Abstract
The mechanical therapy with multiple doses of antibiotics is one of modalities for treatment of periodontal diseases. However, treatments using multiple doses of antibiotics carry risks of generating resistant strains and misbalancing the resident body flora. We present an approach via immunization targeting an outer membrane protein FomA of Fusobacterium nucleatum, a central bridging organism in the architecture of oral biofilms. Neutralization of FomA considerably abrogated the enhancement of bacterial co-aggregation, biofilms and production of volatile sulfur compounds mediated by an interspecies interaction of F. nucleatum with Porphyromonas gingivalis (P. gingivalis). Vaccination targeting FomA also conferred a protective effect against co-infection-induced gum inflammation. Here, we advance a novel infectious mechanism by which F. nucleatum co-opts P. gingivalis to exacerbate gum infections. FomA is highlighted as a potential target for development of new therapeutics against periodontal infection and halitosis in humans.
Keywords: Co-aggregation, Fusobacterium nucleatum, FomA, Porphyromonas gingivalis, Vaccine, Abscesses, Halitosis
1. Introduction
Co-aggregation, an early event of biofilm formation, is characterized as an intra- or inter-species interaction of oral bacteria during the development of oral plaques which function as a mixed-culture biofilm for the growth of a spatially organized and metabolically integrated microbial community [1, 2]. Biofilms form when planktonic cells adhere to surfaces, proliferate, and co-aggregate with other bacteria. During proliferation and co-aggregation, bacteria use amino acids including cysteine and methionine as nutrients and convert them into volatile sulfur compounds (VSCs) [3, 4]. Once plaques were formed, they increase the risk of developing various dental diseases such as caries and periodontitis [5]. Thus, the process of bacterial co-aggregation presents a valuable early target for therapy aimed at suppressing the progress of oral bacterial infections and preventing halitosis and periodontal diseases.
The Gram-negative anaerobe Fusobacterium nucleatum (F. nucleatum) is an oral bacteria that exists as a part of the normal oral microbiome [6]. However, it also has pathogenic potential and is implicated in periodontal diseases as well as halitosis [6, 7]. Additionally, F. nucleatum is thought to act as a “microbial bridge” as it can co-aggregate with early and late colonizers of dental plaque [8]. Evidence also shows that F. nucleatum can enter the bloodstream and cause endocarditis [9], urinary tract infection [10] or preterm birth [11]. Although systemic diseases in association with microbial species in oral biofilm has been reported [12, 13], there are difficulties in establishing a causal role for oral bacteria in systemic conditions. The major outer membrane protein of F. nucleatum, FomA, has been shown to function as a non-specific porin in lipid bilayer membranes [14], and to function as a porin in vivo when recombinantly expressed in Escherichia coli (E. coli) [15]. It is also known that FomA is a voltage-dependent general diffusion porin [14, 15] and a virulence factor which facilitates bacterial evasion of host immune surveillance by binding the Fc fragment of human immunoglobulin G (IgG) [15]. Additionally, FomA has been recognized as a major immunogen of F. nucleatum [16, 17]. Intriguingly, it has been reported that FomA is involved in binding between fusobacteria and Streptococcus sanguis on the tooth-surface and to Porphyromonas gingivalis (P. gingivalis) in the periodontal pockets [18], supporting the view that FomA acts as a receptor protein in co-aggregation with other oral pathogenic bacteria. Thus, FomA is a potential target for the prevention of bacterial co-aggregation.
Classical treatments for periodontal diseases involve not only mechanical and antibiotic therapies but also surveillances on dynamic processes including the periodontopathogenic bacteria and the host responses. Chemical antiseptics are also used for treatments of periodontitis and halitosis. However, most of the chemical antiseptics fail to cure chronic, severe periodontitis and halitosis. Treatments using multiple doses of antibiotics to cure infection-induced periodontitis and halitosis have risks of generating resistant strains and misbalancing the resident body flora [19]. In addition, even though bacteria in the dental biofilm can invade the periodontal tissues, most of bacteria located in the dental biofilm and outside the host tissues are inaccessible to antibiotics. The treatments of periodontitis and halitosis have not been significantly improved during the past forty years due to the lack of focus on the awareness that these diseases are polymicrobial diseases as opposed to mono-infections. Vaccines targeting oral bacteria [such as Streptococcus mutans (S. mutans) for dental caries; P. gingivalis for periodontitis] are currently being evaluated [20, 21]. However, these vaccines cannot combat the enhanced pathogenesis (e.g. co-aggregation/biofilms) by F. nucleatum. Since the plaque biofilm is a common feature for almost all oral bacteria, blocking the bacterial co-aggregation at an early stage in biofilm formation will broadly prevent various biofilm-associated oral diseases including periodontitis and halitosis [22].
In the study, we demonstrate that F. nucleatum FomA is immunogenic, and that mice immunized with FomA produce neutralizing antibodies which prevent bacterial co-aggregation and, also gum abscesses and halitosis associated with co-aggregation. Moreover, immunization with FomA conferred a protective effect on bacteria-induced gum swelling and decreased the production of macrophage-inflammatory protein-2 (MIP-2) cytokine. These findings envision a novel infectious mechanism by which F. nucleatum interacts with P. gingivalis to aggravate oral infections. Moreover, this work has identified FomA as a potential molecular target for the development of drugs and vaccines against biofilm-associated oral diseases.
2. Materials and methods
2.1. Bacterial culture
F. nucleatum (ATCC® 10953) and P. gingivalis (ATCC® 33277) were cultured in 4% (w/v) trypticase soy broth (TSB, Sigma-Aldrich, St. Louis, MO) supplemented with 0.5% (w/v) yeast extract (DIFCO, Detroit, MI), 1.0% (v/v) hemin (Remel, Lenexa, KS) and 0.1% (v/v) vitamin K1 (Remel, Lenexa, KS). Both bacteria were cultured under anaerobic conditions using Gas-Pak (BD, Sparks, MD) at 37°C for 3 days without shaking.
2.2. Biofilm detection
Various dilutions of F. nucleatum [(4 × 108- 4 × 102 colony forming unit (CFU)/0.2 ml] and P. gingivalis [(108-102 CFU)/0.1 ml] were incubated in a 96-well nonpyrogenic polystyrene plate (Supplementary Fig. 1) at 37°C for 36 h under anaerobic conditions. Each well on the plate was gently washed with phosphate-buffered saline (PBS) (pH 7.2) and stained with 0.4% (w/v) crystal violet for 1 min.
2.3. Bacterial co-aggregation
Bacterial co-aggregation recognized as the association of bacterial particles was detected by a Malvern Zetasizer Nano-ZS (Malvern, Worcestershire, UK) which measures the size of bacterial particles in a fluid by detecting the Brownian motion of the particles. The sizes of the particles are measured by observing the scattering of laser light from these particles using the Stokes-Einstein relationship [23]. This method is called dynamic light scattering (DLS). To obtain a pattern of kinetic co-aggregation, F. nucleatum (4 × 109 CFU in 2 ml TSB medium) alone, P. gingivalis (105 CFU in 1 ml TSB medium) alone, or F. nucleatum (4 × 109 CFU in 2 ml TSB medium) plus P. gingivalis (105 CFU in 1 ml TSB medium) were incubated for 1, 3, 6, and 36 h. After that, bacteria were diluted (100 fold) in 400 μl TSB medium. Forty μl of each diluted solution was added into a micro Plastibrand ultraviolet (UV)-cuvette(Brand GMBH, Wertheim, Germany). The size (nm) of co-aggregated bacteria was measured at room temperature by a Malvern Zetasizer Nano-ZS equipped with a 4 mW He-Ne laser (633 nm). Data analysis was performed by Malvern's Dispersion Technology Software (DTS), using a non-negatively constrained least squares fitting algorithm.
