB staining kit and Vector Blue alkaline phosphatase substrate kit (Vector Laboratories Burlingame, CA); DAKO Glycergel and DAKO Fluorescent mounting medium (DAKOcytomation, Carpinteria, CA); Alexafluor 594 labeled goat anti-mouse or anti-rabbit IgG (1:2000) and Alexafluor 488 labeled goat anti-rabbit IgG (1:2000), goat anti-rat IgG (1:500), or goat anti-mouse IgG (1:2000), and nuclear yellow, all from Invitrogen (Carlsbad,CA); rabbit anti-Sox10 (1:5000, kindly provided by Dr. Michael Wegner, Institut für Biochemie, Universität Erlangen-Nürnberg; (Stolt et al., 2003)); rabbit anti-connexin29 (1:100, Zymed/Invitrogen); rabbit anti-p75 (1:500, Chemicon); rabbit anti-myelin basic protein (Biotrend); rabbit anti-S100 (Sigma); rabbit anti-GFAP (DakoCytomation); rabbit anti-P0 (gift of Dr. Marie Filbin, Hunter College, NY).
Cell Culture
Cultures of dissociated spiral ganglia were prepared as previously described in detail (Whitlon et al., 2006; Whitlon et al., 2007). Cultures were maintained with three different medium additions: Control cultures contained BDNF, NT3, 10% FBS and N2 supplement. LIF cultures additionally contained Leukemia Inhibitory Factor; BMP4 cultures replaced LIF with Bone Morphogenetic Protein 4. After two days in culture, medium was changed every other day. For BrdU labeling, BrdU was added to wells two hours after plating and maintained throughout the culture period. Cultures were routinely plated on poly-D-lysine/laminin coated 96 well plates (Whitlon et al. 2007; Whitlon et al. 2006) or on glass or plastic culture slides (Lab-Tek). For some experiments, laminin was replaced by fibronectin or tenascin.
Immunolabeling
Immunocytochemistry: Both enzyme-linked and fluorescent methods were used to label cultures as detailed in Table I. Enzyme-linked procedures were used with cultures in 96 well plastic tissue culture plates. Cells for immunofluorescence were cultured on glass or plastic (Nunc, Lab-Tek) multiwell slides. Neurons were identified using either a mouse or rabbit antibody directed against the neural βIII tubulin. Immunohistochemistry Cochleas were embedded and sectioned at 15 µm as described (Whitlon et al., 2001). After air drying for 2 hours, the slides were treated with 0.1% Triton X-100 in TBS for 2 hours at room temperature in a humid chamber. After rinsing in TBS, the slides were blocked for 1 hour in TBS containing 5% BSA/10% normal goat serum. The primary antibody (anti-βIII tubulin) was then added in 5%BSA/10% normal goat serum/0.1%Triton X-100 and incubated overnight at 4°C. After washing the slides in TBS, the secondary antibody was applied (Alexafluor 488 or 594 labeled goat anti-rabbit IgG or goat anti-mouse IgG; 1:2000) 0.1% Triton X-100 for 4 hours at room temperature in the dark. After washing in TBS the slides were again blocked for 1 hour and the second primary antibody was added and maintained overnight at 4°C. After washing in TBS, the second secondary antibody was added in 0.1% Triton X-100 for 4 hours at room temperature. The slides were washed with TBS and treated for 5 minutes with a .0001% solution of nuclear yellow, washed again in TBS and mounted in DAKO fluorescent mounting medium.
Table I.
Outline of procedures for single and double labeled enzyme linked labeling and double fluorescent labeling of cultures.
| Enzyme-Linked | Fluorescent | ||
|---|---|---|---|
| Single | Double | Double | |
| Fix | 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.4 × 1 hr @ RT | Same | 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.4 × 15 min |
| Wash | TBS | TBST | TBS |
| Quench | TBS/methanol/30% hydrogen peroxide/water (5:1:0.4:3.6) × 10 min @ RT | Same | Skip |
| Wash | TBS | TBST | Skip |
| Solubilize | 0.3% Triton × 2 hr @ RT | Same | Same |
| Wash | 0.01% ovalbumin in TBS or TBST | Same | TBS |
| Block | 0.1% ovalbumin in TBS or 10% NDS+2% NFDM in TBS × 1 hr @ RT | 10% NDS+2% NFDM in TBS for 1 hr @ RT | 5% BSA/10% NGS in TBS × 1 hr @ RT |
| 1° antibody | Diluted in 0.03% Triton in TBS or in 10% NDS+2%NFDM in 0.3% Triton in TBS. 3 days @ 4°C | TuJ1 in blocking solution overnight @ 4°C | TuJ1 or rabbit anti-βIII tubulin diluted in 5% BSA+10% NGS +0.3% Triton overnight @ RT |
| Wash | 0.01% ovalbumin in TBS or in TBST | TBST | TBS |
| 2° antibody | Diluted in 0.3% Triton in TBS or 2% NFDM in 0.3% Triton in TBS × 4 hours @ RT | TBS containing 2% NFDM and 0.3% Triton × 4 hours @ RT | Alexafluor conjugated antibody diluted in 0.1% Triton in TBS × 4 hours @ RT; dark |
| Wash | TBS or TBST | TBST | TBS |
| Visualization | Vectastain DAB staining kit | Same | Skip |
| Mounting | 2–4 drops DAKO Glycergel per well | Skip | Skip |
| Wash | Skip | TBS | |
| Block | 10% NDS+2% NFDM in TBS for 1 hr @ RT | 5% BSA + 10% NGS in TBS × 1 hour @ RT | |
| Second 1° Antibody | Diluted in 10% NDS+2% NFDM+0.3% Triton × overnight @ 4°C | Diluted in the blocking solution × overnight @ RT | |
| Wash | TBST | TBS | |
| Second 2° Antibody | AP labeled antibody diluted in TBS containing 2% NFDM and 0.3% Triton × 4 hours @ RT | Alexafluor conjugated antibody diluted in 0.1 % Triton in TBS × 4 hours @ RT; dark | |
| Wash | TBST, then TBS | TBS | |
| Visualize | AP substrate kit, 15 minutes; @ RT; dark | ||
| Wash | Tris-HCl, pH 8.2, then TBS | ||
| Mount | 2–4 drops Glycergel | ||
| Visualize nuclei | 0.001 % nuclear yellow × 5 min @ RT | ||
| Wash | TBS | ||
| Mount | DAKO fluorescent mounting medium | ||
Abbreviations: NDS, normal donkey serum; NFDM, non-fat dry milk; RT, room temperature; Triton, Triton-X-100; BSA, Bovine serum albumin. Words in italics refer to procedures used only with mouse monoclonal antibodies.
