Abstract
Our understanding of how radiation kills normal and tumour cells has been based on an intimate knowledge of the direct induction of DNA damage and its cellular consequences. What has become clear is that, as well as responses to direct DNA damage, cell–cell signalling — known as the bystander effect — mediated through gap junctions and inflammatory responses may have an important role in the response of cells and tissues to radiation exposure and also chemotherapy agents. This Review outlines the key aspects of radiation-induced intercellular signalling and assesses its relevance for existing and future radiation-based therapies.
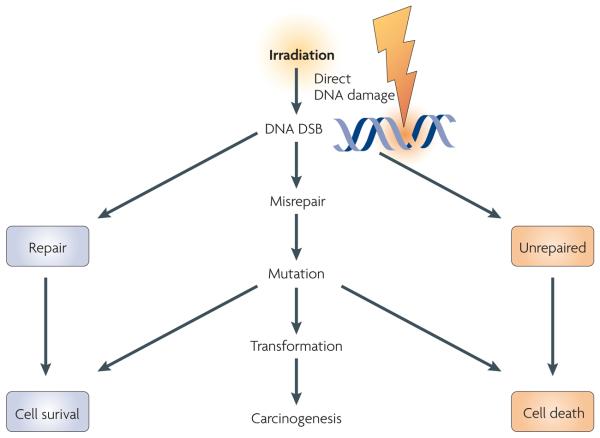
When ionizing radiation interacts with biological material, energy is deposited and chemical bonds are broken. In cells, the basic components of proteins, lipids and nucleic acids can all be damaged. However, a key consequence is that direct damage occurs to DNA within the nucleus, producing a range of lesions of which DNA double strand breaks (DSBs) have a pivotal role in determining whether cells survive radiation exposure1. If DNA damage is not correctly repaired two direct consequences can occur. Residual or unrepaired damage leads directly to chromosomal aberrations, loss of genetic material and cell death. Also, unrepaired or incorrectly repaired (misrepaired) damage can lead to mutations that might result in carcinogenesis or cell death (FIG. 1). The mechanisms underpinning DNA damage and repair processing in irradiated cells have been extensively studied since the discovery of DNA as the genetic material by Watson and Crick over 50 years ago. This has included exhaustive study of the DNA damage sensing and signalling pathways underpinning the DNA damage response that is present in cellular systems2. What is clear from these efforts is that cells have multiple and complex processes for sensing and repairing changes to their genomes to enable future propagation and stability. A series of sensor, transducer and effector proteins give cells important choices in response to radiation-induced DNA damage, such as DNA repair, cell cycle delay and cell death (apoptosis)3.
Figure 1. Direct DNA damage radiation model.
The schematic shows the standard model of DNA damage responses to radiation in biological systems, with direct DNA damage having a central role and the production of DNA double strand breaks (DSBs) leading to downstream biological consequences. Cells have complex pathways for sensing DNA damage and correctly repairing the DNA damage to survive the radiation exposure. If the DNA damage is not repaired, there is a high probability of cell death. DNA damage that is misrepaired can lead to mutations, increasing the probability of transformation and carcinogenesis.
Evidence now shows that, as well as these direct DNA damage-dependent effects, irradiated cells also send signals to their neighbours. These non-irradiated cells respond to signals produced by neighbouring irradiated cells by what has been termed a bystander effect (extensively reviewed in REFs 4-7). The term bystander effect is not new and has been observed in response to a range of other insults including ultraviolet radiation8, photodynamic therapy9, heat10 and chemotherapy agents11. Its observation in response to chemotherapy agents underpins its considerable importance in gene therapy regimens, in which not all tumour cells are targeted and indirect killing of non-targeted cells is required to ensure maximal tumour cell kill12. For example, the archetypal gene therapy model is the herpes simplex virus-thymidine kinase (HSV-TK) system. In this system the HSV-TK gene is transfected into cells and these are incubated with the non-toxic agent ganciclovir, which is converted to a toxic analogue that diffuses and kills neighbouring cells13. This bystander effect involves direct cell–cell communication through gap junctional intercellular communication (GJIC) and requires expression and surface location of CX43 (also known as GJC1) gap junctions14. By contrast, the bystander effect mediated by the thymidine phosphorylase-5′-deoxy-5-fluorouridine suicide gene system involves a factor released into the medium that is independent of GJIC15. So, the paradigm of a bystander response after radiation treatment is not new in other fields; in essence it is a manifestation of intercellular signalling that is either specific or non-specific in its mode of action.
Radiation-induced bystander responses have been observed in a range of cell types, tissue models and in vivo. Although the majority of the evidence for bystander effects has come from cellular studies, a range of other responses have been classified as bystander effects in the literature. In humans, in response to radiotherapy, longer-range effects occurring within or between tissues have also been reported and have been termed abscopal, out-of-field or distant bystander responses16. Radiation-induced bystander responses have been observed, not just from studies with external beam irradiation, but also from approaches using targeted radioisotopes. Several key questions emerge from these observations in terms of their relevance to cancer therapy. First, does an understanding of bystander mechanisms highlight new potential targets for cancer therapies and, if so, can this be modulated to either increase tumour cell killing or protect normal tissues? Second, do these bystander responses, especially after low-dose irradiation, contribute to increased carcinogenic risks associated with radiation exposure?
At a glance.
Radiation-induced bystander responses are defined as the response of cells to their neighbours being irradiated. These have been observed in a range of cell types and measured for a range of end points.
Long-range, abscopal (out-of-field) effects have also been observed after the clinical use of radiation.
The main mechanisms involve direct cell–cell communication by gap junction intercellular communication and release of factors into the medium.
Bystander signalling has a key role in increasing the effectiveness of gene therapy approaches in which common mechanisms involving cytokine signalling and the production of reactive oxygen and nitrogen species have been used to maximize effectiveness.
With the development of suitable strategies, radiation-induced bystander responses may be used to enhance tumour cell kill or protect normal tissues from the damaging consequences of radiation exposure.
The bystander responses
A simple definition of a radiation-induced bystander response is one in which ‘a cell that responds to the fact that its neighbours have been irradiated’. This definition has been expanded by many to include effects related to the production of clastogenic factors and longer-range abscopal effects studied in whole organisms, which are described below.
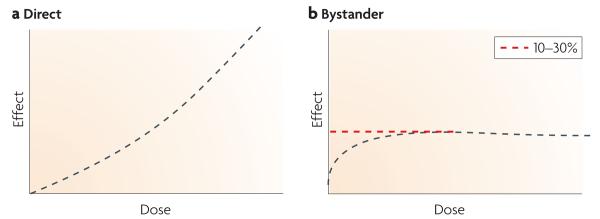
A key characteristic of the bystander responses, in contrast to direct irradiation effects, is the dose–response relationship (FIG. 2). Instead of an increased response with increasing radiation dose, the bystander response becomes saturated at relatively low doses (typically less than 1 Gy)17,18. This saturation means that above a certain dose no additional effect occurs and in practice means that, for any given endpoint, not every cell responds with a bystander effect. Also, it has been proposed that a binary mode of action may occur, with a simple on–off response, the probability of which increases with radiation dose19. It should be emphasized, however, that bystander responses can have a significant role even after high doses (>10 Gy)20. Interestingly, in many models after low-dose exposure, bystander responses have been almost equally effective as the direct response, suggesting that at least under some conditions bystander responses could predominate in overall effectiveness21.
Figure 2. Key aspects of radiation-induced bystander responses.
Typical dose response curves for direct (a) and bystander (b) responses are shown, highlighting the commonly observed saturation of response for bystander effects.
Bystander responses have been observed in most cell types, including lymphocytes22, fibroblasts23, endothelial cells24 and tumour cells25. They have also been observed for a range of end points and there has been considerable debate as to whether these can be termed damaging or protective bystander responses26. These include damage-mediated end points such as DNA damage27, mutations28, transformation29 and cell death30 and also protective responses involving terminal differentiation31, apoptosis32 (removal of damaged cells) and radioadaptive responses33. To some extent the debate over damaging or protective bystander responses is a moot point, as many of the studies have been done in specific cell models which have, in many cases, limited options or routes for coping with stress-mediated signalling. For example, our own work has extensively studied the response of Chinese hamster V79 cells to bystander signals19,21. In response to a stress signal, these cells can attempt DNA repair and, if this is unsuccessful, they accumulate mutations leading to chromosome aberrations and ultimately to cell death.