2.4. Molecular cloning and expression of recombinant FomA
A polymerase chain reaction (PCR) product encoding a putative F. nucleatum FomA (GenBank Accession Number: X72583), an outer membrane protein, was generated using the forward PCR primer (5’-AAAAATTGTCGACGAAACAACCATGAAAAAATTAGCATTAGTATTA-3’) containing a Sal I site (GTCGAC) and the reverse PCR primer (5’- CTGTGAAAGCTTTTAATAATTTTTATCAATTTTAACCTTAGCTAAGC-3’) containing a Hind III site (AAGCTT). The amplified fragment was inserted into an In-Fusion™ Ready pEcoli-6×HN-GFPuv vector (Clontech Laboratories, Inc., Mountain View, CA) which was subsequently transformed into an E. coli BL21(DE3) strain (Stratagene, La Jolla, CA). Luria-Bertani (LB) plates containing ampicillin (50 μg/ml) were used for colony selection. A single colony was isolated and cultured overnight at 37°C with gentle shaking. An aliquot of the overnight culture was diluted 1:100 with LB-medium and incubated at 37°C until reaching optical density at 600 nm of 0.6. Isopropyl-β-D-thiogalactoside (IPTG) (1mM) was added into culture for 4 h. After centrifugation at 10,000 × g at 4°C for 5 min, bacterial pellets were re-suspended with sodium dodecyl sulfate (SDS) loading buffer [125 mM Tris-HCl buffer, pH 6.8, containing 4% (w/v) SDS, 10% (w/v) glycerol, 5% (v/v) 2-mercaptoethanol and 0.002% (w/v) bromophenol blue] and then boiled for 5 min. SDS-polyacrylamide gel electrophoresis (SDS-PAGE, 10%) and subsequent gel staining with coomassie blue were used for detection of protein expression. The fusion protein was purified from IPTG-induced bacteria in denaturing conditions via a standard nickel resin purification protocol (Qiagen, Valencia, CA). In-gel digestion with trypsin and protein identification via nano-liquid chromatography-linear ion trap quadrupole mass spectrometry (Nano-LC-LTQ-MS) analysis (Thermo Electron Corp. Waltham, MA) were performed following the protocols described previously [24].
2.5. Intranasal immunization with an UV-irradiated E. coli vector-based vaccine
After IPTG induction, E. coli harboring the expression vector with inserted FomA gene [E. coli BL21(DE3) FomA] were spread on a sterilized surface and irradiated with UV at total energy of 7,000 J/m2 by an UV cross-linker (Spectronics, Westbury, NY). The viability of UV-irradiated E. coli was determined by observing the growth of bacterial colonies on LB agar plates. For immunization, female ICR (Institute of Cancer Research) mice (3 to 6 weeks old; Harlan, Indianapolis, IN) were intranasally immunized by inoculating 25 μl of UV-irradiated E. coli BL21(DE3) FomA (108 CFU) into the nasal cavity of each mouse for nine weeks at a 3 week-interval. The second and third inoculations were administered in the same manner as the first immunization. Mice immunized with an UV-irradiated E. coli harboring expression vector for green fluorescence protein (GFP) [E. coli BL21(DE3) GFP] (108 CFU) served as a control group.
2.6. Detection of antibodies to FomA
The concentrations of purified recombinant FomA and GFP were determined by a Bradford assay (Bio-Rad, Hercules, CA). The sample (25 μg) was electrophoresed in a 10% (w/v) SDS-PAGE and electrophoretically transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA) for 90 min at a current of 75 V. The membrane was pre-incubated overnight in Tris-buffered saline [with 0.1% (v/v) Tween 20] containing 5% (w/v) skim milk, and then incubated at 4°C overnight with serum (1:1,000 dilution) obtained from mice immunized with UV-irradiated E. coli BL21(DE3) FomA or GFP for 9 weeks. Bound antibodies (IgG) were detected with anti-mouse horseradish peroxidase (HRP)-conjugated IgG (1:5,000 dilution, Promega, Madison, WI). The peroxidase activity was developed with a western lighting chemiluminescence kit (PerkinElmer, Boston, MA).
2.7. Protective effect of E. coli vector-based FomA vaccines
To induce gum swelling and abscesses, the immunized mice were inoculated with live bacteria as previously described [25]. Briefly, an aliquot of 100 μl of live F. nucleatum (4 × 108 CFU/2 ml in PBS), P. gingivalis (103 CFU/1 ml in PBS) or F. nucleatum plus P. gingivalis (4 × 108 CFU plus103 CFU/3 ml in PBS) were suspended in 100 μl of PBS, and then inoculated into the oral cavities of immunized mice every day for 3 days. An aliquot of 30 μl was injected into the gums of lower incisors using a 28-gauge needle. An aliquot of 30 μl was directly dropped into the oral cavity. The remaining 40 μl of aliquot was spread over the surface of the tongue. The change in the gum thickness (millimeter, mm) was measured using a digital caliper (Traceable Digital Caliper, Fisher Scientific, Pittsburgh, PA). For quantification of gum swelling, a transparent piece of parafilm was placed on the top of a swollen site. The swollen area was marked on the transparent parafilm by drawing an area that covered the whole swollen site. The swollen area was calculated using ImageJ software, version 1.40 [National Institutes of Health (NIH), http://rsb.info.nih.gov/ij/] and expressed as mm2. The volume of gum swelling in mm3 was calculated by the formula: Volume = thickness × area. Experiments were performed in triplicate at four mice per group. For histological observation, the gum tissues with abscesses were cross-sectioned, stained with hematoxylin and eosin (H&E) (Sigma diagnostics, St Louis, MO) and viewed on a Zeiss Axioskop2 plus microscope (Carl Zeiss, Thornwood, NY).
2.8. Measurements of MIP-2 production
Bacteria-injected gums of the immunized mice were excised 2 days after the third inoculated with live F. nucleatum (4 × 108 CFU) plus P. gingivalis (103 CFU). After homogenization and centrifugation at 10,000 × g at 4°C for 5 min, MIP-2 quantities in supernatants were measured using an enzyme-linked immunosorbent assay (ELISA) kit according to manufacturer's instructions (BD Biosciences, San Diego, CA). A goat anti-mouse IgG-HRP conjugate (Promega, Madison, WI) (1:5,000 dilution) was added and incubated for 2 h before washing. The HRP activity was determined by reading OD at 490 nm using an OptEIA™ Reagent Set (BD Biosciences, San Diego, CA).
2.9. VSC detection
The VSC production was visualized as brown/dark precipitates of lead sulfides on the surfaces of agar plates as described [25]. F. nucleatum (4 × 109 CFU/2 ml in PBS), P. gingivalis (104 CFU/1 ml in PBS), and F. nucleatum plus P. gingivalis (4 × 109 CFU plus104 CFU/3 ml in PBS) were cultured on a 6-well nonpyrogenic polystyrene plate for 36 h. An oral hydrogen sulfide (H2S)-producing organism (OHO-C, Anaerobe Systems, CA) plate containing lead acetate was used for the detection of VSCs (mainly H2S). After excising the bottom of each well, attached bacteria on one side of each well were positioned on the surface of an OHO-C agar plate and immediately cultured at anaerobic atmosphere at 37°C overnight.
2.10. Neutralization of FomA against bacterial co-aggregation, biofilm formation, VSC production, and gum swelling
Serum was obtained from mice immunized with UV-irradiated E. coli BL21(DE3) FomA (anti-FomA) or GFP (anti-GFP). Complement in the serum was inactivated by heating at 56°C for 30 min. F. nucleatum was neutralized by pre-treating with 2.5% (v/v) inactivated anti-FomA or anti-GFP serum in the medium at 37°C for 2 h. The 2 h incubation did not significantly influence the growth of F. nucleatum (2.66 ± 2.08 × 107 CFU) and P. gingivalis (2.33 ± 1.52 × 107 CFU) (data not shown). Neutralized F. nucleatum mixed with P. gingivalis in a ratio of 4 × 105: 1 was used for the detection of co-aggregation, biofilm formation, and VSC production. For in vivo neutralization, F. nucleatum (4 × 108 CFU) was neutralized with anti-FomA or anti-GFP serum, co-incubated with P. gingivalis (1 × 103 CFU) for 3 h, and then resuspended in an aliquot of 100 μl PBS. After neutralization, co-aggregated bacteria were inoculated into mice to induce gum swelling as described above. The Experiments were performed in triplicate at four mice per group.
2.11. Statistical analyses
Data are presented as mean ± SE. Student t-test was used to assess the significance of independent experiments. The criterion (*p<0.05, **p<0.005, ***p<0.0005) was used to determine statistical significance.