Digital Photography
Cultures and tissue sections were photographed with a Nikon DXM1200 camera attached to a Zeiss Axioscope microscope under phase, partial Nomarski or fluorescence optics. Digital processing of images was limited to reorientation of photographs, converting to grayscale, resizing and contrast enhancement with either Adobe Photoshop CS or Metavue software.
RESULTS
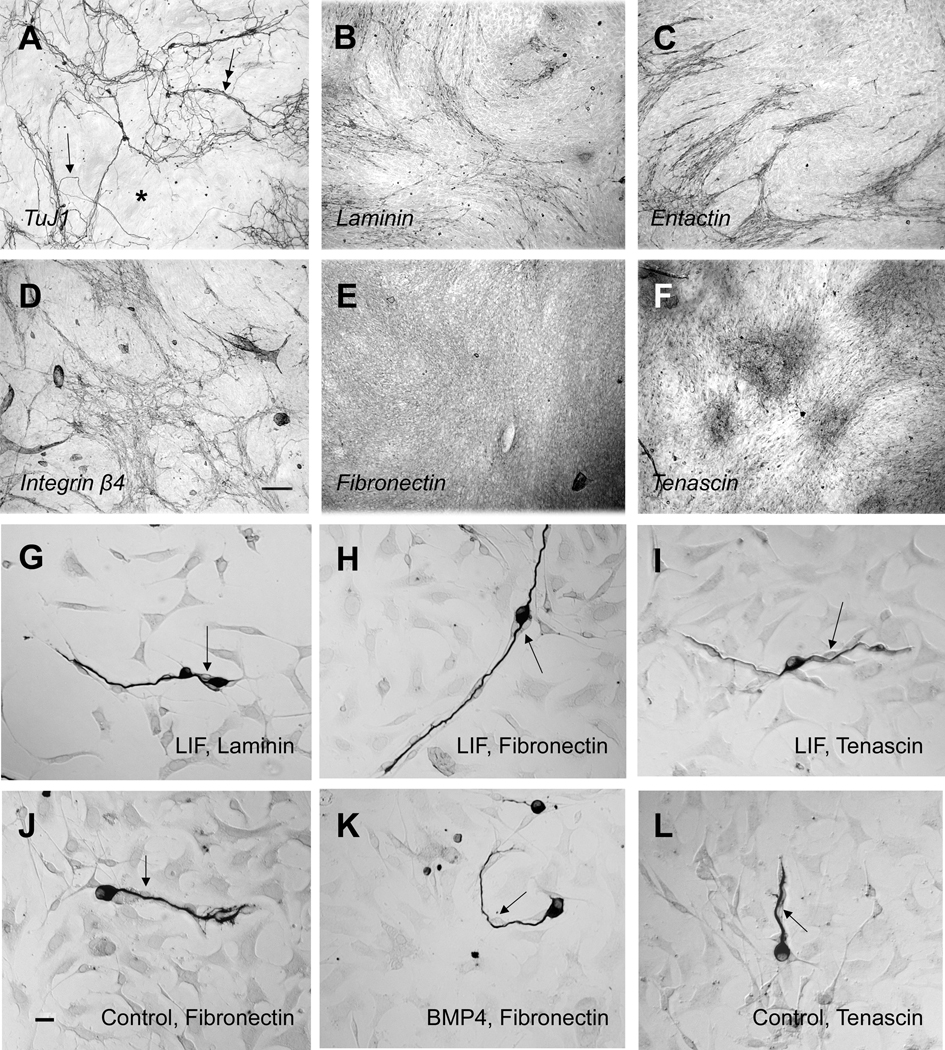
Spiral ganglion cultures maintained for 5–7 days generated a layer of non-neural cells that covered the entire growth surface. The overall pattern of neurite growth atop this surface was non-uniform (Fig. 1A). In some regions of the well, neurons extended long neurites that sometimes grew singly (Fig. 1A, arrow), sometimes grew in bundles (Fig. 1A, double arrow), and sometimes completely bypassed areas of underlying cells (Fig. 1A, asterisk). This behavior suggested that the underlying surface of non-neural cells supporting this neurite growth might also be non-uniform. To determine if the underlying cells elaborated extracellular matrix or cell surface proteins in a non-random pattern, similar cultures were immunolabeled for laminin-1 (Fig. 1B), entactin (Fig. 1C), integrin β4 (Figure 1D), fibronectin (Fig. 1E) or tenascin (Fig. 1F). The patterns formed by laminin, entactin and integrin β4, but not fibronectin or tenascin, were filamentous in appearance and were similar to that created by the growth paths of the neurites. At this stage, fibronectin labeling is nearly uniform in the dish, while tenascin expression forms a patchy pattern of immunostained islands of cells. Double label of a 5-day culture shows that neurites co-localize with cells in the culture that are laminin-1 positive (Figure 2A).