Molecular and cellular bystander mechanisms
Radiation-induced bystander responses have two main mechanisms of action, which mirror gene therapy bystander effects (FIG. 3). For cells in direct contact, bystander signalling can occur through GJIC. Gap junctions are multimeric protein channels between cells that allow transmission of signalling molecules34. Key to these structures are the connexion proteins, which are formed as individual hemi-channels on separated cells merge to form gap junctions as cells physically interact with each other. Typically these pores can allow molecules of up to 1,000–1,500 Da to pass through. Key ions and metabolites that are known to be transmitted through GJIC include ions such as Ca2+, nucleotides, peptides and other secondary messengers35.
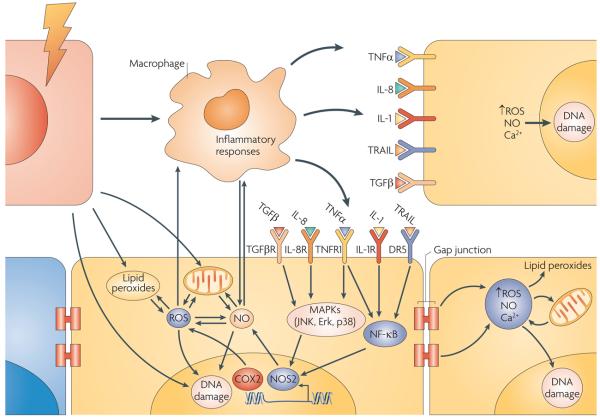
Figure 3. Key pathways affecting bystander signals.
Cells respond to direct radiation (red cell) by producing bystander responses through two key routes. One involves direct cell–cell communication through gap junctions and the second release of cytokine signals into the extracellular matrix. Not all cells respond (for example, the blue cell). In vivo, macrophages may be important mediators, which in response to radiation-induced tissue damage release bystander signals that affect non-irradiated cells (yellow cells). Some of the key pathways and mechanisms are now being elucidated, with roles for cytokine-mediated signalling, signal transduction through MAPKs and nuclear factor-κB (NF-κB) alongside the production of reactive oxygen and nitrogen species. COX2, cyclooxygenase 2; DR5, death receptor 5 (also known as TNFRSF10B); IL, interleukin; JNK, Jun N-terminal kinase; NO, nitric oxide; NOS2, NO synthase 2; ROS, reactive oxygen species; TGFβ, transforming growth factor-β; TGFβR, TGFβ receptor; TNFα, tumour necrosis factor-α; TRAIL, TNF-related apoptosis-inducing ligand.
Much of the early work in characterizing the role of GJIC was performed with confluent cell monolayers that were irradiated under situations in which not every cell was exposed. Individual charged particles deposit energy across cells in the form of tracks consisting of closely spaced ionizations and excitations. As the dose delivered to a cell population is reduced, the number of tracks crossing each cell is reduced until not every cell is irradiated. Using this approach, little and colleagues showed that, under conditions in which only 1% of a cell population was irradiated, 30% showed chromosomal changes in the form of sister chromatid exchanges17. Further work in primary human fibroblasts showed that inhibitors of GJIC using inhibitors such as lindane prevented this signalling in bystander cells36,37. Importantly, GJIC is only available as a signalling option in normal cells and is generally downregulated in tumour cells in which it is a key phenotypical change in carcinogenesis38.
The second route by which bystander responses are mediated is through the release of soluble factors from cells that have been irradiated. These factors can be transferred through cell culture medium from irradiated to non-irradiated cells30. These factors have been extensively studied (reviewed in REF. 39) and they have been postulated to be between 1,000 and 10,000 kDa in size and include lipid peroxide products40, inosine nucleotides41 and cytokines such as tumour necrosis factor-α (TNFα)42, but underlying their actions is the involvement of reactive oxygen species (ROS) such as superoxide radicals. The first report of soluble factors released after radiation exposure was in 1922: serum from irradiated animals was shown to stimulate the growth of lymphoid cells in suspension whereas control serum caused cell degradation43. The effect was evident 1–2 hours after irradiation but was not observed in serum isolated 17 hours after exposure. Further studies in the 1950s and 1960s reported these clastogenic factors in blood samples from radiotherapy patients44 and more recent work has reported these factors were present in blood samples from individuals exposed to radiation from the Chernobyl nuclear power plant accident45.
The mechanisms of bystander signalling are now starting to be elucidated at the molecular level and several key molecules are known to have major roles in some systems (FIG. 3). Not surprisingly, these are central factors involved in stress responses and cell–cell signalling, which are not generally specific to radiation exposure. A range of studies have shown clear evidence for a key role for cytokines, including interleukin 6 (IL-6)46, IL-8 (REF. 47), transforming growth factor-β1 (TGFβ1)48 and TNFα49, ROS50 and reactive nitrogen species25,51. Many aspects of bystander-mediated signalling and response have close parallels to inflammatory responses. For example, recent studies have shown that macrophages, which are key mediators of the inflammatory response in vivo, produce persistent increased levels of oxidative stress after radiation exposure under bystander conditions52. At the molecular level, cyclooxygenase 2 (COX2, also known as PTGS2)-dependent signalling has been shown to be a central player in cellular inflammatory responses and also mediates bystander signalling. The activation of the MAPK pathways is crucial for the action of COX2. Downstream signalling leads to transcription factor activation, including inducible nitric oxide (NO) synthase (iNOS, also known as NOS2) activation leading to the production of reactive nitrogen species. Inhibition of these pathways in bystander cells leads to inactivation of bystander responses, suggesting a potential route for modulating these responses in a clinical setting49. For example, it may be beneficial to activate bystander responses to increase tumour cell killing, or to prevent them in order to protect normal tissues from additional damage.
Overall, the underlying mechanisms involved in the signalling of bystander responses to neighbouring cells and the cell signalling pathways in those cells are being elucidated. However, there is a significant paucity of data on the mechanisms of release of bystander signals from irradiated cells. Using microbeams to target radiation to specific subregions of cells, it was shown that direct DNA damage of an irradiated cell is not required to trigger a bystander response53. Energy deposited in the cytoplasm is capable of producing ROS, which can indirectly lead to nuclear DNA damage54. Therefore, it has been proposed that subcellular targets such as mitochondria could play an important part, either as direct targets for the production of bystander signals or as parts of a signal transduction mechanism55,56. This probably involves the direct release of ROS in response to direct irradiation or indirect triggering of cytochrome c release owing to changes in mitochondrial membrane permeability.
Long-range bystander effects
It is important to determine how the bystander responses manifest in more complex three-dimensional systems and in vivo. A natural progression from studies looking at bystander interactions between different cell types has been the use of intact tissue sections or reconstruct models for bystander signalling57. Some of the earliest work in this area has been done in urothelial models owing to their organized structures and ease of isolation from animal sources and from patients undergoing urology procedures. In pioneering work, Mothersill and colleagues used samples of both human and murine urothelium. Using a cell reporter system for measuring the bystander effect, sections of bladder were irradiated and medium was transferred from these to the reporter system. Significant changes in the survival of the reporter cells were observed58. Measurements in vivo from mice confirmed such changes in survival and provided evidence for a genetic component underlying these responses59.
Belyakov and colleagues used a microbeam approach with both human and porcine urothelial samples. In a series of studies they reported that, as well as a damaging bystander response in locally irradiated sections of tissues, there was increased differentiation of cells after bystander signalling. This response was significantly greater than the number of damaged cells being produced in the population suggesting that, at least in this model, a significant protective bystander response was occurring31,60. In further studies, sections of three-dimensional human skin reconstructs were locally irradiated with helium ions from a microbeam and then 72 hours later the skin was sectioned, and apoptotic and micronucleated cells were scored at different distances away from the irradiated plane. Significant numbers of damaged cells were observed up to 1 mm away from the targeted region61. More recently, these long-range bystander responses have also been extended to a lung reconstruct model in which levels of the phosphorylated histone variant γH2AX, which is used as a marker of DSBs, were found to be increased. Methylation and fractions of senescent cells were also observed to increase62.