3. Results
3.1. Co-aggregation and biofilm formation of F. nucleatum with P. gingivalis
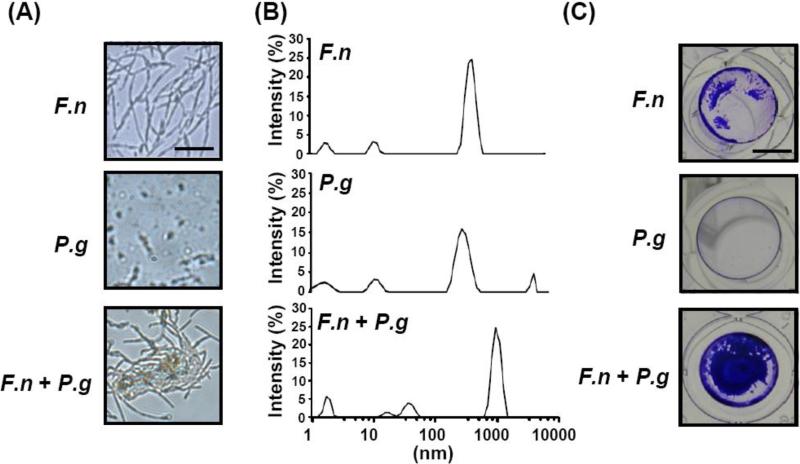
As shown in Supplementary Fig. 1, biofilm enhancement by F. nucleatum reached the maximal level when F. nucleatum (4 × 108 CFU) was co-cultured with P. gingivalis (103 CFU). Light microscopy and the Zetasizer Nano-ZS were employed to examine the bacterial association. The spindle-shaped F. nucleatum [6] and rod-shaped P. gingivalis [26] were clearly observed using light microscopy (Fig. 1A). Many bacterial aggregates were found when F. nucleatum was co-cultured with P. gingivalis for 3 h on a nonpyrogenic polystyrene plate, indicating bacterial co-aggregation occurred. To validate that interspecies co-aggregation is mediated by a physical interaction between two bacteria, the Zetasizer Nano-ZS with dynamic light scattering was utilized to detect the changes in the sizes of bacterial particles or aggregates. F. nucleatum (4 × 108 CFU) alone, P. gingivalis (103 CFU) alone, or F. nucleatum plus P. gingivalis (4 × 108 CFU/103 CFU) were resuspended in TSB medium for 3 h. The particle sizes of F. nucleatum and P. gingivalis ranged from 342-712 nm and 220-615 nm, respectively, as detected by the Zetasizer Nano-ZS (Fig. 1B), are consistent with previous observations using electron microscopy (EM) [18, 27]. Larger particles ranging from 712-1,281 nm were detected when F. nucleatum was mixed with P. gingivalis, supporting the hypothesis that F. nucleatum physically interacts with P. gingivalis to form aggregates. Bacterial co-aggregation is an early event of biofilm formation [28]. To investigate if upstream co-aggregation of F. nucleatum with P. gingivalis can further boost the development of biofilms, F. nucleatum alone, P. gingivalis alone, and F. nucleatum plus P. gingivalis at a ratio of 4 × 105: 1 CFU were cultured on nonpyrogenic polystyrene plates for 36 h. Biofilms formed on the plates were stained with 0.4% (v/v) crystal violet. Biofilm formation by F. nucleatum was tremendously enhanced by the presence of P. gingivalis (Fig. 1C), in agreement with the previous finding that P. gingivalis enhances biofilm formation by F. nucleatum [29]. Notably, the results above support the concept that P. gingivalis co-aggregates with F. nucleatum which leads to an increase in biofilm growth.
Fig. 1.
The co-aggregation of F. nucleatum with P. gingivalis and biofilm enhancement. The detection of bacterial co-aggregation and biofilm formation is described in “Materials and methods”. (A) F. nucleatum (F.n), P. gingivalis (P.g), and F. nucleatum co-aggregated with P. gingivalis (F.n + P.g) were visualized using light microscopy at a magnification of 60×. Bars = 2.5 μm. (B) A Malvern Zetasizer Nano-ZS with a DLS technique was used for measuring the particle sizes of F. nucleatum (F.n), P. gingivalis (P.g), and F. nucleatum co-aggregated with P. gingivalis (F.n + P.g). (C) Compared to F. nucleatum alone (F.n) and P. gingivalis alone (P.g), biofilms were significantly enhanced when F. nucleatum was co-cultured with P. gingivalis (F.n + P.g). Bars = 3 mm.
3.2. Production of neutralizing antibody to FomA via immunization with E. coli-based vaccines
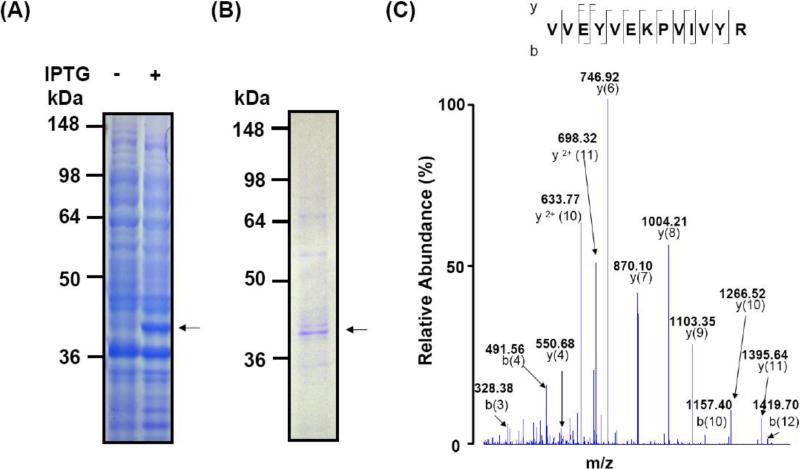
The protein FomA has been recognized as a receptor in co-aggregation of fusobacteria with other oral bacteria [8]. To examine if FomA contributes to the co-aggregation of F. nucleatum with P. gingivalis, we first generated neutralizing antibody to FomA via immunization of ICR mice with E. coli-based vaccines [25]. The gene encoding FomA was amplified by PCR using specific primers and genomic DNA prepared from F. nucleatum. The PCR products were inserted into a pEcoli-6×HN plasmid and expressed in E. coli [E. coli BL21(DE3)]. After IPTG induction, the over-expressed FomA-6×HN fusion protein at approximately 40 kDa molecular weight was detected by SDS-PAGE with coomassie blue staining (Fig. 2A). FomA (Fig. 2B) was purified using a nickel-nitroloacetic acid column. Twenty-eight internal peptides (Supplementary Table 1) derived from expressed FomA were fully sequenced by Nano-LC-LTQ-MS analysis after in-gel trypsin digestion, matching well with those from F. nucleatum FomA (GenBank Accession Number: gi|19713103). An internal peptide (VVEYVEKPVIVYR; 34-46 amino acid residues) of FomA is presented (Fig. 2C), validating the expression of recombinant FomA.
Fig. 2.
Expression, purification, and identification of recombinant FomA. A pEcoli-6×HN-GFPuv vector was inserted with a full PCR amplified FomA gene of F. nucelatum (ATCC 10953). (A) FomA was expressed in E. coli in the absence (-) or presence (+) of 1 mM IPTG. After IPTG induction, FomA (arrows) was successfully expressed in E. coli and shown at approximately 40 kDa on a 10% SDS-PAGE. (B) The recombinant FomA with a 6×HN tag was purified with a Ni-NTA column according to the manufacturer's instructions (Qiagen, Chatsworth, CA). (C) The identity of F. nucelatum FomA was validated by Nano-LC-LTQ-MS analysis (Thermo Electron Corp. Waltham, MA). Twenty eight internal peptides of FomA were fully sequenced. An internal sequence (VVEYVEKPVIVYR) of FomA corresponding to the amino acids 34 to 46 is presented.
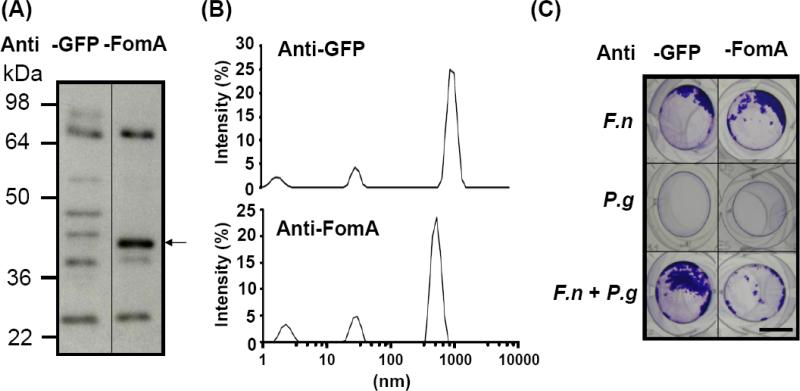
Next, a vaccine was constructed using inactivated whole E. coli particles over-expressing FomA. To assess the immunogenicity of FomA, ICR mice were vaccinated with UV inactivated-E. coli over-expressing FomA or a negative GFP control protein [E. coli BL21(DE3) FomA or GFP] for nine weeks. A strong band appearing at approximately 40 kDa was visualized when purified FomA was reacted with the serum obtained from mice immunized with [E. coli BL21(DE3) FomA], demonstrating the immunogenicity of FomA (Fig. 3A). No immunoreactivity against FomA was detected when serum from mice immunized with [E. coli BL21(DE3) GFP] were used.