Figure 1.
A–F. Examples of 7-day, confluent cultures immunolabeled as shown in italics. G–L. Examples of 42 hour cultures maintained under different growth factor and substrate conditions and immunolabeled with TuJ1 to highlight neurons. (A). The nerve fiber pattern in this longer term culture is non-uniform. Fibers extend in some regions and avoid others (asterisk), growing singly (arrowhead) but also, often growing in bundles (double arrowhead) atop the underlying non-neural cells. (B–D) Endogenously generated laminin-1 (B), entactin (C) or integrin β4 (D) form filamentous, non-uniform patterns in the culture; (E) Fibronectin is expressed fairly homogenously across the nonneural cells. (F) Tenascin immunoreactivity is observed in islands of stain across the culture well. (G–L) Under all tested conditions (control, LIF or BMP4 medium and laminin, fibronectin or tenascin coating of the culture well), neurites grow only in association with a type of non-neural cell that is usually spindle shaped (arrows).Neurites and spindle-shaped cells retain their associations even throughout abrupt changes in the direction of growth (see K).
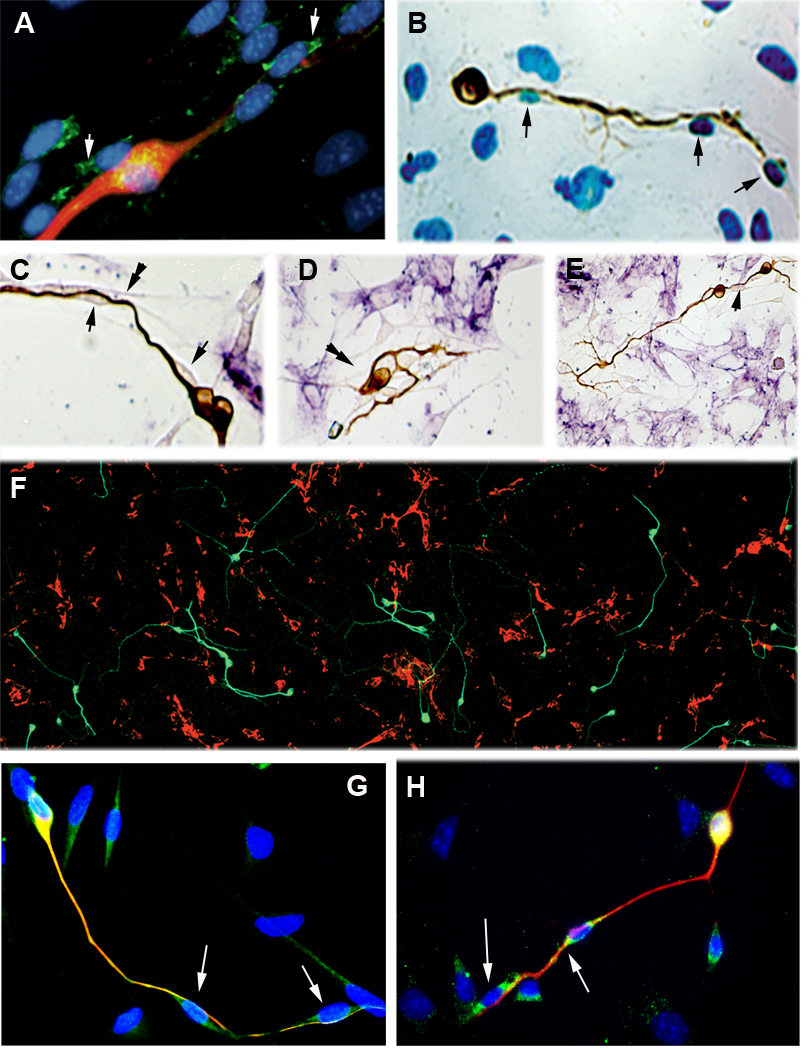
Figure 2.
42 hour cultures. (A) Immunolabeled for neurons (TuJ1, red), Laminin-1 (green). Nuclei (blue). The red neuron is associated with cells that express laminin-1. The cells at the lower right are not immunoreactive for laminin-1. (B) Cells lining the regrowing neurites (brown) incorporate BrdU (blue) into their nuclei (arrow) indicating that they were produced by mitotic division in culture. (C–E). Double immunolabeled for fibronectin (purple) and TuJ1 (brown). Neurites grow on a class of non-neural cells (arrows) that do not express fibronectin. The association of the neruites with these cells is maintained even when the direction of growth changes (double arrowhead). (F) Overview, double immunolabel for fibronectin (red) and TuJ1 (green). Only rarely can TuJ1 labeled neurites been see to cross regions of fibronectin immunoreactivity. (G) Cells lining the neuron (TuJ1, red) are S100-positive (green). Merge (yellow) shows that neurons also express S100. Nuclei, blue. Cells in the upper right do not express S100. (H) Laminin -2 positive cells (green) line the TUJ1-positive neuron (red). Nuclei, blue. Merge (yellow) demonstrates laminin-2 around the neuronal soma.
