Abstract
Cytotoxic T cells (CTLs) are an important component of adaptive immunity. The study of antigen-specific CTLs in vivo is desirable yet difficult. Identification of the class-I-restricted peptide used by CTLs for target recognition is often required for detailed studies, but is generally not known for most antigens. Toxoplasma gondii is a medically important, obligate intracellular parasite and is often used as a model for studies of parasite immunology. No class-I-restricted peptides for CTLs are known. We show here a new and convenient method to detect T. gondii-specific CTLs in vivo. We engineered T. gondii tachyzoites to express the model antigen ovalbumin, for which many useful reagents and transgenic mice are available. Using ovalbumintransgenic T. gondii tachyzoites, antigen-specific CTLs were detected in vivo, and at much earlier time points post-infection than previously reported. This new method has several additional advantages over current methods to detect T. gondii-specific CTLs.
1. Introduction
Adaptive immunity is necessary to fight off infections in an antigen-specific fashion. While peptides recognized by MHC molecules have been identified for a variety of pathogens, specific immunity to many pathogens is through unknown MHC-binding peptides. Toxoplasma gondii is an important human pathogen and useful immunologic tool (Suzuki et al., 1988; Denkers et al., 1993; Gazzinelli et al., 1993b). A few of the major appeals of T. gondii models is that there is an established and convenient mouse model, it stimulates robust immunity, and its genome has been sequenced and is in the final stages of annotation.
A vigorous cell mediated immune response to T. gondii that includes Toxoplasma-specific CTLs and production of interferon (IFN)-γ is necessary to elicit effective immunity (Suzuki et al., 1988; Gazzinelli et al., 1991; Subauste et al., 1991; Denkers et al., 1993; Gazzinelli et al., 1993a; Gazzinelli et al., 1993b; Khan et al., 1994; Purner et al., 1996). Measures of functional CD8+ T. gondii-specific CTLs have been carried out using an in vitro 51Cr release assay. This is a lengthy assay and complex procedure with high potential for failure at many steps. Furthermore, several spleens or lymph nodes are usually pooled to collect enough cells to perform one experiment, preventing measurements from individual mice. 51Cr is a relatively strong gamma ray emitter requiring extra precautions to protect investigators, and making radioactive waste disposal an issue. The assay cannot be used to study CTL function in vivo. Finally, few laboratories have successfully performed this 51Cr release assay to study CTL function in T. gondii infection.
To date, no class I-specific peptides have been identified for T. gondii that have been incorporated into such assays despite considerable efforts. A reliable, convenient assay to study T. gondii specific CTLs without a priori knowledge of the parasite's immunogenic peptides is desirable. Ovalbumin (OVA) has been used as a surrogate antigen owing to the many reagents and sytems available to study OVA-specific immunity. These include class I peptides, pentamers, antibodies recognizing OVA binding to T cells, and mice with transgenic T cell receptors recognizing class I or II-restricted OVA peptides.
We now report the development of a reliable, specific and convenient in vivo CTL assay that can detect T. gondii-specific CTLs using OVA as the surrogate antigen. Experimental data using this system can be evaluated on a single cell basis and in individual mice.
2. Materials and Methods
2.1 T. gondii parasites
T. gondii Me49 strain tachyzoites were originally obtained from Elmer Pfefferkorn (Dartmouth University, Hanover, NH). Human foreskin fibroblasts (HFFs) (ATCC, Manassas, VA) were grown to confluence in T-25 plastic tissue culture flasks with complete R10 medium defined as RPMI 1640 (GIBCO, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (GIBCO), 10 mM N-(2-hydroxyethyl)piperazine-N'-(2-ethanesulfonic acid), 2 mM L-glutamine, and antibiotics. T. gondii tachyzoites were passaged in HFFs until about 90% lysis of the monolayer prior to being transferred to a new flask of confluent HFFs. Cells were tested periodically for Mycoplasma infection by PCR (ATCC Manassas, VA) or by staining with Hoechst 33342 dye (Hoechst Chemicals, Frankfurt, Germany) and were negative. Viable tachyzoites were obtained by lysis of HFFs by forced passage through a 27½ gauge needle. Following staining with Trypan blue, tachyzoites were enumerated using a hemocytometer prior to being intraperitoneally (i.p.) injected into mice in 500 μl sterile phosphate-buffered saline (PBS). Unless otherwise noted, 2000 tachyzoites were used for injection and they were invariably injected within 2 hours following the forced rupture of the HFFs.