In vivo evidence of abscopal bystander responses are limited and in humans rely on anecdotal evidence (reviewed in REF. 16). However, it is interesting to speculate as to the underlying mechanisms involved. It is also important to put this in the context of what we know about how normal tissues respond to radiation exposure. For radiotherapy, when looking at normal tissue complications, the key assumption has been that the degree of effect is related to the volume of tissue irradiated, although many studies have shown that the relationship is much more complex63. Khan et al.64, found that when rat lung was partially irradiated micronucleus formation was observed in other non-irradiated areas of the lung, indicating DNA damage at these non-irradiated sites. Pre-injection of animals with Cu–Zn superoxide dismutase (SOD) or the NOS inhibitor L-NAME led to a reduced response in the shielded area, indicating the involvement of ROS and NO65. This was accompanied by waves of macrophage activation and production of cytokines, including IL-1α, IL-1β, IL-6, TNFα and TGFβ, lasting up to 16 weeks after irradiation66. In mouse models, activated macrophages and T cells were shown to radiosensitize tumour cells through two bystander effect mechanisms. First, activated immune cells secrete cytokines, leading to NOS2 induction and endogenous production of the radiosensitizing molecule NO inside tumour cells. Second, activated macrophages produce NO, which diffuses to and radiosensitizes bystander tumour cells67. If these responses are proved in humans, it may be necessary to incorporate directional and geometrical information into calculations of normal tissue complication probabilities for lung; these are currently not considered in the conventional dose–volume histograms used for therapy68. In humans, abscopal events have also been observed in patients, involving bilateral pneumonitis after unilateral irradiation69, and these may also involve inflammatory responses. The cytokine involvement in these responses in vivo agrees with analyses using in vitro cell culture-based bystander signalling. Indirect macrophage responses have been reported in irradiated tissues and they may be key drivers of bystander signalling at the tissue level70.
Further evidence of long-range bystander responses has come from localized irradiations in mice: irradiation (1 Gy X-rays) of one side of the body led to epigenetic changes in the shielded non-exposed side 1 cm away. Differential changes in the activity of DNA methyltransferases at the non-irradiated site were observed, suggesting that epigenetic regulation was involved in the aetiology of bystander responses71. In a further study, cranial irradiation of a higher dose (20 Gy) was used to determine the extent of distant effects in the spleen, which also showed epigenetic effects72. Further work has reported sex-dependent differences in response in these studies73 and also a potential role for microRNAs74. Recent work has also suggested that these long-range effects may have a role in oncogenesis. In a series of studies in which mice were irradiated with the head shielded, significant levels of DNA DSBs, apoptotic cells and evidence of carcinogenesis were found in the cerebellum of Ptch1 mutant mice, which develop cerebellar tumours resembling human medulloblastoma. These long-range effects involved GJIC in the central nervous system75.
Other long-range interactions have also been reported between normal tissue and tumours in mice. Camphausen et al. irradiated the legs of mice (five fractions of 10 Gy) that had tumours transplanted at the dorsal midline. They observed reduced tumour growth rates when the leg was irradiated, with tumour growth inhibition decreasing when the radiation dose was reduced to 12 fractions of 2 Gy. The response was prevented when the drug α-pifithrin, which blocks p53, was given to the mice76.
Studies with internally deposited radioactive materials have also reported evidence for bystander effects in vivo. When hamsters were injected with the α-particle emitters 239PuO2 or [230Pu]plutonium citrate, which concentrate in the liver, the induction of chromosome aberrations was independent of large changes in the local dose homogeneity when this was altered by injecting a range of particle sizes but maintaining a constant total dose to the liver77. A similar response was observed when the induction of liver tumours was observed78. Thus the authors suggested that the liver was responding to the total energy and total dose to the liver, not to the numbers of cells traversed by an α-particle or the local dose distribution79.
Relevance of bystander responses to therapy
From our understanding of the mechanisms that underpin bystander signalling and the growing evidence for their role in vivo, it is clearly of interest to consider the relevance of bystander responses to cancer therapy.
As defined earlier, the term bystander response is used in the field of gene therapy, in which the requirement to increase cell killing beyond cells that have taken up vectors expressing bioactive or chemotoxic agents is crucial to efficacy. understanding radiation-induced bystander responses may therefore highlight potential new therapeutic approaches that invoke mechanisms related to cell–cell communication of damage-sensing signals and allow amplification of cell killing effects. For example, recently it was shown that NO-dependent signalling is required in tumour cells to undergo radiation-induced bystander responses51. This observation coincided with efforts to use gene therapy approaches to introduce NOS2 into cells for therapeutic gain80 and also to use radiation-inducible promoters81 to drive NOS2 expression. Production of NO will affect larger numbers of cells than those originally transfected, increasing effectiveness. Combining gene therapy with targeted radionuclide therapy might therefore increase cell killing owing to bystander responses82. Clearly, more mechanistic and preclinical information is required, particularly under conditions relevant to radio-therapy, before this can be fully developed, but it does offer a rationale for the development of new approaches based on bystander mechanisms.
Factors modulating bystander responses
For the use of radiation therapy, we know of several key factors that modify response and determine overall efficacy of treatment. These include repair, cell cycle distribution, repopulation, reoxygenation and individual radiosensitivity1. Underpinning these factors are modulators of response, which include radiation quality, dose rate and fractionation schedule1. An important consideration is what role these would have on bystander responses, but in many cases information is limited or completely lacking. However, some clues are emerging; for example, several groups have shown evidence for some of the key DNA damage response and repair processes occurring in bystander cells83,84. Importantly, there may be differential DNA damage responses in direct and bystander cells that could be modulated in future therapies85. For example, in directly irradiated cells the kinases ataxia–telangiectasia mutated (ATM), DNA-dependent protein kinase (DNA-PK) and ataxia–telangiectasia and Rad3-related (ATR) have key roles such that inhibition of these DNA damage sensors increases radiosensitivity. In bystander cells, ATR is important, with ATM acting downstream. In contrast to directly irradiated cells, inhibition of ATR or ATM prevents the killing of bystander cells85.
For radiotherapy, fractionation of the dose delivered to a tumour has long been used to improve the differential effect of killing tumour cells relative to normal cells, as splitting the dose allows time for normal cells to repair damage between fractions1. A similar effect occurs when the dose rate is reduced1. However, almost all studies of bystander responses have used relatively high-dose-rate, single-fraction exposures. One study has looked at the effect of repeated addition of medium from irradiated cells to bystander cells and also the effect of repeated dose exposures to the cells producing bystander signals as a way of mimicking fractionated exposures using a bystander signal. The authors reported that fractionated bystander treatments removed the conventional dose sparing that is observed after fractionated radiations due to the gap between fractions allowing time for DNA repair86. Another study has recently reported differences after changing the dose rate, albeit over a limited range87.
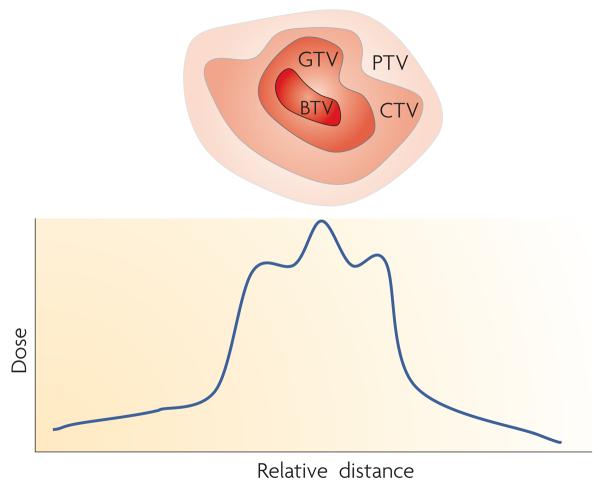
With the significant advances in delivery of external beam radiotherapy, in particular three-dimensional conformal radiotherapy (3DCRT), it is now possible to deliver radiation to tumours with high physical reproducibility88. 3DCRT first involves the identification of the gross tumour volume (FIG. 4), representing the visible tumour (usually identified clinically by cross-sectional imaging by a computed tomography scan, for example). By adding a margin to encompass microscopic tumour extension, the gross tumour volume becomes the clinical target volume. The planning target volume is then used to define the area that should be irradiated. The planning target volume is the clinical target volume plus a margin to accommodate for organ motion and deformation as well as errors in treatment set up. Radiotherapy treatment plans are assessed according to the homogeneity of dose to the planning target volume as well as by the dose to the organs at risk. These criteria are measured using dose–volume histograms. Considerable clinical data now exists for correlating dose–volume histogram criteria with radiation toxicity outcomes89. Currently, little consideration is given to the potential of bystander effects in the aetiology of toxicity.
Figure 4. Defining tumours for external beam targeting.