The association of non-neural cells with regrowing neurites was next examined at an earlier time after plating (42 hours), when the protein coated dish was partially free of cellular covering and the individual neurites were shorter and more easily followed. We questioned whether differences in neuronal morphology, in substrate proteins or in medium composition would result in neurites growing unaccompanied on the substrate coating of the dish. It was shown previously (Whitlon et al., 2006; Whitlon et al., 2007) that when dissociated spiral ganglion is cultured on a poly-D-lysine/laminin substrate under control conditions (see methods), the 42 hour surviving neurons comprise a mixture of three main morphological types: monopolar, bipolar, and neurite-free. Inclusion of LIF in the culture medium increases neuronal survival. The resulting neuronal population has a higher percentage of bipolar neurons and longer neurites than in control cultures. On the other hand, inclusion of BMP4 in the culture medium, while also increasing survival, generates a population with a higher percentage of monopolar and neurite-free neurons, and the neurites are shorter than in control cultures. In the present study, dissociated spiral ganglia were cultured with control, LIF and BMP4 medium on dishes coated with laminin or with fibronectin or tenascin, proteins that are known to support neurite growth from other types of neurons (Rigato et al., 2002; Yong et al., 1988). On all substrates LIF and BMP4 increased survival and produced the same differences in neuronal morphology as described above for laminin growth. In all types of cultures regardless of protein substrates, composition of morphologic types, or growth medium, neurites grew only in association with non neural cells, usually spindle-shaped, rather than directly on the protein coated surface of the culture well (Examples in Figs. 1G–1L).
To determine whether the spindle shaped cells were those that were originally plated or were derived from the plated cells by mitotic division, cells were cultured in the presence of BrdU. The spindle shaped cells incorporated BrdU during the culture period, demonstrating that they were derived from mitotic division in vitro (Figure 2B).
The fairly ubiquitous distribution of fibronectin in the older cultures from Fig. 1E, raised the question of whether during the early stages, regrowth of the nerve fibers was indifferent to the fibronectin positive regions or whether they showed a preference for regions devoid of fibronectin positive cells. Cultures were double labeled with antibodies directed against neurons (TuJ1) and against fibronectin. At 42 hours in culture, most of the cells in the well were positive for fibronectin. Nonetheless, regrowing nerve fibers could rarely be seen in association with fibronectin expressing cell types (Figs. 2C–2F). The growth of nerve fibers and the paths of the fibronectin negative cells were so strongly linked that the association was preserved even when the growth paths made abrupt curves and bends (Fig. 1K;Fig 2C, 2D, double arrows). These observations indicate that given a choice between fibronectin positive and negative cells, neurites associate with fibronectin negative cells.
Based on their spindle shaped morphology, incorporation of BrdU in culture, lack of fibronectin labeling and tight association with neurites, we hypothesized that these were cells derived from Schwann or satellite cells of the original preparation. To determine the identity of the spindle shaped cells, we immunolabeled cells with antibodies directed against proteins that label Schwann cells in the cochlea or other regions of the nervous system – the transcription factor Sox10, the gap junction protein connexin29, the low affinity NGF growth factor receptor P75; S100; laminin-2; GFAP; or myelin basic protein (Britsch et al., 2001; Jessen and Mirsky, 2005; Tang et al., 2006). We triple labeled the cultures for the neuronal marker βIII tubulin, one of the Schwann cell markers above, and a nuclear visualization dye. At 42 hours in culture, no cells were positive for GFAP or myelin basic protein (data not shown).
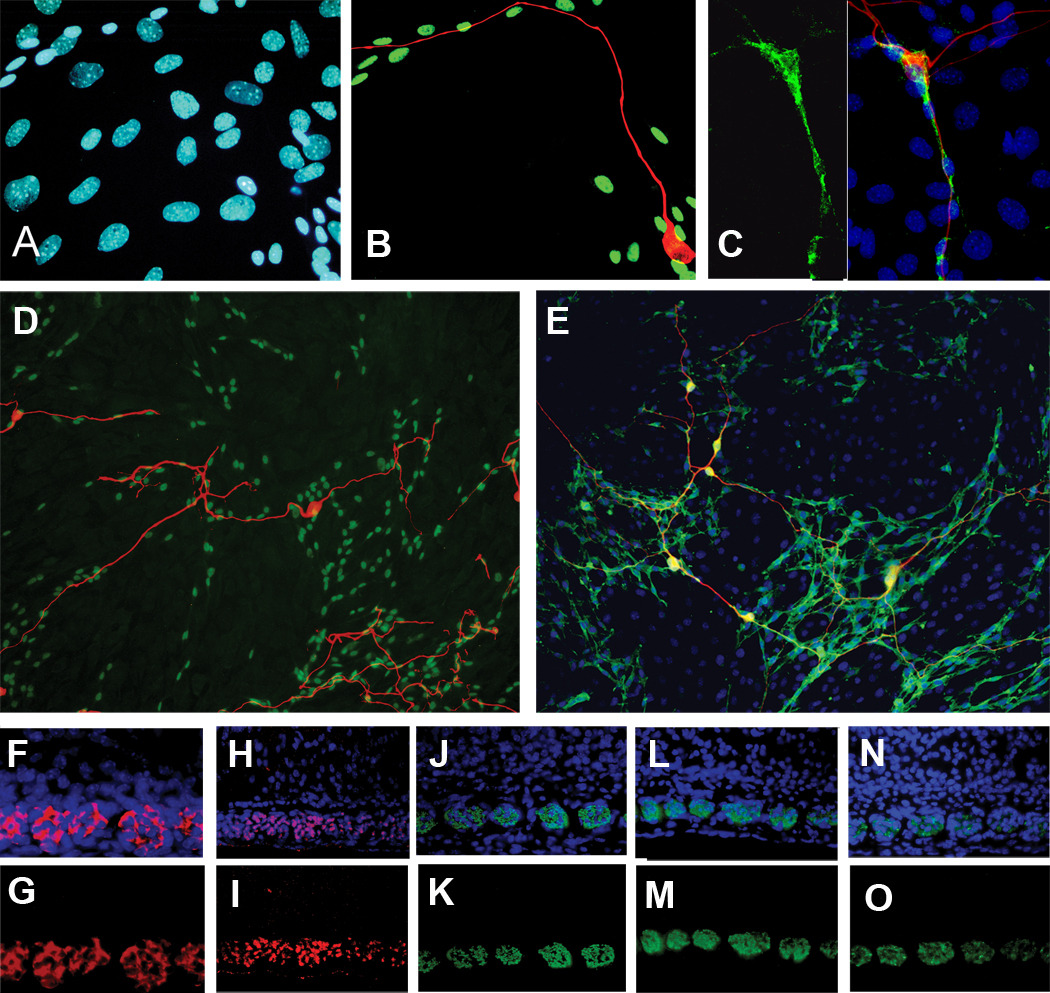
Fig 2G–H and fig 3A–E demonstrate the expressions of Schwann cell markers S100 (Fig. 2G), Laminin-2 (Fig. 2H), Sox10 (Fig. 3A,B,D), connexin29 (Fig. 3C) and P75 (Fig. 3E) in neurite-associated non-neural cells. Nuclear labeling in Fig. 3A (the same microscopical field as Fig. 3B) suggest a variety of cell types displaying different nuclear morphologies. Fig 3B shows a merged view of immunolabeling for Sox10 (green) and βIII tubulin (red). Sox10 is expressed only in a subset of the nuclei shown in Fig. 3A. The red, βIII tubulin-positive neuron extends its neurite preferentially over the Sox10 positive cells. In other cultures, the spindle shaped cells were labeled with antibodies directed against connexin29. Fig. 3C shows the field of nuclei of the cells in the culture (blue) and the small subset of cells that express connexin29 (green) that are spindle shaped and lie along the path of the TuJ1 positive nerve fiber (red). Figures 3D and 3E show older cultures, 5 days in vitro, and demonstrate that a field of cells that express Sox10 (Fig. 5D) or P75 (Fig. 5E) underlie the growing neurites.