Fig. 3.
Suppression of bacterial co-aggregation and biofilm formation using neutralizing antibody to FomA. (A) ICR mice were immunized with UV-irradiated E.coli BL21(DE3) FomA or GFP for nine weeks (three boosts at a three-week interval). Twenty five μg of recombinant FomA was separated via a 10% SDS-PAGE, transferred to a PVDF membrane and reacted with anti-GFP (Anti-GFP) or anti-FomA (Anti-FomA) serum (1:1,000 dilution). Antibodies to FomA (arrow) were detected in mice immunized with E. coli BL21(DE3) FomA, but not GFP. A representative of three separate experiments with similar results was shown. (B) For neutralization, F. nucleatum was pre-incubated with 2.5% (v/v) anti-FomA (Anti-FomA) or anti-GFP serum (Anti-GFP) for 2 h, after complement was inactivated by heating at 65°C for 30 min. The neutralized F. nucleatum (4 × 108 CFU) was mixed with P. gingivalis (103 CFU) in the ratio of 4 × 105 for 3 h and 36 h for co-aggregation and biofilm assays, respectively. After neutralization with anti-FomA or anti-GFP serum, the change in the particle sizes of co-aggregated bacteria was measured by a Malvern Zetasizer Nano-ZS. (C) Biofilms were detected after treatments of F. nucleatum (F.n), P. gingivalis (P.g) and F. nucleatum plus P. gingivalis (F.n + P.g) with in anti-FomA (Anti-FomA) or anti-GFP serum (Anti-GFP). Bars = 3 mm.
3.3. The involvement of FomA in bacterial co-aggregation and biofilms
To examine if FomA participates in bacterial co-aggregation and biofilms, F. nucleatum was neutralized with serum from mice immunized with [E. coli BL21(DE3) FomA] (anti-FomA serum) and then incubated in the presence or absence of P. gingivalis. Neutralization with serum from mice immunized with [E. coli BL21(DE3) GFP] (anti-GFP serum) served as a control. As shown in Fig. 3B, the co-aggregation of anti-GFP serum-neutralized F. nucleatum with P. gingivalis generated a peak signal ranging from 825-1,718 nm on the spectrum of Zetasizer Nano-ZS. The size of aggregate was decreased to 458-825 nm (Fig. 3B) when P. gingivalis was mixed with F. nucleatum neutralized with anti-FomA serum. Similarly, biofilm enhancement was detectable, as expected, in a co-culture of P. gingivalis with F. nucleatum neutralized with anti-GFP serum. However, the enhancement was dramatically abrogated when anti-FomA serum-neutralized F. nucleatum was co-cultured with P. gingivalis (Fig. 3C). These results indicate that a neutralizing antibody to FomA was produced after immunization and confirmed that FomA mediated the co-aggregation and biofilm formation of F. nucleatum with P. gingivalis.
3.4. The contribution of FomA to bacterial co-aggregation mediated gum inflammation
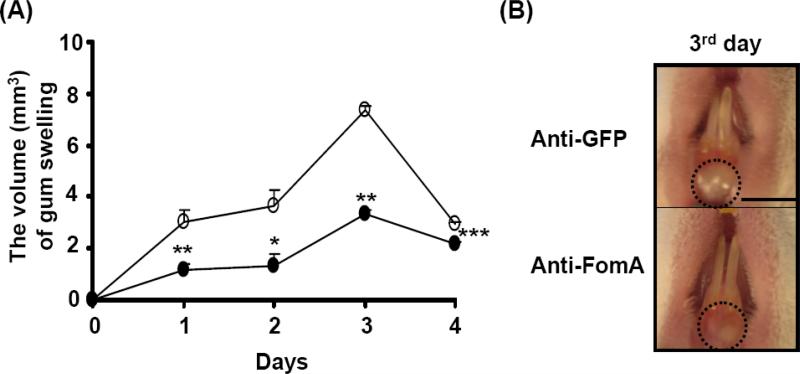
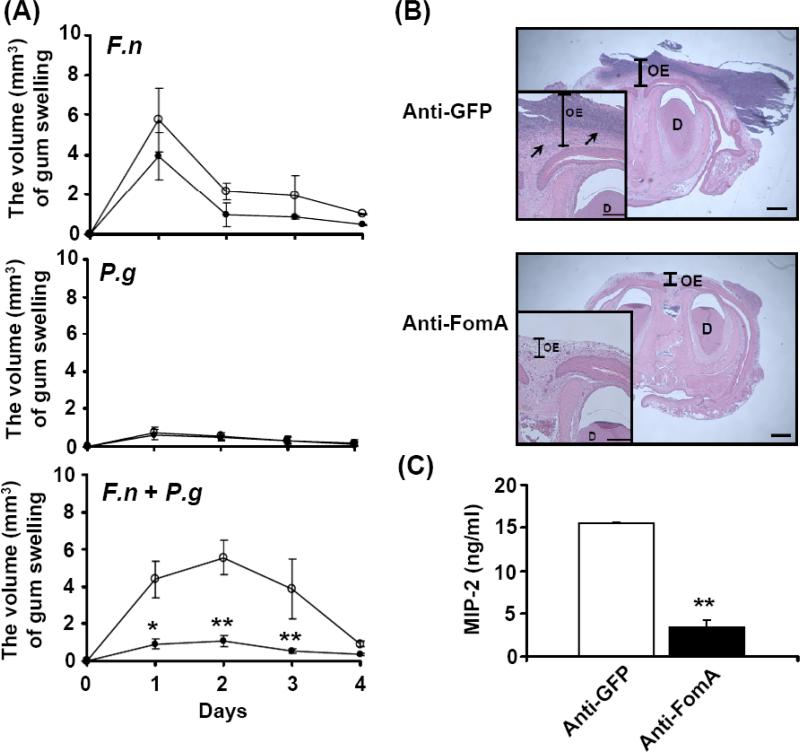
We previously determined that co-injection of F. nucleatum (4 × 108 CFU) with P. gingivalis (103 CFU) into the gums of ICR mice everyday for 3 days induced greater gum swelling than injection of individual bacterium (data not shown), suggesting that bacterial co-aggregation exacerbates gum inflammation. To examine if FomA contributes to the exacerbation of gum inflammation, F. nucleatum (4 × 108 CFU) was neutralized with either anti-FomA or anti-GFP serum [2.5 % (v/v)] prior to mixing with P. gingivalis (103 CFU). To induce gum inflammation, this bacterial mixture was injected into the gums of the lower incisors of naïve ICR mice every day for 3 days. Three days after injection, the severity of gum swelling was recorded for 4 days. Injection of P. gingivalis with anti-GFP serum-neutralized F. nucleatum induced a swollen gum with the volume ranging from 2.95 to 7.36 mm3. The greatest degree of swelling (7.36±0.12 mm3) was observed on the day 3 after recording (Fig. 4A and B). The gum swelling was significantly suppressed when the gum was injected with P. gingivalis along with anti-FomA serum-neutralized F. nucleatum. These results reveal the essential role of FomA in bacterial co-aggregation-induced gum inflammation and further supported FomA as a potential therapeutic target for treatment of bacterial co-aggregation-associated diseases.
Fig. 4.
Abrogation of bacteria-induced gum swelling via passive neutralization of FomA. After pre-treatment of F. nucleatum (4 × 108 CFU) with anti-FomA or anti-GFP serum, bacteria were incubated with P. gingivalis (103 CFU) in PBS for 3 h. Both bacteria (100 μl) were subsequently administrated into gum tissues and oral cavities of ICR mice for 3 days to induce abscesses as described in “Materials and methods”. (A) The volumes of swollen gums administered with P. gingivalis plus F. nucleatum pre-treated with anti-FomA (solid circles) or anti-GFP (open circles) serum were recorded every day for 4 days. The data represent as mean ± SE (n = 4, *p<0.05, **p<0.005, ***p<0.0005 by Student's t-test). (B) The morphologies of swollen (circles) gums administered with P. gingivalis plus F. nucleatum pre-treated with anti-FomA (Anti-FomA) or anti-GFP serum (Anti-GFP) on the day 3 after recording were shown. Bar = 2.2 mm.