For external beam radiotherapy it is usual to define the site of the tumour in terms of a gross tumour volume (GTV), which is where the tumour is located and includes a region of subclinical disease that is partially infiltrated by the tumour. Together this gives the clinical target volume (CTV), which is the volume in which there is a malignancy. The planning target volume (PTV) is then used to define the area that needs to be irradiated. In the future, it may be possible to define regions within the tumour, such as areas of hypoxia, and define a biological target volume (BTV) that could receive additional dose. A simplistic representation of the dose profile across the region is given, showing a low dose area with the PTV and an additional high dose area within the GTV, covering the BTV.
Intensity-modulated radiation therapy (IMRT) is an evolution of 3DCRT in which modulation of the beam intensity in time and space permits shaping of the high-dose radiation volume to conform to complex tumours90. This technique results in the organs at risk being exposed to a lower dose. It could be hypothesized that a larger volume of irradiated tissue could have a higher risk of a significant bystander effect. optimal targeting of the tumour can lead to steep dose gradients in the region of the planning target volume. In the future it may also be possible to apply dose escalation to specific areas within a tumour — for example, those that contain hypoxic or radioresistant regions — which would lead to an improved therapeutic outcome but limit the dose to surrounding normal tissue. This involves the delineation of a biological target volume for delivering additional dose to a tumour91 (FIG. 4), which would generate significant dose gradients within the tumour. The benefits of the complex dose distributions made possible by the use of IMRT can only be fully realized by image-guided radiation therapy. This technology uses a variety of techniques to image target volumes while the patient is being treated on a linear accelerator. By reducing or eliminating set-up error, and ensuring the precision and reproducibility of radiation delivery, image-guided radiation therapy technology enables a reduction in the margins that are added to the clinical target volume to create the planning target volume92. This degree of precision is particularly important when in-field boost volumes such as biological target volume are being treated using IMRT.
Little is known about radiation response within dose gradients and whether bystander responses are involved. In recent elegant work93, Suchowerska and colleagues have started to address the effectiveness of dose gradients. They have measured survival in cells exposed to a 6 MV X-ray intensity-modulated beam, in a three compartment flask such that cells could be irradiated separately in the three sections or share the same medium. They exposed the flasks to a modulated dose defined by a wedge placed in the beam path. Cells that could communicate (that is, shared the same medium) gave poorer survival than predicted after low-dose exposure, but higher than expected survival after high dose exposure. This effect was abolished when communication was prevented, suggesting significant modulation of response in situations in which dose gradients are present. In further studies they compared the effects of a uniform field with those when 25% of a flask was exposed either as a single region or as three parallel stripes. Survival was dependent on a complex interplay between the fraction of shielded cells and the dose to the exposed areas, again indicative of bystander signalling having a significant effect94.
Other examples of localized dose delivery are proton and other heavy ion particle therapies that provide the ability to deliver high radiation doses to precise volumes with resultant high dose gradients, although nothing is known regarding the role of bystander responses in the clinical setting95. Studies in animal models have used microplanar X-ray microbeams as a way of differentially targeting brain tumours with high dose while sparing normal tissue96 and have shown significant evidence for bystander responses97.
The second issue related to the potential role of bystander responses is related to radiation protection and carcinogenesis. In most external beam therapies, multiple beams are delivered from different directions to maximize the dose to the tumour and minimize the exposure to normal tissues. Two consequences of this require discussion with respect to bystander responses. A consequence of the use of IMRT is that a greater volume of normal tissue is exposed to low-dose radiation than in conventional radiotherapy. This is largely a consequence of the use of multiple beams, as well as the increased time required to deliver the prescribed dose. Newer evolutions of modulated beam therapy technology that involve rotational delivery of radiation including Tomotherapy (Tomotherapy, Inc.), Rapid Arc (Varian medical Systems) and volumetric modulated-arc therapy (VMAT; Elekta) all result in larger irradiated volumes than conventional 3DCRT or finite field IMRT. It has been postulated that this large volume of low-dose exposure may lead in the longer term to a 2–3-fold increase in secondary cancer rates. It is clearly of relevance to determine whether these changes in the delivery of radiation have consequences for patient outcomes98.
Radionuclide-mediated responses
Another area in which bystander effects may have a significant role is with the use of therapeutic radionuclides. A range of studies have determined the role of bystander responses after treatments with various radionuclides both in vitro and in vivo. For experimental studies testing for radio-nuclide-induced bystander responses, it is an important challenge to ensure that no radioactivity is incorporated into cells that would otherwise be defined as bystander cells. This is especially crucial given the evidence from external radiation studies showing that bystander responses are essentially a low-dose phenomenon.
The earliest studies on radionuclide-induced bystander responses used 3H-labelled DNA produced by incubation of cells with [3H]thymidine. The mean energy of the β-rays is 5.67 keV and their mean range is 1 μm. Bishayee and colleagues compared the effectiveness of inactivation of radiolabelled cells in small multicellular spheroids typically of 1.6 mm diameter consisting of 4 × 106 V79 cells. They saw greater effectiveness (measured as loss of clonogenic survival) under conditions in which only 50% of the cells were labelled than was predicted from 100% labelled cells, which they concluded was due to a bystander response. They also tested for a role for GJIC using lindane and found evidence for direct cell–cell communication in this model99.
Bystander responses after radionuclide incorporation have also been reported in vivo. In a sophisticated protocol, human colon LS174T adenocarcinoma cells were pre-labelled with [125I]uridine and injected subcutaneously into nude mice with a mixture of non-labelled cells. Under these conditions with 1:1 and 1:5 ratios of labelled to unlabelled cells, significant tumour regression derived from the unlabelled cells was observed100. In further studies, they compared the effects of 125I-labelling with 123I-labelling strategies in the same in vivo tumour model. They reported an inhibitory bystander response for 125I-labelling, but a stimulatory bystander response was observed for 123I-labelling, which was confirmed from in vitro studies. The reasons for these differences are unclear, as both radionuclides produced short range auger electron cascades. Interestingly, however, there are significant (~100-fold) differences in dose rate owing to the differences in half-life (123I has a half-life of 13.3.hours and 125I of 60.5 days).
These discrepancies for different radionuclides in effectively the same biological model are indicative of the need to more carefully compare different radionuclide-mediated bystander responses in comparison with external beam exposure. In a recent defining study, Boyd and colleagues101 compared the effect of an external radiation-mediated bystander response with different radionuclide approaches. In particular, they compared three different halogenated analogues of m-iodobenzylguanidine (MIBG). MIBG is selectively taken up into cells expressing the noradrenaline transporter (NAT, also known as SLC6A2) gene. The authors compared the effectiveness of the β-emitter [131I]MIBG with the auger electron emitter [123I]MIBG and the α-emitter [211At]m-astatobenzylguanidine ([211At]MABG) in two tumour lines transfected with NAT. For external beam irradiation followed by medium transfer onto non-irradiated cells a significant bystander response measured as a loss of clonogenic survival was observed. As found for other studies with external radiation approaches, the degree of bystander response increased at low dose and then saturated at ~60–70% survival in the two cell lines. This was in contrast to the studies with radionuclides for which, although bystander responses were detected, no saturation was observed. For [131I]MIBG, a significant bystander response was detected that increased in proportion to the activity added to the directly exposed cells, leading to killing of 70–80% of the bystander cells. By contrast, treatment of cells with either [123I]MIBG or [211At]MABG led to an increased cell kill in recipient bystander cells up to a maximum of 35–70% but the effect decreased with increasing activity, leading to U-shaped response curves. These studies suggest there may be important linear energy transfer differences in the response of cells to bystander factors produced in response to radionuclide incorporation and that the types of bystander responses induced may be distinct from those observed after external radiation studies. One possibility is that the design of these studies may also be highlighting important dose rate dependencies of bystander responses that have to date not been extensively explored with external radiation approaches.
It is important to speculate on the consequences of the observation of bystander responses after radionuclide treatments for therapy. Significant advances are being made in the use of targeted radionuclides. These include, for example, the ability to target small metastatic regions that are not accessible with conventional external beam approaches and the development of useful biological targeting strategies to give tumour cell specificity102-104.
Earlier studies have predicted that the use of radionuclides that produce electrons that have relatively long ranges and interact with multiple cells would be beneficial owing to radiological crossfire. For example, studies in multicellular spheroids have shown that the effectiveness of [131I]MIBG is twice that observed in cell monolayer studies owing to significant crossfire from the long range of the β-rays105. If recent experimental studies are extrapolated into a tumour killing situation it becomes clear that a radiobiological bystander response as well as crossfire effects could be important in producing additional cell kill. Future therapies involving radionuclides need, a priori, to consider the effect of bystander responses in overall outcome. The suggestion that dose rate may be important needs to be further defined for both external beam and radionuclide exposures, as this may even affect our use and development of brachytherapy approaches. To date we have bystander information on a limited range of radionuclides despite the large range of potential candidates for therapy106. We also do not know the consequences of low-dose exposure to radionuclides under conditions in which bystander responses may occur. If the robust bystander responses reported in vitro translate in vivo, this could affect the use of radionuclides for therapeutic and imaging approaches in the longer term. However, more study of the role of cell–cell communication in a range of biological contexts is required for this to be fully elucidated.