2.2 Transfections
T. gondii strain Me49 tachyzoites were transfected with plasmid ptubP30-OVA/sagCAT, which was a generous gift of Dr. Boris Striepen, University of Georgia, Athens, GA (Gubbels et al., 2005). This plasmid expresses a fusion protein consisting of the complete T. gondii P30 (SAG1, the major surface protein) fused to the C-terminal coding region comprising amino acids 140 – 386 of chicken ovalbumin. Transfections were performed with Nucleofector II hardware (Amaxa, Gaithersburg, MD). Briefly, 2 × 107 tachyzoites were resuspended in complete T cell solution (Amaxa, Gaithersburg, MD) along with 10 g of NotI-linearized ptubP30-OVA/sagCAT. Program X-005 was initiated and cells were then immediately diluted in fresh R10 medium and transferred to a T25 flask containing confluent HFFs. Selection for a polyclonal population of stably transfected tachyzoites was achieved by culturing in the presence of 20 μM chloramphenicol for at least four passages. The resulting tachyzoites were resuspended in PBS and stained with an anti-ovalbumin monoclonal antibody (mAb) (clone ab17291; Abcam, Cambridge, MA) and sorted on a FACSAria (Beckon-Dickinson, San Jose, CA) based on --. Sorted parasites were seeded directly onto confluent HFFs in a 96 well flat bottom plate at a density of one tachyzoite per well.
2.3 Western blotting
Ten million tachyzoites freshly isolated from HFFs were lysed in RIPA buffer (20 mM Tris–HCl pH 8.0, 150 mM NaCl, 1 mM disodium EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1% triton-X100) supplemented with 1 mM phenylmethanesulphonylfluoride or phenylmethylsulphonyl fluoride (PMSF) and protease inhibitor cocktail (Sigma, St. Louis, MO) for 5 min on ice prior to adding Laemmli loading buffer (BioRad, Hercules, CA). Fifty micrograms of parasite protein was separated on 12.5% acrylamide gels by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Following protein transfer to nitrocellulose, immunoblots were blocked in Tris-buffered saline-0.1% Tween (TBST) supplemented with 5% skim milk for 1 h at ambient temperature prior to adding primary mouse anti-ovalbumin antibody (ab17291; 1:10,000 dilution; Abcam) or mouse anti-β-tubulin antibody (1:1000 dilution; gift of Dr. L. David Sibley, Washington University School of Medicine, St. Louis, MO). Following five washes in TBST, a fluorescent goat anti-mouse secondary antibody (RDye-800CW; 1:10,000 dilution; LI-COR Biosciences, Lincoln, NE) was added and proteins were detected by fluorescent imaging (Odyssey infrared imaging system, LI-COR Biosciences, (Lincoln, NE)).
2.4 Flow cytometry
Cells were prepared and stained with fluorochrome-labeled antibodies and fixed in 1% formaldehyde. Anti-mouse monoclonal antibodies (anti-CD3, anti-CD4, and anti-CD8) were purchased from BD Pharmingen (San Jose, CA). Ovalbumin-specific CD8+ T cells were detected by SIINFEKL-loaded pentamers specific for BL6 MHC class I (ProImmune, Oxford, UK). Events were acquired and analyzed on an LSRII using DIVA 6.1.1 software (Becton Dickinson, San Jose, CA).
