Abstract
The use of long-acting glucocorticoids in the treatment of individuals with Congenital Adrenal Hyperplasia (CAH) has been greeted with controversy. Avoidance of dexamethasone therapy is in part due to the mistaken assumptions that dexamethasone is 30-fold more potent than hydrocortisone in suppressing adrenal activity, resulting in the overtreatment and the “growth toxic” label. However, as shown more than 50 years ago, dexamethasone is 80- to 100-fold (or greater) more potent than hydrocortisone in suppressing adrenal androgen production. When children are treated with low doses of dexamethasone once daily in the morning (0.15–0.3 mg/m2/day of dexamethasone versus 10–25 mg/m2/day of hydrocortisone), studies involving infants and children show that normal growth and skeletal maturation can be achieved, along with appropriate suppression of adrenal androgen secretion. Due to its high potency, the potential for overtreatment remains high with dexamethasone. Thus, it is imperative that dexamethasone-treated children be closely monitored.
1. Introduction
The use of long-acting glucocorticoids in the treatment of individuals with Congenital Adrenal Hyperplasia (CAH) has been greeted with controversy [1]. A large part of this controversy rests on the mistaken assumption that prednisone is 5 times more potent than hydrocortisone and dexamethasone is 30 times more potent than hydrocortisone in suppressing adrenal androgen production [2]. These literature entrenched equivalencies are based on anti-inflammatory properties [2]. And when prednisone and hydrocortisone are used at 5- and 30-fold potencies relative to hydrocortisone, respectively, children will be overtreated [3, 4].
As observed more than 50 years ago by Wilkins and coworkers, prednisone and dexamethasone are 15- and 80-fold more potent than hydrocortisone that in suppressing adrenal androgen production, respectively [5]. These relative dose equivalencies have been reaffirmed in clinical studies [6–9], and when prednisone and hydrocortisone are used at the higher potency equivalency ratios, children can be effectively treated with these long-acting glucocorticoids [6–8].
2. Origins of Dexamethasone Therapy of CAH
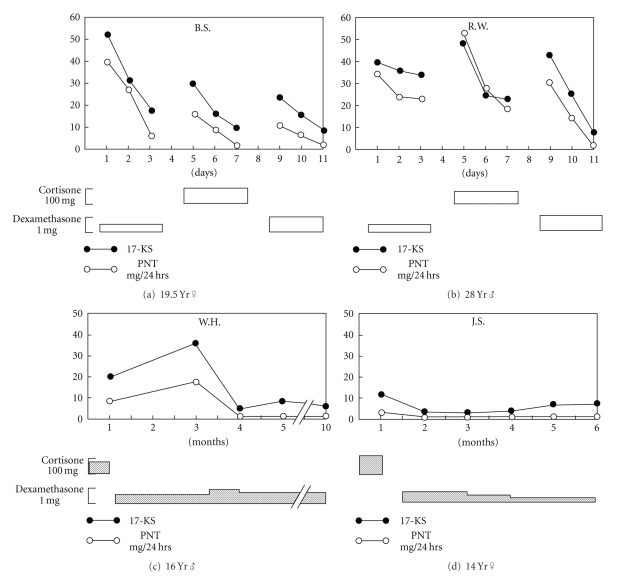
The use of dexamethasone in the treatment of CAH was introduced in 1971 by Hayek et al. [10]. The authors noted “Elucidation of the circadian rhythm of ACTH release, and the demonstration that secretion as reflected by low 8 a.m. cortisol levels can be efficiently suppressed by a single 1-mg dose of dexamethasone, led us to investigate the efficacy of prolonged administration of the this compound once daily as an alternative to the conventional three daily dose in the treatment of patients with CAH”. In detailed acute studies of four patients with CAH, daily administration of dexamethasone resulted in normalization of 17-ketoseteroid and pregnanetriol excretion (Figure 1). The dexamethasone doses used in these studies were 0.2 to 0.5 mg/m2.