Implications for current and future therapies
For the future, further insight into the mechanisms underpinning bystander signalling is required if potential targets for therapy are going to be developed. Alongside this, a crucial appreciation of the relevance of bystander responses in radiation-induced carcinogenesis is required.
The current understanding of the role of radiation-induced bystander responses has to be seen in the context of our knowledge of how cells communicate and their integration in tissue level responses to localized and systemic therapies. For radiation-based therapies this means that molecular pathways and targets outside directly exposed fields could contribute to a therapeutic response. Future therapies will need to be optimized for tissue and tumour level responses to include differential effects that are mediated by intra- and inter-tissue signals if these are to affect treatment outcome. For targeted radionuclide approaches the modulation of bystander responses probably holds out the best opportunities in the near term (FIG. 5). This is not least because an analogy can be strongly made to the relevance of bystander responses in gene therapy. If the approaches taken in the gene therapy area are to be extrapolated to radionuclide therapies, further in vivo studies are needed. This requires not only further insight into molecular mechanisms and the highlighting of specific targets but also a greater appreciation of the interrelationships between specific activity, dose rate and radiological crossfire versus bystander responses. The observation of bystander responses after exposure to chemotherapy agents may also assist in the development of approaches for maximising radionuclide therapies and also enhancing external beam-mediated responses by maximizing their contribution to tumour cell kill.
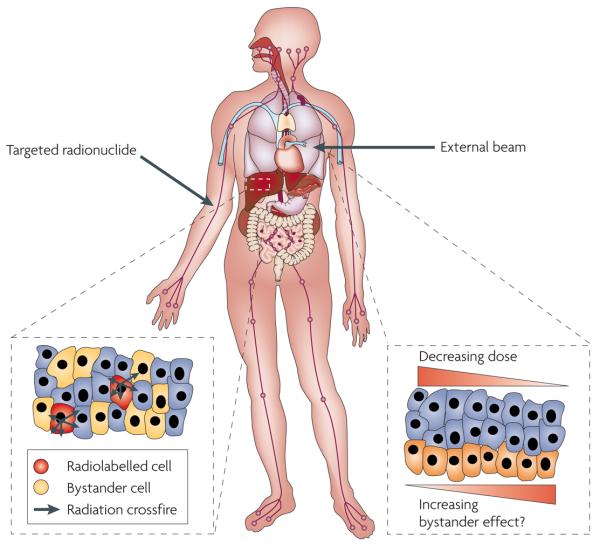
Figure 5. Treating cancer with radiation.
The figure shows the two potential routes by which bystander responses may affect clinical therapies. For external beam therapies (such as intensity-modulated radiotherapy), dose gradient-dependent responses may influence the effect. Tumour heterogeneity may also lead to non-linear responses within the treatment field and to longer-range, abscopal or systemic effects. For radionuclide approaches (such as those tagged to monoclonal antibodies), the signals from a few labelled cells may be amplified by bystander signals within tumours and may also have long-range, abscopal or systemic effects.
For external beam therapies, the challenge is to define the physical ranges of bystander responses at the cellular, tissue and whole body levels and relate these to specific mechanisms. Development of strategies to maximize bystander responses in tumours and to minimize their expression in normal tissues will require further mechanistic information of potential molecular targets so that informed choices can be made. With the continuing developments in the delivery of radiotherapy and combined imaging, the possibility of dose-painting approaches urgently requires information on bystander signalling under conditions in which steep dose gradients exist, in particular IMRT, Tomotherapy and heavy ion particle therapy, to more carefully define their potential effect on both radiation effectiveness and radiation risk (FIG. 5). A key question is the role that intratumoural factors that may affect clinical response — such as hypoxia, proliferation and intrinsic radiosensitivity — affect intercellular and longer-range signalling mechanisms. As radiation is predominantly given to patients as part of multimodality therapies, the role of systemic responses resulting from these treatments and their effect on long-range effects is completely unknown.
The observation that bystander responses predominate after low-dose exposure implies that additional effects would be predicted. There has been considerable debate as to their relevance to radiation risk at low doses with some authors suggesting that they affect the current use of the linear no-threshold hypothesis for risk estimation107. This has led to the proposal that low-dose exposures may be considerably more active than previously thought and could, for example, affect secondary cancer rates after external beam therapies98. However, given the paucity of in vivo data for bystander responses in exposed individuals it is premature to make fundamental predictions, although recent data on carcinogenesis from animal models support an important role in this process. Extensive study is now required in vivo to quantify responses under relevant dose exposure scenarios, not just for environmental and occupational exposures but for clinically relevant dose and dose distributions at the tissue and whole-body level.
Acknowledgements
The authors acknowledge the extensive literature they were not able to cite owing to space limits. They are grateful to Cancer Research UK grant number C1513/A7047, the European Union NOTE project (FI6R 036465) and the US National Institutes of Health (5P01CA095227-02) for funding their work.
Glossary
- Photodynamic therapy
A therapeutic approach in which photosensitive chemicals are taken up into tumours and then activated by laser or other light source to produce damaging free radicals
- Ganciclovir
A synthetic analogue of 2′-deoxyguanosine that becomes phosphorylated when taken up by cells and is incorporated into DNA by DNA polymerase and leads to chain termination of the replicating DNA strands.
- Clastogenic factor
A species that can break chromosomes.
- Sister chromatid exchanges
Exchange of chromosomal material between the chromatids of a chromosome.
- Lindane
A neurotoxin that can inhibit gap-junctional intercellular communication.
- Reconstruct models
In vitro models in which individual cell types can be co-cultured and used to form three-dimensional representations of the original tissue.
- Bilateral pneumonitis
Inflammation of lung tissue in both lungs.
- Radionuclide therapy
The use of radioisotopes tagged to molecules or proteins for treating cancer
- Dose rate
The amount of dose delivered per unit time.
- Intensity-modulated radiation therapy
IMRT. An advanced mode of radiotherapy that uses multiple modulated beams in which the intensity is varied to allow maximal conformation of the beam delivery to the tumour in three dimensions.
- Linear accelerator
A device for the acceleration of subatomic particles that can produce electron beams for radiotherapy.
- Heavy ion particle therapy
The use of accelerated beams of high-atomic-mass elements (for example, carbon) for therapy.
- Microplanar X-ray microbeams
Parallel beams of X-rays only a few micrometres across that are used for localized irradiation.
- Auger electron cascades
Decay of radioactive isotopes by K-shell electron capture leads to the Auger effect, resulting in the loss of several orbital electrons.
- Linear no-threshold hypothesis
A model used for radiation protection that aims to describe the relationship between radiation dose and risk, a linear relationship that has no dose threshold for increased risk.