2.5 Mice
Female 4-8 week old C57BL/6 (Jackson Labs, Bar Harbor, ME) were housed in standard microisolator cages and fed commercial chow and water ad libitum. Mice were sacrificed by CO2 asphyxiation and spleens were harvested on day 11 post-infection with T. gondii infection unless otherwise noted. All experiments involving mice were approved by Institutional Animal Care and Use Committee at the University of Texas Health Sciences Center San Antonio.
2.6 In vivo cell depletion
Naïve and infected mice were injected with 500 μg of either anti-CD4 (clone GK1.5) or anti-CD8 (clone 53-6.7) monoclonal antibodies (Biolegend, San Diego, CA) 24 hours prior to sacrifice.
2.7 Cytotoxic T lymphocytes (CTL) detection and in vivo CTL assay
Splenocytes from naïve mice were mechanically disrupted into single cell suspensions and the erythrocytes were lysed using red cell lysis buffer (Sigma, St. Louis, MO). Incubations were then performed in RPMI 1640. Splenocyte suspensions were either loaded with SIINFEKL or no peptide for 1 hour at 37°C. The SIINFEKL-loaded or untreated splenocytes were labeled with CFSE (Invitrogen, Carlsbad, CA) at either 5.0 μM (CFSEhi) or 0.5 μM (CFSElo) respectively, for 10 minutes at 37° C. Cells were washed with RPMI 1640 supplemented with 10% FBS. The SIINFEKL-loaded or control cells were counted using a Vi-Cell automated cell counter (Beckman Coulter, Fullerton, CA) and mixed in a 1:1 ratio. 2 × 107 cells were injected intravenously (i.v.) into T. gondii-infected or naïve mice. Spleens from these mice were collected at times specified and analyzed by flow cytometry. Percent killing was calculated using the equation (1-((CFSEhi/CFSElo of naïve mice)/(CFSEhi/CFSElo of T. gondii infected mice)) × 100).
2.8 Statistical analysis
Differences in cell surface molecule expression were determined by the X2 test and in other variables, by unpaired, two-tailed t-test, with p< 0.05 being considered significant. All results shown are representative of experiments that were repeated at least five times with similar results.
3. Results
3.1 Generation of ovalbumin-expressing transgenic T. gondii tachyzoites
Transgenic T. gondii tachyzoites were grown as polyclonal cultures for six weeks in HFFs. At that time, tachyzoites were freshly isolated from HFFs and sorted by flow cytometry based on --. OVA expression in the purified, polyclonal population transfected with plasmid ptubP30-OVA/sagCAT was confirmed by Western blot (Fig. 1). In contrast, OVA was undetectable in the parental wild type Me49 strain (Fig. 1). The OVA-expressing parasite is referred to as Me49-OVA and the wild type parental Me49 strain is referred to as Me49-GFP. OVA expression in polyclonal Me49-OVA tachyzoites was stable for more than six months (Fig 1).
Figure 1.
OVA expression in transgenic T. gondii. Intracellular parasites were isolated from HFFs by forced rupture using a 27 gauge needle. Parasite protein was prepared as described in methods. 50 μg of T. gondii protein was separated by SDS-PAGE. T. gondii β-tubulin was used for normalization. Ova protein is detected in Me49-Ova strain just after purification and remains at the same level after 6 months of passage in HFFs. No Ova protein is detected in parental Me49.