Figure 1.
Responses of patients with congenital adrenocortical hyperplasia syndrome to cortisone in three daily doses as contrasted with those to single dose of dexamethasone at midnight. Black dots: values for daily excretion of urinary 17-ketosteroid; open circles: 24 hr excretion of pregnanetriol. Amount of medication in each period is shown by height of bars below abscissal scale. Patients (a) and (b) had “incomplete” form of 21-hydroxylase deficiency; (c) and (d) had “complete” salt-wasting variant. From [10].
3. University of Wales College of Medicine Studies of Dexamethasone
In the 1980s Hughes and colleagues at the University of Wales College of Medicine (Cardiff, UK) published an elegant series of publications relating to dexamethasone use in CAH [9, 11–14].
When applied to girls with CAH, menarche or regular menses occurred when plasma testosterone concentrations became normal using a single daily dexamethasone dose of 0.25–0.75 mg/day. As observed by Wilkins [5], it was noted “The potency of this glucocorticoid in suppressing adrenal steroid biosynthesis relative to cortisol is about 80 : 1. The onset of regular, ovulatory menstrual cycles, as judged by daily salivary progesterone profiles, was achieved within 2-3 years of menarche using this treatment regimen. Such patients have a good prognosis for normal fertility.” [12].
The use of dexamethasone was further detailed in another group of patients with CAH, in which hormonal control was monitored by frequent, serial measurements of saliva 17OH-progesterone (17OHP) concentrations [13]. Data showed that, when used, dexamethasone therapy resulted in adequate adrenal suppression [13].
Dexamethasone pharmacokinetics was studied in CAH using a radioimmunoassay [14]. The authors observed that dexamethasone remained in the circulation for about 16 hours after administration, with a half-life of about 4 hours. Thus the pharmacological effects of dexamethasone may extend beyond the time in circulation. Suppression of the hypothalamo-pituitary-adrenal axis also correlated with plasma dexamethasone levels [14]. There were considerable interindividual differences in dexamethasone clearance, which was postulated to explain the differing dose and dose schedule requirements necessary to achieve adequate therapeutic control in the clinical management of CAH [14].
Changes in 17OHP concentrations were also reported for 13 patients with CAH given a single dose of dexamethasone (0.01 mg/kg) given in the morning [9]. Treatment resulted in rapid drop in 17OHP levels, which remained low throughout the day until the onset of an abrupt nocturnal rise between 24.00 and 05.00 hr [9].
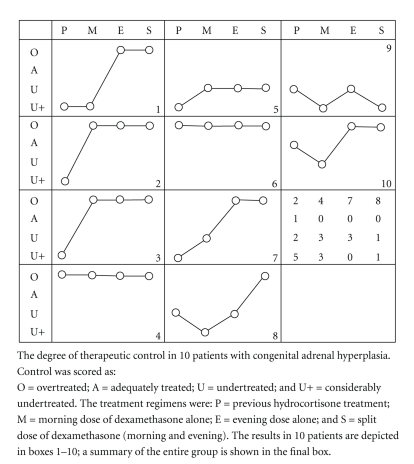
Results of short-term dexamethasone therapy in 10 patients with CAH (12–29 years) were later reported [11]. Three crossover dosage regimens were tested. A starting dose of 5 mcg per/kg (~0.2 mg/m2) was used [11]. Importantly results indicated that when the same dose was given in the evening, as compared to the morning, patients were observed to show clinical signs of overtreatment (Figure 2) [11]. These observations show that when dexamethasone is used at night, as by some [15], there is a greater risk for overtreatment than when the dose is given in the morning. At present, we do not know whether the potential risk of overtreatment with dexamethasone use in the evening can be mitigated with using lower doses or whether this phenomenon reflects a non-dose-related, time of day effect.
Figure 2.
Response to dexamethasone therapy as related to time of day of treatment. Note that treatment in the evening increased the likelihood of overtreatment. From [11].