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
NAT |Ptch1
UniProtKB: http://www.uniprot.org
ATM | ATR | COX2 | GJC1 | IL-1α, IL-1β | IL-6 | IL-8 | NOS2 | p53 | TGFβ1 | TNFα
FURTHER INFORMATION
Kevin M. Prise's homepage: http://www.qub.ac.uk/research-centres/CentreforCancerResearchCellBiology/
References
- 1.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Lippincott William & Wilkins; Philadelphia: 2006. [Google Scholar]
- 2.Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol. Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Jackson SP. Detecting, signalling and repairing double-strand breaks. Biochem. Soc. Trans. 2001;29:655–661. doi: 10.1042/0300-5127:0290655. [DOI] [PubMed] [Google Scholar]
- 4.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat. Res. 2003;159:567–580. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. Good starting point for a review of cellular data on radiation-induced bystander responses. [DOI] [PubMed] [Google Scholar]
- 5.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat. Res. 2003;159:581–596. doi: 10.1667/0033-7587(2003)159[0581:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Hamada N, Matsumoto H, Hara T, Kobayashi Y. Intercellular and intracellular signaling pathways mediating ionizing radiation-induced bystander effects. J. Radiat. Res. (Tokyo) 2007;48:87–95. doi: 10.1269/jrr.06084. [DOI] [PubMed] [Google Scholar]
- 7.Mothersill C, Seymour CB. Radiation-induced bystander effects — implications for cancer. Nature Rev. Cancer. 2004;4:158–164. doi: 10.1038/nrc1277. [DOI] [PubMed] [Google Scholar]
- 8.Bagdonas S, Dahle J, Kaalhus O, Moan J. Cooperative inactivation of cells in microcolonies treated with UVA radiation. Radiat. Res. 1999;152:174–179. [PubMed] [Google Scholar]
- 9.Dahle J, et al. The bystander effect in photodynamic inactivation of cells. Biochim. Biophys. Acta. 2000;1475:273–280. doi: 10.1016/s0304-4165(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 10.DeVeaux LC, Durtschi LS, Case JG, Wells DP. Bystander effects in unicellular organisms. Mutat. Res. 2006;597:78–86. doi: 10.1016/j.mrfmmm.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 11.Alexandre J, Hu Y, Lu W, Pelicano H, Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67:3512–3517. doi: 10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- 12.Ramesh R, Marrogi AJ, Munshi A, Abboud CN, Freeman SM. In vivo analysis of the ‘bystander effect’: a cytokine cascade. Exp. Hematol. 1996;24:829–838. [PubMed] [Google Scholar]
- 13.Mesnil M, Piccoli C, Tiraby G, Willecke K, Yamasaki H. Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc. Natl Acad. Sci. USA. 1996;93:1831–1835. doi: 10.1073/pnas.93.5.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMasters RA, et al. Lack of bystander killing in herpes simplex virus thymidine kinase-transduced colon cell lines due to deficient connexin43 gap junction formation. Hum. Gene Ther. 1998;9:2253–2261. doi: 10.1089/hum.1998.9.15-2253. [DOI] [PubMed] [Google Scholar]
- 15.Denning C, Pitts JD. Bystander effects of different enzyme–prodrug systems for cancer gene therapy depend on different pathways for intercellular transfer of toxic metabolites, a factor that will govern clinical choice of appropriate regimes. Hum. Gene Ther. 1997;8:1825–1835. doi: 10.1089/hum.1997.8.15-1825. [DOI] [PubMed] [Google Scholar]
- 16.Kaminski JM, et al. The controversial abscopal effect. Cancer Treat. Rev. 2005;31:159–172. doi: 10.1016/j.ctrv.2005.03.004. A good review of clinical data supporting long-range or abscopal radiation-induced bystander responses. [DOI] [PubMed] [Google Scholar]
- 17.Nagasawa H, Little JB. induction of sister chromatid exchanges by extremely low doses of α-particles. Cancer Res. 1992;52:6394–6396. A defining paper of radiation-induced bystander responses in cellular models. [PubMed] [Google Scholar]
- 18.Belyakov OV, Malcolmson AM, Folkard M, Prise KM, Michael BD. Direct evidence for a bystander effect of ionizing radiation in primary human fibroblasts. Br. J. Cancer. 2001;84:674–679. doi: 10.1054/bjoc.2000.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schettino G, Folkard M, Michael BD, Prise KM. Low-dose binary behavior of bystander cell killing after microbeam irradiation of a single cell with focused CK X rays. Radiat. Res. 2005;163:332–336. doi: 10.1667/rr3319. [DOI] [PubMed] [Google Scholar]
- 20.Shareef MM, et al. Role of tumor necrosis factor-α and TRAIL in high-dose radiation-induced bystander signaling in lung adenocarcinoma. Cancer Res. 2007;67:11811–11820. doi: 10.1158/0008-5472.CAN-07-0722. [DOI] [PubMed] [Google Scholar]
- 21.Schettino G, et al. Low-dose studies of bystander cell killing with targeted soft X rays. Radiat. Res. 2003;160:505–511. doi: 10.1667/rr3060. [DOI] [PubMed] [Google Scholar]
- 22.Kadhim MA, et al. Bystander-mediated genomic instability in human lymphocytes after single α-particle irradiation. Radiat. Res. 2004;161:110–111. doi: 10.1667/0033-7587(2001)155[0122:ltgiih]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Prise KM, Belyakov OV, Folkard M, Michael BD. Studies of bystander effects in human fibroblasts using a charged particle microbeam. Int. J. Radiat. Biol. 1998;74:793–798. doi: 10.1080/095530098141087. The first direct evidence of radiation-induced bystander effect using a microbeam to irradiate individual cells. [DOI] [PubMed] [Google Scholar]
- 24.Gaugler MH, et al. Intestinal epithelial cell dysfunction is mediated by an endothelial-specific radiation-induced bystander effect. Radiat. Res. 2007;167:185–193. doi: 10.1667/rr0702.1. [DOI] [PubMed] [Google Scholar]
- 25.Shao C, Furusawa Y, Aoki M, Matsumoto H, Ando K. Nitric oxide-mediated bystander effect induced by heavy-ions in human salivary gland tumour cells. Int. J. Radiat. Biol. 2002;78:837–844. doi: 10.1080/09553000210149786. [DOI] [PubMed] [Google Scholar]
- 26.Coates PJ, Lorimore SA, Wright EG. Damaging and protective cell signalling in the untargeted effects of ionizing radiation. Mutat. Res. 2004;568:5–20. doi: 10.1016/j.mrfmmm.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Asaad N, Held KD. Medium-mediated intercellular communication is involved in bystander responses of X-ray-irradiated normal human fibroblasts. Oncogene. 2005;24:2096–2103. doi: 10.1038/sj.onc.1208439. [DOI] [PubMed] [Google Scholar]
- 28.Huo L, Nagasawa H, Little JB. HPRT mutants induced in bystander cells by very low fluences of alpha particles result primarily from point mutations. Radiat. Res. 2001;156:521–525. doi: 10.1667/0033-7587(2001)156[0521:hmiibc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Sawant SG, Randers-Pehrson G, Geard CR, Brenner DJ, Hall EJ. The bystander effect in radiation oncogenesis: I. Transformation in C3H 10T1/2 cells in vitro can be initiated in the unirradiated neighbors of irradiated cells. Radiat. Res. 2001;155:397–401. doi: 10.1667/0033-7587(2001)155[0397:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Mothersill C, Seymour C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of irradiated cells. Int. J. Radiat. Biol. 1997;71:421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- 31.Belyakov OV, Folkard M, Mothersill C, Prise KM, Michael BD. Bystander-induced differentiation: A major response to targeted irradiation of a urothelial explant model. Mutat. Res. 2006;597:43–49. doi: 10.1016/j.mrfmmm.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Lyng FM, Seymour CB, Mothersill C. Initiation of apoptosis in cells exposed to medium from the progeny of irradiated cells: a possible mechanism for bystander-induced genomic instability? Radiat. Res. 2002;157:365–370. doi: 10.1667/0033-7587(2002)157[0365:ioaice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Iyer R, Lehnert BE. Low dose, low-LET ionizing radiation-induced radioadaptation and associated early responses in unirradiated cells. Mutat. Res. 2002;503:1–9. doi: 10.1016/s0027-5107(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 34.Herve JC, Bourmeyster N, Sarrouilhe D, Duffy HS. Gap junctional complexes: from partners to functions. Prog. Biophys. Mol. Biol. 2007;94:29–65. doi: 10.1016/j.pbiomolbio.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Oyamada M, Oyamada Y, Takamatsu T. Regulation of connexin expression. Biochim. Biophys. Acta. 2005;1719:6–23. doi: 10.1016/j.bbamem.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Azzam EI, de Toledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of α particles. Radiat. Res. 1998;150:497–504. [PubMed] [Google Scholar]