3.5. Vaccination targeting FomA against bacteria-infected gum inflammation
To evaluate if FomA can be a valuable target for the development of vaccines against periodontal infection, mice were immunized with UV inactivated-E. coli BL21(DE3) FomA or GFP for 9 weeks. To induce inflammation, the gums of lower incisors in the immunized mice were challenged with live F. nucleatum (4 × 108 CFU) alone, P. gingivalis (103 CFU) alone, and F. nucleatum plus P. gingivalis (4 × 108/103 CFU) every day for 3 days. The severity of bacteria-induced gum swellings was measured daily for 4 days after 3-day challenge. Vaccination with E. coli BL21(DE3) FomA or GFP did not make a significant difference in of the amount of gum swelling induced by the injection of F. nucleatum alone or P. gingivalis alone (Fig. 5A). However, compared to the mice immunized with E. coli BL21(DE3) GFP, the amount of gum swelling induced by co-injection of F. nucleatum and P. gingivalis was considerably attenuated in the mice immunized with E. coli BL21(DE3) FomA. Histological examination by H&E staining illustrated the gum inflammation with thickened gum epithelium and gramulomatsis. In addition, there was greater inflammation caused by bacterial co-injection in the GFP-immunized mice than in the FomA-immunized mice (Fig. 5B). Previous studies have shown that the induction of pro-inflammatory cytokines plays a crucial role in the pathogenesis of periodontal infection [30]. To determine whether immunization with FomA alters the level of bacterial co-injection-induced pro-inflammatory cytokines, MIP-2 cytokine in swollen gums was quantified by ELISA. On day 2 following a 3-day challenge with both F. nucleatum and P. gingivalis, a significant elevation in the level of MIP-2 (15,528.88 ± 68.3 pg/ml) was detected in the GFP-immunized mice, while 77.7% (3,465.55 ± 763 pg/ml) less MIP-2 was measured in the FomA-immunized mice (Fig. 5C). Besides,CD11b, a prominent marker of inflammatory cells including macrophages was used to further analyze the severity of gum inflammation. A significant decrease in CD11b positive cells in swollen gum was detected in the FomA-immunized mice compared to the GFP-immunized mice (Supplementary Fig. 2). These results clearly demonstrate that vaccines targeting FomA efficiently prevent gum inflammation in mice caused by co-infection of F. nucleatum and P. gingivalis.
Fig. 5.
Vaccination with FomA against oral bacteria-induced gum inflammation. A gum pocket model [25] with abscesses and swollen tissues was used for evaluation of the in vivo efficacy of vaccination. (A) After inoculation with live bacteria F. nucleatum (4 × 108 CFU) (F.n), P. gingivalis (103 CFU) (P.g) or F. nucleatum plus P. gingivalis (4 × 108 CFU plus 103 CFU) (F.n + P.g) for 3 days, the severity of gum swelling (mm3) in the mice immunized with E. coli BL21(DE3) FomA (solid circles) or GFP (open circles) was measured daily for 4 days. (B) The H&E-stained gum tissue sections of lower incisors [magnification 4× and 20× (inserted panels)] displayed gum inflammation as indicated by an increase in the thickness of oral epithelium (OE) and gramulomatous reaction (arrows) in the mice immunized with E. coli BL21(DE3) GFP (Anti-GFP). Gum inflammation was significantly suppressed in mice immunized with E. coli BL21(DE3) FomA (Anti-FomA). D: dentin. Bars = 1 mm. Bars (inserted panels) = 0.2 mm. (C) Compared to vaccination with E. coli BL21(DE3) GFP (Anti-GFP), the bacteria-induced MIP-2 production was significantly diminished by vaccination with E.coli BL21(DE3) FomA (Anti-FomA). Data represent mean ± SE of five separate experiments (**p< 0.005 by Student's t-test).
3.6. The inhibition of co-aggregation-induced VSC production by antibody to FomA
F. nucleatum is one of the predominant organisms associated with halitosis, and this bacterium produces high levels of VSCs [7]. The plaque biofilm is considered to be the principle source generating such VSCs [3]. Results in Fig. 1 indicated that co-aggregation of F. nucleatum with P. gingivalis augments biofilm formation. Thus, we next examined if bacterial co-aggregation could increase VSC production and if inhibition of F. nucleatum FomA can efficiently suppress the co-aggregation-induced VSC production.
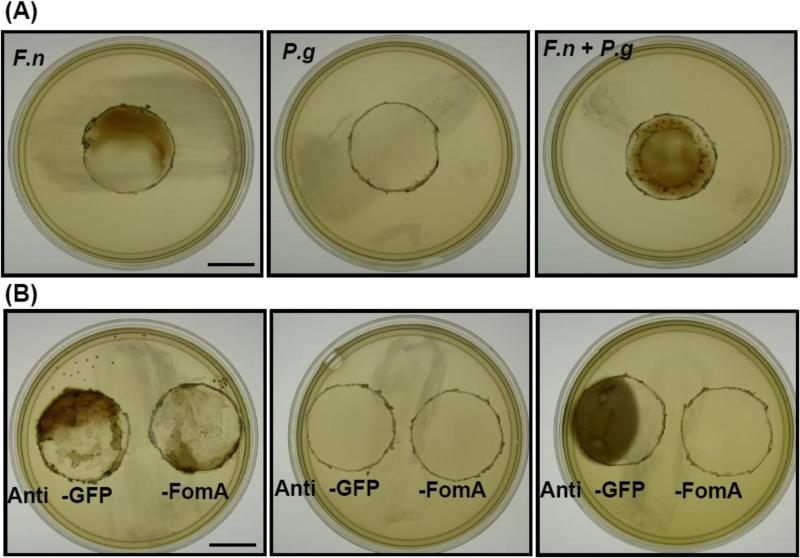
VSC production of F. nucleatum alone, P. gingivalis alone, and F. nucleatum plus P. gingivalis (4 × 109/104 CFU) were detected on lead acetate-contained agar plates. F. nucleatum (4 × 109 CFU), but not P. gingivalis (104 CFU), produced VSCs (Fig. 6A). The co-culture of F. nucleatum (4 × 109 CFU) with P. gingivalis (104 CFU) markedly enhanced VSC production (Fig. 6A), supporting the hypothesis that bacterial co-aggregation intensifies the emission of VSCs. To explore the involvement of FomA in VSC production, F. nucleatum was neutralized with either anti-FomA or anti-GFP serum [2.5 % (v/v)] (Fig. 3 and 4) and then co-cultured with P. gingivalis. After treatment with anti-FomA or anti-GFP serum, 104 CFU of P. gingivalis alone was insufficient to produce detectable VSCs although P. gingivalis has been shown to be a VSCs-producing bacterium [31]. The VSC production of F. nucleatum was slightly reduced after treatment with anti-FomA, but not anti-GFP serum (Fig. 6B). After treatment with anti-GFP serum, co-aggregated F. nucleatum and P. gingivalis retained the capability of producing VSCs. In contrast, bacterial co-aggregation-induced VSC production was entirely suppressed when F. nucleatum was neutralized with anti-FomA serum (Fig. 6B). This clearly demonstrates the ability of an antibody to FomA to prevent VSC production mediated by bacterial co-aggregation.
Fig. 6.
Blockage of VSC production using neutralizing antibodies to FomA. Detection of VSC production of F. nucleatum (F.n), P. gingivalis (P.g) and F. nucleatum plus P. gingivalis (F.n + P.g) in the absence (A) or presence of (B) anti-FomA or anti-GFP serum. VSC production by bacteria on the OHO-C agar plates is described in “Materials and methods”. Bars = 1.5 cm.