3.2 Me49-OVA infection elicits OVA-specific CD8+ T cells in infected mice
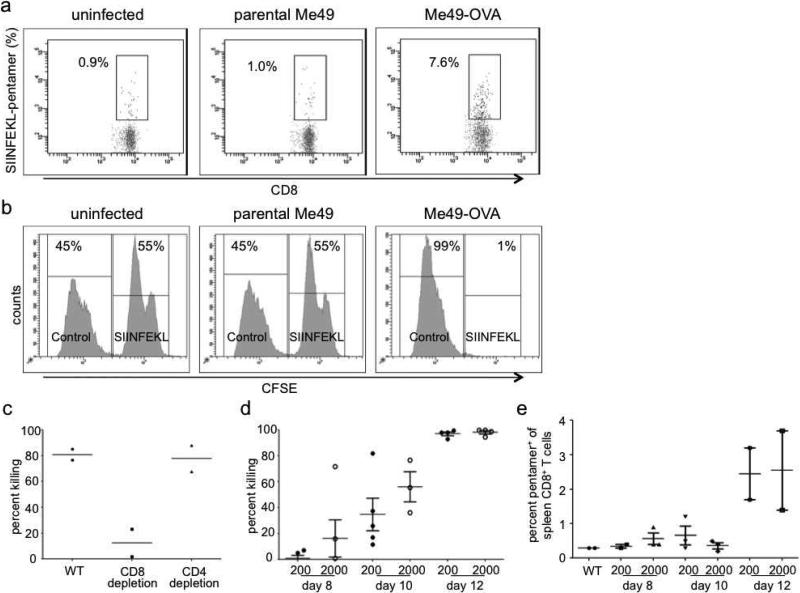
We used OVA-specific class I pentamers to detect OVA-specific CD3+CD8+pentamer+ cells on day 14 following Me49-OVA infection (Fig. 2a). In contrast, infection with wild type Me49 elicited no significant CD3+CD8+pentamer+ cell population (Fig. 2a).
Figure 2.
C57BL/6 mice were infected with either parental Me49 or Me49-OVA by i.p. administration and sacrificed at the indicated time points. A. Mice infected with 2000 Me49-OVA tachyzoites had increased pentamer+ cells while mice infected with a corresponding number of parental Me49 tachyzoites show no increase in pentamer+ cells on day 14 post-infection. B. An in vivo CTL assay was performed as described in panel A. Only the Me49-OVA-infected mice show specific CTL activity (depletion of SIINFEKL pulsed target cells). C. Depletion of CD8+ cells decreased in vivo killing compared to depletion of CD4+ T cells or no depletion. Thus, in vivo cytotoxicity by this method is largely through CD8+ cells. D-E. C57BL/6 mice were infected with either 200 (D) or 2000 (E) Me49-OVA tachyzoites by i.p. administration and were sacrificed at the indicated time points. In vivo killing assay shows increased killing between days 8 and 12 while pentamer staining is not detectable until day 12.
3.3 Me49-OVA infection elicits OVA-specific cytotoxicity detectable in vivo in infected mice
To test whether generation of CD3+CD8+pentamer+ cells correlated with functional CTL activity, we infected mice with Me49-OVA. On day 13 after infection, SIINFEKL peptide-loaded and control target cells were prepared as described and injected i.v. into mice. Mice were sacrificed 16-20 h after adoptive transfer of target cells (day 14 post-infection). Target cells loaded with SIINFEKL peptide were depleted in vivo in T. gondii infected mice but not in uninfected mice (Fig. 2b) suggesting OVA-specific elimination by CTLs. To confirm the specificity of this result, we administered SIINFEKL-loaded and control target cells to mice infected with the parental Me49 strain. No depletion of the target cells was observed (Fig. 2b). Finally, OVA-specific cytotoxicity increased with the prevalence of CD3+CD8+pentamer+ cells (Fig. 2b). Taken, together, these data are consistent with generation and detection of OVA-specific cytotoxicity following Me49-OVA infection.
3.4 Depletion of CD8+ cells abrogates OVA-specific CTL activity in vivo
In addition to CD8+ CTLs, NK cells, killer dendritic cells, and CD4+ CTLs have also been reported to mediate cytotoxic functions against T. gondii (Hauser and Tsai, 1986; Hunter et al., 1994). To identify the OVA-specific effector cell population, we infected mice with Me49-OVA, and depleted either CD4+ or CD8+ cells using specific depleting antibodies on day 10 post-infection, and assessed in vivo cytotoxicity on day 11. When CD8+ cells were depleted, killing of the target cells was significantly reduced. Reduced cytotoxicity was not observed when CD4+ cells were depleted (Fig. 2c) suggesting that OVA-specific CD8+ CTLs are responsible for killing in vivo.