- 37.Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from α-particle irradiated to nonirradiated cells. Proc. Natl Acad. Sci. USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. Direct evidence for a role of GJIC in radiation-induced bystander signalling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trosko JE, Ruch RJ. Cell-cell communication in carcinogenesis. Front. Biosci. 1998:D208–D236. doi: 10.2741/a275. [DOI] [PubMed] [Google Scholar]
- 39.Emerit I. Reactive oxygen species, chromosome mutation, and cancer: possible role of clastogenic factors in carcinogenesis. Free Radic. Biol. Med. 1994;16:99–109. doi: 10.1016/0891-5849(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 40.Emerit I, Khan SH, Esterbauer H. Hydroxynonenal, a component of clastogenic factors? Free Radic. Biol. Med. 1991;10:371–377. doi: 10.1016/0891-5849(91)90045-5. [DOI] [PubMed] [Google Scholar]
- 41.Auclair C, Gouyette A, Levy A, Emerit I. Clastogenic inosine nucleotide as components of the chromosome breakage factor in scleroderma patients. Arch. Biochem. Biophys. 1990;278:238–244. doi: 10.1016/0003-9861(90)90253-u. [DOI] [PubMed] [Google Scholar]
- 42.Emerit I, et al. Superoxide-mediated clastogenesis and anticlastogenic effects of exogenous superoxide dismutase. Proc. Natl Acad. Sci. USA. 1996;93:12799–12804. doi: 10.1073/pnas.93.23.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy JB, Liu JH, Sturm E. Studies on X-ray effects: IX. The action of serum from X-rayed animals on lymphoid cells in vitro. J. Exp. Med. 1922;35:373–384. doi: 10.1084/jem.35.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hollowell JG, Littlefield G. Chromosome damage induced by plasma of X-rayed patients: an indirect effect of X-ray. Proc. Soc. Exp. Biol. Med. 1968;129:240–244. doi: 10.3181/00379727-129-33295. [DOI] [PubMed] [Google Scholar]
- 45.Emerit I, et al. Transferable clastogenic activity in plasma from persons exposed as salvage personnel of the Chernobyl reactor. J. Cancer Res. Clin. Oncol. 1994;120:558–561. doi: 10.1007/BF01221035. [DOI] [PubMed] [Google Scholar]
- 46.Chou CH, et al. Radiation-induced hepatitis B virus reactivation in liver mediated by the bystander effect from irradiated endothelial cells. Clin. Cancer Res. 2007;13:851–857. doi: 10.1158/1078-0432.CCR-06-2459. [DOI] [PubMed] [Google Scholar]
- 47.Narayanan PK, LaRue KE, Goodwin EH, Lehnert BE. α particles induce the production of interleukin-8 by human cells. Radiat. Res. 1999;152:57–63. [PubMed] [Google Scholar]
- 48.Iyer R, Lehnert BE. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000;60:1290–1298. [PubMed] [Google Scholar]
- 49.Zhou H, et al. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc. Natl Acad. Sci. USA. 2005;102:14641–14646. doi: 10.1073/pnas.0505473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lehnert BE, Goodwin EH. Extracellular factor(s) following exposure to α-particles can cause sister chromatid exchanges in normal human cells. Cancer Res. 1997;57:2164–2171. [PubMed] [Google Scholar]
- 51.Shao C, Stewart V, Folkard M, Michael BD, Prise KM. Nitric oxide-mediated signaling in the bystander response of individually targeted glioma cells. Cancer Res. 2003;63:8437–8442. [PubMed] [Google Scholar]
- 52.Coates PJ, Robinson JI, Lorimore SA, Wright EG. Ongoing activation of p53 pathway responses is a long-term consequence of radiation exposure in vivo and associates with altered macrophage activities. J. Pathol. 2008;214:610–616. doi: 10.1002/path.2321. [DOI] [PubMed] [Google Scholar]
- 53.Shao C, Folkard M, Michael BD, Prise KM. Targeted cytoplasmic irradiation induces bystander responses. Proc. Natl Acad. Sci. USA. 2004;101:13495–13500. doi: 10.1073/pnas.0404930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu LJ, et al. Targeted cytoplasmic irradiation with α particles induces mutations in mammalian cells. Proc. Natl Acad. Sci. USA. 1999;96:4959–4964. doi: 10.1073/pnas.96.9.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nugent SM, et al. Increased mitochondrial mass in cells with functionally compromised mitochondria after exposure to both direct γ radiation and bystander factors. Radiat. Res. 2007;168:134–142. doi: 10.1667/RR0769.1. [DOI] [PubMed] [Google Scholar]
- 56.Tartier L, Gilchrist S, Burdak-Rothkamm S, Folkard M, Prise KM. Cytoplasmic irradiation induces mitochondrial-dependent 53BP1 protein relocalization in irradiated and bystander cells. Cancer Res. 2007;67:5872–9. doi: 10.1158/0008-5472.CAN-07-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mothersill C. Development of primary tissue culture techniques for use in radiobiology. Radiat. Res. 1998;150:S121–125. [PubMed] [Google Scholar]
- 58.Mothersill C, et al. Individual variation in the production of a ‘bystander signal’ following irradiation of primary cultures of normal human urothelium. Carcinogenesis. 2001;22:1465–1471. doi: 10.1093/carcin/22.9.1465. [DOI] [PubMed] [Google Scholar]
- 59.Mothersill C, et al. Genetic factors influencing bystander signaling in murine bladder epithelium after low-dose irradiation in vivo. Radiat. Res. 2005;163:391–399. doi: 10.1667/rr3320. [DOI] [PubMed] [Google Scholar]
- 60.Belyakov OV, Folkard M, Mothersill C, Prise KM, Michael BD. A proliferation-dependent bystander effect in primary porcine and human urothelial explants in response to targeted irradiation. Br. J. Cancer. 2003;88:767–774. doi: 10.1038/sj.bjc.6600804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belyakov OV, et al. Biological effects in unirradiated human tissue induced by radiation damage up to 1 mm away. Proc. Natl Acad. Sci. USA. 2005;102:14203–14208. doi: 10.1073/pnas.0505020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sedelnikova OA, et al. DNA double-strand breaks form in bystander cells after microbeam irradiation of three-dimensional human tissue models. Cancer Res. 2007;67:4295–4302. doi: 10.1158/0008-5472.CAN-06-4442. [DOI] [PubMed] [Google Scholar]
- 63.Travis EL. Semin. Radiat. Oncol. 2001;11:184–196. doi: 10.1053/srao.2001.25243. [DOI] [PubMed] [Google Scholar]
- 64.Khan MA, Hill RP, Van Dyk J. Partial volume rat lung irradiation: an evaluation of early DNA damage. Int. J. Radiat. Oncol. Biol. Phys. 1998;40:467–476. doi: 10.1016/s0360-3016(97)00736-0. [DOI] [PubMed] [Google Scholar]
- 65.Khan MA, Van Dyk J, Yeung IW, Hill RP. Partial volume rat lung irradiation; assessment of early DNA damage in different lung regions and effect of radical scavengers. Radiother. Oncol. 2003;66:95–102. doi: 10.1016/s0167-8140(02)00325-0. [DOI] [PubMed] [Google Scholar]
- 66.Calveley VL, Khan MA, Yeung IW, Vandyk J, Hill RP. Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int. J. Radiat. Biol. 2005;81:887–899. doi: 10.1080/09553000600568002. [DOI] [PubMed] [Google Scholar]
- 67.De Ridder M, et al. IFN-γ+ CD8+ T lymphocytes: possible link between immune and radiation responses in tumor-relevant hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 2008;71:647–651. doi: 10.1016/j.ijrobp.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 68.Moiseenko VV, Battista JJ, Hill RP, Travis EL, Van Dyk J. In-field and out-of-field effects in partial volume lung irradiation in rodents: possible correlation between early DNA damage and functional endpoints. Int. J. Radiat. Oncol. Biol. Phys. 2000;48:1539–1548. doi: 10.1016/s0360-3016(00)00802-6. [DOI] [PubMed] [Google Scholar]
- 69.Morgan GW, Breit SN. Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int. J. Radiat. Oncol. Biol. Phys. 1995;31:361–369. doi: 10.1016/0360-3016(94)00477-3. [DOI] [PubMed] [Google Scholar]
- 70.Coates PJ, Rundle JK, Lorimore SA, Wright EG. Indirect macrophage responses to ionizing radiation: implications for genotype-dependent bystander signaling. Cancer Res. 2008;68:450–456. doi: 10.1158/0008-5472.CAN-07-3050. A paper highlighting the important role of macrophage responses in radiation-induced bystander signalling. [DOI] [PubMed] [Google Scholar]
- 71.Koturbash I, et al. Irradiation induces DNA damage and modulates epigenetic effectors in distant bystander tissue in vivo. Oncogene. 2006;25:4267–4275. doi: 10.1038/sj.onc.1209467. [DOI] [PubMed] [Google Scholar]