4. Discussion
Co-aggregation initiated by interaction and/or adherence of pathogenic bacteria is often an essential first step in the infectious process. The ability of oral bacteria to interact with one another, or to co-aggregate, may be an important factor in their ability to colonize and function as pathogens in the periodontal pocket [18] P. gingivalis and F. nucleatum are among the most frequently isolated bacterial species from both suppurative apical periodontitis and abscesses of dental origin [32]. The interaction of F. nucleatum and P. gingivalis appeared to be mediated by an adhesion protein identified as the outer membrane protein FomA on F. nucleatum and a carbohydrate receptor on P. gingivalis [18, 33], although only a few studies have shown a role for FomA in the pathogenesis of periodontal diseases and halitosis [25]. Our data demonstrate for the first time that F. nucleatum co-opts P. gingivalis via FomA to enhance co-aggregation, biofilm formation, gum inflammation, and VSC production.
Co-aggregation between F. nucleatum and P. gingivalis strains has been previously observed using either a macroscopic visual co-aggregation assay, based on radioactive labeling of bacteria, or using fluorochromes and confocal microscopy [32]. Although assaying co-aggregation by detecting visible clusters of bacteria is a common method, one main disadvantage of the method is the inability to dynamically quantify the co-aggregation. This method also lacks the capability of verifying the physical interactions among bacteria although bacterial clusters can be observed. On the other hand, the use of Malvern Zetasizer Nano-ZS equipped with DLS provides the ability to detect an increase in particle sizes derived from the physical aggregation of multiple particles [32]. Although F. nucleatum is a spindle-shaped bacterium, a size distribution between 342 to 712 nm is detected by the DLS analysis of Malvern Zetasizer Nano-ZS. Size analysis of the co-aggregation of F. nucleatum and P. gingivalis using Malvern Zetasizer Nano-ZS showed the presence of larger aggregates (712-1,281 nm) (Fig. 1B), verifying the physical interaction between two bacteria. Although we observed larger aggregates in the co-culture of bacteria on nonpyrogenic polystyrene plates (Fig. 1A), these larger aggregates were undetectable by Malvern Zetasizer Nano-ZS. Possible explanations include that the Malvern Zetasizer Nano-ZS has a limitation that restricts its ability to detect particle sizes greater than 6,000 nm. It is also possible that bacteria on the nonpyrogenic polystyrene plates formed larger aggregates than those bacteria suspended in the bacterial medium during Malvern Zetasizer Nano-ZS analysis.
It is worthwhile to note that only few P. gingivalis (103 CFU) are needed to trigger the enhancement of bacterial co-aggregation between F. nucelatum (4 x 108 CFU) and P. gingivalis (Supplementary Fig. 1). This result is consistent with recent findings that a low dose of P. gingivalis (106 CFU) synergistically enhances the pathogenicity of F. nucleatum (109 CFU) in a murine model using subcutaneously implanted chambers [32, 34]. Thus, besides the physical interaction among bacteria, bacterial co-aggregation may also be strengthened by quorum sensing mechanisms [35]. Although FomA has been proven to participate in the co-aggregation process by directly binding to various oral bacteria [18, 36], it is worthwhile to determine in the future if FomA is regulated by quorum sensing.
The gene encoding FomA was cloned into an E. coli vector-based system [37] for generation of vaccines against bacteria-induced gum inflammation (Fig. 5) and production of antibodies against VSC emission (Fig. 6). The E. coli vector-based system has been used in our laboratory to develop various non-invasive vaccines [37]. The E. coli vector (E. coli intact particle) has all E. coli components and exhibits an excellent and natural adjuvant effect that accelerates the evaluation of protein immunogenicity [38]. Most E. coli strains are harmless and are part of the normal flora in human. In addition, an UV-irradiated and non-pathogenic E. coli BL21(DE3) strain was used in this study to construct vaccines targeting FomA. The fact that F. nucleatum is not an indigenous bacterium in murine oral cavities has hindered the development of animal models of abscesses and halitosis for evaluation of vaccines and drugs against oral infections. In humans, gum pockets appear in an empty space between the root of the tooth and the top edge of the gum. These pockets trap bacteria and are the perfect incubators for bacteria to grow biofilm and produce VSCs. An oral colonization model in which bacteria are administered directly into the mouse oral cavity using PBS with carboxymethylcellulose [39, 40] has been commonly used for studying oral infections. Undoubtedly, the model represents the natural route of oral infection. However, the ability to quantify the bacterial colonization is limited due to the uneven distribution of infected sites. Furthermore, unlike humans, mice do not physically secrete abundant saliva [41]. Thus, it may be inappropriate to use this model for studying the in vivo effect of vaccine-induced secretory immunoglobulin A (S-IgA) on bacterial colonization. Alternatively, injection of F. nucleatum and P. gingivalis into gum tissues of ICR mice recapitulates a model of infection in a gum pocket [22], validating our use of this model for quantification of gum inflammation (Figs. 4 and 5) in this study.
It has been shown that prior exposure of mice to F. nucleatum modulates host response to P. gingivalis [42]. All the T-cell clones derived from mice immunized with F. nucleatum followed by P. gingivalis were T-helper type 2 (Th2) subsets, while those from mice immunized with P. gingivalis alone belonged to T-helper type 1 (Th1) subsets based on the flow cytometric analysis and cytokine profiles [43]. Other studies have shown that exposure of mice to F. nucleatum prior to P. gingivalis interfered with the opsonophagocytosis function of sera against P. gingivalis [42]. However, our results demonstrated that mice immunized with E. coli BL21(DE3) FomA did not increase the severity of P. gingivalis-induced gum swelling (Fig. 5A), suggesting that vaccination with F. nucleatum FomA may not alter the host susceptibility to other oral bacteria. After injection of F. nucleatum and P. gingivalis into the gum tissues of immunized mice, gum tissues were homogenized for counting CFUs. The 11.19 ± 0.37 × 104 CFU and 8.36 ± 1.28 × 104 CFU of bacteria were recovered from GFP- and FomA-immunized mice, respectively, suggesting that the antibody to FomA did not influence the bacterial growth but significantly neutralized the bacteria-induced gum inflammation (Fig. 5).
Although halitosis, characterized by the emission of VSCs, is a multifactorial disease, more than 90% of cases of halitosis originate from oral bacterial infections [44]. The disease, which is afflicting up to 50% of the U.S. population, has no appropriate therapeutic modalities that specifically suppress bacteria-induced pathogenesis. VSCs in oral cavities are produced via digestion of amino acids by bacterial enzymes such as L-cysteine desulfhydrase and METase [45]. However, there are several reasons for avoiding molecules involved in the pathways of amino acids metabolism as therapeutic targets. First, VSCs are not the only source of bad breath. Second, various oral bacteria use different systems to degrade amino acids from diverse sources [46]. Furthermore, most amino acid catabolic enzymes are located within bacteria where antibodies cannot easily reach them. On the other hand, biofilm formation, a key source of oral malodor, is a common feature for most of oral bacteria. Thus, bacterial co-aggregation, an early event of biofilm growth, was selected as a target for development of therapeutics against halitosis in this study. Our data demonstrated that bacteria co-aggregation increased the VSC production (Fig. 6), revealing the possibility that bacteria utilize amino acids as nutrients and convert them to VSCs during co-aggregation [47]. Although it is still not clear how FomA mediates the production of VSCs, it has been known that bacterial pore-forming proteins (porins) can act as major routes of uptake for various nutrients including amino acids [48, 49]. Thus, it is possible that non-specific FomA porin may be responsible for uptake of cysteine and methionine that can eventually be converted to VSCs. Recently, it has also been found that H2S stimulated the production of pro-inflammatory cytokines, including tumor necrosis factor-α, interleukin (IL)-1β, and IL-6 in human U937 monocytes [50]. The finding provides a possibility that bacterial co-aggregation elevates the VSC production which increases the release of pro-inflammatory cytokines and subsequently leads to a greater degree of gum swelling/inflammation.
Antibodies (IgG and IgA) to oral strains of F. nucleatum are detectable and elevated in patients with chronic periodontitis [51]. No reports have demonstrated that FomA is antigenic in the sera of halitosis patients, however. In addition to IgG, S-IgA in saliva was detectable in mice immunized with UV-inactivated E. coli over-expressing FomA (Supplementary Fig. 3A). An in vitro assay demonstrated the ability of the S-IgA to FomA to neutralize the F. nucleatum-induced VSC production and biofilm formation (Supplementary Fig. 3B and C). Although S-IgA in saliva may not obtain access to bacteria accumulated within gum pockets, it is worth investigating whether S-IgA can eliminate the halitosis generated from plaque biofilms on the surface of mouse incisors and/or oral epithelium. Furthermore, since both IgG in serum and S-IgA in saliva were measurable in FomA-immunized mice, determination of other IgG subclasses (such as IgG1 and IgG2a) [25] and cell-mediated immunity may increase understanding of the potency of FomA-targeted vaccines. A qualitative and quantitative examination of biofilm formation in vivo is still a challenge. Recently, a novel combination of measurements using an integrated nuclear magnetic resonance and confocal laser scanning microscope have been developed to study the processes occurring within biofilm communities [52]. These techniques may provide new tools for evaluation of the effects of vaccination on biofilm formation in vivo.