4. Long-Term Treatment of CAH with Dexamethasone
In the late 1970s and 1980s, Crawford and colleagues began to routinely use dexamethasone in treating children with CAH. In 2000, this experience was reported for 26 boys and girls with CAH [7]. Thirteen boys and 8 girls were diagnosed with 21-hydroxylase deficiency; 4 boys and 1 girl were diagnosed with 11-hydroxylase deficiency.
Based on the bone ages (BAs) at the onset of dexamethasone therapy, patients were arbitrarily divided into 2 groups: those children in whom the BA was within 2 years of the chronological age (CA) at therapy onset (BA = CA; 13 males and 6 females) and those children in whom the BA was 2 years greater than the CA at therapy onset (BA ≫ CA; 4 males and 3 females). Biochemical monitoring was performed by assessment of urinary 17-ketosteroid and pregnanetriol excretions, along with measurement of circulating 17OHP levels.
A 0.1 mg/mL elixir of generic dexamethasone was used. Doses were administered between 7:00 and 9:00 a.m. using a dosing syringe. When the patients were ill, parents were instructed to give a double dose in the morning and the same double dose in the evening for the duration of illness plus one day.
Patients were considered undertreated when 17-ketosteroid secretion rates increased above the normal ranges for age (1–8 years,0.5–2.0 mg/day; 8–12 years, 2.5–8 mg/day; 12–16 years, 8–22 mg/day). Patients were considered overtreated when the growth rate slowed, the face became round, there was an increase in body hair, or the body weight increased more than expected for changes in height. In addition, if the morning 17-hydroxyprogesterone values were within or lower than the normal range for age (<200 ng/dL) and the ACTH concentration was <120 pg/mL, overtreatment was suspected.
Most patients were well controlled on single-morning dose, ranging between 0.24 and 0.33 mg/m2/day (0.27 ± 0.01 mg/m2/day). Two patients required daily doses of 0.44 and 0.71 mg/m2/day, divided among a morning dose (2/3) and an evening dose (1/3). Five patients required relatively lower doses of dexamethasone. Compared with standard hydrocortisone doses, which can range from 10–25 mg/m2/day [1], we thus found dexamethasone to be about 80–100 fold more potent than hydrocortisone.
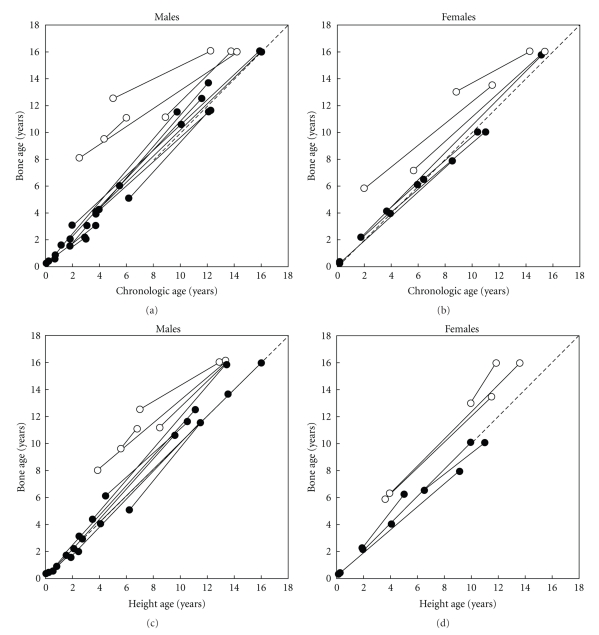
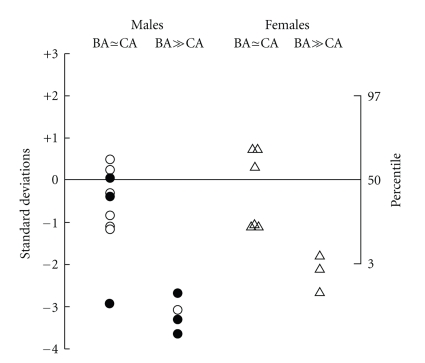
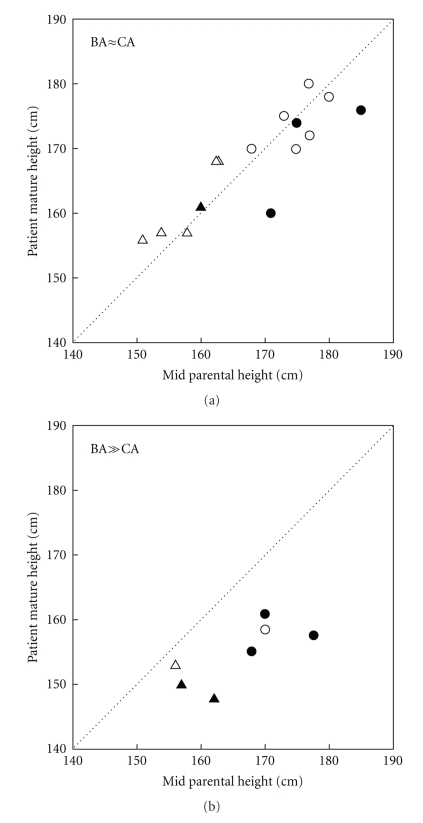