- 72.Koturbash I, et al. Role of epigenetic effectors in maintenance of the long-term persistent bystander effect in spleen in vivo. Carcinogenesis. 2007;28:1831–1838. doi: 10.1093/carcin/bgm053. [DOI] [PubMed] [Google Scholar]
- 73.Koturbash I, et al. Radiation-induced bystander effects in vivo are sex specific. Mutat. Res. 2008;642:28–36. doi: 10.1016/j.mrfmmm.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 74.Koturbash I, et al. Sex-specific microRNAome deregulation in the shielded bystander spleen of cranially exposed mice. Cell Cycle. 2008;7:1658–1667. doi: 10.4161/cc.7.11.5981. [DOI] [PubMed] [Google Scholar]
- 75.Mancuso M, et al. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc. Natl Acad. Sci. USA. 2008;105:12445–12450. doi: 10.1073/pnas.0804186105. Studies in vivo showing evidence of long-range bystander responses leading to carcinogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Camphausen K, et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 2003;63:1990–1993. [PubMed] [Google Scholar]
- 77.Brooks AL, Retherford JC, McClellan RO. Effect of 239PuO2 particle number and size on the frequency and distribution of chromosome aberrations in the liver of the Chinese hamster. Radiat. Res. 1974;59:693–709. [PubMed] [Google Scholar]
- 78.Brooks AL, et al. The induction of liver tumors by 239Pu citrate or 239PuO2 particles in the Chinese hamster. Radiat. Res. 1983;96:135–151. [PubMed] [Google Scholar]
- 79.Barcellos-Hoff MH, Brooks AL. Extracellular signaling through the microenvironment: a hypothesis relating carcinogenesis, bystander effects, and genomic instability. Radiat. Res. 2001;156:618–627. doi: 10.1667/0033-7587(2001)156[0618:esttma]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 80.Wang Z, et al. Adenoviral gene transfer of the human inducible nitric oxide synthase gene enhances the radiation response of human colorectal cancer associated with alterations in tumor vascularity. Cancer Res. 2004;64:1386–1395. doi: 10.1158/0008-5472.can-03-1307. [DOI] [PubMed] [Google Scholar]
- 81.Worthington J, et al. Use of the radiation-inducible WAF1 promoter to drive iNOS gene therapy as a novel anti-cancer treatment. J. Gene Med. 2004;6:673–680. doi: 10.1002/jgm.567. [DOI] [PubMed] [Google Scholar]
- 82.Boyd M, et al. An efficient targeted radiotherapy/gene therapy strategy utilising human telomerase promoters and radioastatine and harnessing radiation-mediated bystander effects. J. Gene Med. 2004;6:937–947. doi: 10.1002/jgm.578. [DOI] [PubMed] [Google Scholar]
- 83.Burdak-Rothkamm S, Short SC, Folkard M, Rothkamm K, Prise KM. ATR-dependent radiation-induced γH2AX foci in bystander primary human astrocytes and glioma cells. Oncogene. 2007;26:993–1002. doi: 10.1038/sj.onc.1209863. [DOI] [PubMed] [Google Scholar]
- 84.Little JB, Nagasawa H, Li GC, Chen DJ. Involvement of the nonhomologous end joining DNA repair pathway in the bystander effect for chromosomal aberrations. Radiat. Res. 2003;159:262–267. doi: 10.1667/0033-7587(2003)159[0262:iotnej]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 85.Burdak-Rothkamm S, Rothkamm K, Prise KM. ATM acts downstream of ATR in the DNA damage response signalling of bystander cells. Cancer Res. 2008;68:7059–7065. doi: 10.1158/0008-5472.CAN-08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mothersill C, Seymour CB. Bystander and delayed effects after fractionated radiation exposure. Radiat. Res. 2002;158:626–633. doi: 10.1667/0033-7587(2002)158[0626:badeaf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 87.Gow MD, Seymour CB, Byun SH, Mothersill CE. Effect of dose rate on the radiation-induced bystander response. Phys. Med. Biol. 2008;53:119–132. doi: 10.1088/0031-9155/53/1/008. [DOI] [PubMed] [Google Scholar]
- 88.Fenwick JD, Tome WA, Soisson ET, Mehta MP, Rock Mackie T. Tomotherapy and other innovative IMRT delivery systems. Semin. Radiat. Oncol. 2006;16:199–208. doi: 10.1016/j.semradonc.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 89.Milano MT, Constine LS, Okunieff P. Normal tissue tolerance dose metrics for radiation therapy of major organs. Semin. Radiat. Oncol. 2007;17:131–140. doi: 10.1016/j.semradonc.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 90.Veldeman L, et al. Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet Oncol. 2008;9:367–375. doi: 10.1016/S1470-2045(08)70098-6. [DOI] [PubMed] [Google Scholar]
- 91.Ling CC, et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int. J. Radiat. Oncol. Biol. Phys. 2000;47:551–560. doi: 10.1016/s0360-3016(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 92.Verellen D, et al. Innovations in image-guided radiotherapy. Nature Rev. Cancer. 2007;7:949–960. doi: 10.1038/nrc2288. [DOI] [PubMed] [Google Scholar]
- 93.Suchowerska N, Ebert MA, Zhang M, Jackson M. In vitro response of tumour cells to nonuniform irradiation. Phys. Med. Biol. 2005;50:3041–3051. doi: 10.1088/0031-9155/50/13/005. [DOI] [PubMed] [Google Scholar]
- 94.Claridge Mackonis E, et al. Cellular response to modulated radiation fields. Phys. Med. Biol. 2007;52:5469–5482. doi: 10.1088/0031-9155/52/18/001. A paper discussing the role of bystander responses to modulated beams used in IMRT. [DOI] [PubMed] [Google Scholar]
- 95.Palm A, Johansson KA. A review of the impact of photon and proton external beam radiotherapy treatment modalities on the dose distribution in field and out-of-field; implications for the long-term morbidity of cancer survivors. Acta Oncol. 2007;46:462–473. doi: 10.1080/02841860701218626. [DOI] [PubMed] [Google Scholar]
- 96.Dilmanian FA, et al. Interlaced x-ray microplanar beams: a radiosurgery approach with clinical potential. Proc. Natl Acad. Sci. USA. 2006;103:9709–9714. doi: 10.1073/pnas.0603567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dilmanian FA, et al. Tissue-sparing effect of x-ray microplanar beams particularly in the CNS: is a bystander effect involved? Exp. Hematol. 2007;35:69–77. doi: 10.1016/j.exphem.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 98.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int. J. Radiat. Oncol. Biol. Phys. 2006;65:1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 99.Bishayee A, Hill HZ, Stein D, Rao DV, Howell RW. Free radical-initiated and gap junction-mediated bystander effect due to nonuniform distribution of incorporated radioactivity in a three-dimensional tissue culture model. Radiat. Res. 2000;155:1–10. doi: 10.1667/0033-7587(2001)155[0335:friagj]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xue LY, Butler NJ, Makrigiorgos GM, Adelstein SJ, Kassis AI. Bystander effect produced by radiolabeled tumor cells in vivo. Proc. Natl Acad. Sci. USA. 2002;99:13765–13770. doi: 10.1073/pnas.182209699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boyd M, et al. Radiation-induced biologic bystander effect elicited in vitro by targeted radiopharmaceuticals labeled with alpha-, beta-, and auger electron-emitting radionuclides. J. Nucl. Med. 2006;47:1007–1015. Comparison of effectiveness of different radionuclides at inducing bystander responses in comparison with external beam therapy. [PubMed] [Google Scholar]
- 102.McCready VR, O'Sullivan JM. Future directions for unsealed source radionuclide therapy for bone metastases. Eur. J. Nucl. Med. Mol. Imaging. 2002;29:1271–1275. doi: 10.1007/s00259-002-0914-2. [DOI] [PubMed] [Google Scholar]
- 103.Dearling JL, Pedley RB. Technological advances in radioimmunotherapy. Clin. Oncol. (R. Coll. Radiol.) 2007;19:457–469. doi: 10.1016/j.clon.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 104.DeNardo SJ, Denardo GL. Targeted radionuclide therapy for solid tumors: an overview. Int. J. Radiat. Oncol. Biol. Phys. 2006;66:S89–S95. doi: 10.1016/j.ijrobp.2006.03.066. [DOI] [PubMed] [Google Scholar]
- 105.Boyd M, Cunningham SH, Brown MM, Mairs RJ, Wheldon TE. Noradrenaline transporter gene transfer for radiation cell kill by 131I meta-iodobenzylguanidine. Gene Ther. 1999;6:1147–52. doi: 10.1038/sj.gt.3300905. [DOI] [PubMed] [Google Scholar]
- 106.Boswell CA, Brechbiel MW. Development of radioimmunotherapeutic and diagnostic antibodies: an inside-out view. Nucl. Med. Biol. 2007;34:757–778. doi: 10.1016/j.nucmedbio.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brenner DJ, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc. Natl Acad. Sci. USA. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]