Overall, we have demonstrated that FomA is a necessary component for co-aggregation of F. nucleatum with P. gingivalis. Bacterial co-aggregation resulted in an enhancement of biofilm formation and VSC production in vitro and gum inflammation in vivo. Blocking FomA with a neutralizing antibody significantly attenuated this enhancement. Vaccination targeting FomA effectively suppressed co-infection-induced gum swelling and the production of MIP-2 cytokine. These results strongly suggested that FomA is critical mediator for bacterial co-aggregation and its associated pathogenicities. Inhibition of co-aggregation by inactivation of F. nucleatum FomA will prevent the progress of oral infections at an early stage. F. nucleatum and P. gingivalis have been implicated in the pathogenesis of several diseases [5], including urinary tract infections, bacteremia, pericarditis, and disorders of the oral cavity such as pulpal infections, alveolar bone abscesses, periodontal disease and halitosis. The immunization approach developed in this study will benefit patients with diseases mentioned above. Most importantly, the concept of blocking bacterial co-aggregation and biofilm formation forms a model system for the study of other biofilm-related pathogenic phenotypes, including those that develop in skin ulcers and other chronic infections.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grants (R01-AI067395-01, R21-R022754-01, R21-I58002-01 and 1R41AR056169-01). We thank Dan MacLeod for critical review.
Abbreviations
- ATCC
American Type Culture Collection
- CFU
colony forming unit
- DLS
dynamic light scattering
- DTS
dispersion technology software
- E. coli
Escherichia coli
- ELISA
enzyme-linked immunosorbent assay
- EM
electron microscopy
- FITC
fluorescein isothiocyanate
- F. nucleatum
Fusobacterium nucleatum
- FomA
outer membrane protein of Fusobacterium nucleatum
- GFP
green fluorescent protein
- H&E
hematoxylin & eosin
- HRP
horseradish peroxidase
- H2S
hydrogen sulfide
- ICR
Institute of Cancer Research
- IgG
immunoglobulin G
- IL
interleukin
- IPTG
isopropyl-β-D-thiogalactoside
- LB
Luria-Bertani
- MIP-2
macrophage-inflammatory protein-2
- NIH
National Institutes of Health
- OE
oral epithelium
- OHO-C
oral hydrogen sulfide-producing organism plate
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PVDF
polyvinylidene fluoride
- P. gingivalis
Porphyromonas gingivalis
- SDS
sodium dodecyl sulfate
- SDS-PAGE
SDS-polyacrylamide gel electrophoresis
- S-IgA
secretory immunoglobulin A
- S. mutans
Streptococcus mutans
- Th1
T-helper type 1
- Th2
T-helper type 2
- TSB
trypticase soy broth
- VSCs
volatile sulfur compounds
- UV
ultraviolet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bradshaw DJ, Marsh PD, Watson GK, Allison C. Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration. Infect Immun. 1998;66(10):4729–32. doi: 10.1128/iai.66.10.4729-4732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh PD. Dental plaque as a microbial biofilm. Caries Res. 2004;38:204–11. doi: 10.1159/000077756. [DOI] [PubMed] [Google Scholar]
- 3.Yaegaki K, Sanada K. Volatile sulfur compounds in mouth air from clinically healthy subjects and patients with periodontal disease. J Periodontal Res. 1992;27(4 Pt 1):233–8. doi: 10.1111/j.1600-0765.1992.tb01673.x. [DOI] [PubMed] [Google Scholar]
- 4.Scully C, Rosenberg M. Halitosis. Dent Update. 2003;30(4):205–10. doi: 10.12968/denu.2003.30.4.205. [DOI] [PubMed] [Google Scholar]
- 5.Liu PF, Zhu WH, Huang CM. Vaccines and photodynamic therapies for oral microbial-related diseases. Curr Drug Metab. 2009;10(1):90–4. doi: 10.2174/138920009787048365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolstad AI, Jensen HB, Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin Microbiol Rev. 1996;9(1):55–71. doi: 10.1128/cmr.9.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang MS, Kim BG, Chung J, Lee HC, Oh JS. Inhibitory effect of Weissella cibaria isolates on the production of volatile sulphur compounds. J Clin Periodontol. 2006;33(3):226–32. doi: 10.1111/j.1600-051X.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- 8.Rickard AH, Gilbert P, High NJ, Kolenbrander PE, Handley PS. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 2003;11(2):94–100. doi: 10.1016/s0966-842x(02)00034-3. [DOI] [PubMed] [Google Scholar]
- 9.Elkaim R, Dahan M, Kocgozlu L, Werner S, Kanter D, Kretz JG, et al. Prevalence of periodontal pathogens in subgingival lesions, atherosclerotic plaques and healthy blood vessels: a preliminary study. J Periodontal Res. 2008;43(2):224–31. doi: 10.1111/j.1600-0765.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 10.Shenker BJ, Datar S. Fusobacterium nucleatum inhibits human T-cell activation by arresting cells in the mid-G1 phase of the cell cycle. Infect Immun. 1995;63(12):4830–6. doi: 10.1128/iai.63.12.4830-4836.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004;72(4):2272–9. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senpuku H, Sogame A, Inoshita E, Tsuha Y, Miyazaki H, Hanada N. Systemic diseases in association with microbial species in oral biofilm from elderly requiring care. Gerontology. 2003;49(5):301–9. doi: 10.1159/000071711. [DOI] [PubMed] [Google Scholar]
- 13.Paju S, Scannapieco FA. Oral biofilms, periodontitis, and pulmonary infections. Oral Dis. 2007;13(6):508–12. doi: 10.1111/j.1601-0825.2007.1410a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleivdal H, Benz R, Jensen HB. The Fusobacterium nucleatum major outer-membrane protein (FomA) forms trimeric, water-filled channels in lipid bilayer membranes. Eur J Biochem. 1995;233(1):310–6. doi: 10.1111/j.1432-1033.1995.310_1.x. [DOI] [PubMed] [Google Scholar]
- 15.Kleivdal H, Benz R, Tommassen J, Jensen HB. Identification of positively charged residues of FomA porin of Fusobacterium nucleatum which are important for pore function. Eur J Biochem. 1999;260(3):818–24. doi: 10.1046/j.1432-1327.1999.00220.x. [DOI] [PubMed] [Google Scholar]
- 16.Mallison SM, 3rd, Smith JP, Schenkein HA, Tew JG. Accumulation of plasma cells in inflamed sites: effects of antigen, nonspecific microbial activators, and chronic inflammation. Infect Immun. 1991;59(11):4019–25. doi: 10.1128/iai.59.11.4019-4025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakken V, Aaro S, Hofstad T, Vasstrand EN. Outer membrane proteins as major antigens of Fusobacterium nucleatum. FEMS Microbiol Immunol. 1989;1(8-9):473–83. doi: 10.1111/j.1574-6968.1989.tb02438.x. [DOI] [PubMed] [Google Scholar]
- 18.Kinder SA, Holt SC. Localization of the Fusobacterium nucleatum T18 adhesin activity mediating coaggregation with Porphyromonas gingivalis T22. J Bacteriol. 1993;175(3):840–50. doi: 10.1128/jb.175.3.840-850.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liebana J, Castillo AM, Alvarez M. Periodontal diseases: microbiological considerations. Med Oral Patol Oral Cir Bucal. 2004;9(Suppl):82–91. 75–82. [PubMed] [Google Scholar]
- 20.Smith DJ. Dental caries vaccines: prospects and concerns. Crit Rev Oral Biol Med. 2002;13(4):335–49. doi: 10.1177/154411130201300404. [DOI] [PubMed] [Google Scholar]
- 21.Sharma DC, Prasad SB, Karthikeyan BV. Vaccination against periodontitis: the saga continues. Expert Rev Vaccines. 2007;6(4):579–90. doi: 10.1586/14760584.6.4.579. [DOI] [PubMed] [Google Scholar]