3.5 Effect of time from primary challenge and inoculum size on detection of OVA-specific CTLs
Day 14 following T. gondii infection is usually chosen as the earliest time after primary challenge to sacrifice mice for CTL studies (Kasper et al., 1995). Other methods have used secondary boosting methods to study CTLs (Hakim et al., 1991; Subauste et al., 1991; Ely et al., 1999). To understand better when CTLs become detectable in vivo using this system, we infected mice with either a low (200) or high (2000) inoculum of Me49-OVA tachyzoites and sacrificed them on days 8, 10, or 12 post-infection. Using our in vivo cytotoxicity assay, we were able to detect OVA-specific cytotoxicity as early as day 8 post-infection (Fig. 2d). In contrast, OVA-specific CD8+ cells could only be detected by pentamer staining as early as day 12 post-infection (Fig. 2e). As expected, a higher initial inoculum of 2000 Me49-OVA tachyzoites elicited greater killing of OVA-peptide-loaded cells compared to infection with 200 tachyzoites. By day 12 following infection, >90% of all target cells were eliminated, with a higher prevalence of CD3+CD8+pentamer+ cells (Fig. 2d), using either the higher or lower inoculum of Me49-OVA tachyzoites (Fig. 2e).
4. Discussion
Most antigen-specific studies in T. gondii use whole parasite lysate (Denkers et al., 1993; Scanga et al., 2002; Pepper et al., 2008) or various length peptides derived from major surface antigen 1 (SAG1) (Velge-Roussel et al., 1997; Siachoque et al., 2006). While these studies have given us some insights into T. gondii-specific immune responses, identification of T. gondii-specific class I and class II restricted peptides have been elusive. We incorporated ovalbumin as a surrogate antigen into T. gondii tachyzoites because many tools to study OVA-specific immune response are readily available from commercial vendors. The incorporation of OVA into T. gondii tachyzoites has several immediate benefits. First, transgenic mice are readily available whose T cells recognize a specific MHC-I OVA-peptide (OT-1 mice) or an MHC-II OVA-peptide (OT-II mice). Second, pentamers and specific peptides are available that allow additional studies of OVA-specific CD8+ T cells. Third, MHC class II restricted peptides and antibodies against the MHC/OVA peptide complex are available that allow study of CD4+ T cell-mediated immunity.
We confirmed that CD8+ T cells are the primary cells responsible for the killing of OVA-pulsed target cells in this T. gondii infection model. Although the majority of OVA-specific lysis was mediated by CD8+ cells, some killing of the target cells was nonetheless observed when CD8+ cells were depleted, most likely explanation because CD8+ cells cannot be completely depleted. However, we cannot rule out the possibility that some target cells were killed by alternative mechanisms.