During treatment with dexamethasone, growth was observed for an average of 7 years. In the patients with comparable skeletal and chronological ages at the onset of therapy, the height and skeletal maturation increased proportionately during dexamethasone treatment (Figure 3). The children without significant BA advancement at the onset of dexamethasone therapy, who had completed growth, had final heights that were similar to midparental heights (Figures 4 and 5). For the boys and girls who were still growing, predicted adult heights were similar to midparental heights (Figures 4 and 5). Individuals with advanced bone ages at therapy onset had mature and predicted adult heights that were well below the 5th percentile and midparental heights (Figures 4 and 5). Importantly height projections increased while being on dexamethasone treatment.
Figure 3.
(a) and (b) Changes in skeletal maturation during dexamethasone therapy. Bone ages (BAs) are depicted at the onset of dexamethasone therapy and at the end of the observation period before epiphyseal fusion. The stippled diagonal line represents the line of identity between BA and chronological age (CA). ● indicates BA within 2 years of CA at therapy onset; o indicates BA > 2 years of CA at therapy onset. (c) and (d) Changes in skeletal maturation relative to height during dexamethasone therapy. BAs and HAs are depicted at the onset of dexamethasone therapy and at the end of the observation period or just before epiphyseal fusion. The stippled diagonal line represents the line of identity between BAs and height ages (HAs). ● indicates BA within 2 years of CA at therapy onset; o, BA > 2 years of CA at therapy onset. From [7].
Figure 4.
Mature heights (solid symbols) or predicted adult heights (open symbols) after treatment with dexamethasone. Heights are given as SDS or percentile. ∆ indicates females; ° indicates males. From [7].
Figure 5.
Mature heights (solid symbols) or predicted adult heights (open symbols) of children treated with dexamethasone, compared with midparental heights corrected for sex. The stippled diagonal line represents the line of identity between patients' and midparental heights. (a) depicts patients with BA within 2 years of CA at therapy onset; (b) depicts patients with BA > 2 years of CA at therapy onset. ∆ indicates females; ° indicates males. From [7].
During treatment with dexamethasone, 17-ketosteroid excretion rates were 1.92 ± 0.21 mg/24 hours from 0 to 4 years, 1.92 ± 0.21 mg/24 hours from 4 to 8 years, 3.42 ± 0.5 mg/24 hours from 8 to 12 years, and 6.7 ± 0.76 mg/24 hours from 12 to 16 years of age.