- 22.Schaudinn C, Gorur A, Keller D, Sedghizadeh PP, Costerton JW. Periodontitis: an archetypical biofilm disease. J Am Dent Assoc. 2009;140(8):978–86. doi: 10.14219/jada.archive.2009.0307. [DOI] [PubMed] [Google Scholar]
- 23.Einstein A. Investigations on the theory of the Brownian movement. Dover, NY: 1956. [Google Scholar]
- 24.Martin A, Clynes M. Comparison of 5 microplate colorimetric assays for in vitro cytotoxicity testing and cell proliferation assays. Cytotechnology. 1993;11(1):49–58. doi: 10.1007/BF00749057. [DOI] [PubMed] [Google Scholar]
- 25.Liu PF, Haake SK, Gallo RL, Huang CM. A novel vaccine targeting Fusobacterium nucleatum against abscesses and halitosis. Vaccine. 2009;27(10):1589–95. doi: 10.1016/j.vaccine.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akifusa S, Ansai T, Yu W, Wachi M, Nagai K, Takehara T. Characterization of the Porphyromonas gingivalis FtsZ containing a novel GTPase activity. Curr Microbiol. 2002;44(4):267–72. doi: 10.1007/s00284-001-0102-9. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto AC, Gaetti-Jardim JE, Arana-Chavez VE, Avila-Campos MJ. Influence of subinhibitory concentrations of antimicrobials on hydrophobicity, adherence and ultra-structure of Fusobacterium nucleatum. Braz J Microbiol. 2002;33:178–84. [Google Scholar]
- 28.Metzger Z, Blasbalg J, Dotan M, Tsesis I, Weiss EI. Characterization of coaggregation of Fusobacterium nucleatum PK1594 with six Porphyromonas gingivalis strains. J Endod. 2009;35(1):50–4. doi: 10.1016/j.joen.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Saito Y, Fujii R, Nakagawa KI, Kuramitsu HK, Okuda K, Ishihara K. Stimulation of Fusobacterium nucleatum biofilm formation by Porphyromonas gingivalis. Oral Microbiol Immunol. 2008;23(1):1–6. doi: 10.1111/j.1399-302X.2007.00380.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim do Y, Jun JH, Lee HL, Woo KM, Ryoo HM, Kim GS, et al. N-acetylcysteine prevents LPS-induced pro-inflammatory cytokines and MMP2 production in gingival fibroblasts. Arch Pharm Res. 2007;30(10):1283–92. doi: 10.1007/BF02980269. [DOI] [PubMed] [Google Scholar]
- 31.Washio J, Sato T, Koseki T, Takahashi N. Hydrogen sulfide-producing bacteria in tongue biofilm and their relationship with oral malodour. J Med Microbiol. 2005;54(Pt 9):889–95. doi: 10.1099/jmm.0.46118-0. [DOI] [PubMed] [Google Scholar]
- 32.Metzger Z, Lin YY, Dimeo F, Ambrose WW, Trope M, Arnold RR. Synergistic pathogenicity of Porphyromonas gingivalis and Fusobacterium nucleatum in the mouse subcutaneous chamber model. J Endod. 2009;35(1):86–94. doi: 10.1016/j.joen.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Haake SK, Wang X. Cloning and expression of FomA, the major outer-membrane protein gene from Fusobacterium nucleatum T18. Arch Oral Biol. 1997;42(1):19–24. doi: 10.1016/s0003-9969(96)00105-7. [DOI] [PubMed] [Google Scholar]
- 34.Metzger Z, Featherstone LG, Ambrose WW, Trope M, Arnold RR. Kinetics of coaggregation of Porphyromonas gingivalis with Fusobacterium nucleatum using an automated microtiter plate assay. Oral Microbiol Immunol. 2001;16(3):163–9. doi: 10.1034/j.1399-302x.2001.016003163.x. [DOI] [PubMed] [Google Scholar]
- 35.Frias J, Olle E, Alsina M. Periodontal pathogens produce quorum sensing signal molecules. Infect Immun. 2001;69(5):3431–4. doi: 10.1128/IAI.69.5.3431-3434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ. Communication among oral bacteria. Microbiol Mol Biol Rev. 2002;66(3):486–505. doi: 10.1128/MMBR.66.3.486-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu YT, Lin SB, Huang CP, Huang CM. A novel immunogenic spore coat-associated protein in Bacillus anthracis: characterization via proteomics approaches and a vector-based vaccine system. Protein Expr Purif. 2008;57(1):72–80. doi: 10.1016/j.pep.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Shi Z, Kong FK, Jex E, Huang Z, Watt JM, et al. Topical application of Escherichia coli-vectored vaccine as a simple method for eliciting protective immunity. Infect Immun. 2006;74(6):3607–17. doi: 10.1128/IAI.01836-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67(6):2804–9. doi: 10.1128/iai.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Momoi F, Hashizume T, Kurita-Ochiai T, Yuki Y, Kiyono H, Yamamoto M. Nasal vaccination with the 40-kilodalton outer membrane protein of Porphyromonas gingivalis and a nontoxic chimeric enterotoxin adjuvant induces long-term protective immunity with reduced levels of immunoglobulin E antibodies. Infect Immun. 2008;76(6):2777–84. doi: 10.1128/IAI.01502-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin AL, Johnson DA, Wu Y, Wong G, Ebersole JL, Yeh CK. Measuring short-term gamma-irradiation effects on mouse salivary gland function using a new saliva collection device. Arch Oral Biol. 2001;46(11):1085–9. doi: 10.1016/s0003-9969(01)00063-2. [DOI] [PubMed] [Google Scholar]
- 42.Choi J, Borrello MA, Smith E, Cutler CW, Sojar H, Zauderer M. Prior exposure of mice to Fusobacterium nucleatum modulates host response to Porphyromonas gingivalis. Oral Microbiol Immunol. 2001;16(6):338–44. doi: 10.1034/j.1399-302x.2001.160604.x. [DOI] [PubMed] [Google Scholar]
- 43.Choi JI, Borrello MA, Smith ES, Zauderer M. Polarization of Porphyromonas gingivalis-specific helper T-cell subsets by prior immunization with Fusobacterium nucleatum. Oral Microbiol Immunol. 2000;15(3):181–7. doi: 10.1034/j.1399-302x.2000.150306.x. [DOI] [PubMed] [Google Scholar]
- 44.Feller L, Blignaut E. Halitosis: a review. Sadj. 2005;60(1):17–9. [PubMed] [Google Scholar]
- 45.Yoshimura M, Nakano Y, Fukamachi H, Koga T. 3-Chloro-DL-alanine resistance by L-methionine-alpha-deamino-gamma-mercaptomethane-lyase activity. FEBS Lett. 2002;523(1-3):119–22. doi: 10.1016/s0014-5793(02)02958-7. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez AB, Rodriguez-Inda G, Morlet-Andreu R. [Microbiological aspects of halitosis and its treatment]. Rev ADM. 1983;40(6):178–80. [PubMed] [Google Scholar]
- 47.Yoshimura M, Nakano Y, Yamashita Y, Oho T, Saito T, Koga T. Formation of methyl mercaptan from L-methionine by Porphyromonas gingivalis. Infect Immun. 2000;68(12):6912–6. doi: 10.1128/iai.68.12.6912-6916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welte W, Nestel U, Wacker T, Diederichs K. Structure and function of the porin channel. Kidney Int. 1995;48(4):930–40. doi: 10.1038/ki.1995.374. [DOI] [PubMed] [Google Scholar]
- 49.Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976;71(3):877–84. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- 50.Zhi L, Ang AD, Zhang H, Moore PK, Bhatia M. Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J Leukoc Biol. 2007;81(5):1322–32. doi: 10.1189/jlb.1006599. [DOI] [PubMed] [Google Scholar]
- 51.Falkler WA, Jr., Lai R, Vincent JW, Dober L, Spiegel C, Hayduk S. The ELISA system for measuring antibody reactive to Fusobacterium nucleatum in the sera of patients with chronic periodontitis. J Periodontol. 1982;53(12):762–6. doi: 10.1902/jop.1982.53.12.762. [DOI] [PubMed] [Google Scholar]
- 52.McLean JS, Ona ON, Majors PD. Correlated biofilm imaging, transport and metabolism measurements via combined nuclear magnetic resonance and confocal microscopy. Isme J. 2008;2(2):121–31. doi: 10.1038/ismej.2007.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.