Figure 3.
A–B, 42 hour culture, same microscopical field. (A) Blue nuclei depict the locations of all cells in the field. (B) Green nuclei show that a subset of cells that express Sox10 lie along the growth path of the neuron (red). (C) Left, connexin29 labeling. Right, same microscopical field, a neuron (red) associates with spindle shaped cells that express connexin29 (green) amid a field of blue nuclei. No other cells in the field are positive for connexin29; (D). 5-day culture. Red, TuJ1 (neurons), green, subset of nuclei expressing Sox10. (Nuclei of confluent cell layer are unlabeled in this image for clarity). Nerve fibers grow only on the field of Sox10 positive cells. (E) 5-day culture. Red, TuJ1 (neurons), Green P75, Yellow, merge. Nuclei, blue. Nerve fibers grow only on the region of cells expressing P75. Neurons are immunopositive for P75. (F–O): Cross sections of the radial bundles in fixed cochleas. (F,G) Postnatal day 3. Same microscopical field: blue, nuclei; red, P75. (H,I) Postnatal day 9. Same microscopical field: blue, nuclei; red Sox10; pink, nuclei expressing Sox10. (J,K) Postnatal day 9. Same microscopical field. blue, nuclei; green, P0. (L,M) Postnatal day 9. Same microscopical field. Blue, nuclei; green, myelin basic protein. (N,O) Postnatal day 9. Same microscopical field. Blue nuclei; green, connexin29.
To validate the Schwann cell markers in vivo, we show that in histologic cross sections of radial bundles in the P3 or P9 cochleas, P75 (Figs 3F,G), Sox10 (Figs 3H,I), and connexin29 (Figs 3N,O), label the same regions as the myelin markers P0 (Figs 3J,K) and myelin basic protein (Figs 3L,M.).
DISCUSSION
The association with and possible dependence of neurite growth on non-neural cells has not been well studied in the cochlea. Here we examined the relationships between regrowing auditory neurites and non-neural cells in vitro. We approached the problem with a little used method – that of plating dissociated tissue without further purification of cells - and relied on immunocytochemical labeling for cellular characterization. After spiral ganglia and surrounding tissue were dissociated and plated, the cells were permitted to find their own cellular associations among the mixed cell types. In this “choice” experiment, in which all cell types from the region of the spiral ganglion were included and different matrix proteins were supplied as potential growth substrates, all regrowing neurites preferentially associated with a particular subclass of spindle-shaped, non-neural cells, which this work further characterized and identified.
Finding that the spindle-shaped, neurite associated non-neural cells lacked fibronectin immunoreactivity and incorporated BrdU in vitro, we hypothesized that they were derived in culture from the original Schwann or satellite cells within the cochlear ganglia and spiral lamina. However, Schwann cells have not been extensively studied in the cochlea (Rio et al., 2002; Tang et al., 2006; Hurley et al., 2007) and there have been even fewer characterizations of cochlear Schwann cells in vitro (Hansen et al., 2001). A small number of Schwann cell markers validated in other regions of the nervous system (Zorick and Lemke, 1996; Jessen and Mirsky, 2005)– such as P75, connexin29, S100, myelin basic protein, Sox10 and P0 – have also been demonstrated in the cochlea by immunolabeling or in situ hybridization (Gestwa et al., 1999; Knipper et al., 1999; Watanabe et al., 2000; Coppens et al., 2001; Tang et al., 2006; Hurley et al., 2007), but not all of these are specific to Schwann cells in this organ. For example, P75, S100 and Sox10 have also been localized to cells in the cochlear epithelium and/or spiral ligament (Foster et al., 1994; Gestwa et al., 1999; Knipper et al., 1999; Watanabe et al., 2000). These cell types do not contaminate our cultures because both of these regions were carefully dissected away before dissociating cells. In contrast, connexin29 immunoreactivity is expressed strongly only by Schwann or satellite cells in the cochlea (Eiberger et al., 2006; Tang et al., 2006). It is interesting that in intact cochleas, only weak GFAP labeling is observed in the spiral ganglion or spiral lamina, even at older ages (Rio et al., 2002) since GFAP is a later marker for Schwann cells in the sciatic nerve (Mirsky et al., 2008). Strong expression of GFAP is also a marker for CNS astrocytes (Bignami et al., 1972), and its absence in our cultures indicates low, if any, contamination by astrocytes. Taken together, the P75, Sox10, laminin-1, laminin-2 and connexin29 labeling of our cells, their mitotic division in vitro, and their lack of GFAP or fibronectin labeling, indicates that the neurite associated cells in our cultures descended from Schwann or satellite cells in the original dissociated preparation of the spiral ganglion and surrounding tissue.