Recognizing that few clinicians now rely on 24-hour urine collections to assess adrenal control, we now measure circulating levels of 17-hydroxyprogesterone, androstenedione, and testosterone late in the day to assess control of adrenal androgen production. Using this monitoring approach, we now aim for late afternoon 17-hdroxyprogesterone levels between 250 and 1,000 ng/dL, and androstenedione and testosterone levels up to 2-fold above normal.
5. Treatment of CAH with Dexamethasone Beginning in Infancy
Following studies of dexamethasone in older children, we applied dexamethasone to the treatment of infants with CAH [8]. Three boys and 5 girls were identified as having CAH by newborn screening and treated with dexamethasone from birth until about 7 years of age, when data were analyzed.
Dexamethasone was administered at an initial dose of 0.1 mg each morning (0.1 mg/mL elixir of generic dexamethasone). Doses were administered between 7:00 and 9:00 a.m. using a dosing syringe. Laboratory tests were obtained between 3:00 and 5:00 p.m. for determination of circulating concentrations of 17-hydroxyprogesterone, androstenedione, testosterone, renin, and electrolytes. Doses were increased when the 17-hdroxyprogesterone levels were above 1,000 ng/dL and the androstenedione and testosterone levels were more than 2-fold above normal. Doses were decreased when the 17-hydroxyprogesterone levels were less than 250 ng/dL and the androstenedione and testosterone levels were normal or below normal, or there was excessive weight gain assessed at home (>0.5 kg in 2 weeks). When dose adjustments were made, doses were generally changed by 10%. Laboratory testing was repeated 2 months after dose changes.
The average dose initially used for treating the infants with dexamethasone was 0.25 mg/m2/day. As the infants became older, most remained on a dexamethasone dose within 30% of the absolute dose started in infancy. At the end of the followup period, the average daily dose of dexamethasone was 0.18 mg/m2/day. Thus, as in the older children, dexamethasone was estimated to be 80–100 fold more potent than hydrocortisone.
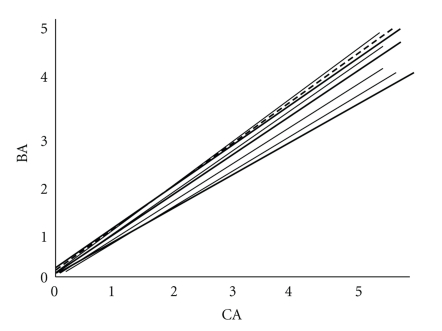
When the linear growth was evaluated, growth rate Z scores were 1.1 ± 0.1. At an average of 6.5 years of age height Z scores were 0.5 ± 0.2 and the average body mass index (BMI) was 18.2 ± 1.5. The mean bone age was 5.1 ± 1.5 years, with a bone age to chronologic age ratio of 0.9 ± 0.6 years (Figure 6). Thus, based on growth and skeletal maturation parameters, the linear growth in dexamethasone-treated children appears normal. Long-term followup of these children though will be needed to ascertain long-term outcomes more than one decade from now.
Figure 6.
Bone age (BA) versus chronological age (CA) in children treated with dexamethasone for CAH from birth. Dashed line is the line of unity. From [8].
When circulating androgen levels were assessed, they were measured at the end of the day. Testing was performed at this time as over the years we assessed circulating hormone levels at different times of the day, and we found that late afternoon levels correlated best with dose adjustments that allowed us to optimize growth and normal weight gain. At late afternoon, circulating testosterone and androstenedione levels were close to, or 2-fold above, the normal range. The 17-hydroxyprogesterone levels were 10- to 20-fold above normal.
6. Treatment of Children with Nonclassical CAH with Dexamethasone
In addition to treating children with classical CAH, we have treated a cohort of 8 boys with nonclassical forms of CAH who presented with bone ages that were more than 4 years advanced relative to chronological ages. All children had 21-hydoxylase deficiency.