Our system allows detailed, in vivo immune studies using the model pathogen T. gondii. For example, CTL function in distinct anatomic compartments can be visualized because the number of cells required for such studies is low. For example, we have detected OVA-specific immunity in peritoneal exudates cells in addition to spleen (not shown). Further, after day 11 of infection in this system, CTL activity is easily detectable after only a 2-hour in vivo incubation (not shown). Thus, investigators can quantify CTL activity by using sub-maximal in vivo incubations, in addition to addressing fundamental yes-no questions regarding CTL activity. Finally, reagents are also available to study GFP-specific immunity, allowing additional studies of antigen-specific responses with Me49-GFP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Black MW, Boothroyd JC. Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev. 2000;64:607–23. doi: 10.1128/mmbr.64.3.607-623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkers EY, Sher A, Gazzinelli RT. CD8+ T-cell interactions with Toxoplasma gondii: implications for processing of antigen for class-I-restricted recognition. Res Immunol. 1993;144:51–7. doi: 10.1016/s0923-2494(05)80099-9. [DOI] [PubMed] [Google Scholar]
- Ely KH, Kasper LH, Khan IA. Augmentation of the CD8+ T cell response by IFN-gamma in IL-12-deficient mice during Toxoplasma gondii infection. J Immunol. 1999;162:5449–54. [PubMed] [Google Scholar]
- Gazzinelli RT, Denkers EY, Sher A. Host resistance to Toxoplasma gondii: model for studying the selective induction of cell-mediated immunity by intracellular parasites. Infect Agents Dis. 1993a;2:139–49. [PubMed] [Google Scholar]
- Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–92. [PubMed] [Google Scholar]
- Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts [see comments]. Proc Natl Acad Sci U S A. 1993b;90:6115–9. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels MJ, Striepen B, Shastri N, Turkoz M, Robey EA. Class I major histocompatibility complex presentation of antigens that escape from the parasitophorous vacuole of Toxoplasma gondii. Infect Immun. 2005;73:703–11. doi: 10.1128/IAI.73.2.703-711.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim FT, Gazzinelli RT, Denkers E, Hieny S, Shearer GM, Sher A. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J Immunol. 1991;147:2310–6. [PubMed] [Google Scholar]
- Hauser WE, Jr., Tsai V. Acute toxoplasma infection of mice induces spleen NK cells that are cytotoxic for T. gondii in vitro. J Immunol. 1986;136:313–9. [PubMed] [Google Scholar]
- Hunter CA, Subauste CS, Van Cleave VH, Remington JS. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994;62:2818–24. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LH, Matsuura T, Khan IA. IL-7 stimulates protective immunity in mice against the intracellular pathogen, Toxoplasma gondii. J Immunol. 1995;155:4798–804. [PubMed] [Google Scholar]
- Khan IA, Ely KH, Kasper LH. Antigen-specific CD8+ T cell clone protects against acute Toxoplasma gondii infection in mice. J Immunol. 1994;152:1856–60. [PubMed] [Google Scholar]
- Khan IA, Kasper LH. IL-15 augments CD8+ T cell-mediated immunity against Toxoplasma gondii infection in mice. J Immunol. 1996;157:2103–8. [PubMed] [Google Scholar]
- Pepper M, Dzierszinski F, Wilson E, Tait E, Fang Q, Yarovinsky F, Laufer TM, Roos D, Hunter CA. Plasmacytoid dendritic cells are activated by Toxoplasma gondii to present antigen and produce cytokines. J Immunol. 2008;180:6229–36. doi: 10.4049/jimmunol.180.9.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purner MB, Berens RL, Nash PB, van Linden A, Ross E, Kruse C, Krug EC, Curiel TJ. CD4-mediated and CD8-mediated cytotoxic and proliferative immune responses to Toxoplasma gondii in seropositive humans. Infect Immun. 1996;64:4330–8. doi: 10.1128/iai.64.10.4330-4338.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- Siachoque H, Guzman F, Burgos J, Patarroyo ME, Gomez Marin JE. Toxoplasma gondii: Immunogenicity and protection by P30 peptides in a murine model. Exp Parasitol. 2006 doi: 10.1016/j.exppara.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Subauste CS, Koniaris AH, Remington JS. Murine CD8+ cytotoxic T lymphocytes lyse Toxoplasma gondii-infected cells. J Immunol. 1991;147:3955–9. [PubMed] [Google Scholar]
- Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–8. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Velge-Roussel F, Moretto M, Buzoni-Gatel D, Dimier-Poisson I, Ferrer M, Hoebeke J, Bout D. Differences in immunological response to a T. gondii protein (SAG1) derived peptide between two strains of mice: effect on protection in T. gondii infection. Mol Immunol. 1997;34:1045–53. doi: 10.1016/s0161-5890(97)00133-8. [DOI] [PubMed] [Google Scholar]