In 42 hour dissociated cultures grown on poly-D-lysine/laminin coated plastic multiwell dishes, neurites have such a strong preference for the spindle shaped, fibronectin-negative Schwann cells that they rarely associate with the extracellular matrix protein coating of the culture dish (laminin, fibronectin or tenascin) or with fibronectin positive cells. A similar association is detected on matrix coated glass, but there seem to be fewer cells lining the nerve fibers at the time of assay, although in the absence of electron microscopy, it is difficult to say whether fine processes from the Schwann cells extend beneath the entire length of the neurite. The present work does not answer the question of which comes first in the progression, neurite extension or Schwann cell association. At the times we assay the cultures, 42 hours, 5 and 7 days, we rarely, if ever, see growth cones or the ends of nerve fibers devoid of contact with Schwann cells. Such a question is better answered with time-lapse studies than with the strictly defined assay times used here. This work also raises, but cannot presently answer the question of the derivation of the spindle shaped cells. Are the progenitors in contact with the neuron at the time of plating and able therefore to divide and travel down the growing nerve fiber; or, are the progenitors recruited to the nerve fiber?
Neurites and glial cells collaborate to permit the progression of neurite growth and regeneration (Mirsky and Jessen, 1999; Chen et al., 2005). The present results are consistent with the view that Schwann cells in these cultures, whatever their derivation, arrive at the growing tip of the neurite, thereby creating a permissive local environment for further extension of the nerve fiber. In general, during development throughout the nervous system in vivo, “guidance” of growing nerve fibers is thought to depend largely on the interactions that growing nerve growth cones have with their microenvironments. The consistent association of regrowing spiral ganglion nerve fibers with Schwann cells in the culture supports the view that actual “pathfinding” in vitro may be an indirect process. Neurites may find Schwann cell surfaces a permissive growth environment, but the pathway they traverse seems more to do with the cues discerned by the Schwann cells than the interactions of the neurites with other elements of the culture environment. This view would be consistent with the work of Wanner et al. (2006a) who found that in developing E14 rat embryos, membranes of Schwann cell surfaces covered a constant 80% of the neuronal growth cone surface as it was nearing its target in the forelimb. It was concluded that at the nerve front it was the Schwann cell precursor, rather than the growth cone itself, that was most exposed to the extracellular environment.
In cochleas, when the hair cell targets of spiral ganglion neurons are destroyed by acoustic overstimulation, antibiotics or other drug toxicity, some of the disconnected spiral ganglion neurons die – the exact proportion differs among species (Teufert et al., 2006). The peripheral fibers of the remaining neurons degenerate, perhaps as far as to the cell soma. In humans, enough of these once bipolar neurons remain connected to the brain stem to permit the functionality of cochlear implants, which rely on electrical stimulation of the neurons or their central fibers to generate auditory information for the brain to decode. Inducing the regrowth of the peripheral nerve fibers toward a cochlear implant has been suggested as a way to decrease the power requirement and increase the frequency selectivity of the implant (Roehm and Hansen, 2005). In this regard, it is encouraging that even adult spiral ganglion neurons have the capacity to sprout to some extent after noise damage or in the presence of infused neurotrophins. In four separate studies of this limited sprouting in vivo, cells with the appearance of Schwann cells were detected in association with the nerve fibers (Bohne and Harding, 1992; Lawner et al., 1997; Wise et al., 2005; Glueckert et al., 2008). Further studies will be required to determine the role of Schwann cells in encouraging, supporting or directing neurite growth and regeneration in the inner ear.
Both positive effects of Schwann cells and negative or non-permissive effects of other cells play a role in sculpting the neuronal fiber patterns in nervous tissue. Having in hand an in vitrosystem in which Schwann cells and neurites interact in a microenvironment that is populated by other cells derived from the spiral ganglion will aid in the discovery of biochemical mechanisms that support spiral ganglion neurite regeneration as well as potential non-permissive mechanisms that may interfere with neurite regrowth.
Acknowledgments
We thank Dr. Michael Wegner (Sox10) and Dr. Marie Filbin (P0) for their generous donation of antibodies for this study. We appreciate the valuable comments on the manuscript made by Dr. James Bartles and Dr. Rhona Mirsky. This work was supported by NIH grant #DC00653, The Hugh Knowles Leadership Fund, and the Department of Otolaryngology, Northwestern University.