Dexamethasone was administered as an initial dose of 0.2 mg/m2 per day each morning. A 0.1 mg/mL elixir of generic dexamethasone was used. Testing and dose adjustment were made as detailed above. At the end of the followup period of 5 years, the average daily dose of dexamethasone was 0.15 mg/m2/day.
When the linear growth was evaluated, growth rate Z scores were 1.0 ± 0.1. The change in bone age to chronological age was 0.67 ± 0.05 years. Later afternoon 17OHP levels were 682 ± 157 ng/dL, testosterone 125 ± 37 ng/dL, and androstenedione 58 ± 10 ng/dL.
7. Summary
Following the discovery of hydrocortisone as a treatment of CAH by Fuller Albright and colleagues at Massachusetts General Hospital [16] and Lawson Wilkins and coworkers at Johns Hopkins University [17], thousands of lives have been saved and children born with CAH now have a bright future. Although we know a lot about the condition, CAH remains a complicated condition to treat, warranting close vigilance [1]. Recognizing the inherent limitations and inconvenience of using short-acting compounds, there is a natural desire for longer-acting drugs to allow once-a-day dosing and avoid the wide swings in circulating adrenal androgen levels associated with hydrocortisone therapy. Dexamethasone fills this need as a long-acting preparation, which can be used for pennies a day. As such, efforts to develop a long-acting hydrocortisone preparation [18], which will come at dramatically higher price point than dexamethasone, can be viewed as superfluous.
Dexamethasone use is not for the casual practitioner. Due to its high potency, the potential for overtreatment remains high. Dilute preparations (0.1 mg/mL), rather than small pills that are cut, or more concentrated preparations, are recommended. Dosing syringes are essential for the delivery of the prescribed dose; medicine-droppers, teaspoons, and medication cups have no role in dexamethasone therapy. Although it is tempting to give the dose at night to suppress morning adrenal androgen production, nighttime dosing of dexamethasone is associated with overtreatment [11]. Thus, the morning is the favored time for administration. Most importantly, irrespective of the initial dose of dexamethasone (or hydrocortisone) used, it is essential that treatment be tailored to the individual child based on patterns of growth, maturation, and circulating androgen levels.
In applying dexamethasone therapy to children with CAH, the past and more recent lessons of steroid potency must be engaged [4]. The mistaken assumptions that prednisone is 5 times more potent than hydrocortisone and dexamethasone is 30 times more potent than hydrocortisone in suppressing adrenal androgen production need to be abandoned, as prednisone and dexamethasone are 10–15 and 80–100 fold (or greater) more potent than hydrocortisone, in suppressing adrenal androgen production, respectively [4]. In failing to do so, children will be overtreated, perpetuating the myth of the “growth toxic” glucocorticoid.
References
- 1.Clayton PE, Oberfield SE, Martin Ritzén E, et al. Consensus statement on 21-hydroxylase deficiency from the Lawson Wilkins Pediatric Endocrine Society and the European Society for Pediatric Endocrinology. Journal of Clinical Endocrinology and Metabolism. 2002;87(9):4048–4053. doi: 10.1210/jc.2002-020611. [DOI] [PubMed] [Google Scholar]
- 2.Physicians Desk Reference. Oradell, NJ, USA: Edward Barnhart; 2009. [Google Scholar]
- 3.Bonfig W, Bechtold S, Schmidt H, Knorr D, Schwarz HP. Reduced final height outcome in congenital adrenal hyperplasia under prednisone treatment: deceleration of growth velocity during puberty. Journal of Clinical Endocrinology and Metabolism. 2007;92(5):1635–1639. doi: 10.1210/jc.2006-2109. [DOI] [PubMed] [Google Scholar]