ABBREVIATIONS
- BDNF
brain derived neurotrophic factor
- BMP4
bone morphogenetic protein 4
- BrdU
bromodeoxyuridine
- BSA
bovine serum albumin
- FBS
fetal bovine serum
- GFAP
glial fibrillary acidic protein
- IgG
immunoglobulin G
- LIF
leukemia inhibitory factor
- MBP
myelin basic protein
- NT3
neurotrophic factor 3
- P
postnatal day
- P0
protein zero
- P75
neurotrophin receptor
- S100
an acidic calcium binding protein
- Sox10
SRY-related high-mobility-group box 10 transcription factor
- TBS
Tris buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bignami A, Eng LF, Dahl D, Uyeda C. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Bohne BA, Harding GW. Neural regeneration in the noise-damaged chinchilla cochlea. Laryngoscope. 1992;102:693–703. doi: 10.1288/00005537-199206000-00017. [DOI] [PubMed] [Google Scholar]
- Britsch S, Goerich D, Riethmacher D, Peirano R, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Gene Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, McDonald D, Cheng C, Magnowski B, Durand J, Zochodne DW. Axon and Schwann cell partnership during nerve regrowth. J Neuropath Exper Neurol. 2005;64:613–622. doi: 10.1097/01.jnen.0000171650.94341.46. [DOI] [PubMed] [Google Scholar]
- Cheng C, Zochodne DW. In vivo proliferation, migration, and phenotypic changes of Schwann cells in the presence of myelinated fibers. Neuroscience. 2002;115:321–329. doi: 10.1016/s0306-4522(02)00291-9. [DOI] [PubMed] [Google Scholar]
- Coppens AG, Kiss R, Heizmann CW, Schafer B, Poncelet L. Immunolocalization of the calcium binding S100A1, S100A5 and S100A6 proteins in the dog cochlea during postnatal development. Brain Res Dev Brain Res. 2001;126:191–199. doi: 10.1016/s0165-3806(00)00153-x. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Filbin MT. Overcoming inhibitors in myelin to promote axonal regeneration. J Neurol Sci. 2005;233:43–47. doi: 10.1016/j.jns.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, He Z, Filbin M. Myelin associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Eiberger J, Kibschull M, Strenzke N, Schober A, Bussow H, Wessig C, Djahed S, Reucher H, Koch DA, Lautermann J, Moser T, Wingerhager E, Willecke K. Expression pattern and functional characterization of connexin29 in transgenic mice. Glia. 2006;53:601–611. doi: 10.1002/glia.20315. [DOI] [PubMed] [Google Scholar]
- Fallon JR. Neurite guidance by non-neuronal cells in culture: Preferential outgrowth of peripheral neurites on glial as compared to nonglial cell surfaces. J Neurosci. 1985;5:3169–3177. doi: 10.1523/JNEUROSCI.05-12-03169.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Drescher MJ, Hatfield JS, Drescher DG. Immunohistochemical localization of S-100 protein in auditory and vestibular end organs of the mouse and hamster. Hear Res. 1994;74:67–76. doi: 10.1016/0378-5955(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Gestwa G, Wiechers B, Zimmermann U, Praetorius M, Rohbock K, Kopschall I, Zenner HP, Knipper M. Differential expression of trkB.T1 and trkB.T2, truncated trkC, and p75(NGFR) in the cochlea prior to hearing function. J Comp Neurol. 1999;414:33–49. doi: 10.1002/(sici)1096-9861(19991108)414:1<33::aid-cne3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Glueckert R, Bitsche M, Miller JM, Zhu Y, Prieskorn DM, Altschuler RA, Schrott-Fischer A. Deafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain derived neurotrophic factor and acidic fibroblast growth factor. J Comp Neurol. 2008;507:1602–1621. doi: 10.1002/cne.21619. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Vijapurkar Y, Koland JG, Green SH. Reciprocal signaling between spiral ganglion neurons and Schwann cells involves neuregulin and neurotrophins. Hear Res. 2001;161:87–98. doi: 10.1016/s0378-5955(01)00360-4. [DOI] [PubMed] [Google Scholar]
- Hurley PA, Crook JM, Shepherd RK. Schwann cells revert to non-myelinating phenotypes in the deafened rat cochlea. Eur J Neurosci. 2007;26:1813–1821. doi: 10.1111/j.1460-9568.2007.05811.x. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nature Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Kleitman N, Simon D, Schachner M, Bunge R. Growth of embryonic retinal neurite elicited by contact with Schwann cell surfaces is blocked by antibodies to L1. Exper Neurol. 1988a;102:298–306. doi: 10.1016/0014-4886(88)90223-3. [DOI] [PubMed] [Google Scholar]
- Kleitman N, Wood P, Johnson MI, Bunge RP. Schwann cell surfaces but not extracellular matrix organized by Schwann cells support neurite outgrowth from embryonic rat retina. J Neurosci. 1988b;8:653–663. doi: 10.1523/JNEUROSCI.08-02-00653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M, Gestwa L, Ten Cate W-J, Lautermann J, Brugger H, Maier H, Zimmermann U, Rohbock K, Kopschall I, Wiechers B, Zenner HP. Distinct thyroid hormone-dependent expression of TrKB and p75NGFR in nonneuronal cells during the critical TH-dependent period of the cochlea. J Neurobiol. 1999;38:335–356. [PubMed] [Google Scholar]
- Lawner BE, Harding GW, Bohne BA. Time course of nerve-fiber regeneration in the noise-damaged mammalian cochlea. Int J Dev Neurosci. 1997;15:601–617. doi: 10.1016/s0736-5748(96)00115-3. [DOI] [PubMed] [Google Scholar]
- Letourneau PC, Shattuck TA, Roche FK, Takeichi M, Lemmon V. Nerve growth cone migration on Schwann cells involves the calcium-dependent adhesion molecule, N-cadherin. Dev Biol. 1990;138:430–442. doi: 10.1016/0012-1606(90)90209-2. [DOI] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Mirsky R, Jessen KR. The neurobiology of Schwann cells. Brain Pathol. 1999;9:293–311. doi: 10.1111/j.1750-3639.1999.tb00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky R, Woodhoo A, Parksinson DB, Arther-Farraj P, Bhaskaran A, Jessen KR. Novel signals controlling embryonic Schwann cell development, myelination and dedifferentiation. J Periph Nerv Sys. 2008;13:122–135. doi: 10.1111/j.1529-8027.2008.00168.x. [DOI] [PubMed] [Google Scholar]
- Morris JK, Maklad A, Hansen LA, Feng F, Sorensen C, Lee K-F, Macklin WB, Fritzsch B. A disorganized innervation of the inner ear persists in the absence of erbB2. Brain Res. 2006;1091:186–189. doi: 10.1016/j.brainres.2006.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigato F, Garwood J, Calco V, Heck N, Faivre-Sarrailh C, Faissner A. Tenascin-C promotes neurite outgrowth of embryonic hippocampal neurons through the alternatively spliced fibronectin type III BD domains via activation of the cell adhesion molecule F3/contactin. J. Neurosci. 2002;22:6596–6609. doi: 10.1523/JNEUROSCI.22-15-06596.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio C, Dikkes P, Liberman MC, Corfas G. Glial fibrillary acidic protein expression and promoter activity in the inner ear of developing and adult mice. J Comp Neurol. 2002;442:156–162. doi: 10.1002/cne.10085. [DOI] [PubMed] [Google Scholar]
- Roehm PC, Hansen MR. Strategies to preserve or regenerate spiral ganglion neurons. Cur Opin Otolaryngol Head Neck Surg. 2005;13:294–300. doi: 10.1097/01.moo.0000180919.68812.b9. [DOI] [PubMed] [Google Scholar]
- Schneider-Schaulies J, von Brunn A, Schachner M. Recombinant peripheral myelin protein P0 confers both adhesion and neurite outgrowth-promoting properties. J Neurosci Res. 1990;27:286–297. doi: 10.1002/jnr.490270307. [DOI] [PubMed] [Google Scholar]
- Seilheimer B, Schachner M. Studies of adhesion molecules mediating interactions between cells of peripheral nervous system indicate a major role for L1 in mediating sensory neuron growth on Schwann cells in culture. J Cell Biol. 1988;107:341–351. doi: 10.1083/jcb.107.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Stock E, Chaboissier MC, Schedl A, Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Gene Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Zhang Y, Chang Q, Ahmad S, Dahlke S, Yi H, Chen P, Paul DL, Lin X. Connexin29 is highly expressed in cochlear Schwann cells, and it is required for the normal development and function of the auditory nerve of mice. J Neurosci. 2006;26:1991–1999. doi: 10.1523/JNEUROSCI.5055-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufert KB, Linthicum FH, Jr, Connell SS. The effect of organ of Corti loss on ganglion cell survival in humans. Otol Neurotol. 2006;27:1146–1151. doi: 10.1097/01.mao.0000232006.16363.44. [DOI] [PubMed] [Google Scholar]
- Wanner IB, Wood PM. N-cadherin mediates axon-aligned process growth and cell-cell interaction in rat Schwann cells. J Neurosci. 2002;22:4066–4079. doi: 10.1523/JNEUROSCI.22-10-04066.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner IB, Mahoney J, Jessen KR, Wood PM, Bates M, Bunge MB. Invariant mantling of growth cones by Schwann cell precursors characterize growing peripheral nerve fronts. Glia. 2006a;54:424–438. doi: 10.1002/glia.20389. [DOI] [PubMed] [Google Scholar]
- Wanner IB, Guerra NK, Mahoney J, Kumar A, Wood PM, Mirsky R, Jessen KR. Role of N-cadherin in Schwann cell precursors of growing nerves. Glia. 2006b;54:439–459. doi: 10.1002/glia.20390. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Takeda K, Katori Y, Ikeda Y, Oshima T, Yasumoto K, Saito H, Takasaka T, Shibahara S. Expression of the Sox10 gene during mouse inner ear development. Brain Res Mol Brain Res. 2000;84:141–145. doi: 10.1016/s0169-328x(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Whitlon D, Szakaly R, Greiner M. Cryoembedding and sectioning of cochleas for immunocytochemistry and in situ hybridization. Brain Res Protoc. 2001;6:159–166. doi: 10.1016/s1385-299x(00)00048-9. [DOI] [PubMed] [Google Scholar]
- Whitlon DS, Grover M, Tristano J, Williams T, Coulson MT. Culture conditions determine the prevalence of bipolar and monopolar neurons in cultures of dissociated spiral ganglion. Neuroscience. 2007;146:833–840. doi: 10.1016/j.neuroscience.2007.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlon DS, Ketels KV, Coulson MT, Williams T, Grover M, Edpao W, Richter CP. Survival and morphology of auditory neurons in dissociated cultures of newborn mouse spiral ganglion. Neuroscience. 2006;138:653–662. doi: 10.1016/j.neuroscience.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G, O'Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- Yong VW, Horie H, Kim SU. Comparison of six different substrata on the plating efficiency, differentiation and survival of human dorsal root ganglion neurons in culture. Dev. Neurosci. 1988;10:222–230. doi: 10.1159/000111972. [DOI] [PubMed] [Google Scholar]
- Zorick T, Lemke G. Schwann cell differentiation. Curr Opin Cell Biol. 1996;8:870–876. doi: 10.1016/s0955-0674(96)80090-1. [DOI] [PubMed] [Google Scholar]