- 4.Rivkees SA. Lost lessons of glucocorticoid potency and the treatment of children with congenital adrenal hyperplasia. Journal of Pediatric Endocrinology and Metabolism. 2008;21(4):297–299. doi: 10.1515/jpem.2008.21.4.297. [DOI] [PubMed] [Google Scholar]
- 5.Wilkins L. The Diagnosis and Treatment of Endocrine Disorders in Childhood. Springfield, Ill, USA: Charles C. Thomas; 1950. [Google Scholar]
- 6.Punthakee Z, Legault L, Polychronakos C. Prednisolone in the treatment of adrenal insufficiency: a re-evaluation of relative potency. Journal of Pediatrics. 2003;143(3):402–405. doi: 10.1067/S0022-3476(03)00294-4. [DOI] [PubMed] [Google Scholar]
- 7.Rivkees SA, Crawford JD. Dexamethasone treatment of virilizing congenital adrenal hyperplasia: the ability to achieve normal growth. Pediatrics. 2000;106(4):767–773. doi: 10.1542/peds.106.4.767. [DOI] [PubMed] [Google Scholar]
- 8.Rivkees SA, Stephenson K. Low-dose dexamethasone therapy from infancy of virilizing congenital adrenal hyperplasia. International Journal of Pediatric Endocrinology. 2010;2010:4 pages. doi: 10.1155/2009/274682. Article ID 274682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen SX, Young MC, Hinohosa-Sandoval M, Hughes IA. 17OH-progesterone response to acute dexamethasone administration in congenital adrenal hyperplasia. Hormone Research. 1989;32(4):136–141. doi: 10.1159/000181275. [DOI] [PubMed] [Google Scholar]
- 10.Hayek A, Crawford JD, Bode HH. Single dose dexamethasone in treatment of congenital adrenocortical hyperplasia. Metabolism. 1971;20(9):897–901. doi: 10.1016/0026-0495(71)90052-7. [DOI] [PubMed] [Google Scholar]
- 11.Young MC, Hughes IA. Dexamethasone treatment for congenital adrenal hyperplasia. Archives of Disease in Childhood. 1990;65(3):312–314. doi: 10.1136/adc.65.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes IA, Read GF. Menarche and subsequent ovarian function in girls with congenital adrenal hyperplasia. Hormone Research. 1982;16(2):100–106. doi: 10.1159/000179489. [DOI] [PubMed] [Google Scholar]
- 13.Hughes IA, Read GF. Control in congenital adrenal hyperplasia monitored by frequent saliva 17OH-progesterone measurements. Hormone Research. 1984;19(2):77–85. doi: 10.1159/000179870. [DOI] [PubMed] [Google Scholar]
- 14.Young MC, Cook N, Read GF, Hughes IA. The pharmacokinetics of low-dose dexamethasone in congenital adrenal hyperplasia. European Journal of Clinical Pharmacology. 1989;37(1):75–77. doi: 10.1007/BF00609429. [DOI] [PubMed] [Google Scholar]
- 15.Ross RJM, Rostami-Hodjegan A. Timing and type of glucocorticoid replacement in adult congenital adrenal hyperplasia. Hormone Research. 2005;64(supplement 2):67–70. doi: 10.1159/000087757. [DOI] [PubMed] [Google Scholar]
- 16.Bartter FC, Forbes AP, Leaf A. Congenital adrenal hyperplasia associated with the adrenogenital syndrome: an attempt to correct its disordered hormonal pattern. The Journal of Clinical Investigation. 1950;29(6):p. 797. [PubMed] [Google Scholar]
- 17.Wilkins L, Lewis RA, Klein R, Rosenberg E. The suppression of androgen secretion by cortisone in a case of congenital adrenal hyperplasia. Bulletin of the Johns Hopkins Hospital. 1950;86(4):249–252. [PubMed] [Google Scholar]
- 18.Verma S, Vanryzin C, Sinaii N, et al. A pharmacokinetic and pharmacodynamic study of delayed- and extended-release hydrocortisone (ChronocortTM) vs. conventional hydrocortisone (CortefTM) in the treatment of congenital adrenal hyperplasia. Clinical Endocrinology. 2009;72(4):441–447. doi: 10.1111/j.1365-2265.2009.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]